Abstract
Planning a response to an outbreak of a pandemic strain of influenza is a high public health priority. Three research groups using different individual-based, stochastic simulation models have examined the consequences of intervention strategies chosen in consultation with U.S. public health workers. The first goal is to simulate the effectiveness of a set of potentially feasible intervention strategies. Combinations called targeted layered containment (TLC) of influenza antiviral treatment and prophylaxis and nonpharmaceutical interventions of quarantine, isolation, school closure, community social distancing, and workplace social distancing are considered. The second goal is to examine the robustness of the results to model assumptions. The comparisons focus on a pandemic outbreak in a population similar to that of Chicago, with ≈8.6 million people. The simulations suggest that at the expected transmissibility of a pandemic strain, timely implementation of a combination of targeted household antiviral prophylaxis, and social distancing measures could substantially lower the illness attack rate before a highly efficacious vaccine could become available. Timely initiation of measures and school closure play important roles. Because of the current lack of data on which to base such models, further field research is recommended to learn more about the sources of transmission and the effectiveness of social distancing measures in reducing influenza transmission.
Keywords: influenza antiviral agents, mitigation, prophylaxis, social distancing, transmission
The ongoing epidemic of highly pathogenic H5N1 influenza infection in global avian populations has made influenza pandemic preparedness a top public health priority. The interventions being considered fall into two broad classes: medical interventions and nonpharmaceutical interventions (NPIs). Medical interventions include the use of antiviral agents for case treatment, targeted prophylaxis of their known contacts, and prophylactic vaccination. NPIs include social distancing, infection control, and travel restrictions. Social distancing measures include isolation of diagnosed cases, quarantine of households of diagnosed cases, closing of schools, and reducing contacts at workplaces or in the community more generally. Many NPIs were used in U.S. cities during the 1918 pandemic, and appeared relatively successful in some instances, although retrospective assessment is difficult (1–3).
Fundamental to the dynamics of an epidemic is the basic reproduction number, R0, and the generation time, Tg, of the pathogen (4). R0 is the average number of secondary cases produced by each primary case at the start of an epidemic in a previously unaffected population, and Tg is the average time between infection of an index case and infection of the secondary cases they produce. Although the R0 of a future newly emergent influenza strain is unknown, previous estimates are 1.89 from the pandemic in 1968 in Hong Kong (5), and 1.5–1.7 in 1957 in Great Britain (6). The reproductive number of the first wave of the 1918 pandemic A(H1N1) in the United States was estimated as 2–3 (7) and 1.7–2.0 (6). Based on past experience, one might assume for a newly emergent pandemic influenza that R0 = 1.7–2.0 and Tg is as short as 3 days. Hence, although an influenza pandemic may be explosive, it is also potentially containable, because reducing transmission by as much as half might achieve an R0 < 1.
Epidemic models represent a powerful tool for gaining insight into how the dynamics of an epidemic are affected by interventions (8). Small- (9, 10) and large-scale (6, 11, 12) individual-based stochastic simulations have previously examined the potential effectiveness of various interventions. However, different research studies seldom examine the same interventions, so results are difficult to compare.
In this article, three groups supported in part by the National Institutes of General Medical Sciences MIDAS network coordinated their efforts to use their own stochastic simulation models to examine the same set of intervention strategies. The intervention scenarios and baseline R0 values examined were selected in consultation with government employees working with the Homeland Security Council and the Department of Health and Human Services in the United States, and thus are particularly relevant for the U.S. pandemic plan. One research group is a collaboration of investigators at the University of Washington and Fred Hutchinson Cancer Research Center in Seattle and the Los Alamos National Laboratories (UW/LANL) (9, 11). One group is a collaboration of investigators at Imperial College and the University of Pittsburgh (Imperial/Pitt) (6). The third group is at the Virginia Bioinformatics Institute of the Virginia Polytechnical Institute and State University (VBI) (13, 14).
Intervention Options
We considered a set of interventions consisting of antiviral treatment and household isolation of identified cases, prophylaxis and quarantine of their household contacts, closure of schools, social distancing in the workplace, and social distancing in the community at large. Because these interventions are combinations of targeted and general interventions, we call them targeted-layered containment (TLC) approaches. We examined different levels of ascertainment of symptomatic influenza cases, compliance with the interventions, and cumulative illness attack rate thresholds for initiating interventions.
Initiating the Interventions.
Each baseline scenario has a common threshold for all interventions, which varies across the scenarios from 1% to 0.01% cumulative illness attack rate of symptomatic cases.
Ascertainment of Cases.
Ascertainment of cases is key for targeted interventions, especially the use of influenza antivirals, case isolation, and quarantine of contacts. Rapid, specific diagnosis will be important. We assume that only 67% percent of influenza infections are symptomatic. We considered two levels of ascertainment of symptomatic influenza cases, namely, 60% and 80%. We assume no asymptomatic influenza infections are ascertained. These levels of ascertainment and pathogenicity correspond to ascertaining 40% and 54% of influenza infections. Interventions within the households of ascertained cases include the following:
Treatment of ascertained cases. All ascertained cases are treated with one course of antiviral drug for 5 days beginning one day after the onset of symptoms. In the UW/LANL model, 5% of treated cases stop taking the drug after 1 day.
Targeted antiviral prophylaxis (TAP) of household contacts. All household contacts receive one course (10 days) of prophylaxis beginning 1 day after the onset of symptoms of the index case. In the UW/LANL model, 5% of individuals who receive prophylaxis stop taking drug after 2 days.
Home isolation of cases. Ascertained cases are isolated in the home, but not isolated from the people with whom they live, with a compliance rate of 60% or 90%.
Quarantine of household contacts. Household contacts of ascertained cases are quarantined within the home for 10 days with a compliance rate of 30%, 60%, or 90%.
School Closure.
All schools, including primary, middle, and high schools, are closed at a particular threshold community cumulative illness attack rate. Once the schools are closed, children are expected to stay at home with a certain compliance rather than to increase community contacts. Compliance is modeled by the reduction in community contacts achieved—assumed to be 30%, 60%, or 90%. In the UW/LANL model, day care centers and small play groups of preschool children are also closed, and the same compliance rates apply. The other two models do not explicitly model day care centers and small play groups.
Liberal Leave Policy.
All symptomatic individuals retire to the home from the workplace one day after becoming ill.
Workplace Social Distancing.
At a particular threshold community cumulative illness attack rate, workplace contacts are reduced by a certain percent. In the baseline combination scenarios, the workplace contacts are reduced by 50%. Workplaces are not closed. Social distancing in the workplace might eventually be accomplished by staggering the arrivals of workers at work, encouraging people to work at home, or other measures.
Community Social Distancing.
Community social distancing represents policies resulting in fewer public activities, such as closing theaters, reducing visits to restaurants, shops, and other public locations, and banning mass gatherings. After a particular threshold attack rate, contacts within the community are reduced by a certain percent, 50% in the baseline combination scenarios. The three models differ in their implementation of community social distancing [see supporting information (SI) Text].
Although we have taken pains to ensure that the models represent the same situations, as described here and in the SI Text, there are subtle model-dependent differences in implementation.
The baseline scenario without intervention, scenario 1, and the five main TLC scenarios are summarized in Table 1. Scenario 2 is the least stringent intervention considered. In scenario 2, interventions are initiated after 1% of the population has developed symptomatic influenza, 60% of clinical cases are ascertained, compliance with quarantine and children staying home after school closing is 30%, and compliance with isolation is 60%. Scenarios 3 and 5 initiate interventions at an illness attack rate threshold of 0.1% and 60% of cases are ascertained, and differ primarily in assuming 60% versus 90% compliance with interventions. Scenarios 4 and 6 initiate interventions earlier at a threshold of 0.01% illness attack rate and 80% of cases are ascertained, and differ primarily in assuming 60% versus 90% compliance. Scenario 6 is the most stringent TLC intervention considered.
Table 1.
The combined scenarios of targeted layered containment
| Intervention | Scenario(Compliance %/Ascertainment %) |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 Base case |
2 30/60 |
3 60/60 |
4 60/80 |
5 90/60 |
6 90/80 |
||||||
| Achieved | Compliance | Achieved | Compliance | Achieved | Compliance | Achieved | Compliance | Achieved | Compliance | ||
| Symptomatic cases ascertained | 60 | 60 | 80 | 60 | 80 | ||||||
| In ascertained case household | |||||||||||
| Threshold | – | 1.0 | 0.1 | 0.01 | 0.1 | 0.01 | |||||
| Index case treated | – | 100 | * | 100 | 100 | 100 | 100 | ||||
| Contacts prophylaxed (TAP) | – | 100 | * | 100 | * | 100 | * | 100 | * | 100 | * |
| Home isolation of cases | † | 60 | 60 | 60 | 90 | 90 | |||||
| Quarantine of contacts | – | 30 | 60 | 60 | 90 | 90 | |||||
| School closure | – | 100 | 100 | 100 | 100 | 100 | |||||
| Threshold | – | 1.0 | 0.1 | 0.01 | 0.1 | 0.01 | |||||
| Children kept home‡ | – | 30 | 60 | 60 | 90 | 90 | |||||
| Workplace distancing | – | 50 | 50 | 50 | 50 | 50 | |||||
| Threshold | – | 1.0 | 0.1 | 0.01 | 0.1 | 0.01 | |||||
| Liberal leave | † | 100 | 100 | 100 | 100 | 100 | |||||
| Community social distancing | – | 50 | 50 | 50 | 50 | 50 | |||||
| Threshold | – | 1.0 | 0.1 | 0.01 | 0.1 | 0.01 | |||||
All numerical values are percentages.
*UW/LANL model assumes 5% stop taking drug after 1 day.
†In all three models, a proportion of symptomatic people retire to home even without intervention.
‡Compliance is % reduction in contacts or contact probabilities outside home.
Sensitivity Analyses.
We undertook the following sensitivity analyses based on scenario 2:
Use scenario 2, but vary the percent of workplace and community social distancing between 0 and 50%.
Vary the threshold from 0.0001% to 10% community illness attack rate for all interventions in scenario 2.
Vary the school closing threshold from 0.0001% to 10% community illness attack rate separately from the 1% threshold for other interventions in scenario 2.
Use scenario 2, but with antivirals used only for treatment of ascertained cases, with no prophylaxis of household contacts.
Follow scenario 2, but use only nonpharmaceutical interventions, with no antivirals used at all.
The UW/LANL and the Imperial/Pitt groups used their U.S. population models to undertake national-scale simulations of the full TLC as in scenario 2. UW/LANL also explored two further interventions with fewer layers in the U.S. population. One is partial TLC with antiviral treatment of ascertained cases but no prophylaxis, no school closure, and no liberal work leave. The other is with only 50% community social distancing and 50% reduction in long-distance travel and nothing else.
Transmissibility and Case Fatality Ratio.
One uncertainty of a future pandemic strain is how transmissible it will be. It is generally expected that the R0 in a new pandemic will be <2, and previously published articles have explored interventions in this range of R0. Interventions work better at lower R0 values. Here, the focus was on where interventions would break down, which required studying improbably high R0 values. We were interested in examining interventions at R0 values near 2.0, 2.4, and 3.0. At the lower R0, the UW/LANL and VBI models used a value of 2.1, and the Imperial/Pitt model used 1.9. This is referred to in the text as 1.9 (2.1). A few scenarios at an R0 of 1.6 that are reported in the text.
Another uncertainty of a future pandemic is the case fatality ratio. The estimated 2 in 100 case fatality ratio in the 1918 pandemic (7, 15) is two orders of magnitude larger than the estimated 2 in 10,000 in the 1957 and 1968 pandemics (16). Because the number of deaths that occur will be a fairly linear function of the number of cases and the case fatality ratio, we present only the illness attack rates, and not the number of deaths.
Some Aspects of the Simulation Models
All three models are stochastic, spatially structured, individual-based discrete time simulations. The social structures of the three models are constructed somewhat differently (see SI Text). The UW/LANL basic model is as described in ref. 11, the VBI model in ref. 14, the Imperial/Pitt model in ref. 6. See also refs. 9, 12, and 13.
The Chicago area models include the 8.6 million people between latitudes 41.2°N and 42.5°N, and longitudes 87.2°W and 88.5°W, extending slightly across the Wisconsin and Indiana borders. The large-scale simulations of the United States include ≈281 million people. Each model represents individuals mixing within households, schools, and workplaces, with mixing in the wider community represented differently (see SI Text and Table 3). Transmission can occur in any of the mixing groups represented in the respective models. All three models were calibrated to have age-specific attack rate patterns between those of the 1957 and 1968 pandemics. A few infections are introduced throughout the epidemic in each model as described in SI Text.
Table 3.
Percentage of infections by place and scenario, RR0 = 1.9 (2.1) in the Chicago population
| Scenario 1. No intervention |
Scenario 2 |
Scenario 3 |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Imperial | UW | VBI | Imperial | UW | VBI | Imperial | UW | VBI | |
| Illness attack rates | 42.4 | 46.8 | 44.7 | 7.3 | 2.8 | 3.9 | 1.1 | 0.31 | 1.3 |
| Places | |||||||||
| Home | 33.1 | 39.4 | 41.1 | 48.3 | 58 | 45.9 | 50.4 | 59 | 36.9 |
| Work | 21.8 | 14.5 | 28.6 | 12.9 | 10 | 27.8 | 13.5 | 10 | 18.7 |
| School | 16.0 | 18.8 | 23.3 | 11.7 | 11 | 9.6 | 9.0 | 11 | 2.7 |
| Day care | – | 1.1 | – | – | 0 | – | – | 0 | – |
| Play group | – | 0.8 | – | – | 0 | – | – | 0 | – |
| College | – | – | 3.3 | – | – | 12.3 | – | – | 40.0 |
| Shopping | – | – | 2.0 | – | – | 2.4 | – | – | 1.0 |
| Neighborhood | – | 17.7 | – | – | 15 | – | – | 15 | – |
| Neighborhood clusters | – | 7.7 | – | – | 5 | – | – | 4 | – |
| Other/Community | 29.0 | 0 | 1.7 | 26.6 | 0 | 2.0 | 23.8 | 0 | 0.8 |
| Totals | |||||||||
| Primary Groups* | 70.9 | 72.7 | 93.0 | 72.9 | 79 | 83.3 | 72.9 | 79 | 58.3 |
| Community† | 29.0 | 25.4 | 3.7 | 26.6 | 20 | 4.4 | 23.8 | 19 | 1.8 |
*Includes home, school, workplace, and for the UW/LANL model, day care and play groups.
†Includes groups subject to community social distancing.
In a stochastic, individual-based model, the chance that any susceptible individual will be infected by a contact with an infected person is random and related to the transmission probability for the situation of the contact. Antiviral prophylaxis is assumed to reduce the probability of becoming infected by a contact by 0.3, and if infected, to reduce the probability of developing illness by 0.60. Antiviral treatment or prophylaxis is assumed to reduce the probability of an infected person transmitting by 0.62 (17). Social distancing can lower the number of effective contacts or the transmission probabilities. Many other aspects of each simulation model occur stochastically; for example, whether a person develops symptoms, or whether a person complies with an intervention strategy. In these large populations with the continual seeding of infectives from outside, there is not much variability in the results. The Imperial/Pitt results are based on an average of ten realizations and those of the UW/LANL model on an average of five realizations. The VBI model is much more computer-intensive than the other two, so the results are based mostly on one realization. The results of a variability study are in the SI Text, SI Fig. 5, and SI Table 5.
Natural History.
The natural history within the human host of a future pandemic strain is unknown. All three models have the infectiousness developing before the onset of symptoms, but more of the infectiousness occurs before symptoms in the Imperial/Pitt model than in the other two. This results in a generation time for the UW/LANL and VBI models of ≈3.2 days, longer than ≈2.6 days in the Imperial/Pitt model. All three models assume that asymptomatic people are 50% as infectious per contact as symptomatic cases and that the probability of developing symptoms if infected (pathogenicity) is 67% (18).
Even in the absence of intervention, all three models assume that clinical disease affects individual behavior. In the UW/LANL and VBI models, 80%, 75%, and 50% of preschool children, school age children, and adults, respectively, with symptomatic influenza withdraw to the home from preschool, school, and work, within the first three days of illness onset. In the Imperial/Pitt model, 90% of symptomatic children do not attend school, 50% of symptomatic adults do not attend work, and community contacts of all symptomatic individuals are reduced by 50%.
Results
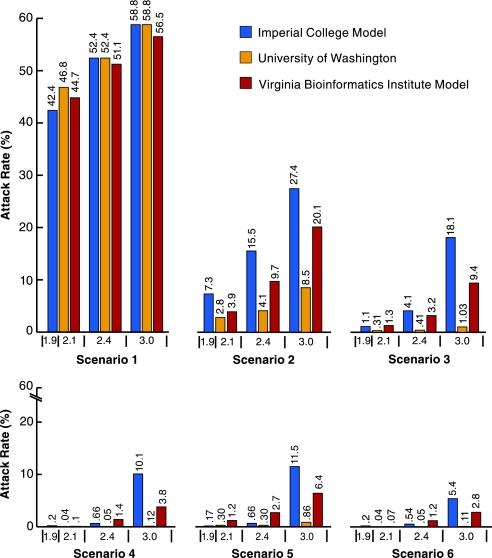
Table 2 and Fig. 1 show theresults. Increasing attack rates correspond to higher R0 values. In the absence of intervention, the three models produce similar illness attack rates, in the range 42.4–46.8% at an R0 of 1.9 (2.1), increasing to the range 56.5–58.8% at an R0 of 3.0. At the lowest R0, in all three models, all five baseline intervention scenarios are effective at reducing the illness attack rates. In scenario 2, at an R0 of 1.9 (2.1), the UW/LANL model achieves a 94% reduction in cases, the VBI model achieves a 91% reduction, and the Imperial/Pitt model achieves an 83% reduction. Although, in scenario 2 at the lower R0, the absolute values of the illness attack rates of the three models range over a factor of 2.6 from 2.8% to 7.3%, the relative effectiveness of the intervention in all three models is high, with the Imperial/Pitt model being the least optimistic. At lower thresholds, higher ascertainment, and higher compliance, the TLC combination is even more effective. At R0 of 1.6 (not in table), the UW/LANL and Imperial/Pitt models produce illness attack rates of 34.7% and 32.0% with no intervention, and 1.9% and 4.5% under the scenario 2 intervention, corresponding to 94% and 85% reductions, respectively.
Table 2.
Illness attack rates (%) and (antiviral courses per 1,000) using scenarios described in Table 1 in the Chicago population
| Scenario% compliance/ascertainment | Intervention threshold, % |
R0 = 1.9 (2.1) |
R0 = 2.4 |
R0 = 3.0 |
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Imperial | UW | VBI | Imperial | UW | VBI | Imperial | UW | VBI | ||
| 1 | NA | 42.4(0) | 46.8(0) | 44.7(0) | 52.4(0) | 52.4(0) | 51.1(0) | 58.8(0) | 58.8(0) | 56.5(0) |
| 2 30/60 | 1 | 7.3(104) | 2.8(38) | 3.9(59.1) | 15.5(239) | 4.1(61.2) | 9.7(140.4) | 27.4(421) | 8.5(138.4) | 20.1(275.1) |
| 3 60/60 | 0.1 | 1.1(17.9) | 0.31(4.3) | 1.3(29.7) | 4.1(69.8) | 0.41(6.2) | 3.2(70.6) | 18.1(300) | 1.03(17.1) | 9.4(189) |
| 4 60/80 | 0.01 | 0.22(4.6) | 0.040.76) | 0.10(3.2) | 0.66(14.2) | 0.05(1.1) | 1.4(41.7) | 10.1(210) | 0.12(2.5) | 3.8(105.9) |
| 5 90/60 | 0.1 | 0.17(3.2) | 0.30(4.1) | 1.2(28.8) | 0.66(14.2) | 0.30(5.8) | 2.7(59.4) | 11.5(192) | 0.86(14.2) | 6.4(132.2) |
| 6 90/80 | 0.01 | 0.20(4.2) | 0.04(0.76) | 0.07(2.3) | 0.54(9.4) | 0.05(1.0) | 1.2(35.5) | 5.4(112) | 0.11(2.1) | 2.8(80.2) |
The Imperial/Pitt model results are based on an average of 10 realizations, the UW/LANL results on an average of 5 realizations, and the VBI results mostly on one realization.
Fig. 1.
Influenza illness attack rates for three R0 values without intervention and with five scenarios of TLC intervention by using the three different models (Chicago population). See Table 1 for a description of scenarios. The R0 values of 1.9 and 2.1 are considered as a single comparison.
At the higher R0 of 3.0, the UW/LANL model has an 85% reduction, whereas the VBI and Imperial/Pitt models achieve more modest reductions of 64% and 53%. At the higher R0, the UW/LANL model is more optimistic than the Imperial/Pitt and VBI models. Part of the difference between the effectiveness of the UW/LANL model and that of the Imperial/Pitt model can be explained by differences in their natural history assumptions. The Imperial/Pitt model has more of the infectiousness earlier so that targeted interventions will have less effect. The difference in the effectiveness of the UW/LANL and VBI models is partly explained by the difference in community social distancing. The VBI model does not close colleges and also has a smaller percentage of the transmissions in the community at large, so that social distancing does not play such a large role. Despite these differences, at the R0 ≈2 or below, the probable range of a pandemic virus, the effectiveness of the interventions in the three models is similar.
All three models have a large and fairly similar proportion of the infections occurring at home and school (Table 3). The Imperial/Pitt and UW/LANL models have similar amounts of transmissions in the combined school and workplaces, whereas the proportion is substantially higher in the VBI model. The amount in the neighborhoods and neighborhood clusters in the UW/LANL model is similar to that in the community at large in the Imperial/Pitt model. The proportion of infections occurring in the households tends to go up as other sources of infection are closed. Because colleges are not closed in the VBI model, they take on an added importance as a source of infection. The effects of home and school interventions are more robust across the three models than the effects of community social distancing. SI Fig. 6 shows the relative contributions of each activity type in the VBI model to interhousehold transmission in the absence of intervention and in scenario 2.
Fig. 2.
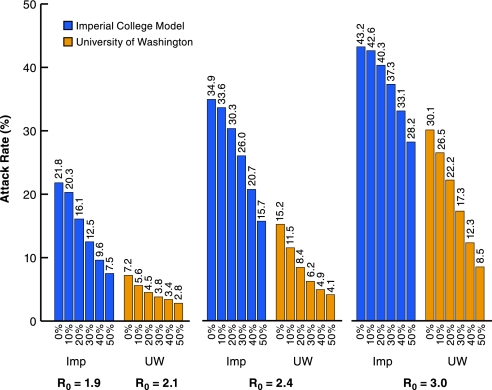
Sensitivity analysis for workplace and community social distancing. Scenario 2, with community and workplace social distancing being varied between 0% and 50%, and three R0 values (Chicago population). Only the UW/LANL and Imperial/Pitt models were used. The VBI model is insensitive to changes in this aspect of community social distancing.
Both the UW/LANL and Imperial/Pitt models show an increasing effectiveness in reducing attack rates as community social and workplace distancing increase from 0% to 50%. The corresponding attack rates do not vary in the VBI, so are not shown, but they are the same as in Table 2. The community and workplace social distancing likely play a larger relative role in the Imperial/Pitt model than in the UW/LANL model because the faster natural history makes the targeted interventions based on case ascertainment relatively less effective.
The VBI model is insensitive to the degree of community social distancing, because, as seen in Table 3, only a small proportion of infections occur outside home, school, workplace, and college. It is relatively insensitive to the degree of workplace social distancing, because in that model, workplace social distancing is achieved by reducing the maximum size of workplaces, which does not affect small workplaces.
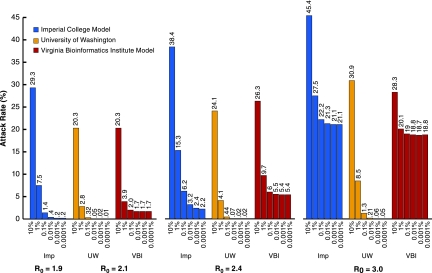
At R0 of 1.9 (2.1), waiting to implement interventions until reaching a 10% illness attack rate would effect a much smaller reduction in illness attack rates (Fig. 3). However, there is not much improvement by initiating interventions before a threshold illness attack rate of 0.1%. At R0 of 3, in the UW/LANL model, the lower threshold allows the intervention combination to be highly effective, whereby it achieves only ≈60% reduction in illness attack rates in the Imperial/Pitt and VBI models. Again, the combination of natural history and community structure of the UW/LANL model make it more optimistic at higher R0 values, whereas all models have similar sensitivity to threshold choice at R0 ≈ 2.
Fig. 3.
Sensitivity to changing thresholds for all interventions simultaneously for the three models. Scenario 2 and three R0 values, with threshold for triggering all measures being varied between 10% and 0.0001% cumulative illness attack rates. Chicago population.
The sensitivity analysis varying only the school closing threshold shows that if schools are closed before the other measures are instituted, the effectiveness of the intervention will be a little greater, but perhaps not enough to warrant the social disruption of early closure of schools (see SI Fig. 7).
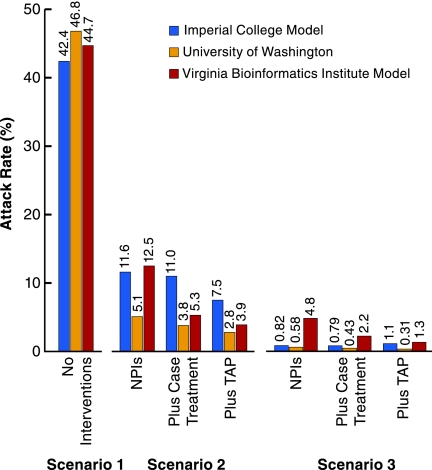
In all three models, most of the reduction in the attack rates appears to come from the NPIs (Fig. 4). In scenario 2, the UW/LANL model achieves 94%, the Imperial/Pitt model 88%, and the VBI model 78% of the illness attack reduction with just the NPIs compared with the baseline scenario 2 that uses antiviral treatment and household prophylaxis.
Fig. 4.
Comparison of no intervention with intervention scenarios 2 and 3 using just NPIs, NPI with addition of just treatment of ascertained cases (Plus Case Treatment), and NPI with addition of treatment of ascertained cases and targeted antiviral prophylaxis (Plus TAP) of their household contacts. Scenario 1: no intervention; scenario 2: just NPI, with treatment only; with TAP (base case scenario 2); scenario 3: just NPI, with treatment only; with TAP and treatment (base case scenario 3); R0 of 1.9 (2.1). Chicago population.
Table 4 shows results of the UW/LANL and Imperial/Pitt national models of the U.S. population with the full TLC intervention of scenario 2 at the lower R0. In both models, the illness attack rates are substantially reduced. The partial TLC strategy includes just treatment and isolation of ascertained cases without prophylaxing and quarantining contacts, closing schools, or recommending liberal leave from work for all symptomatic cases. In a third scenario, there is a 50% reduction in community social distancing, such as closing theaters or reduced activities in public places, and a 50% reduction in long-distance travel, not even closing schools. Although the partial TLC strategy still can cut the attack rates in half, the intervention with just community social distancing and 50% reduction in long-distance travel has a much smaller effect, ≈17% reduction in illness attack rates.
Table 4.
U.S. national illness (infection) attack rates using three national intervention strategies in the U.S. population models
| Illness (infection) | Attack rate, % | |
|---|---|---|
| Scenario | UW/LANL | Imperial/Pitt |
| No intervention | 47 (70) | 42 (63) |
| Social distancing* | 39 (58) | – |
| Partial scenario 2† | 23 (35) | – |
| Full TLC (scenario 2)‡ | 0.13 (0.20) | 0.30 (0.45) |
Threshold is an illness attack rate of 1/1,000 nationally for all interventions except school closure. School closure is implemented locally at the local threshold of 1/1,000 illness attack rate. Otherwise similar to scenario 2 (30/60) when applicable. UW/LANL model R0 = 2.1; Imperial/Pitt model R0 = 1.9.
*Only 50% community social distancing and 50% reduction in long distance travel, nothing else.
†Scenario 2, 50% reduction in long distance travel; but no TAP, treatment only, no school closure, no liberal leave.
‡Scenario 2, school closure at local threshold; 50% reduction in long-distance travel.
Discussion
Using three different models, we have examined targeted layered containment strategies based on social distancing, rapid case ascertainment, and targeted prophylaxis that, in theory, might be effective in reducing transmission of pandemic influenza. Timely intervention reduces the final number of influenza illnesses.
Especially at values of R0 ≈ 2 or below, the more probable values for a pandemic strain, the interventions are similarly, although not identically, effective in all three models. At the lower R0, all three models show considerable effectiveness of the suite of NPIs. School closure plays an important role in all three models.
The policy implications have two main aspects. The first is how these results can inform pandemic planning now. If one could achieve these levels of compliance, ascertainment, and social distancing, then there would be a possibility of considerably mitigating a pandemic until a vaccine were available. However, whether the ascertainment and compliance levels modeled here are realistic has yet to be demonstrated. Whether public health officials would actually choose to implement such measures will eventually depend on the lethality and transmissibility (R0) of the pandemic strain. Flexibility in the response plans for different eventualities will be important.
The second aspect for policy is the need for further field research to quantify the natural history of influenza, the sources of influenza transmission, and the feasibility and effectiveness of social distancing measures. Further understanding of the contact structures, such as workplaces and schools, their contribution to the overall transmission of influenza, and how amenable they are to social distancing measures are central to judging which social distancing measures would be effective and worth the social cost.
We caution against overinterpretation of the modeling results, even where the three models suggest similar effectiveness of interventions. Because of the uncertainties in the models, the results need to be viewed more as helping to structure thinking about pandemic planning, rather than being predictive of the precise effectiveness of different policies.
Other simulation results (6, 9, 11) have demonstrated that use of even poorly matched, low-efficacy vaccines would greatly enhance the effectiveness of other intervention measures. Thus, the development and stockpiling of vaccines should be a high priority. When the next pandemic unfolds, it will be important to have the capability to implement real-time surveillance and epidemiological analysis, including characterizing the new virus, predicting the epidemic trajectory, and if necessary, refining intervention strategies.
Supplementary Material
Acknowledgments.
We thank Richard J. Hatchett and Rajeev V. Venkayya for formulating scenarios of potential interest to the White House Homeland Security Council; Karla Atkins, Keith Bisset, Jiangzhou Chen, Laxminarayana Ganapathi, Achla Marathe, Madhav Marathe, Henning Mortveit, Douglas Roberts, and Paula Stretz (all VBI model) and Simon Cauchemez (Imperial/Pittsburgh model) for helping in developing the original models; and Irene A. Eckstrand for her support and encouragement. This work was supported in part by National Institute of General Medical Sciences MIDAS network Grants U01-GM070749, U01-GM070694, U01-GM070698, and U01-GM070708.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0706849105/DC1.
References
- 1.Markel H, Stern AM, Navarro A, Michalsen JR, Monto AS, DiGiovanni C., Jr [Last accessed January 31, 2007];Emerg Infect Dis 12. 2006 doi: 10.3201/eid1212.060506. Available at http://www.cdc.gov/ncidod/EID/vol12no12/06–0506.htm. [DOI] [PMC free article] [PubMed]
- 2.Hatchett RJ, Mecher CE, Lipsitch M. Proc Natl Acad Sci USA. 2007;104:7582–7587. doi: 10.1073/pnas.0610941104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bootsma MCJ, Ferguson NM. Proc Natl Acad Sci USA. 2007;104:7588–7593. doi: 10.1073/pnas.0611071104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fraser C, Riley S, Anderson RM, Ferguson NM. Proc Natl Acad Sci USA. 2004;101:6146–6151. doi: 10.1073/pnas.0307506101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rvachev LA, Longini IM. Math Biosci. 1985;75:3–22. [Google Scholar]
- 6.Ferguson NM, Cummings DAT, Fraser C, Cajka JC, Cooley PC, Burke DS. Nature. 2006;442:448–252. doi: 10.1038/nature04795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mills CE, Robins JM, Lipsitch M. Nature. 2004;432:904–906. doi: 10.1038/nature03063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Committee on Modeling Community Containment for Pandemic Influenza and Institute of Medicine. Modeling community containment for pandemic influenza: A letter report. [Last accessed December 1, 2007];2007 Available at: http://books.nap.edu/catalog/11800.html#orgs.
- 9.Longini IM, Nizam A, Xu S, Ungchusak K, Hanshaoworakul W, Cummings DAT, Halloran ME. Science. 2005;309:1083–1087. doi: 10.1126/science.1115717. [DOI] [PubMed] [Google Scholar]
- 10.Longini IM, Halloran ME, Nizam A, Yang Y. Am J Epidemiol. 2004;159:623–633. doi: 10.1093/aje/kwh092. [DOI] [PubMed] [Google Scholar]
- 11.Germann TC, Kadau K, Longini IM, Macken CA. Proc Natl Acad Sci USA. 2006;103:5935–5940. doi: 10.1073/pnas.0601266103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ferguson NM, Cummings DA, Cauchemez S, Fraser C, Riley S, Meeyai A, Iamsirithaworn S, Burke DS. Nature. 2005;437:209–214. doi: 10.1038/nature04017. [DOI] [PubMed] [Google Scholar]
- 13.Eubank S, Guclu H, Kumar VSA, Marathe MV, Srinivasan A, Toroczkai Z, Wang N. Nature. 2004;429:180–184. doi: 10.1038/nature02541. [DOI] [PubMed] [Google Scholar]
- 14.Lewis B, Beckman R, Kumar VS, Chen J, Stretz P, Bissett K, Mortveit H, Atkins K, Marathe A, et al. Blacksburg, VA: Virginia Bioinformatics Institute, Virginia Tech; 2007. Simulated pandemic influenza outbreaks in Chicago. Technical Report NDSSL-TR-07-004. [Google Scholar]
- 15.Murray CJL, Lopez AD, Chin B, Feehan D, Hill KH. Lancet. 2007;368:2211–2218. doi: 10.1016/S0140-6736(06)69895-4. [DOI] [PubMed] [Google Scholar]
- 16.Kilbourne ED. The Influenza Viruses and Influenza. New York: Academic; 1975. [Google Scholar]
- 17.Yang Y, Longini IM, Halloran ME. Appl Stat. 2006;55:317–330. doi: 10.1111/j.1467-9876.2006.00539.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Halloran ME, Hayden FG, Yang Y, Longini IM, Monto AS. Am J Epidemiol. 2007;165:212–221. doi: 10.1093/aje/kwj362. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.