Abstract
It is of adaptive value for a plant to prepare its defenses when a threat is detected, and certain plant volatiles associated with insect damage, such as cis-jasmone (CJ), are known to switch-on defense metabolism. We used aphid and aphid parasitoid responses to Arabidopsis thaliana as a model system for studying gene expression and defense chemistry and its impact at different trophic levels. Differential responses to volatiles of induced Arabidopsis occurred for specialist and generalist insects: the generalist aphid, Myzus persicae, was repelled, whereas the specialist, Lipaphis erysimi, was attracted; the generalist aphid parasitoid Aphidius ervi was attracted, but the specialist parasitoid Diaeretiella rapae was not affected. A. ervi also spent longer foraging on induced plants than on untreated ones. Transcriptomic analyses of CJ-induced Arabidopsis plants revealed that a limited number of genes, including a gene for a cytochrome P450, CYP81D11, were strongly up-regulated in the treated plants. We examined transgenic Arabidopsis lines constitutively overexpressing this gene in bioassays and found insect responses similar to those obtained for wild-type plants induced with CJ, indicating the importance of this gene in the CJ-activated defense response. Genes involved in glucosinolate biosynthesis and catabolism are unaffected by CJ and, because these genes relate to interactions with herbivores and parasitoids specific to this family of plants (Brassicaceae), this finding may explain the differences in behavioral response of specialist and generalist insects.
Keywords: induced defense, plant volatile, plant–insect interaction, stress response
Induced defense occurs when a plant becomes more resistant to insect pests or pathogens after a signal causes a change in its metabolism (1, 2). Such signals are known as “elicitors” or “plant activators” and are produced naturally by attacking organisms (3, 4). Elicitors of plant defense typically originate from the attacking insect or pathogen itself; however, plants can also detect signals that indicate that a neighboring plant is being attacked (5), and these plant volatile signals can also induce defense. There is evidence that plants use volatile compounds for within-plant signaling, rather than relying solely on transport in the vascular system (6). Genes associated with the production of defense metabolites are up-regulated in induced plants. In addition to the switching-on of direct defense traits against herbivores, indirect defense is activated in tritrophic interactions (7), whereby the plants become more attractive to natural enemies of the phytophagous insects (8–10), which has been shown to confer fitness advantages to the plant (11). Synthetic chemicals can be used to induce defense when applied artificially to the plant, and we have found that certain plant-produced volatiles, such as cis-jasmone (CJ), that are emitted in larger quantities after insect damage, can activate plant defense against phytophagous insects (2, 12, 13). The ecological implication of this response is that plants detect volatiles from neighboring plants that have been exposed to insect attack and up-regulate their defense systems accordingly, as suggested by Karban et al. (5).
CJ is released naturally from insect-damaged plants. Cotton leaves damaged by Spodoptera exigua larvae emit CJ (14), and it is systemically released from undamaged leaves (15). Cotton buds damaged by Helicoverpa zea larvae (16) emit CJ, and it is also emitted from Nicotiana in response to oral secretions from Manduca sexta larvae (17) and by maize plants exposed to oral secretions of Spodoptera littoralis (18). Recently, our understanding of the biosynthetic pathway that leads to CJ has been improved (19) by elucidation of a novel pathway from 12-oxo-phytodienoic acid via isomerization to iso-12-oxophytodienoic acid, which then undergoes three cycles of β-oxidation and decarboxylation to yield CJ.
CJ is structurally related to jasmonic acid and methyl jasmonate, which are well known to activate plant defense (20, 21). However, transcriptomic analyses of gene expression in Arabidopsis have shown that a unique, and more limited, set of genes is up-regulated by CJ treatment compared with methyl jasmonate treatment (refs. 12 and 22 and M.C.M., P. Verrier, J.A.P., and J.A.N., unpublished data). CJ is well suited for use as an artificial inducing agent. Induction with CJ offers the opportunity to activate defense based on volatile chemical signals without unduly influencing other important plant physiological processes that could compromise the investigation. Our earlier work on CJ activation of crop plants showed insect behavioral effects in wheat, for example. CJ induction made plants less favorable for the grain aphid, Sitobion avenae, by reducing aphid settlement, growth, and development (13) and attracting the parasitoid Aphidius ervi (23). Effects on aphid growth and development are partly due to the production of hydroxamic acids in wheat (24), but the mechanism underlying differential emission of volatiles that affects aphid settlement has not been elucidated.
The next step is to determine the genetic basis for the chemical ecology of such multitrophic interactions by examining modulation of gene expression resulting from CJ treatment. The underlying genetics and biochemistry of secondary metabolism in the model plant, Arabidopsis, are well known (25), and its well defined genome sequence makes it an ideal plant system for conducting gene expression studies. Therefore, Arabidopsis was chosen for the present study, in which we investigate the multitrophic interactions between CJ-induced plants and aphids, and their hymenopteran parasitoids, and compare the effect on those insects specializing on the Brassicaceae with the effect on more generalist insects. The system we used involved Arabidopsis (ecotype Col-0) at the first trophic level, the specialist mustard aphid Lipaphis erysimi and the generalist aphid Myzus persicae (Homoptera: Aphididae) at the second trophic level, and the specialist parasitoid Diaeretiella rapae and the generalist parasitoid A. ervi (Hymenoptera: Braconidae) at the third trophic level.
Results and Discussion
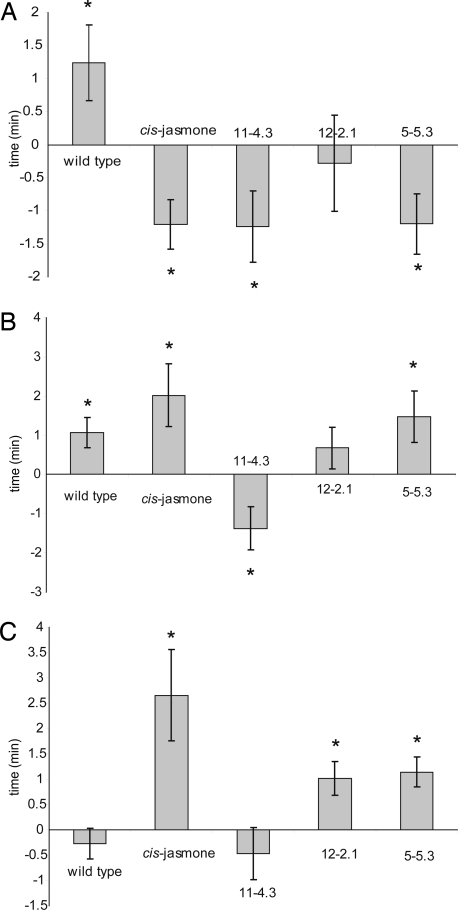
CJ treatment caused alterations in the volatiles emitted by Arabidopsis, which were associated with altered behavioral responses of the generalist insects tested. In an olfactometer bioassay, M. persicae was repelled by volatiles of treated plants, whereas it was attracted to untreated Arabidopsis volatiles (Fig. 1A), and A. ervi was attracted to the blend of volatiles emitted from treated plants but not to those emitted from untreated plants (Figs. 1C and 2A). Both the generalist aphid M. persicae and the specialist aphid L. erysimi showed significant attraction to untreated wild-type Arabidopsis volatiles. For M. persicae, this behavioral response was reversed with CJ treatment; instead of being attracted, these aphids were significantly repelled by the volatiles of the induced plants (Fig. 1A). In contrast, L. erysimi still showed significant attraction to volatiles of CJ-induced plants (Fig. 1B), suggesting that the specialist insects use different semiochemicals for host plant recognition.
Fig. 1.
Olfactometer responses of M. persicae (A), L. erysimi (B), and A. ervi (C). Corrected responses are shown (mean time spent in the control arms was subtracted from time spent in the treated arm for each replicate). Asterisks indicate where time spent in the treated arm was significantly different from time spent in the control arm (P < 0.05).
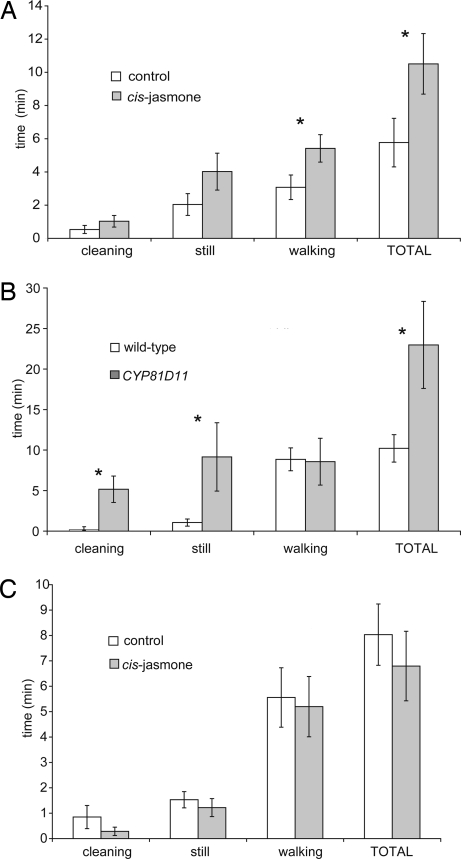
Fig. 2.
Foraging bioassay with A. ervi on CJ-treated Arabidopsis (A), A. ervi on CYP81D11-transformed Arabidopsis (B), and D. rapae on CJ-treated Arabidopsis (C). Asterisks indicate where treated and control responses were significantly different (P < 0.05).
The generalist aphid parasitoid A. ervi was not attracted to wild-type Arabidopsis volatiles (Fig. 1C) but was attracted to CJ-induced volatiles. This indicated that potentiation of the Arabidopsis volatile blend was required in order to elicit a behavioral response from the generalist A. ervi. No potentiation of the volatile blend was needed for the specialist parasitoid D. rapae, which was strongly attracted to volatiles of wild-type untreated Arabidopsis. Although D. rapae was also attracted to volatiles from CJ-induced plants, there was no increase in attraction when compared with the strong response to the wild-type untreated volatiles (data not shown). Foraging A. ervi spent significantly more time on CJ-induced Arabidopsis plants than on control plants (P = 0.025) and spent significantly more time walking (P = 0.021) (Fig. 2A). However, CJ had no significant effect on the foraging behavior of the specialist parasitoid D. rapae (Fig. 2C), confirming the findings of the olfactometer bioassays (Fig. 1), which showed a clear effect of CJ induction with A. ervi but not with D. rapae. We used uninfested plants in the bioassay because earlier experiments with aphid-infested plants that had been induced with CJ indicated that the arrestment effect of encountering aphids was overriding (data not shown).
The effects of CJ defense activation on Arabidopsis gene expression were investigated by means of transcriptomic analyses. Plants induced with CJ were compared with plants treated in a similar way with methyl jasmonate. High-quality mRNA was used as a template for probe synthesis and hybridized against the Arabidopsis Functional Genomics Consortium 14K Arabidopsis cDNA microarray (46). Pairwise comparisons of different treatments were carried out: control vs. CJ, control vs. methyl jasmonate, and CJ vs. methyl jasmonate. As a result, ≈30 transcripts were identified as being solely up-regulated on exposure to CJ and not with methyl jasmonate. To validate these observations, expression of a subset of the CJ-induced genes was examined by Northern blotting, which strongly confirmed the data obtained from the microarray analyses (M.C.M., P. Verrier, J.A.P., and J.A.N., unpublished data). On the validated list were transcripts derived from genes annotated as cytochromes P450, a 4-methyl-5(2-hydroxyethyl)thiazole monophosphate biosynthase (At3g14990), and an oxophytodienoic acid reductase gene, OPR1 (At1g76680). Two cytochrome P450 genes—CYP81D11 (At3g28740) and CYP72A13 (At3g14660)—were highly up-regulated, with the former showing the strongest induction by CJ. CYP81D8 and genes in the CYP89 family also showed some moderate up-regulation, but of particular interest was the observation that none of the CYP genes associated with glucosinolate biosynthesis were up-regulated.
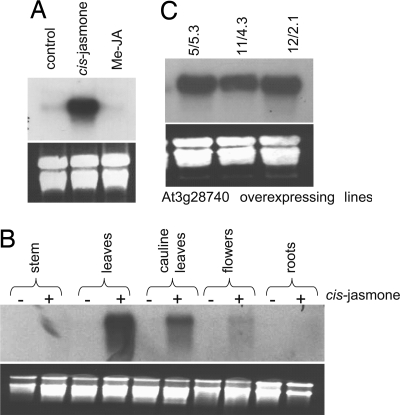
Although the CYP81D11 transcript was strongly up-regulated by CJ, almost no transcripts could be detected in the absence of this signal nor under a range of different treatments and developmental stages (Fig. 3 A and B; also determined by expression profiling using the Arabidopsis expression profile database at https://www.genevestigator.ethz.ch). In particular, CYP81D11 was not induced by the structurally related compound methyl jasmonate (Fig. 3A), whereas CJ treatment resulted in high-level expression in aerial tissues, specifically in rosette and cauline leaves (Fig. 3B). Most importantly, it was observed that CJ-induced expression was independent of COI1, the F-box protein through which methyl jasmonate modulates gene expression, indicating the distinct nature of CJ-induced gene expression (M.C.M., J. Ward, P. Verrier, J.A.P., and J.A.N., unpublished data). This precise regulation of expression led us to investigate the specific role of this cytochrome P450 in modulating plant–insect interactions, in particular in multitrophic interactions with aphids and their parasitoids in Arabidopsis. Transgenic Arabidopsis lines were generated that constitutively overexpressed the CYP81D11 ORF, using the cauliflower mosaic virus 35S promoter. A number of lines were selected on the basis of constitutive high-level expression of this transgene, as determined by Northern blotting. After further genetic characterization for Mendelian segregation and stable transmission of transgenic expression, three different T3 lines (5-5.3, 11-4.3, and 12-2.1, representing different transgenic events) were selected for subsequent bioassay experiments (Fig. 3C). Thus, these lines express CYP81D11 at very high (although differing) levels in all aerial tissues in the absence of CJ.
Fig. 3.
Northern blotting analysis of CYP81D11 (At3g28740) expression by Arabidopsis wild-type and overexpressing plants. (A) Wild-type plants were exposed to CJ or methyl jasmonate for 20 h. RNA was subsequently extracted from leaves, and At3g28740 expression was analyzed by Northern blotting. (B) Tissue-specific expression of At3g28740 in Arabidopsis wild-type after ± exposure to CJ for 20 h. (C) Transgenic Arabidopsis lines constitutively overexpressing CYP81D11 (At3g28740) confirmed by Northern blotting. RNA was isolated from rosette leaves from homozygous T3 plants used in subsequent bioassays.
Insect behavioral responses to volatiles emitted from transformed plants overexpressing the CYP81D11 gene (CYP81D11 OE plants) (Fig. 1) were broadly similar to those observed with CJ-treated plants. For the generalist insects, responses to these plants were different from those observed when the insects were exposed to volatiles of wild-type untreated plants. As with the CJ treatment, CYP81D11 OE had little effect on the specialist insects (except for an anomalous response of L. erysimi to volatiles from line 11-4.3). Volatiles emitted from CYP81D11 OE plants in lines 11-4.3 and 5-5.3 elicited a repellent response with M. persicae, which was similar to the response observed with CJ-induced volatiles (Fig. 1A). Volatiles from line 12-2.1 were not significantly repellent, but the attraction observed with the wild-type volatiles was switched off. With the specialist L. erysimi, variable responses to the CYP81D11 OE plant volatiles were observed (Fig. 1B): line 5-5.3 elicited an attractive response; no response in terms of time spent was observed to line 12-2.1 volatiles (although significantly more entries were made into the treated olfactometer arm); and volatiles of line 11-4.3 elicited a repellent response. The repellent effect of line 11-4.3 volatiles possibly indicated the presence of a different volatile or volatiles in the blend that is repellent even to specialists on Brassicaceae. The generalist aphid parasitoid A. ervi was not attracted to wild-type Arabidopsis volatiles (Fig. 1C) but was attracted to volatiles from two of three of the transformed lines (12-2.1 and 5-5.3). Volatiles from line 11-4.3 were not attractive, again suggesting that some difference exists in this line compared with the other two. The Northern blot is less intense for line 11-4.3 (Fig. 3C), suggesting that CYP81D11 gene expression is lower (although this remains to be confirmed by quantitative PCR). D. rapae was strongly attracted to volatiles of wild-type untreated Arabidopsis. Although D. rapae was also attracted to volatiles of CYP81D11 OE plants, the attraction was very similar to the response to the wild-type untreated volatiles (data not shown). Foraging A. ervi spent significantly longer foraging on CYP81D11 OE line 5-5.3 (Fig. 2B). Significantly more time was spent keeping still, indicating that there was arrestment on the treated plants. The increase in time spent walking that occurred with CJ-treated plants was not observed. In contrast, no clear effect was observed with similarly produced CYP72A1 OE transgenic plants.
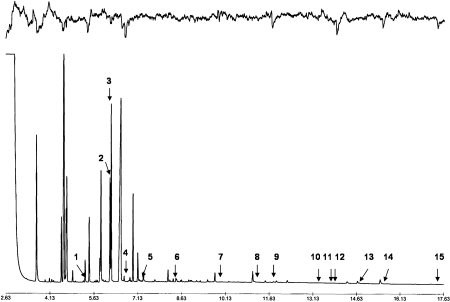
Volatiles emitted from CJ-treated plants and CYP81D11 OE plants were compared with those emitted from wild-type untreated plants by using gas chromatography coupled with mass spectrometry (GC-MS). With both CJ and the CYP81D11 OE transgenics, we noted increases in emission of (Z)-3-hexen-1-ol, ethylbenzene, 4-ethyltoluene, 1-octen-3-ol, 6-methyl-5-hepten-2-one, heptyl isothiocyanate, and an unidentified ester and decreases in emission of 4-methylthiobutyl cyanide and 4-methylthiobutyl isothiocyanate. However, to explain insect behavior, it is important to determine which volatiles are actually perceived by the insects. To address this, we used GC coupled with electroantennography (GC-EAG), with the antenna of A. ervi as a highly sensitive biological detector that was exposed to bulked samples of CJ-treated and CYP81D11 OE plants (Fig. 4). With CJ-treated plants, two compounds—(Z)-3-hexen-1-ol and 4-methylthiobutyl isothiocyanate—were associated with EAG activity and were present in sufficient levels to allow identification by GC-MS. The other electrophysiological responses occurred at retention times where there was insufficient material present to identify the compounds by GC-MS. However, consistent peaks of EAG activity were observed at retention times corresponding to methyl salicylate, caryophyllene, and 4,8,12-trimethyl-(EE)-trideca-1,3,7,11-tetraene (TMTT). With a bulked sample of volatiles from CYP81D11 OE plants (line 5-5.3) (Fig. 4), there were 15 electrophysiological responses, but 10 of these were below the threshold for sensitivity for the GC-MS detector. Tentative identifications were made for these volatiles on the basis of retention times, and again one of the EAG responses to this sample and to the CJ-treated sample occurred at a retention time that matched the retention index for TMTT.
Fig. 4.
GC-EAG with CYP81D11-induced Arabidopsis and A. ervi. Upper trace, response of antenna; lower trace, FID response. Electrophysiologically active peaks are marked with arrows. Tentative identifications based on retention indices and GC-MS: (1) (E)-2-pentenal, (2) (Z)-3-hexenal, (3) hexanal, (4) unidentified, (5) ethylbenzene. Tentative identifications based on retention index only, because of the small amount of material: (6) benzaldehyde/α-pinene, (7) 4-pentyl isothiocyanate/(E)-2-octen-1-ol, (8) unknown, (9) benzathiazole, (10) α-cubebene, (11) isolongifolene/bourbonene, (12) unidentified, (13) 2-tridecanone/germacrene D, (14) 4,8,12-trimethyl-(E,E)-trideca-1,3,7,11-tetraene, (15) unknown.
The consistent electrophysiological responses to CJ-induced and CYP81D11 OE Arabidopsis volatiles at retention times where quantities of phytochemical were below the threshold of the GC-MS detector and no GC peak was visible indicated the very high sensitivity of the insect antenna to the compounds involved. The insect olfactory system is renowned for its sensitivity (26), and volatiles relevant to insect behavior detected in GC-EAG studies often are not the compounds present in the largest quantities. G protein amplification allows insects to detect low numbers of semiochemical molecules, whereas current detection thresholds using GC and GC-MS are many orders of magnitude higher. Nevertheless, (Z)-3-hexen-1-ol was identified as being electrophysiologically active with A. ervi and is particularly interesting because it is known to attract A. ervi in wind tunnel experiments (27). Its levels were increased with both CJ treatment and CYP81D11 OE. Furthermore, (Z)-3-hexen-1-ol has been shown to trigger defense responses in maize (28), which is further evidence for its role as a stress-associated semiochemical.
GC-MS analytical studies showed that the volatile profiles of the three CYP81D11 OE lines were very similar [see supporting information (SI) Fig. S1]. However, despite this similarity, at the behavioral level different responses were observed for L. erysimi and A. ervi with line 11-4.3 (Fig. 1). This outcome again shows how insect behavioral responses can be far more sensitive than current analytical chemistry techniques. The only compound that was emitted in significantly larger amounts in line 11-4.3 was 4-hydroxy-4-methyl-2-pentanone, which, when tested on its own in the olfactometer, was not repellent to L. erysimi. Again, the most likely explanation is that trace amounts of repellent compounds undetectable by GC-MS influenced insect behavior with this line.
Logically, it might be expected that products from genes induced by plant activators would include enzymes involved in the generation of herbivore repellents and foraging stimulants for predators and parasitoids. The cytochromes P450 may fulfill such a role, and the experiments reported here provide evidence for this. It is known that CYP79B2 is up-regulated after aphid feeding in Arabidopsis but not after piercing with a sterile needle (29, 30). A link between CYP genes and volatile production has also been demonstrated in a study in which a CYP79D2 from cassava that catalyses production of valine- and isoleucine-derived glucosinolates was successfully introduced into Arabidopsis (31). Preliminary work with CJ-induced cotton has shown that release of TMTT is greatly increased, and TMTT has been found to explain repulsion of Aphis gossypii (T.J.A.B and M.A.B., unpublished data), but in Arabidopsis we have not yet proven the role of this compound because there was insufficient material for confirmation by GC-MS (although electrophysiological responses of A. ervi occurred at the appropriate retention time). We hypothesized that CYP genes could be involved directly in TMTT biosynthesis. The other cytochrome P450 gene up-regulated by CJ in Arabidopsis, CYP72A13, is, in sequence terms, closely related to the secologanin synthase gene CYP72A1 (32), which synthesizes secologanin from loganin by a reaction mechanism that we realized is identical to that required for TMTT synthesis from the precursor geranyllinalool proposed by Boland et al. (33) (SI Fig. S2). We therefore produced Arabidopsis lines overexpressing CYP72A13; however, these have not shown the behavioral differences expected nor have we detected higher levels of TMTT (data not presented). It thus appears that involvement of CYP genes in TMTT biosynthesis is a hypothesis that would be better tested in other plant species, such as cotton, in future research.
It appears that specialized aphids are adapted to cope with the induced defenses of their host plants and thus are less influenced by induction of defense. Although the volatiles induced by CJ appear to affect generalist insects more than specialist ones, this does not preclude the involvement of other semiochemicals affecting the multitrophic interactions of the specialist insects in Arabidopsis because specialists use glucosinolate catabolites that are not influenced by CJ. Specialist insects may rely on cues that are more specific to their particular aphid–host plant complex. For example, it is known that D. rapae is attracted to 3-butenyl isothiocyanate (34, 35), but we did not find any change in the emission levels of this compound with CJ treatment or CYP81D11 OE.
Hymenopteran parasitoids are an important agent of natural mortality of aphids (36, 37), and their foraging behavior is influenced by semiochemical cues from the aphid host plants (27, 38). The findings of the present study support the hypothesis that parasitoids use induced plant volatiles as a factor determining patch residence times (39). Induction of crop plants with CJ could provide a means of enhancing parasitoid activity, and hence biological control. In previous work focused on crop plants (23), we have shown that the time spent foraging by A. ervi was significantly increased on wheat induced by CJ. This suggests that a widely occurring aspect of plant defense metabolism might be modified by CJ activation, which would enhance the performance of A. ervi by providing volatile cues that lead to attraction and arrestment. Increased attraction of A. ervi to CJ-treated bean plants in a wind tunnel (12) supports the idea that volatile cues from the plant play a role in attracting parasitoids to induced plants and further extends the range of plants for which CJ-induced volatile production elicits behavioral effects on A. ervi.
Chemical ecology deals with chemical mechanisms controlling intra- and interspecific interactions among living organisms. Here we have considered a plant-derived semiochemical, CJ, and how it acts as a signal altering gene expression and volatile production in the model plant Arabidopsis, with consequent changes in interactions at the second trophic level with aphid herbivores and at the third trophic level with aphid parasitoids. The cytochrome P450 gene CYP81D11 was shown to play an important role in this interaction, given that it was up-regulated in CJ-treated plants and that insect behavioral responses to plants overexpressing this gene were similar to the responses to induced plants.
Materials and Methods
Plant Cultivation.
Seeds were stratified for 2 days at 4°C to ensure uniform germination before being placed in growth chambers under constant light (60.58 μmol·m−2·s−1) at 22°C. After ≈10 days, plants were transferred into soil: one plant per 5-cm-diameter pot. Plants were then cultivated in a SANYO growth chamber under long day conditions with a 16-h light and 8-h dark period. During the light period (350 μmol of photons per m2 per s1), the temperature was kept at 23°C with a relative humidity of 75%. During the dark period, the temperature was 18°C with a relative humidity of 80%.
Treatment of Plants.
CJ was purchased from Avocado Research Chemicals, and methyl jasmonate was purchased from Nippon Zeon. For exposure to either CJ or methyl jasmonate, Arabidopsis thaliana plants were grown to the rosette stage, placed into 3.7-liter airtight containers, and exposed to 1.5 μg of CJ or methyl jasmonate, respectively. These compounds were applied to a small piece of filter paper (no.1; Whatman) that had been attached to the lid of the container; the container was sealed with parafilm, and the compounds were left to evaporate for 20 h. Control plants were sealed in an identical tank with no treatment. After 20 h, leaves were harvested and snap-frozen in liquid nitrogen.
For parasitoid foraging bioassays, Arabidopsis plants obtained from the glasshouse were sprayed using a hydraulic nozzle (Lurmark 015-F110) mounted on a variable-speed spray track at 1 ms−1 in an indoor spray facility. The CJ was formulated in a 0.1% aqueous solution of a nonionic surfactant, Ethylan BV (EBV) (Akcros Chemicals) and applied at a rate equivalent to 50 g ha−1 in 200 liters ha−1. Control plants were sprayed with 0.1% aqueous EBV and were kept in a separate glasshouse to avoid contamination by CJ-treated plants. Plants were treated at least 48 h prior to bioassay.
Air Entrainment.
The volatile chemicals from the headspaces of wild-type and transgenic plants were collected by entrainment onto Porapak Q (60/80 mesh, 0.05 g) contained in a glass GC inlet liner between glass-wool plugs. Leaves from the Arabidopsis plants were harvested and dropped into liquid nitrogen immediately to minimize degradation of plant tissue. They were then ground to a powder, and the volatiles entrained as they were allowed to warm to room temperature, with a final warming to 40°C for 30 min. The volatiles were collected on Porapak and eluted with 500 μl redistilled diethyl ether.
Aphid and Parasitoid Olfactometer Bioassay.
Behavioral assays used a Perspex four-arm olfactometer (40) lit from above by diffuse, uniform lighting and maintained at 23°C. The bottom of the apparatus was lined with filter paper (no. 1; Whatman), and air was drawn through the four arms toward the center at 350 ml min−1. Single alate aphid virginoparae or female parasitoids were introduced into the central chamber, and the time spent and number of entries into each arm were recorded by using specialist software (OLFA; Exeter Software) over a 160-min period. The apparatus was rotated one-quarter turn every 2 min to eliminate directional bias. Aliquots (10 μl each) of entrainment samples were applied to a filter paper strip, and the solvent was allowed to evaporate for 30 s. The filter paper was then placed at the end of the treated side arm. The three control arms were similarly treated with 10 μl of redistilled diethyl ether alone on filter paper. The percentage of time spent in each of the four arms, of the total time spent in all four arms, was calculated. Data were transformed by using a logit transformation. Transformed data were then compared with a test mean of −1.099 (logit transformation of 25%), using a one-sample t test (GenStat ver. 10; VSN International).
Parasitoid Foraging Bioassay.
Differences in the densities of searching parasitoids between patches of plants in the field are mainly due to differences in parasitoid leaving rates, rather than differences in arrival rates (41). Thus, the foraging bioassay used here recorded behaviors of parasitoids released on the plant and time spent before leaving it. Experienced parasitoids were used. A. ervi were reared on pea aphid on bean to ensure that it would behave as a generalist and that it had not become “specialized” by imprinting on brassica-feeding aphids. D. rapae was reared on M. persicae on Chinese cabbage. The experimental procedure was similar to that described in ref. 38. Individual walking female parasitoids were released directly onto the center of individual Arabidopsis plants at growth stage 3.50 (50% of final size) (42). Treated and control plants were alternated to eliminate any effect of time of day on foraging behavior. Experiments were conducted at 22°C. Direct observations of foraging behavior were then made as the parasitoids searched the plants, and Noldus Observer 4.1 software was used to record the behavioral observations. Time spent walking, still, and cleaning was recorded, as well as total time spent before the parasitoid left the plant. An observation was terminated when a parasitoid flew away from the plant, which was considered as the foraging “patch.” Time spent on treated and control plants was compared by using a paired t test (GenStat).
Generation of CYP81D11 OE and CYP72A13 OE Plants.
The clone pda04666 containing the cDNA for At3g28740 was obtained from RIKEN. Primers RVP450sense (5′-ACGGATATCATGTCATCAACAAAGACAAT-3′) and SacIP450sense (5′-ACGGAGCTCTTATGGACAAGAAGCATCTA-3′) were used to amplify the insert, and the amplification product was cloned by using the TOPO cloning kit from Invitrogen. The ORF of the PCR product was confirmed through sequencing, excised from the TOPO vector by EcoRV and SacI (New England Biolabs), and subsequently cloned into pJD330, from which the glucuronidase gene had been removed by SalI and SacI digestion. The SalI restriction site was rendered compatible with the EcoRV by treatment with Klenow. At3g28740 under the control of the CaMV35S promoter was removed from pJD330 by using HindIII and EcoRI and cloned into the expression vector BIN19. Clone C105322 containing the cDNA for At3g14660 was obtained from the Arabidopsis Biological Resource Center (Ohio State University, Columbus, OH). The insert was amplified with the primers At3g14660SalIF 5′-GTCGACATGGAGATATCAGTTGCATC-3′ and At3g14660SalIR 5′-GTCGACTTAGAGCTTGTGCAAGATAA-3′ and cloned into TOPO (Invitrogen) for verification of the ORF. The insert was excised by SalI, cloned into BIN19-S35, and checked for correct orientation before being used for transformation of plants. Transformation of Arabidopsis (Col-0) was achieved by using the floral dip method, as described in ref. 43. Transgenic plants were selected on Kanamycin-containing plates, and transgene expression was confirmed by Northern blotting.
Microarray Studies.
The effects of CJ on gene expression in Arabidopsis were analyzed using the Stanford Arabidopsis microarray facility, provided by the Arabidopsis Functional Genomics Consortium, in which the effects of CJ would be tested against a control consisting of plants treated in a similar way with methyl jasmonate. Thus, intact 8-week-old Arabidopsis, ecotype Columbia, were exposed for 24 h in sealed boxes (3.7 liters) to methyl jasmonate or CJ as a vapor from 1 μl (≈1 mg) of undiluted material. The extracted messenger RNA was hybridized to the Stanford array, giving the following comparisons: control vs. CJ, control vs. methyl jasmonate, and CJ vs. methyl jasmonate. Approximately 30 genes were up-regulated by 24-h exposure to CJ. Confirmation of this up-regulation was obtained for a subset of the initially recognized genes by differential expression to CJ, using Northern blotting.
Electrophysiology.
EAG recordings were made using Ag–AgCl glass electrodes filled with saline solution (composition as in ref. 44 but without glucose). A female parasitoid was chilled in ice and the head was removed. The head was placed inside the indifferent electrode, and the tips of the two antennae were inserted into the recording electrode. The signals were passed through a high-impedance amplifier (UN-06; Syntech) and analyzed using a customized software package (Syntech). The coupled GC-EAG system, in which the effluent from the GC column is simultaneously directed to the antennal preparation and the GC detector, has been described previously (45). Separation of the volatiles was achieved on a Hewlett-Packard 6890 gas chromatograph equipped with a cold on-column injector and a flame ionization detector (FID). The column used was 50 m × 0.32-mm i.d. HP-1. The oven temperature was maintained at 30°C for 2 min and then programmed at 5o/min to 100°C, followed by 10o/min to 250°C. Single-cell recordings from neuronal cells associated with the olfactory receptors on the distal primary rhinarium of alate virginoparous M. persicae were made by using tungsten microelectrodes. The indifferent electrode was placed in the first or second antennal segment, and the recording electrode was then brought into contact with the rhinarium until impulses were recorded.
GC.
Volatiles were analyzed on a Hewlett-Packard 6890 gas chromatograph equipped with a cold on-column injector, a FID, and a 50 m × 0.32-mm i.d. HP-1 bonded phase fused silica capillary column. The oven temperature was maintained at 30°C for 2 min and then programmed at 5°C/min to 150°C, followed by 10°C/min to 150°C. The carrier gas was hydrogen.
Coupled GC-MS.
A capillary GC column (50 m × 0.32-mm i.d., HP-1) fitted with an on-column injector was directly coupled to a mass spectrometer (MAT-95 XP; Thermo-Finnigan). Ionization was by electron impact at 70 eV and 200°C. The oven temperature was maintained at 30°C for 5 min and then programmed at 5o/min to 250°C.
Acknowledgments.
Rothamsted Research receives grant-aided support from the Biotechnology and Biological Sciences Research Council of the U.K., and this work was supported in part by the U.K. Department for Environment, Food and Rural Affairs.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0710305105/DCSupplemental.
References
- 1.Karban R, Kuc J. Induced resistance against pathogens and herbivores: An overview. In: Agrawal AA, Tuzun S, Bent E, editors. Induced Plant Defence Against Pathogens and Herbivores. APS Press: St. Paul, MN; 1999. pp. 1–16. [Google Scholar]
- 2.Chamberlain K, Pickett JA, Woodcock CM. Plant signalling and induced defence in insect attack. Mol Plant Pathol. 2000;1:67–72. doi: 10.1046/j.1364-3703.2000.00009.x. [DOI] [PubMed] [Google Scholar]
- 3.Mattiacci L, Dicke M, Posthumus MA. β-Glucosidase: An elicitor of herbivore-induced plant odor that attracts host-searching parasitic wasps. Proc Natl Acad Sci USA. 1995;92:2036–2040. doi: 10.1073/pnas.92.6.2036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Alborn HT, et al. An elicitor of plant volatiles from beet armyworm oral secretion. Science. 1997;276:945–949. [Google Scholar]
- 5.Karban R, Baldwin IT, Baxter KJ, Laue G, Felton GW. Communication between plants: Induced resistance in wild tobacco plants following clipping of neighboring sagebrush. Oecologia. 2000;125:66–71. doi: 10.1007/PL00008892. [DOI] [PubMed] [Google Scholar]
- 6.Heil M, Silva Bueno JC. Within-plant signaling by volatiles leads to induction and priming of an indirect plant defense in nature. Proc Natl Acad Sci USA. 2007;104:5467–5472. doi: 10.1073/pnas.0610266104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vet LEM, Dicke M. Ecology of infochemical use by natural enemies in a tritrophic context. Annu Rev Entomol. 1992;37:141–172. [Google Scholar]
- 8.Farmer EE. Surface-to-air signals. Nature. 2001;411:854–856. doi: 10.1038/35081189. [DOI] [PubMed] [Google Scholar]
- 9.Kessler A, Baldwin IT. Defensive function of herbivore-induced plant volatile emissions in nature. Science. 2001;291:2141–2144. doi: 10.1126/science.291.5511.2141. [DOI] [PubMed] [Google Scholar]
- 10.Whitfield J. Making crops cry for help. Nature. 2001;410:736–737. doi: 10.1038/35071188. [DOI] [PubMed] [Google Scholar]
- 11.Tooker JF, Hanks LM. Tritrophic interactions and reproductive fitness of the prairie perennial Silphium laciniatum Gillette (Asteraceae) Environ Entomol. 2006;35:537–545. [Google Scholar]
- 12.Birkett MA, et al. New roles for cis-jasmone as an insect semiochemical and in plant defense. Proc Natl Acad Sci USA. 2000;97:9329–9334. doi: 10.1073/pnas.160241697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bruce TJA, et al. cis-Jasmone treatment induces resistance in wheat plants against the grain aphid, Sitobion avenae (Fabricius) (Homoptera : Aphididae) Pest Manage Sci. 2003;59:1031–1036. doi: 10.1002/ps.730. [DOI] [PubMed] [Google Scholar]
- 14.Loughrin JH, Manukian A, Heath RR, Tumlinson JH. Volatiles emitted by different cotton varieties damaged by feeding beet armyworm larvae. J Chem Ecol. 1995;21:1217–1227. doi: 10.1007/BF02228321. [DOI] [PubMed] [Google Scholar]
- 15.Röse USR, Tumlinson JH. Systemic induction of volatile release in cotton: How specific is the signal to herbivory? Planta. 2005;222:327–335. doi: 10.1007/s00425-005-1528-2. [DOI] [PubMed] [Google Scholar]
- 16.Röse USR, Tumlinson JH. Volatiles released from cotton plants in response to Helicoverpa zea feeding damage on cotton flower buds. Planta. 2004;218:824–832. doi: 10.1007/s00425-003-1162-9. [DOI] [PubMed] [Google Scholar]
- 17.Lou Y, Baldwin IT. Manduca sexta recognition and resistance among allopolyploid Nicotiana host plants. Proc Natl Acad Sci USA. 2003;100:14581–14586. doi: 10.1073/pnas.2135348100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Degen T, Dillmann C, Marion-Poll F, Turlings CJ. High genetic variability of herbivore-induced volatile emission within a broad range of maize inbred lines. Plant Physiol. 2004;135:1928–1938. doi: 10.1104/pp.104.039891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dabrowska P, Boland W. iso-OPDA: An early precursor of cis-jasmone in plants. ChemBioChem. 2007;18:2281–2285. doi: 10.1002/cbic.200700464. [DOI] [PubMed] [Google Scholar]
- 20.Farmer EE, Ryan CA. Interplant communication: Airborne methyl jasmonate induces synthesis of proteinase inhibitors in plant leaves. Proc Natl Acad Sci USA. 1990;87:7713–7716. doi: 10.1073/pnas.87.19.7713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Thaler JS, Stout MJ, Karban R, Duffey SS. Exogenous jasmonates simulate insect wounding in tomato plants (Lycopersicon esculentum) in the laboratory and field. J Chem Ecol. 1996;22:1767–1781. doi: 10.1007/BF02028503. [DOI] [PubMed] [Google Scholar]
- 22.Pickett JA, et al. cis-Jasmone as an allelopathic agent in inducing plant defence. Allelopath J. 2007;19:109–117. [Google Scholar]
- 23.Bruce TJA, Pickett JA, Smart LE. cis-Jasmone switches on plant defence against insects. Pestic Outlook. 2003;14:96–98. [Google Scholar]
- 24.Moraes MCB, et al. Developments in aspects of ecological phytochemistry: The role of cis-jasmone in inducible defence systems in plants. Phytochemistry. 2007;68:2937–2945. doi: 10.1016/j.phytochem.2007.09.025. [DOI] [PubMed] [Google Scholar]
- 25.Kliebenstein DJ. Secondary metabolites and plant/environment interactions: A view through Arabidopsis thaliana tinged glasses. Plant Cell Environ. 2004;27:675–684. [Google Scholar]
- 26.Angioy AM, Desogus A, Barbarossa IT, Anderson P, Hansson BS. Extreme sensitivity in an olfactory system. Chem Senses. 2003;28:279–284. doi: 10.1093/chemse/28.4.279. [DOI] [PubMed] [Google Scholar]
- 27.Du YJ, et al. Identification of semiochemicals released during aphid feeding that attract parasitoid Aphidius ervi. J Chem Ecol. 1998;24:1355–1368. [Google Scholar]
- 28.Farag MA, et al. (Z)-3-Hexenol induces defense genes and downstream metabolites in maize. Planta. 2005;220:900–909. doi: 10.1007/s00425-004-1404-5. [DOI] [PubMed] [Google Scholar]
- 29.Reymond P, Weber H, Damond M, Farmer EE. Differential gene expression in response to mechanical wounding and insect feeding in Arabidopsis. Plant Cell. 2000;12:707–719. doi: 10.1105/tpc.12.5.707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kusnierczyk A, et al. Transcriptional responses of Arabidopsis thaliana ecotypes with different glucosinolate profiles after attack by polyphagous Myzus persicae and oligophagous Brevicoryne brassicae. J Exp Bot. 2007;58:2537–2552. doi: 10.1093/jxb/erm043. [DOI] [PubMed] [Google Scholar]
- 31.Mikkelsen MD, Halkier BA. Metabolic engineering of valine- and isoleucine-derived glucosinolates in Arabidopsis expressing CYP79D2 from cassava. Plant Physiol. 2003;131:773–779. doi: 10.1104/pp.013425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Irmler S, et al. Indole alkaloid biosynthesis in Catharanthus roseus: New enzyme activities and identification of cytochrome P450 CYP72A1 as secologanin synthase. Plant J. 2000;24:797–804. doi: 10.1046/j.1365-313x.2000.00922.x. [DOI] [PubMed] [Google Scholar]
- 33.Boland W, Gäbler A, Gilbert M, Feng Z. Biosynthesis of C-11 and C-16 homoterpenes in higher plants: Stereochemistry of the C-C-bond cleavage reaction. Tetrahedron. 1998;54:14725–14736. [Google Scholar]
- 34.Bradburne RP, Mithen R. Glucosinolate genetics and the attraction of the aphid parasitoid Diaeretiella rapae to Brassica. Proc R Soc London Ser B. 2000;267:89–95. doi: 10.1098/rspb.2000.0971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Blande JD, Pickett JA, Poppy GM. A comparison of semiochemically mediated interactions involving specialist and generalist Brassica-feeding aphids and the braconid parasitoid Diaeretiella rapae. J Chem Ecol. 2007;33:767–779. doi: 10.1007/s10886-007-9264-7. [DOI] [PubMed] [Google Scholar]
- 36.Schmidt MH, et al. Relative importance of predators and parasitoids for cereal aphid control. Proc R Soc London Ser B. 2003;270:1905–1909. doi: 10.1098/rspb.2003.2469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Powell W, Pickett JA. Manipulation of parasitoids for aphid pest management: Progress and prospects. Pest Manage Sci. 2003;59:149–155. doi: 10.1002/ps.550. [DOI] [PubMed] [Google Scholar]
- 38.Umoru PA, Powell W, Clark SJ. Effect of pirimicarb on the foraging behaviour of Diaeretiella rapae (Hymenoptera: Braconidae) on host-free and infested oilseed rape plants. Bull Entomol Res. 1996;86:193–201. [Google Scholar]
- 39.Tentelier C, Fauvergue X. Herbivore-induced plant volatiles as cues for habitat assessment by a foraging parasitoid. J Anim Ecol. 2007;76:1–8. doi: 10.1111/j.1365-2656.2006.01171.x. [DOI] [PubMed] [Google Scholar]
- 40.Pettersson J. An aphid sex attractant. 1. Biological studies. Entomol Scand. 1970;1:63–73. [Google Scholar]
- 41.Budenberg WJ, Powell W, Clark SJ. The influence of aphids and honeydew on the leaving rate of searching aphid parasitoids from wheat plants. Entomol Exp Appl. 1992;63:259–264. [Google Scholar]
- 42.Turner SR, Somerville CR. Collapsed xylem phenotype of Arabidopsis identifies mutants deficient in cellulose deposition in the secondary cell wall. Plant Cell. 1997;9:689–701. doi: 10.1105/tpc.9.5.689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Clough SJ, Bent AF. Floral dip: A simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J. 1998;16:735–743. doi: 10.1046/j.1365-313x.1998.00343.x. [DOI] [PubMed] [Google Scholar]
- 44.Maddrell SHP. Secretion by the Malphigian tubules of Rhodnius: The movement of ions and water. J Exp Biol. 1969;51:71–97. [Google Scholar]
- 45.Wadhams LJ. In: Chromatography and Isolation of Insect Hormones and Pheromones. McCaffery AR, Wilson ID, editors. Plenum: London; 1990. pp. 289–298. [Google Scholar]
- 46.Wu S, Ramonell K, Gollub J, Somerville SC. Plant gene expression profiling with DNA microarrays. Plant Physiol Biochem. 2001;39:917–926. [Google Scholar]