Abstract
Light is an important environmental factor for regulation of mood. There is a high frequency of seasonal affective disorder in high latitudes where light exposure is limited, and bright light therapy is a successful antidepressant treatment. We recently showed that rats kept for 6 weeks in constant darkness (DD) have anatomical and behavioral features similar to depressed patients, including dysregulation of circadian sleep–waking rhythms and impairment of the noradrenergic (NA)-locus coeruleus (LC) system. Here, we analyzed the cell viability of neural systems related to the pathophysiology of depression after DD, including NA-LC, serotoninergic-raphe nuclei and dopaminergic-ventral tegmental area neurons, and evaluated the depressive behavioral profile of light-deprived rats. We found increased apoptosis in the three aminergic systems analyzed when compared with animals maintained for 6 weeks in 12:12 light-dark conditions. The most apoptosis was observed in NA-LC neurons, associated with a significant decrease in the number of cortical NA boutons. Behaviorally, DD induced a depression-like condition as measured by increased immobility in a forced swim test (FST). DD did not appear to be stressful (no effect on adrenal or body weights) but may have sensitized responses to subsequent stressors (increased fecal number during the FST). We also found that the antidepressant desipramine decreases these neural and behavioral effects of light deprivation. These findings indicate that DD induces neural damage in monoamine brain systems and this damage is associated with a depressive behavioral phenotype. Our results suggest a mechanism whereby prolonged limited light intensity could negatively impact mood.
Keywords: apoptosis, constant darkness, depression, forced swim test, prefrontal cortex
Depression is associated with decreased function in the noradrenergic (NA) locus coeruleus (LC), serotoninergic (5-HT) dorsal raphe (DR) and median raphe (MnR), and dopaminergic (DA) ventral tegmental area (VTA) systems (1–4). Behaviorally, depression is characterized by lethargy, feelings of helplessness, and profound alterations of sleep–wake rhythms (5). In at least some cases, depression is associated with decreased light availability (e.g., seasonal affective disorder) and a blunted amplitude and phase delay of circadian rhythms (5). Also, degeneration of NA fibers from LC has been associated with stress-induced depression in rats (6, 7). Recently, we showed that, as compared with animals on a 12:12 light-dark (LD) schedule, rats kept for 6 weeks in constant darkness (DD) exhibit decreased NA-LC fibers and boutons in the frontal cortex, a delayed onset of active/rest periods, and a decreased circadian amplitude of the sleep–waking rhythm (8). These results raise the possibility that the absence of light could contribute to depression, perhaps in part through effects on monoamine systems. Here, we evaluated the integrity of brain NA, 5-HT, and DA neurons, and depressive behavioral profiles, of animals kept in long-term DD to test the hypothesis that decreased function in one or more of these neural systems promotes depression after limited light exposure. The present data demonstrate that the absence of light not only damages monoamine systems, but also induces depression-like behavioral symptoms, indicating the involvement of a light-dependent mechanism in the etiology of depression. We show the effect of light deprivation on depression-associated behaviors in animals and on neural systems involved in the pathophysiology of depression.
Results
Long-Term DD Causes Apoptotic Responses in NA-LC, 5-HT-Raphe, and DA-VTA Neurons.
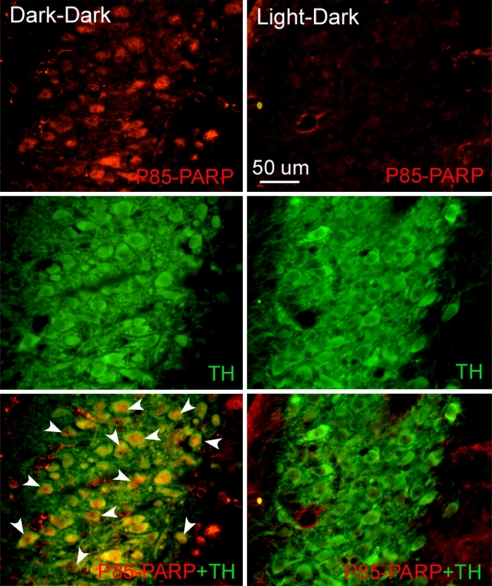
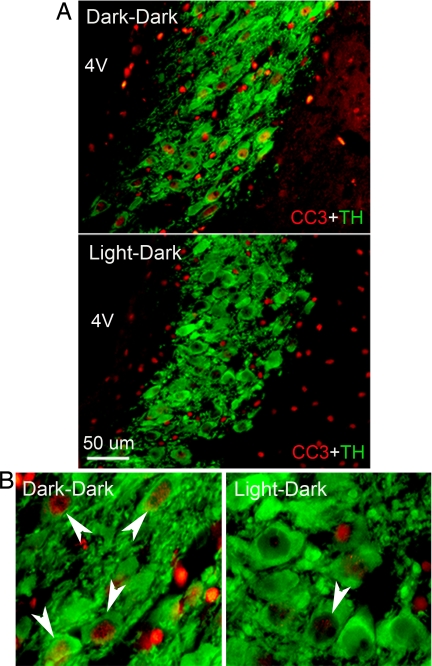
A series of immunohistochemical and morphological assays were performed to examine possible apoptosis or damage in monoamine neurons after long-term DD. Staining with an antibody against the p85 fragment of (ADP-ribose) polymerase (PARP) revealed inclusions of intense immunofluorescence that were localized principally in the nuclei of tyrosine hydroxylase-positive (TH+) neurons [Fig. 1 and supporting information (SI) Fig. 5] and in the nuclei or cytoplasm of 5-HT-positive (5-HT+) neurons (SI Fig. 6). Immunoreactive PARP fragmentation was increased in somata of the three aminergic systems at all of the levels analyzed, but caudal and central NA-LC areas showed the highest apoptotic indices after light deprivation. The percentages of NA (TH+)-LC cells that were also positive for cleaved PARP increased ≈15- to 50-fold in DD compared with LD animals [rostral LC: 54.1 ± 3.3 vs. 1.0 ± 1.0, t(8) = 15.19, P < 0.0001; mid-LC: 85.8 ± 5.3 vs. 2.2 ± 1.5, t(8) = 15.01, P < 0.0001; caudal LC: 74.3 ± 3.3 vs. 4.5 ± 2.1, t(8) = 17.62, P < 0.0001] (Fig. 1). The percentage of DA (TH+)-VTA cells that were cleaved PARP-positive increased ≈7-fold in DD animals compared with LD subjects [51.9 ± 17.2 vs. 8.5 ± 5.1; t(6) = 2.417, P < 0.05] (SI Fig. 5), and the corresponding percentages for 5-HT neurons in DD compared with LD subjects increased ≈3- to 5-fold [rostral DR: 74.6 ± 6.0 vs. 25.4 ± 5.0, t(6) = 6.293, P < 0.001; caudal DR: 34.4 ± 9.5 vs. 19.0 ± 10.5, t(6) = 1.081, P = 0.32; rostral MnR: 77.3 ± 13.1 vs. 20.7 ± 6.9, t(6) = 3.822, P < 0.01; caudal MnR: 38.4 ± 9.1 vs. 7.8 ± 5.9, t(6) = 2.828, P < 0.05] (SI Fig. 6). At least for the NA-LC, the neural damage caused by DD was associated with impending cell death, as indicated by increased caspase-3 (CC3) expression in the nucleus of TH+ cells [rostral LC: 35.4 ± 10.7 vs. 6.0 ± 3.1, t(8) = 2.861, P < 0.05; mid-LC: 62.0 ± 6.9 vs. 5.5 ± 1.6, t(8) = 8.747, P < 0.0001; caudal LC: 43.6 ± 10.7 vs. 4.8 ± 2.2, t(8) = 3.951, P < 0.01] (Fig. 2). There was no CC3 staining in either DD or LD animals for either 5-HT raphe or DA-VTA neurons. We did not detect distinct chromatolysis or other morphological signs of necrosis (such as changes in the shape of the somata or clumping of heterochromatin) in monoamine neurons of DD rats stained for Nissl substance (data not shown). All neuronal staining for cleaved PARP and CC3 appeared to occur in TH+ cells in LC, and primarily also in monoaminergic cells in VTA and raphe; some nonmonoaminergic neurons were also PARP- or CC3-positive in the latter two regions.
Fig. 1.
Representative photomicrographs showing cleaved PARP staining through the mid-LC of a light-deprived rat (dark–dark) and a control (light–dark) subject. (Top and Middle) Double immunostaining using an antibody against the p85 fragment of PARP (red) and an antibody against TH (green) revealed that DD increases apoptosis in NA-LC neurons. Regions of overlap give rise to a yellow signal in the merged image (Bottom). Arrowheads indicate the apoptotic response. Coronal sections, 20 μm thickness, medial is to the left, dorsal is upward.
Fig. 2.
Low-power (A) and high-power (B) photomicrographs of double immunostaining for CC3 (red) and TH (green) in frontal sections through the mid-LC of a light-deprived rat (dark–dark) and a control light–dark subject. Note the increased CC3 activation in NA neurons of rats maintained in DD; arrowheads indicate the apoptotic response. 4V = fourth ventricle. Coronal sections, 20 μm thickness, medial is to the left, dorsal is upward.
DD Decreases NA-Immunoreactive Boutons in Prefrontal Cortex.
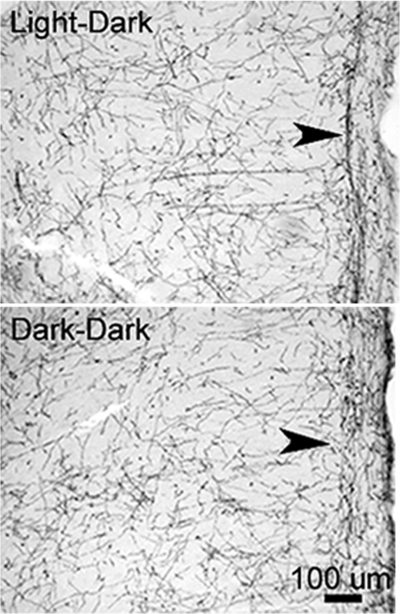
In agreement with the substantial apoptotic response of NA neurons, we found a reduction in dopamine-β-hydroxylase-positive (DβH+) fibers and boutons in prefrontal cortex (PFC) of DD rats. The loss of terminals appeared to be greatest in layer I (Fig. 3). DβH+ boutons of prelimbic and cingulate cortices decreased in DD subjects by 39% [t(9) = 2.359, P < 0.05], whereas the density of cortical 5-HT+ and TH+ boutons did not change significantly, although a tendency toward a decrease was found in DD subjects (Table 1). A similar result occurred in frontal cortex, which had a 52% decrease in NA-LC boutons [2.33 ± 0.46 vs. 1.12 ± 0.06, t(5) = 3.087, P < 0.05] and no significant change in the density of 5-HT boutons (n = 7 for each lighting condition), in agreement with our previous study (8).
Fig. 3.
Photomicrographs of frontal sections through the PFC stained for DβH showing DβH-positive fibers in rats maintained in darkness for 6 weeks and control rats similarly housed but in light–dark conditions. Note that there are fewer NA fibers present in the light-deprived rats, particularly in superficial layers (arrowheads). Coronal sections, 40 μm thickness, midline is at right, dorsal is upward.
Table 1.
Effect of lighting conditions on the density of monoaminergic cortical boutons
Mean number (±SEM) of NA (DβH-IR), 5-HT (5-HT-IR) and DA (TH-IR) boutons counted in the PFC. The drawing at left shows the area in PFC (Cg1: cingulate cortex, area 1; PRL: prelimbic cortex) used for analysis of the density of boutons. The gray box indicates the frame used for counting boutons. Rats kept in DD (n = 5) during 6 weeks exhibited fewer NA boutons in the PFC as compared with animals in LD conditions (n = 6). Light deprivation did not significantly affect the number of TH-IR or 5-HT-IR boutons. Counting frame area: 400 μm2. *, P < 0.05. AI, agranular insular cortex; AOM, anterior olfactory nu, medial; AOV, anterior olfactory nu, ventral; Cl, claustrum; Den, dorsal endopiriform nu; DTr, dorsal transition zone; DTT, dorsal tenia tecta; LO, lateral orbital cortex; M1, primary motor cortex; M2, secondary motor cortex; MO, medial orbital cortex; Pir, piriform cortex; VO, ventral orbital cortex; VTT, ventral tenia tecta (34).
Rats Kept in DD Show a Depressive Behavioral Phenotype.
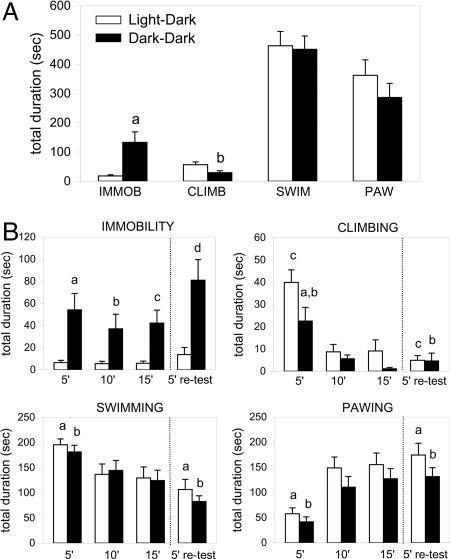
When compared with LD rats, we noted that DD rats were less irritable and aggressive during handling. The latency to immobility (IMMOB) was shorter in DD compared with LD subjects during the 15-min forced swim test (FST) [14.3 ± 4.1 s vs. 154.7 ± 57.6 s; t(36) = 2.429, P < 0.01] and the 5-min retest [36.1 ± 19.4 s vs. 126.2 ± 28.7 s; t(36) = 2.602, P < 0.01]. Light-deprived rats also showed a significant increase in IMMOB time throughout the 15-min FST [+635%, t(36) = 3.184, P < 0.01] and during the 5′ retest [+484%, t(36) = 3.416, P < 0.01] (Fig. 4B); this was characterized primarily by an increase in the number of IMMOB episodes of ≈300% during the 15-min FST [32 ± 4 vs. 8 ± 1, t(36) = 5.375, P < 0.0001] and ≈400% during the retest [15 ± 2 vs. 3 ± 1, t(36) = 5.453, P < 0.0001]. The mean duration of IMMOB episodes was also longer in DD than LD animals during the 15-min FST (+151%, P = 0.08) and retest (+289%, P = 0.06). An ANOVA revealed that lighting condition was the factor that significantly contributed to these results (F1,36 = 10.026, P < 0.001). Post hoc analysis showed that in DD animals IMMOB increased significantly at each 5-min point of the 15-min test (0–5 min = +729%, P < 0.0001; 5–10 min = +564%, P < 0.001; 10–15 min = +612%, P < 0.001), indicating that rats did not develop passive coping behavior in response to the inescapable situation, but had a preexisting behavioral deficit (Fig. 4B). To test whether increased IMMOB was induced by an acute effect of darkness we extended the dark period of rats kept for 6 weeks in LD by 2 h (12:14) for 1 day and tested their depressive-like behavioral phenotype during the last hour of darkness. No difference in IMMOB was observed during the 15-min FST as compared with 12:12 LD subjects tested under light (total duration: LD 12:12 = 18.1 ± 3.9 s, n = 19; LD 12:14 = 11.8 ± 8.9 s, n = 4; mean duration of episodes: LD 12:12 = 1.9 ± 0.3 s, n = 19; LD 12:14 = 1.9 ± 1.2 s, n = 4). This result indicates that the depressive profile was induced by long-term DD. Animals housed in DD conditions showed less climbing (CLIMB) during the 15-min FST [−48%, t(36) = 2.270, P < 0.05], resulting from a significant reduction in the mean duration of episodes by 44% [t(36) = 3.094, P < 0.001] (Fig. 4A). A two-way ANOVA revealed a significant effect of lighting conditions (F1,36 = 1.734, P < 0.01) and time (F2,72 = 27.97, P < 0.0001) on CLIMB. Also, CLIMB in DD subjects decreased progressively to become practically nil at the end of the 15-min test, whereas LD rats continued to climb during the entire test (Fig. 4B). During the 5-min FST retest, both groups climbed less than during the first 5 min of the 15-min FST [DD: −80%, t(18) = 2.953, P < 0.01; LD: −88%, t(18) = 6.492, P < 0.0001], and they spent a similar time (4.94 ± 2.13 s vs. 4.61 ± 3.52 s), with a similar duration and number of CLIMB episodes (Fig. 4B). Throughout the 15-min FST the swimming (SWIM) and pawing (PAW) times were similar in LD and DD groups, indicating that the increased IMMOB in DD animals was correlated exclusively with a decrease in CLIMB activity (Fig. 4A). Moreover, an ANOVA revealed a significant main effect for SWIM and PAW over time (F2,72 = 15.23, P < 0.0001 and F2,72 = 49.35, P < 0.0001, respectively). During the first FST session, 32% of LD animals dove underwater once or more, submerging their head or total body, whereas only 16% of the DD animals did so. This behavior was observed only during the first 5 min of the swim test (except for one LD rat that dove during the 5- to 10-min period). For animals that exhibited diving, the number of diving episodes and time spent on each dive was similar between lighting conditions but the behavior appeared to be much more energetic in LD than in DD rats.
Fig. 4.
Effect of continuous darkness on FST-associated behaviors. Values represent mean (± SEM) time of IMMOB, CLIMB, SWIM, and PAW during the entire 15-min FST period (A) and for 5-min blocks during the 15-min FST and the 5-min retest (B). Light deprivation (dark–dark) induced a significant IMMOB increase and CLIMB decrease during the 15-min FST (a, P < 0.01; b, P < 0.05). At each 5-min point of the 15-min FST and retest the light-deprived group showed more IMMOB (a, P < 0.0001; b, P < 0.001; c, P < 0.001; d, P < 0.01), and CLIMB time was less during the first 5 min of the initial FST (a, P < 0.05). In both groups, CLIMB was practically absent during the retest and was significantly less than the first 5 min of the 15-min FST (c, P < 0.0001; b, P < 0.01). As compared with the first 5 min of the previous FST, during the retest PAW time increased and the SWIM time decreased for both groups (a and b, P < 0.0001). No differences in SWIM and PAW times were observed between lighting conditions during the retest. There were 19 rats per group.
Locomotor Activity Is Increased by DD.
We examined whether the differences in FST performance between lighting conditions emerged from differences in overall activity caused by the circadian activity phase at which the test took place. For this, we evaluated the motor activity of LD and DD animals in a locomotor box at the same clock time as when the FST was conducted. Compared with the LD group, animals housed in DD showed increased locomotor activity that reached significance only during the last 30 min of a 1-h test [+70%, t(16) = 2.250, P < 0.05].
DD Does Not Affect Body or Adrenal Weights but Increases Sensitization to Stress.
As described (9), 6 weeks in DD did not appear to be stressful for nocturnal rats. It did not affect body weight gain [LD: +224 ± 13 g; DD: 233 ± 16 g; t(22) = 0.46, not significant (NS)] nor adrenal weights (left+right) [LD: 0.070 ± 0.003 g; DD: 0.060 ± 0.004 g; t(17) = 2.021, NS]. However, during the first FST, DD animals produced significantly more fecal pellets than LD rats [LD: 6 ± 1, n = 18; DD: 8 ± 1, n = 17; t(34) = 2.324, P < 0.05]. During the FST retest both groups defecated less than during the previous test [LD: 4 ± 1; DD: 5 ± 0; t(17) = 3.828, P < 0.001; DD: −38%; t(16) = 4.896, P < 0.0001], but there was no difference between LD and DD groups.
Desipramine (DMI) Decreases Neural and Behavioral Effects of DD.
We treated DD rats with DMI (10) to test whether an antidepressant could reverse the above effects of light deprivation (for methods, see SI Text). The number of PARP+ or CC3+ NA-LC neurons was ≈8- to 10-fold higher in rats kept for 9 weeks in DD compared with LD [t(12) = 24.60, P < 0.0001; t(10) = 8.312; P < 0.0001, for cleaved PARP and CC3, respectively]. Also, fewer DβH fibers were found in PFC of the DD group (most noticeably in layer I). Many of these were tortuous, swollen, intensely stained axons, with fewer boutons [−35%, t(9) = 2.773, P < 0.05]. Chronic administration of DMI (19 ± 1 mg/kg per day; see SI Text) in weeks 6–9 of DD treatment attenuated the apoptotic index in LC of DD rats [cleaved PARP: −78%, t (8) = 18.03, P < 0.0001; CC3: −55%, t(6) = 3.602; P < 0.01] and promoted NA axonal regrowth in DD rats without affecting bouton density in PFC (1.62 ± 0.25, n = 7 vs. 1.68 ± 0.17, n = 6). In the FST with DD rats, DMI decreased IMMOB time [−54%, t(12) = 2.718, P < 0.05] and improved escape behaviors by increasing time spent in SWIM [+38%, t(12) = 2.619, P < 0.05].
Discussion
The present study shows that long-term DD causes profound changes in NA-LC, 5-HT-raphe, and DA-VTA neurons that include increased apoptotic markers and loss of cortical NA fibers/boutons; these changes were associated with behavioral indices of depression such as increased IMMOB and reduced escape behaviors (CLIMB) during a FST.
LC is the only source of NA in neocortex (11), and the decrease of NA fibers/boutons in PFC (most noticeable in layer I) could reflect fiber or neuron degeneration, or some dysfunction of intact LC-NA neurons (e.g., loss of DβH in terminals). We found that DD activates the molecular machinery for apoptosis in NA-LC, 5-HT-raphe, and DA-VTA neurons. PARP is a key DNA-binding protein that supports enzymatic DNA repair. Once PARP is cleaved by caspase enzymes, DNA repair is inhibited and apoptosis becomes irreversible (12). After rats were in DD for 6 weeks we observed a dramatic increase in the expression of cleaved PARP in NA-LC, 5-HT-DR/MnR, and DA-VTA neurons when compared with these neurons in rats maintained on a LD schedule. These results confirm that DD leads to DNA damage, presumably decreasing the synthesis of cellular proteins that are necessary for normal neural function. We also found that many NA-LC cells had increased activation of CC3, a marker of impending neuronal death (13). However, we saw no indication of necrosis or chromatolysis after DD, indicating that this time point may represent the first stages in the process of apoptosis (12). The mechanisms that induce DNA damage under DD remain to be determined.
Repeated mild stress induces NA-LC axonal sprouting in cortex (14), whereas intense stress damages or otherwise decreases cortical LC terminals (15), accompanied by a depression-like behavioral state in animals (6, 7). Thus, to analyze the effect of DD on mood, we first evaluated its potential stress effect. Light deprivation did not affect body weight gain or adrenal weight as compared with LD controls, in agreement with a previous study that also found no changes in plasma adrenocorticotropin hormone or corticosterone after DD treatment for 6 weeks (9). These results indicate that the LC damage caused by DD is not a result of chronic stress.
Most studies using animal models of depression measure signs of helplessness that are caused by exposure to an unnatural stressor such as forced walking (6, 7) or forced SWIM (16). A more authentic animal model would be based on spontaneous despair behavior; we measured this in our study by using a modified FST without the stressors that could contribute to an escape deficit (16). We modified the usual FST by filling the tank with warm water, habituating rats to handling, and providing the rats with a flotation aid. Under these conditions, LD rats showed almost no IMMOB on the first FST, indicating that the escape deficit in the DD group was primarily caused by the DD treatment. Furthermore, the use of flotation aids also allowed more confident scoring by eliminating movements related to drowning that may be scored during a traditional FST as IMMOB events (17, 18).
CLIMB and SWIM times are positively correlated with NA-LC and 5-HT status, respectively (17). In agreement with this, we found that DD rats had substantially decreased DβH+ fibers/boutons and decreased CLIMB without changes in 5-HT fibers or SWIM times.
We examined whether the differences in FST performance between lighting conditions were caused by locomotor activity differences in the two lighting conditions. DD increased (rather than decreased) ambulatory activity in a novel environment, indicating that the increased IMMOB described here was not caused by a deficit in motor activity or response to salient stimuli. Moreover, it is unlikely that the anatomical and behavioral depressive-like features observed in DD were caused by alterations in sleep because in DD rats there was no overall change in the total daily amounts of sleep or wakefulness (8).
We predict that light-deprived rats could be a useful tool for validating new antidepressant therapies. Our results showing that DMI can decrease the effects of DD treatment on immobilization in the FST are consistent with this possibility.
The mechanism by which DMI attenuated the behavioral effects of DD is unclear. This compound selectively and substantially increases NA availability (10) and decreased apoptosis in NA-LC neurons and apparent NA fiber loss in PFC (present results), indicating that effects on the NA system may be involved. This conclusion is consistent with previous results showing that DMI can reverse NA fiber loss in a rat depression model (19).
The beneficial effect of bright light on mood, the elevated risk of depression with limited light exposure/intensity, and the comorbidity between mood disorders and abnormal circadian rhythms highlight the potential participation of the suprachiasmatic nucleus and its entrainment by light in the neurobiological mechanisms leading to mood disorders (5). We recently showed that the amplitude of the suprachiasmatic nucleus-dependent circadian sleep–waking rhythm is decreased in rats after DD, or after lesions of LC projections, and is associated with a loss of NA-LC terminals in the frontal cortex of DD subjects (8). We propose that the absence of light modifies the suprachiasmatic nucleus influence over the LC, raphe, VTA, and similar structures that regulate the sleep–wake cycle and mood (20–22).
Previous studies showed that specific lesions limited to the NA-LC by N- (2-chloroethyl)-N-ethyl-2-bromobenzylamine (DSP-4) had no effect on, or tended to decrease, IMMOB during a FST (18, 23). The discrepancy between these and the present results may occur either because systems other than those studied here are involved in the depressive phenotype that follows DD (e.g., histamine) (24), or DSP-4 lesions at the short survival time used in those previous studies might have affected non-LC systems (25, 26).
The FST has been questioned as a model of despair. It has been argued that increased IMMOB reflects “learning to be immobile,” or fatigue, rather than behavioral despair (27, 28). In our study, DD animals showed a similar increase in IMMOB time in the beginning and throughout all of the 15-min FST. This finding is inconsistent with the possibility that this behavior reflects development of a coping strategy or fatigue during the FST, but rather that DD rats had a preexisting behavioral deficit. The NA-LC impairment caused by DD may result in an inability to cope with acute stressful situations such as the FST, causing the animal to exhibit helplessness as expressed by an escape deficit (29). We interpret our results to suggest that DD rats were predisposed to exhibit a passive coping strategy at the outset of the first FST session, consistent with a depressive phenotype.
Increased anxiety is a common phenomenon in depression, and is related to central NA dysfunction (4, 30). The number of fecal boli excreted during the FST is an index of emotionality (31) and a trait stress response in animal models of anxiety (32). Exposed for the first time to a FST, DD rats defecated more than LD rats, which might indicate dysregulation of stress responses (30) after DD. We hypothesize that an absence of light may not be stressful by itself, but may sensitize the autonomic nervous system to stressors.
Finally, subtle damage in PFC (where we observe a loss of NA fibers and boutons in DD rats) could produce many behavioral changes, including altered decision-making and attention. Moreover, the NA-LC is importantly involved in decision- and attention-related functions (33). Thus, it would not be surprising for the dysfunction in LC described in DD rats to produce cognitive impairments, another hallmark of depression (2, 4).
Our results indicate that light stimulation may be required for intact functioning of LC, raphe, and VTA neurons and maintenance of their related behavioral roles (e.g., in mood, sleep, decision-making, attention). Future studies are needed to determine the threshold for DD for developing such abnormalities, and whether subsequent light exposure can reverse these deficits. We predict that DD may be less deleterious for nocturnal wild rats living in burrows and adapted to long periods of darkness (i.e., Norway rats), than for rats born and raised in constant LD conditions. In addition, nocturnal animals may be less sensitive to DD than diurnal animals (36), so that deleterious effects of DD may occur in humans with less extreme light environments than used here.
Materials and Methods
Subjects and Housing.
Adult Sprague–Dawley male rats (200–250 g) were individually placed in transparent cages (47 cm long × 26 cm wide × 21 cm high) and maintained in a ventilated chamber (23 ± 1°C) with free access to food and water. Multiple cages were situated together throughout the treatment period to allow social contact and avoid extreme sensory deprivation. We showed previously that 6 weeks of DD caused a significantly greater impairment of NA-LC function than 3 weeks of DD (8). Therefore, animals were kept during 6 weeks either in a 12:12 LD schedule (lights on 0500 h, 190–250 lux inside the cage), or in DD (0 lux). Cages were exchanged with clean cages weekly, and animals were handled at randomized times twice per week under white light (≈200 lux, LD group) or red light (1–2 lux, DD group). The University of Pennsylvania Institutional Animal Care and Use Committee approved all procedures.
Histology.
A group of animals kept in LD or DD conditions for 6 weeks, and not subjected to behavioral testing, was deeply anesthetized with sodium pentobarbital (60 mg/kg, i.p.) and perfused with 150 ml of saline followed by 500 ml of 0.1 M PBS (pH 7.4) containing 0.25% glutaraldehyde, 4% paraformaldehyde, and 0.12% picric acid. The brain was removed, postfixed overnight in the fixative without glutaraldehyde at 4°C, and immersed in 0.1 M PBS containing 30% sucrose. Brains were frozen with CO2 and cut on a cryostat in coronal sections. Sections from LD vs. DD animals were processed together to reduce variability of staining and allow comparisons between groups.
PARP immunodetection.
Apoptosis was assessed by incubating overnight at room temperature 20-μm-thick free-floating sections of LC, DR/MnR or VTA in a mixture of rabbit antiserum against the p85 fragment of PARP (1:100; Promega) and mouse antiserum against TH (1:1,000; Incstar) for LC and VTA, or mouse antiserum against 5-HT (1:20,000; gift from Lucienne Leger, Université Claude Bernard, Lyon, France) for raphe nuclei. After washes, sections were placed for 90 min in a mixture of Alexa Fluor 594 donkey anti-rabbit IgG (red, 1:400) and Alexa Fluor 488 donkey anti-mouse IgG (green, 1:400; both from Molecular Probes) at room temperature.
CC3.
Free-floating 20-μm-thick sections of LC, DR/MnR, and VTA were incubated with rabbit antiserum against cleaved CC3 (1:100; Cell Signaling) and mouse antiserum against TH (1:1,000; Incstar) for LC and VTA, or mouse antiserum against 5-HT for raphe nuclei (as described above). After washes, sections were placed for 60 min in a mixture of Alexa Fluor 594 donkey anti-rabbit IgG (red, 1:200) and Alexa Fluor 488 donkey anti-mouse IgG (green, 1:200, both from Molecular Probes) at room temperature.
Nissl staining.
Necrosis was assessed by analyzing the cell nuclei for changes in Nissl staining after light deprivation. Twenty-micrometer-thick free-floating sections of LC, DR/MnR, or VTA were immersed in hematoxylin QS for a few seconds, washed in distilled water, dehydrated, cleared in xylene, and coverslipped.
Immunostaining of Aminergic Fibers in PFC.
Free-floating 40-μm-thick sections through PFC were incubated overnight at room temperature in mouse anti-DβH primary antibody (1:1,000; Chemicon), mouse anti-5-HT primary antibody (1:200,000; gift from Lucienne Leger), or mouse anti-TH primary antibody (1:1,000; Chemicon, for DA). After washes the sections were incubated for 90 min in biotinylated donkey anti-mouse IgG (1:1,000; Jackson ImmunoResearch) followed by avidin-biotin-peroxidase complex (ABC; 1:1,000; VECTASTAIN Elite kit, Vector Laboratories). Immunolabeling was visualized by incubation in 22% 3,3′-diaminobenzidine-4HCl (Sigma) containing 4% nickel ammonium sulfate (only for DβH) and 1% H2O2 in 0.05 M Tris·HCl buffer (pH 7.6) for 6–10 min at room temperature. One wash in Tris and extensive washes with 0.1 M PBS stopped the reaction.
Quantification of Apoptotic Neurons.
Quantification of DNA-damaged neurons was done by using Openlab image processing software (Improvision) on a Macintosh computer that was linked to a microscope (Leica DMR-XA) and digital camera (Princeton Instruments). Color images of the areas of interest were taken and saved to disk. Labeled neurons were manually counted on a high-resolution monitor with a point counter tool on the saved digitized image. This tool simultaneously marked and counted each cell so that no cells could be counted twice and the total number of cells counted was available. Only those neurons whose nuclei could be visualized were considered for analysis. Neurons were counted in one section of each rat at each of three rostrocaudal levels of LC (−9.8 to −10.3 mm from bregma), at two levels of DR and MnR nuclei (−7.3 to −8 mm from bregma), and at one level of rostral-mid-VTA (−5 to −5.8 mm from bregma) (34) by two observers blind to the experimental conditions. An apoptotic index was defined as the percentage of TH+ or 5-HT+ neurons that were doubly labeled for PARP-p85 or CC3 fragment.
Quantification of Monoaminergic Boutons in the PFC.
We quantified the density of monoaminergic boutons in PFC to evaluate the functional status of these systems. Immunohistochemically stained boutons (discrete and uniform bead-like enlargements along fibers) were counted with a computer-assisted quantification system that included a Leica DMR-BE microscope and a computer equipped with Stereo Investigator (MicroBrightField). The dissector counting technique was used, which randomly counts objects in a defined volume of a structure (35). After the area of interest was outlined (width: 800 μm; length: 2,200 μm), boutons were counted in cingulate-prelimbic cortex (+3.70 mm from bregma; ref. 34) by using a ×100 oil immersion objective by an observer blind to the experimental condition. The stage was moved in the z axis to count boutons between 1 and 5 μm from the cut surface of the section, in a 20-μm × 20-μm counting frame that was moved randomly across the selected cortical area. The number of sampling sites was previously determined to produce the minimum coefficient error (<0.2). Thirty sampling sites were analyzed to quantify DβH+ boutons, and 20 sampling sites were used for 5-HT+ and TH+ boutons. The number of boutons per μm3 was obtained by dividing the total number of boutons by the number of sampling sites multiplied by the dissector volume.
Behavioral Testing.
The FST and locomotor activity were tested between 0700 and 1100 h (LD group) and 1600 and 1900 h (DD group), times of day that would correspond to the rest period for each group as determined in our previous study (8).
FST.
At the end of 6 weeks of LD or DD housing, animals were individually submitted to a modified FST. Luminance during the FST was 70–80 lux inside the tank for the LD group and 1 lux for the DD group. Rats were fitted with a “bubble wrap” flotation aid on the midscapular area and placed in a cylindrical Plexiglas tank (38.5 cm high × 30.5 cm diameter; INSTECH) filled with warm water (30°C). The tank was placed inside a black cylinder. Thus, visual stimulation during the FST was also minimal for LD subjects and would not be substantially different from DD animals. Rats were submitted to the swim test during 15 min on the first day and retested for 5 min 24 h later. A digital video camera mounted over the tank recorded behaviors during both FSTs. The following measures were scored on a video monitor from taped images by two observers blind to the experimental conditions of the animals: (i) IMMOB: the animal was completely motionless, no movements of limb, tail, or head; (ii) CLIMB: front paw movements against the tank wall bringing part of the body out of the water; (iii) PAW: gentle movement of only one front paw against the wall, while the rest of the body remained motionless; and (iv) diving: immersion of the head or total body. SWIM was characterized by SWIM around the tank or crossing the cylinder by using the paws or tail without elevating the body out of the water. Individual swim epochs were often very short events, making reliable scoring as separate events impossible. Therefore, we deduced SWIM time by subtracting the cumulative time for other behaviors from the total duration of the FST. Immediately after the FST, each animal was removed from the water, towel-dried, and returned to its home cage. The water was changed and the cylinder was cleaned between each rat. We determined the numbers and durations of episodes for each behavior during the FST. For that, the beginning and end of each behavioral epoch was marked with a digital pulse that was led to a computer, allowing scoring and analyses of behavioral data.
Locomotor activity.
Rats kept for 6 weeks in LD or DD conditions, and not subjected to the FST, were placed individually in Plexiglas enclosures of 37 cm × 39 cm × 30 cm during the same clock time and under the same lighting conditions as the FST. These enclosures contained four photobeams placed 3 cm above the floor and connected via an interface (MED Associates) to a computer that recorded photocell beam interruptions. During a 1-h session, three beams broken in a series was scored as horizontal locomotor activity.
Physiological Parameters.
Body weight was measured at the beginning of the adaptation to lighting conditions, and 3 and 6 weeks later, before behavioral tests. The number of feces was counted at the end of the 15-min FST and retest. Also, a group of animals kept in LD or DD conditions for 6 weeks, and not tested for behavior, were deeply anesthetized with sodium pentobarbital (60 mg/kg, i.p.), and their right and left adrenal glands were removed and weighed.
Statistical Analysis.
Results are expressed as mean ± SEM, and they were statistically evaluated for significance by using an unpaired Student's t test except for the temporal analysis of the 15-min FST. For that, we used a two-way ANOVA (lighting conditions × time) with repeated measures over time (each 5-min time point of the 15-min FST) followed by post hoc comparisons (Newman–Keuls test) when the significant main effects were indicated.
Supplementary Material
Acknowledgments.
We thank Yan Zhu for immunofluorescence tissue processing and assistance with neuronal counting; Steven Arnold for use of stereology facilities; Julie Leu for advice on apoptotic techniques; Leslie Ramsey, Mona Tarun Vakil, and Natalya Biskup for assistance during the FST; and Horacio de la Iglesia and John Neumaier (University of Washington, Seattle) for comments on the manuscript. This work was supported by Public Health Service Grants NS 24698 and DA 017289, and a Young Investigator Award from the Mental Health Research Association (National Alliance for Research on Schizophrenia and Depression).
Footnotes
The authors declare no conflict of interest.
Some of the data contained herein were presented at the 34th Annual Meeting of the Society for Neuroscience, October 23–27, 2004, San Diego; the 35th Annual Meeting of the Society for Neuroscience, November 12–16, 2005, Washington, DC; and the 39th Winter Conference on Brain Research, January 21–27, 2006, Steamboat Springs, CO.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0703615105/DC1.
References
- 1.Garlow SJ, Nemeroff CB. In: Neurobiology of Mental Illness. Charrney DS, Nestler EJ, editors. New York: Oxford Univ Press; 2004. pp. 440–460. [Google Scholar]
- 2.Harro J, Oreland L. Depression as a spreading adjustment disorder of monoaminergic neurons: A case for primary implication of the locus coeruleus. Brain Res Brain Res Rev. 2001;38:79–128. doi: 10.1016/s0165-0173(01)00082-0. [DOI] [PubMed] [Google Scholar]
- 3.Klimek V, Schenck JE, Han H, Stockmeier CA, Ordway GA. Dopaminergic abnormalities in amygdaloid nuclei in major depression: A postmortem study. Biol Psychiatry. 2002;52:740–748. doi: 10.1016/s0006-3223(02)01383-5. [DOI] [PubMed] [Google Scholar]
- 4.Ressler KJ, Nemeroff CB. Role of serotoninergic and noradrenergic systems in the pathophysiology of depression and anxiety disorders. Depress Anxiety Suppl. 2000;1:2–19. doi: 10.1002/1520-6394(2000)12:1+<2::AID-DA2>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 5.Duncan WC., Jr Circadian rhythms and the pharmacology of affective illness. Pharmacol Ther. 1996;71:253–312. doi: 10.1016/s0163-7258(96)00092-7. [DOI] [PubMed] [Google Scholar]
- 6.Kitayama I, et al. Degeneration of locus coeruleus axons in stress-induced depression model. Brain Res Bull. 1994;35:573–580. doi: 10.1016/0361-9230(94)90171-6. [DOI] [PubMed] [Google Scholar]
- 7.Kitayama I, et al. Long-term stress degenerates, but imipramine regenerates, noradrenergic axon s in the rat cerebral cortex. Biol Psychiatry. 1997;42:687–696. doi: 10.1016/s0006-3223(96)00502-1. [DOI] [PubMed] [Google Scholar]
- 8.Gonzalez MMC, Aston-Jones G. Circadian regulation of arousal: Role of the noradrenergic locus coeruleus system and light exposure. Sleep. 2006;29:1327–1336. doi: 10.1093/sleep/29.10.1327. [DOI] [PubMed] [Google Scholar]
- 9.Vernikos-Danellis J, Winget CM, Hetherington NW. Diurnal rhythm of the pituitary-adrenocortical response to stress: Effect of constant light and constant darkness. Life Sci Space Res. 1970;8:240–246. [PubMed] [Google Scholar]
- 10.Benmansour S, et al. Regulation of the norepinephrine transporter by chronic administration of antidepressants. Biol Psychiatry. 2004;55:313–316. doi: 10.1016/s0006-3223(03)00676-0. [DOI] [PubMed] [Google Scholar]
- 11.Aston-Jones G, Shipley MT, Grzanna R. In: The Rat Nervous System. Paxinos G, editor. New York: Academic; 1995. pp. 183–213. [Google Scholar]
- 12.Jellinger KA. Cell death mechanisms in Parkinson's disease. J Neural Transm. 2000;107:1–29. doi: 10.1007/s007020050001. [DOI] [PubMed] [Google Scholar]
- 13.Thornberry NA, Lazebnik Y. Caspases: Enemies within. Science. 1998;281:1312–1316. doi: 10.1126/science.281.5381.1312. [DOI] [PubMed] [Google Scholar]
- 14.Nakamura S, Sakaguchi T, Aoki F. Electrophysiological evidence for terminal sprouting of locus coeruleus neurons following repeated mild stress. Neurosci Lett. 1989;100:147–152. doi: 10.1016/0304-3940(89)90675-7. [DOI] [PubMed] [Google Scholar]
- 15.Nakamura S, Kitayama I, Murase S. Electrophysiological evidence for axonal degeneration of locus coeruleus neurons following long-term forced running stress. Brain Res Bull. 1991;26:759–763. doi: 10.1016/0361-9230(91)90172-g. [DOI] [PubMed] [Google Scholar]
- 16.Drugan RC, et al. Impact of water temperature and stressor controllability on swim stress-induced changes in body temperature, serum corticosterone, and immobility in rats. Pharmacol Biochem Behav. 2005;82:397–403. doi: 10.1016/j.pbb.2005.09.011. [DOI] [PubMed] [Google Scholar]
- 17.Detke MJ, Rickels M, Lucki I. Active behaviors in the rat forced swimming test differentially produced by serotonergic and noradrenergic antidepressants. Psychopharmacology. 1995;121:66–72. doi: 10.1007/BF02245592. [DOI] [PubMed] [Google Scholar]
- 18.Cryan JF, Page ME, Lucki I. Noradrenergic lesions differentially alter the antidepressant-like effects of reboxetine in a modified forced swim test. Eur J Pharmacol. 2002;436:197–205. doi: 10.1016/s0014-2999(01)01628-4. [DOI] [PubMed] [Google Scholar]
- 19.Nakamura S. Antidepressants induce regeneration of catecholaminergic axon terminals in the rat cerebral cortex. Neurosci Lett. 1990;111:64–68. doi: 10.1016/0304-3940(90)90345-a. [DOI] [PubMed] [Google Scholar]
- 20.Aston-Jones G, Sheng C, Zhu Y, Oshinsky M. A neural circuit for circadian regulation of arousal. Nat Neurosci. 2001;4:732–738. doi: 10.1038/89522. [DOI] [PubMed] [Google Scholar]
- 21.Deurveilher S, Semba K. Indirect projections from the suprachiasmatic nucleus to major arousal-promoting cell groups in rat: Implications for the circadian control of behavioral state. Neuroscience. 2005;130:165–183. doi: 10.1016/j.neuroscience.2004.08.030. [DOI] [PubMed] [Google Scholar]
- 22.Fite KV, Wu PS, Bellemer A. Photostimulation alters c-Fos expression in the dorsal raphe nucleus. Brain Res. 2005;1031:245–252. doi: 10.1016/j.brainres.2004.10.054. [DOI] [PubMed] [Google Scholar]
- 23.Harro J, et al. Dose-dependent effects of noradrenergic denervation by DSP-4 treatment on forced swimming and beta-adrenoceptor binding in the rat. J Neural Transm. 1999;106:619–629. doi: 10.1007/s007020050184. [DOI] [PubMed] [Google Scholar]
- 24.Lamberti C, Ipponi A, Bartolini A, Schunak W, Malmberg-Aiello P. Antidepressant-like effects of endogenous histamine and of two histamine H1 receptor agonists in the mouse forced swim test. Br J Pharmacol. 1998;123:1331–1336. doi: 10.1038/sj.bjp.0701740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fety R, Misere V, Lambas-Señas L, Renaud B. Central and peripheral changes in catecholamine-synthesizing enzyme activities after systemic administration of the neurotoxin DSP-4. Eur J Pharmacol. 1986;124:197–202. doi: 10.1016/0014-2999(86)90145-7. [DOI] [PubMed] [Google Scholar]
- 26.Hallman H, Sundström E, Jonsson G. Effects of the noradrenaline neurotoxin DSP-4 on monoamine neurons and their transmitter turnover in rat CNS. J Neural Transm. 1984;60:89–102. doi: 10.1007/BF01245027. [DOI] [PubMed] [Google Scholar]
- 27.Borsini F, Meli A. Is the forced swimming test a suitable model for revealing antidepressant activity? Psychopharmacology. 1988;94:147–160. doi: 10.1007/BF00176837. [DOI] [PubMed] [Google Scholar]
- 28.Sillaber I, Holsboer F. In: Neurobiology of Mental Illness. Charrney DS, Nestler EJ, editors. New York: Oxford Univ Press; 2004. pp. 380–396. [Google Scholar]
- 29.Stanford SC. Central noradrenergic neurons and stress. Pharmacol Ther. 1995;68:297–342. doi: 10.1016/0163-7258(95)02010-1. [DOI] [PubMed] [Google Scholar]
- 30.Charney DS, Bremner JD. In: Neurobiology of Mental Illness. Charney DS, Nestler EJ, editors. New York: Oxford Univ Press; 2004. pp. 605–627. [Google Scholar]
- 31.Armario A, Gavalda A, Marti O. Forced swimming test in rats: Effect of desipramine administration and the period of exposure to the test on struggling behavior, swimming, immobility, and defecation rate. Eur J Pharmacol. 1988;158:207–212. doi: 10.1016/0014-2999(88)90068-4. [DOI] [PubMed] [Google Scholar]
- 32.Fuchs E, Flügge G. In: Neurobiology of Mental Illness. Charrney DS, Nestler EJ, editors. New York: Oxford Univ Press; 2004. pp. 546–557. [Google Scholar]
- 33.Aston-Jones G, Cohen JD. Adaptive gain and the role of the locus coeruleus-norepinephrine system in optimal performance. J Comp Neurol. 2005;493:99–110. doi: 10.1002/cne.20723. [DOI] [PubMed] [Google Scholar]
- 34.Paxinos G, Watson C. In: The Rat Brain in Stereotaxic Coordinates. Paxinos G, Watson C, editors. London: Academic; 1998. [Google Scholar]
- 35.West MJ. Stereological methods for estimating the total number of neurons and synapsis: issues of precision and bias. Trends Neurosci. 1999;22:S1–S6. doi: 10.1016/s0166-2236(98)01362-9. [DOI] [PubMed] [Google Scholar]
- 36.Jiao Y-Y, Lee TM, Rusak B. Brain Res. 1999;817:93–103. doi: 10.1016/s0006-8993(98)01218-9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.