Abstract
Humoral memory to an antigen (Ag) is maintained for several decades in the form of memory B cells and serum Ab. In fact, plasma cells (PCs) that secrete Ab are known to be long-lived and could be solely responsible for maintaining the long-lived Ab titers. Alternatively, it has been proposed that the PC compartment is maintained for long periods by the differentiation of memory cells into long-lived PCs as a result of nonspecific stimulation. This model predicts accelerated decay of PC numbers in the absence of memory cells for the same Ag. To address this prediction, we have developed a mouse model system that combined the ability to deplete B cells with the ability to detect Ag-specific memory and PCs. After establishing an immune response, we depleted Ag-specific memory B cells with an anti-hCD20 mAb and determined the effect on the PC compartment over 16 weeks. Using a combination of surface markers, we demonstrated that memory B cells remained depleted over the course of the experiment. However, despite this absence of memory cells for an extended duration, PC numbers in spleen and bone marrow did not decline, which indicates that the PC compartment does not require a significant contribution from memory B cells for its maintenance and instead that PCs are sufficiently long-lived to maintain Ab titers over a long period without renewal. This observation settles an important controversy in B cell biology and has implications for the design of vaccines and for B cell depletion therapy in patients.
Keywords: CD20, antibody-forming cell, serum antibody, B cell depletion
Immune response to a T-dependent antigen (Ag) leads to activation and differentiation of B cells into plasma cells (PCs) and memory cells. It is thought that the combination of serum Ab and memory B cells together provide long-lasting immunity to a variety of pathogens. Memory B cells are known to be long-lived, although they undergo homeostatic self-renewal (1–3). However, how serum Ab is maintained for long periods is less clear. In some cases, chronic exposure to Ag could be responsible, although it is unlikely that it explains long-lived Ab to transient Ags such as tetanus toxoid and lymphocytic choriomeningitis virus (LCMV) (4, 5). A second alternative is that long-lived PCs are sufficient to maintain long-lived Ab titers for a lifetime (6–8). Indeed, there is substantial evidence that PCs have long half-lives, estimated to be 138 days in mice (9). A third possibility is that memory cells renew the long-lived PC compartment on a regular basis (10). The stimulation of these memory cells to differentiate could be stochastic, from cross-reactive specific Ags or from nonspecific innate immune [e.g., Toll-like receptor (TLR)] signals. In support of this latter idea are several pieces of evidence. First, the measured half-lives of PCs suggest that they need to be renewed to maintain the very long-standing Ab titers that can ensue after a single immunization (11). Further, memory cells are more sensitive to TLR and possibly B cell receptor signals and are prone to differentiate into Ab-forming cells (AFCs) in response to a variety of stimuli (12, 13). Critically for this argument, Bernasconi et al. (10) found a tight correlation between the frequency of circulating memory cells specific for a given Ag and the serum Ab titers for that same Ag in humans. They interpreted this finding in causal terms in that the result was consistent with the notion that such memory cells supported the PC compartment (which in turn secreted the serum Ab). Otherwise, they argued, one would not expect to find such a close relationship between memory cell frequency and serum Ab level. However, it is quite difficult to test such a proposition in humans.
If memory cells were to contribute to PCs on a regular basis, then depletion of memory cells (but not PCs) should have a significant effect on PC numbers and Ab titers. Anticipating such a possibility, Slifka and Ahmed (7) had used sublethal irradiation to eliminate memory B cells, which they demonstrated by a clonal expansion assay. They were able to detect PCs and serum Ab for >1 year, although the levels declined continuously in the interim; interestingly, this decline was more pronounced in the irradiated compared with the nonirradiated mice. This decline could be because irradiation is not a specific way to eliminate B cells and had other undesirable effects, perhaps on bone marrow (BM) stromal cells that provide PC niches and on PCs themselves. Thus, this experiment, although demonstrating long PC half-lives, could be interpreted in favor of either model for long-lived serum Ab maintenance.
The question of how the PC compartment turns over with respect to memory B cells is not only of basic interest in B cell biology but also is important in the context of B cell depletion therapy that is being used for treatment of lymphoma and autoimmune diseases (14, 15). In patients, B cell depletion has been mainly accomplished by using rituximab, a chimeric mAb to human CD20 (hCD20), which depletes mature B cells including (CD27+) memory B cells (16, 17). The extent of depletion in secondary lymphoid tissues is poorly understood, although secondary Ab responses are impaired after treatment (18–20). Preestablished Ab titers to certain vaccine Ags have not been affected in the short-term in patients, although titers of several autoantibodies decline after treatment (21, 22). Because the source of such autoantibodies is unclear, as is the extent of depletion of memory cells and other potential precursors (17, 23), it is not possible to make conclusions about memory cell and PC relationships in patients at this time. Indeed, dissecting this relationship further in patients will be difficult if not impossible until the extent of memory cell depletion in lymphoid tissues can be reliably assessed. Thus, a murine model is needed to explore the question further. Anti-CD20-mediated B cell depletion murine models developed by our group (22) and others (24, 25) are ideal for such studies.
To address the role of memory B cells in PC maintenance, we crossed our hCD20 transgenic (Tg) mice (22) with the B1-8 knockin mice (26). The B1-8 allele, in combination with endogenous Vλ1, confers specificity to the (4-hydroxy-3-nitrophenyl) acetyl (NP) hapten, resulting in a population of ≈2% B cells with NP specificity. The hCD20 Tg mice express hCD20 exclusively on B cells. Using the hCD20 Tg system, we can deplete all populations of B cells, in particular memory B cells, using an anti-hCD20 mAb. This treatment should not affect the PC pool, which does not express hCD20 (27). The presence of the B1-8 allele allowed us to generate a robust population of NP-specific memory B cells that was amenable to reproducible quantitation upon immunization with NP-chicken gamma globulin (NP-CGG). In contrast, immunization of WT mice leads to a variable and small number of memory B cells (≈40,000 per spleen), which would be difficult to assess, particularly after depletion (28). Furthermore, unlike previous studies that used functional assays that depend on various external factors, we directly enumerated the expanded Ag-specific cells, using IgG1 isotype expression, BrdU labeling, and newly defined surface markers to identify memory B cells (3). We found that depletion of memory B cells for prolonged periods of time did not have any detectable effect on the PC population, indicating that a continuous input from memory B cells is not required for maintenance of PCs and humoral memory for the duration and conditions studied.
Results
Experimental Design.
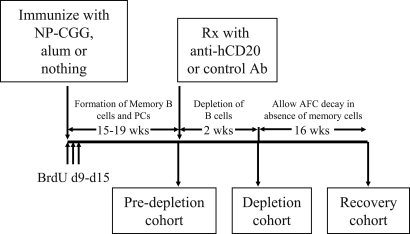
The experimental design is depicted in Fig. 1. To test the hypothesis that maintenance of PCs is independent of memory B cells, we crossed hCD20 Tg mice (22) with B1-8 knockin mice (26) to create hCD20 × B1-8 Tg mice. The hCD20 × B1-8 Tg mice were immunized with NP-CGG. After allowing 15–19 weeks for the memory B cell pool to become stably established (refs. 2 and 3 and as confirmed in the “predepletion cohort”), we treated the mice with anti-hCD20 mAb for 2 weeks to deplete B cells. Using this strategy, we expected to deplete memory B cells but not PCs because memory B cells are CD20+, whereas PCs are not. We confirmed this outcome in a subset of mice (“depletion cohort”). Moreover, a cohort of the immunized mice was injected with BrdU during the peak of germinal center response (day 9–day 15). With this strategy, all of the cells that are dividing during the labeling period take up BrdU (2, 3). However, once the labeling is stopped, BrdU is lost from the cells that continue to divide and is retained only by cells that stopped dividing. Therefore, remaining BrdU+ cells >15 weeks after labeling represent long-lived cells that were generated during the initial response (Fig. 1). We then waited 16 weeks after the termination of depletion therapy to allow time for the lack of putative memory cell input into the PC compartment to manifest, potentially as reduced numbers of PCs in the depleted cohort. Prior data (9) had suggested that this time frame should be more than sufficient to observe detectable effects. We then analyzed the depleted mice for recovery of naïve and memory B cells 16 weeks after depleting the memory B cells (“recovery cohort”) and most importantly evaluated the effect of long-term absence of memory B cells on AFCs.
Fig. 1.
Experimental design for determining the effect of memory B cell depletion on PCs. The hCD20 × B1-8 mice were immunized with NP-CGG. After 15–19 weeks, the immunized mice were treated with anti-hCD20 mAb 2H7 for 2 weeks. During the recovery period of 16 weeks, the AFCs were allowed to decay in the absence of any input from memory B cells. Instead of anti-hCD20 mAb, the control-treated mice (Ctrl-Rx) were given mouse gamma globulin. Alum-treated and unimmunized mice received no further treatment. BrdU was injected in a cohort of unimmunized, alum-treated, and NP-CGG-treated mice every day from day 9 through day 15 (d9-d15) after immunization. Cohorts of mice were analyzed 15–19 weeks after immunization (predepletion cohort), immediately after anti-hCD20 therapy (depletion cohort), and 16 weeks after termination of therapy (recovery cohort) for numbers of total B cells, memory B cells, and PCs.
Generation of Memory Cells and PCs in hCD20 × B1-8 Tg Mice.
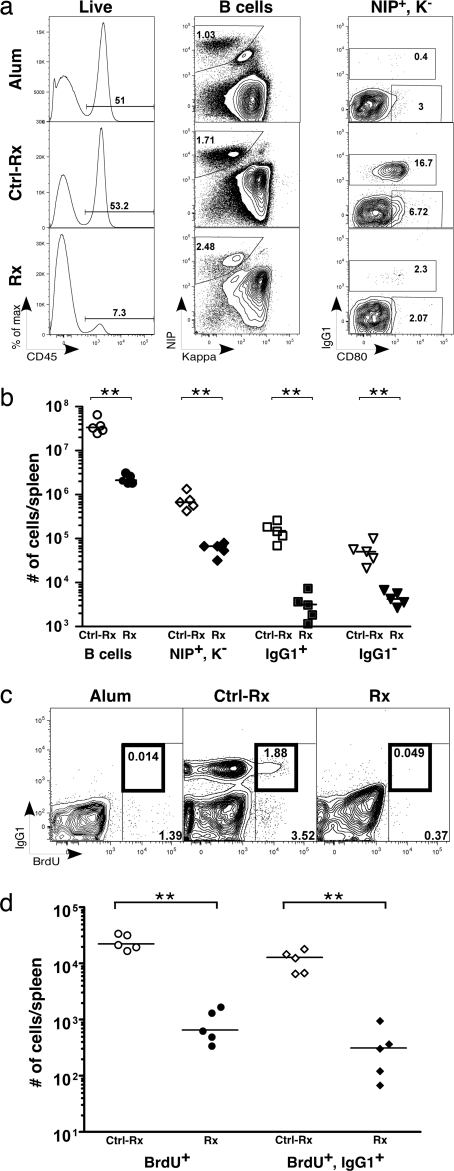
Immunization of hCD20 × B1-8 double Tg mice led to a significant increase in the frequency of NIP+/Kappa− (NIP+) B cells (Fig. 2a, Middle, Ctrl-Rx mice, and b) when analyzed 17–21 weeks later. Among NIP+ B cells, there was an increase in the frequency and numbers of NIP+/Kappa−/IgG1+ (IgG1+) B cells and NIP+/Kappa−/IgG1−/CD80hi (IgG1−) B cells (Fig. 2 a, Right, Ctrl-Rx mice, and b) compared with the alum-treated (Fig. 2a, Alum mice) or unimmunized mice (data not shown). Because the GC response has ended by this time (refs. 3 and 28; discussed below), the IgG1+ B cells are deemed “true” memory B cells. Moreover, in agreement with a recent report on the identity of memory B cells, a significant fraction (≈45%) of IgG1+ cells express high levels of CD73 and CD80 (ref. 3 and data not shown). Although the IgG1− subset includes some contaminating naïve B cells, it also expanded as a result of immunization, indicating that it is substantially comprised of memory B cells of IgM and/or other non-IgG1 isotypes (3). This notion was further confirmed by coexpression of CD73 and CD80 on the majority of IgG1− cells (data not shown). Additionally, the presence of mutations in Vλ1, a hallmark of memory, was confirmed in both Ag-specific populations in the immunized mice (see Methods and data not shown). These results were in agreement with published studies (3, 29, 30). Also in accord with published data (2, 3), in the mice that were labeled with BrdU during the peak of GC response, ≈4% of NIP+ cells were BrdU+, and ≈50% of them were IgG1+ (Fig. 2 c, Ctrl-Rx mice, and d) and CD80hi (data not shown).
Fig. 2.
Depletion of memory B cells immediately after treatment with anti-hCD20 mAb 2H7 (depletion cohort). (a) Representative FACS plots showing the gating strategy for identification of memory B cells in alum-treated (Alum, n = 5; Top); immunized, control mouse gamma globulin-treated (Ctrl-Rx, n = 5; Center), and immunized, 2H7-treated (Rx, n = 5; Bottom) mice. The parent gates are shown on Top. (b) Total numbers of B cells, NIP+/Kappa− cells, NIP+/IgG1+ cells, and NIP+/IgG1− cells in spleens of Ctrl-treated (open symbols) and 2H7-treated (filled symbols) mice. (c) Representative FACS plots of NIP+ cells from alum-treated, Ctrl-treated, and 2H7-treated mice stained with IgG1 and BrdU. The percentages of total BrdU+ (gate with thin line) and BrdU+/IgG1+ (gate with thick line) are shown. (d) Comparison of total numbers of NIP+/BrdU+ and NIP+/BrdU+/IgG1+ memory subsets in spleens of Ctrl-treated (open symbols) and 2H7-treated (filled symbols) mice. (b and d) Horizontal lines represent median. **, P < 0.01, Mann–Whitney U test.
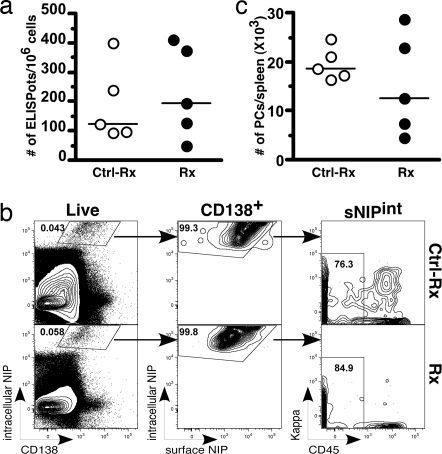
A stable pool of Ag-specific PCs, as revealed by the ELISPot AFC assay, was also observed in immunized mice (Fig. 3a, Ctrl-Rx mice). A phenotypic Ag-specific PC population, defined as CD138+/intracellular NIPhi/surface NIPint/CD45−, was also noted, comprising ≈0.022% of total splenocytes 17–21 weeks after immunization (Fig. 3b, Ctrl-Rx mice). It should be noted that at this time point, only 0.2% of NIP+ cells are PNAhi/CD95+ (data not shown), indicating that, as reported (3, 28), the GC response had essentially ceased, and few if any additional memory cells or PCs were being generated.
Fig. 3.
Short-term effect of depletion of B cells on PCs (depletion cohort). (a) Comparison of the number of NP-specific ELISPots per million splenocytes from Ctrl-treated mice (open symbols, n = 5) and 2H7-treated mice (filled symbols, n = 5). (b) Representative FACS plots showing phenotyping of PCs as CD138+/intracellular NIPhi/surface NIPint/CD45− in the Ctrl-Rx (Upper) and 2H7 Rx (Lower) immunized mice. (c) Total number of PCs, identified by FACS, in spleens of Ctrl-treated (open symbols) and 2H7-treated (filled symbols) mice. (a and c) Horizontal lines represent median.
Depletion of Ag-Specific Memory B Cells with Anti-hCD20 Ab Treatment.
After establishing the presence of Ag-specific memory cells and PCs, a cohort of immunized mice was treated with 2 mg per week of the murine anti-hCD20 mAb, 2H7, for 2 weeks, i.p. (Fig. 1). Treatment with 2H7 resulted in ≈94% depletion of splenic B cells (Fig. 2 a, Rx mice, and b; P = 0.0079) compared with control mice treated with mouse gamma globulin (Fig. 2 a, Ctrl-Rx mice, and b). This result is in accord with previous data using similar systems (22, 24, 25). NIP+ B cells were also depleted to a comparable extent (90%, P = 0.0079; Fig. 2a, Rx mice, and b). Interestingly, anti-hCD20 treatment resulted in almost complete depletion of both IgG1+ (98%, P = 0.0079) and IgG1− (92%, P = 0.0079) subsets of memory B cells (Fig. 2b). Extensive depletion of Ag-specific BrdU+ cells (97%, P = 0.0079) and IgG1+/BrdU+ cells (98%, P = 0.0079; Fig. 2 c and d) confirmed these results. Thus, in our model system, the extent of depletion of memory B cells was comparable with that of naïve B cells and possibly exceeded it, perhaps because of resistance of certain naïve B cell subsets, chiefly MZ B cells (25).
Short-Term Effect of B Cell Depletion Therapy on AFCs.
Because most if not all long-lived PCs are believed to down-regulate CD20 expression (27), we expected little if any immediate effect on these cells. Indeed, B cell depletion caused no reduction in NP-specific splenic AFCs in the ELISpot assay (Fig. 3a). Similarly, the average number of splenic PCs, identified by FACS (Fig. 3b), was similar in the treated mice (P = 0.5476) immediately after depletion to that in the control treated mice (Fig. 3 b and c). Taken together, these results demonstrate that initial depletion of B cells had minimal effect on splenic PCs.
Long-Term Effect of B Cell Depletion on Memory Cells.
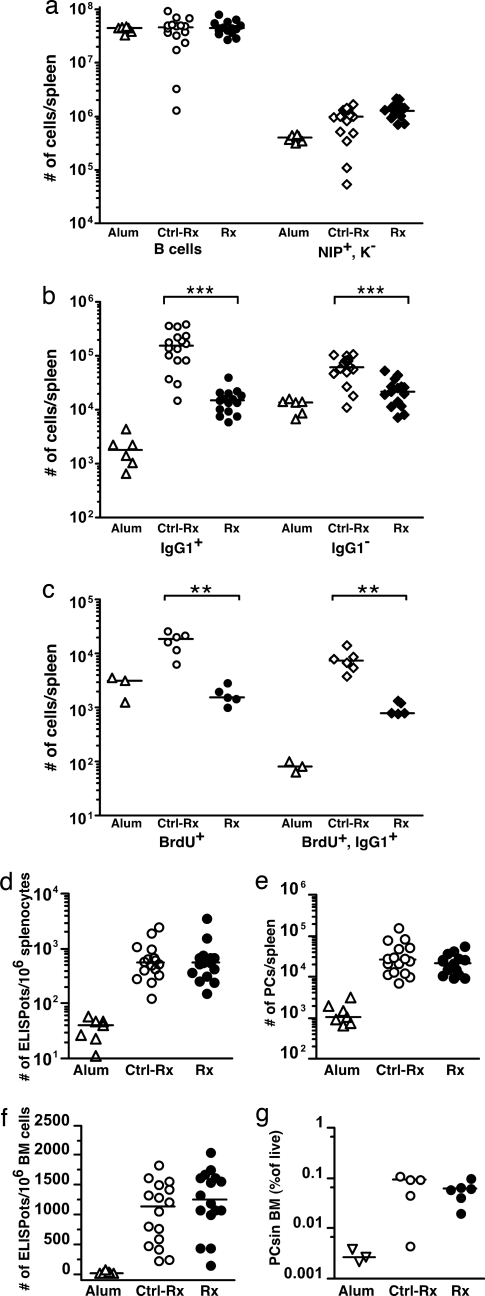
Sixteen weeks after termination of treatment, the numbers of total splenic B cells (P = 0.8279) and naïve NIP+ cells (P = 0.0695) were restored to levels similar to those in control-treated, immunized mice (Fig. 4a). In contrast, depletion remained substantial, with a 90% reduction of Ag-specific IgG1+ cells compared with controls (P < 0.0001) and 65% depletion of Ag-specific IgG1− cells (P = 0.0005; Fig. 4b), although the number of splenic memory B cells in the recovery cohort was slightly higher than the depletion cohort. Most likely, the apparently better reconstitution of the IgG1− B cell subset is attributable to inclusion in the FACS gate of some naïve cells, which recover almost completely during this time period (see above). Furthermore, NIP+/BrdU+ cells (92% depletion; P = 0.0043) and NIP+/BrdU+/IgG1+ cells (89% depletion; P = 0.0043) remained depleted (Fig. 4c). That memory cells were irreversibly depleted was as expected but nonetheless rules out that the memory cell compartment can renew itself to any significant extent after depletion.
Fig. 4.
Long-term effect of depletion of B cells on memory B cells and PCs (recovery cohort). (a–c) Total numbers of B cells and NIP+/Kappa− cells (a), NIP+/IgG1+ cells and NIP+/IgG1− cells (b), and NIP+/BrdU+ cells and NIP+/BrdU+/IgG1+ cells (c) in spleens of alum-treated (open triangles, n ≥ 3), Ctrl-treated (open symbols, n ≥ 5), and 2H7-treated (filled symbols, n ≥ 5) mice. (d) Comparison of the number of NP-specific ELISPots per million splenocytes from alum-treated (open triangles, n = 7), Ctrl-treated (open symbols, n = 16), and 2H7-treated (filled symbols, n = 15) mice. (e) Total number of PCs, identified by FACS, in spleens of alum-treated (open triangles, n = 7), Ctrl-treated (open symbols, n = 16), and 2H7-treated (filled symbols, n = 15) mice. (f) Comparison of the number of NP-specific ELISPots per million BM cells from alum-treated (open triangles, n = 7), Ctrl-treated (open symbols, n = 16), and 2H7-treated (filled symbols, n = 15) mice. (g) Percentage of PCs, identified by FACS, in the BM of alum-treated (open triangles, n = 3), Ctrl-treated (open symbols, n = 5), and 2H7-treated (filled symbols, n = 6) mice. (a–g) Horizontal lines represent median. **, P < 0.01; ***, P ≤ 0.0005, Mann–Whitney U test.
Long-Term Effect of B Cell Depletion Therapy on AFCs.
If memory cells are required for continuous generation of PCs, a long-term absence of memory cells should lead to reduced numbers of PCs. To test this possibility, we evaluated the number of AFCs 16 weeks after completion of B cell depletion in immunized mice. Strikingly, even after prolonged depletion of memory B cells, the number of splenic AFCs, determined by NP-specific ELISPot assays, was indistinguishable from control-treated mice, in which memory B cells remained (Fig. 4d; P = 0.9100). This finding was confirmed by the presence of similar numbers of intracellular NIPhi/CD138+/surface NIPint/CD44hi/CD45− cells in the treated and control-treated groups of mice (Fig. 4e; P = 0.3530). Most importantly, the numbers of long-lived AFCs in the BM of treated mice were comparable with those of control mice, as assessed by NP-specific ELISpot assay (Fig. 4f; P = 0.2821) and FACS (Fig. 4g; P = 0.6623). These data indicate that the PC compartments of both spleen and BM remain intact in the absence of memory cells for extended periods of time, probably because of their long-lived nature.
Discussion
Although the t½ of serum IgG is on the order of a few weeks, serum titers of Ab are maintained long after exposure to an Ag. This paradox is explained in part by constant secretion of Ab from the long-lived PC pool. Some have suggested that this alone is sufficient to explain the longevity of serum Ab titers after a single exposure to Ag (6–8). Alternatively, because PCs appear to have a finite life span, it has been suggested that their numbers are maintained by continued differentiation from the memory B cell pool, perhaps by nonspecific stimulation (10). Here, we attempted to distinguish the two possibilities by depleting Ag-specific memory B cells for extended periods and analyzing the effect on PCs. Our data clearly show that a 2-week treatment of hCD20 × B1-8 Tg mice with anti-CD20 mAb resulted in effective depletion of both mature and memory B cells, whereas PCs were not significantly affected. Strikingly, numbers of PCs did not decrease over 4 months, even in the absence of memory B cells, indicating that the PCs do not need to be continuously generated from memory B cells. Our data differ from a previous analysis of LCMV-infected mice, which were irradiated to deplete memory B cells; PC numbers in such mice declined 4-fold in 4 months (7). Given our results, this decline could be attributed to the pleiotropic effects of radiation on other components of immune system. In our experiments, we specifically targeted B cells (including memory B cells) with anti-hCD20 mAb, minimizing any undesirable side effects. Another advantage of our approach is that we directly enumerated memory B cells using surface markers in FACS instead of functional assays as in previous studies. Doing so ensures that our results are not confounded by factors external to the memory cells per se. In this regard, we were able to show depletion of not only IgG1+ memory cells but also IgG1− memory cells that presumptively included IgM+ cells, now appreciated to be a substantial component of murine (and human) memory (3, 16, 31). To do this, we used CD80 expression, supplemented with CD73 expression (data not shown), both of which are elevated on IgG and IgM memory cells. Furthermore, the almost complete elimination of NP-specific cells that were labeled with BrdU during the immunization phase and that contained both IgM and IgG cells (ref. 3, Fig. 2d, and data not shown) argues that our results cannot be explained by an unexpected failure to detect a memory cell subset.
A potential explanation for sustained levels of PCs even upon depletion of memory B cells could be an increase in levels of B cell-activating factor of the TNF family (BAFF), which was seen in humans as a result of B cell depletion (32, 33). BAFF and APRIL (a proliferation inducing ligand) are known to promote the survival of B cells and PCs, respectively (34). BAFF elevations alone, as would transiently occur after B cell depletion, are unlikely to promote abnormal PC survival. Most likely, APRIL is sufficient to promote PC survival because inhibition of BAFF and APRIL led to reduction in BM PC numbers in a B cell maturation Ag-dependent fashion (35), whereas inhibition of BAFF alone did not affect long-lived PC levels and the Ab they secreted (M. Tomayko, M. Cancro, and M.J.S., unpublished observations). Whereas BAFF levels increase 2- to 3-fold during rituximab-mediated depletion of B cells in lupus and rheumatoid arthritis patients, APRIL levels actually decrease in lupus patients and remain unchanged in rheumatoid arthritis (32, 33). Moreover, BAFF levels return to pretreatment levels with recovery of B cells. In mice, B cell recovery after depletion occurs in several weeks (25). These considerations together suggest that it is unlikely that BAFF levels would play a significant role in maintenance of PC pool in our work.
Over a 4-month period because we saw no reduction in PC numbers even after averaging results from ≥15 individual mice from each group, it is clear that the actual PC half-life is substantially longer. This point holds regardless of whether memory cells are present. For example, were the half-life to be 32 weeks, we should have seen at least a 30% decrement in PC numbers in 16 weeks. Thus, it seems that in mice PCs likely are able to live as long as the life of the animal, and the PC compartment does not require any significant contribution from memory cells to maintain serum Ab titers. Nonetheless, we cannot rule out the possibility that a much longer follow-up period would have revealed a very slow decay of PCs in depleted mice.
Our data show that there is no need to invoke memory cell recruitment for PC renewal. However, they do not rule out that such recruitment can occur under certain circumstances, for example, after PC depletion (although a physiologic situation for this has yet to be defined). It has been suggested that a small fraction of memory B cells divide every day (1, 36). The reported slow decay of BrdU+ memory cells in the face of stable overall numbers of these cells (3) and as also seen in our control-treated mice, is most consistent with homeostatic memory cell renewal resulting in dilution of the label over time. This renewal has been suggested to be consistent with some differentiation of memory cells into AFCs in vivo (10). Moreover, in vitro data suggest that dividing memory cells can differentiate into PCs upon nonspecific stimulation or bystander T help. Therefore, it is possible that stimulation of TLR4 and TLR9 may result in proliferation and differentiation of memory cells even under physiological conditions in vivo. However, such nonspecific stimulation of memory cells in WT mice has recently been shown to generate short-lived plasmablasts without their recruitment to the long-lived PC compartment in BM (37). In fact, the Ag-specific long-lived PC pool was shown, if anything, to contract as a consequence of nonspecific stimulation, which suggests that spontaneous turnover of memory cells is unlikely to be a major factor in preservation of serum Ab titers.
It is well established that long-lived PCs play an important role in sustaining humoral memory. However, the total number of “survival niches” in BM is thought to be constant and is estimated to support a maximum of 106 PCs per mouse (38). Moreover, to maintain protective serum Ab titers, the repertoire of humoral memory is estimated to include ≈1,000 different specificities. Therefore, to explain how additional long-lived PCs can be generated with each new infection, a model has been proposed in which old PCs are displaced from BM at a rate slow enough not to lose the old specificities (38, 39). The newly generated plasmablasts are then recruited into the long-lived PC pool caused by the opening of the niches. In our experiments, we did not attempt to address this issue, which although related, is independent from the relationship between memory cells and PCs.
Unexpectedly, during the course of the experiment, we noticed an increase in total AFC numbers in the NP-specific ELISpot of the immunized mice as time progressed. Total splenic AFC numbers in both control treated (open symbols) and anti-hCD20 treated (filled symbols) immunized mice were ≈4-fold higher at the recovery time point (Fig. 4d) compared with the depletion time point (Fig. 3a). Considering that, as just demonstrated, differentiation from memory cells does not increase PC numbers, a possible explanation is that not all of the PCs were fully mature at the depletion time point (even though we waited 15–19 weeks after immunization), and as time progressed, more PCs matured. Alternatively, it is possible that developed PCs divide at a very slow rate at least for some time after initial formation, which could explain the increased numbers.
The finding that differentiation of memory B cells into PCs is not a major pathway for maintenance of the PC pool implies that the immune system has evolved two independent strategies to maintain long-term memory: PCs and memory B cells. Each has its own signals for formation and its own signals and niches for survival (39). This strategy represents a more robust defense against immunosuppressive interventions, whether pathogen-mediated or iatrogenic, than if the PC compartment were to rely on the memory compartment for its renewal. However, in the case of Ag-specific restimulation, the memory compartment could potentially contribute further to the long-term PC compartment, thus shaping it further, although the main product of restimulation of memory cells is a short-lived AFC (37).
With regard to clinical application, our data suggest that depletion of memory B cells with rituximab should have no effect on long-lived PC numbers, which is in agreement with the fact that total serum IgG and Abs to microbes and vaccine Ags either decline slowly or not at all even after prolonged B cell depletion (21, 22). To the extent that some decay of Ig levels occurs with B cell depletion, it could be attributed to a component derived from short-lived plasmablasts (which are reliant on CD20+ precursors for renewal), a limit on the life span (albeit a long one) of established PCs, and/or an effect of niche displacement caused by subsequent immunization (39). The maintenance of the serum Ab component of humoral memory despite B cell depletion could account for the low infectious complication rate in chronically depleted individuals. Indeed, the lack of infections in such B cell-depleted individuals highlights the two-pronged and independent strategy of the immune system to achieve long-term humoral immunity.
Methods
Mice and Immunization.
The human CD20 (hCD20) Tg (22) and B1-8 knockin mouse strains (26) on BALB/c backgrounds were crossed to generate hCD20 × B1-8 Tg mice. The F1 mice from these crosses were immunized i.p. at 8–15 weeks of age with either 50 μg of alum-precipitated NP-CGG or alum precipitate alone as control. All animal experiments were approved by the Yale Institutional Animal Care and Use Committee.
FACS Analysis.
For FACS analysis, NIP-haptenated PE (NIP-PE), and APC (NIP-APC) reagents and mAbs against murine CD80 (1610A1), Kappa (187.1), and CD44 (Pgp-1) were prepared as described in ref. 40. Abs against IgG1 (A85-1), CD73 (Ty/23), CD19 (1D3), CD138 (281-2), CD45 (RA3-6B2), CD95 (Jo-2) were purchased from BD Biosciences. Abs against BrdU (PRB-1; Molecular Probes), Lambda (Southern Biotech), streptavidin (SA)-PE/Cy7 (eBiosciences), and PNA-FITC (Vector Laboratories) were also purchased. Samples were stained and analyzed on LSRII or FACSAria (BD Biosciences).
Immunodepletion.
The mAb against hCD20, 2H7, was used for B cell depletion. 2H7 is a mouse IgG2b that binds an epitope on hCD20 similar to that bound by rituximab and therefore, mimics rituximab treatment of humans. 2H7 was purified from culture supernatants (the hybridoma was a generous gift from E. Clark, University of Washington, Seattle) as described in ref. 22. Mice were injected i.p. with 2 mg per week of either 2H7 or control mouse IgG (Rockland Immunochemicals) in sterile PBS for 2 weeks, administered twice a week.
BrdU Administration and Detection.
A cohort of immunized and alum-treated mice were labeled with BrdU by injecting 3 mg/ml BrdU (Sigma–Aldrich) i.p., every 24 h from day 9 through day 15 after immunization. BrdU was detected, at the time of sacrifice, as described in ref. 41.
ELISPot.
NP-specific AFCs were quantitated by ELISpot assay as described in ref. 40, with plates coated with NP26-BSA and visualized with polyclonal anti-λ-alkaline phosphatase Ab (Southern Biotech) and bromo-4-chloro-3-indolyl phosphate substrate (AMRESCO).
Sorting and Sequence Analysis.
To analyze for presence of mutations in λ chain, the memory subsets were sorted and sequenced as described in ref. 3.
Statistical Analysis.
The Mann–Whitney two-tailed U test was used to determine statistical significance.
Acknowledgments.
We thank Dr. Klaus Rajewsky for B1-8 knockin mice; Jinping Wang, Jonathan Shupe, Cuiling Zhang, and Haowei Wang for expert technical assistance; Anja Hauser and Kim Good for helpful discussions; Geoff Lyon of the Yale Cell Sorting Facility for help with sorting; and Michelle Horniak, Terrence Hunt, and the rest of the staff of the Yale Animal Resource Center for excellent animal care. This work was supported by National Institutes of Health Grants AI43603 and AR44077 (to M.J.S.). A.A. was supported by an Arthritis Foundation Postdoctoral Fellowship.
Footnotes
The authors declare no conflict of interest.
References
- 1.Schittek B, Rajewsky K. Maintenance of B-cell memory by long-lived cells generated from proliferating precursors. Nature. 1990;346:749–751. doi: 10.1038/346749a0. [DOI] [PubMed] [Google Scholar]
- 2.Anderson SM, Hannum LG, Shlomchik MJ. Memory B cell survival and function in the absence of secreted antibody and immune complexes on follicular dendritic cells. J Immunol. 2006;176:4515–4519. doi: 10.4049/jimmunol.176.8.4515. [DOI] [PubMed] [Google Scholar]
- 3.Anderson SM, Tomayko MM, Ahuja A, Haberman AM, Shlomchik MJ. New markers for murine memory B cells that define mutated and unmutated subsets. J Exp Med. 2007;204:2103–2114. doi: 10.1084/jem.20062571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gatto D, et al. Regulation of memory antibody levels: The role of persisting antigen versus plasma cell life span. J Immunol. 2007;178:67–76. doi: 10.4049/jimmunol.178.1.67. [DOI] [PubMed] [Google Scholar]
- 5.Zinkernagel RM, et al. On immunological memory. Annu Rev Immunol. 1996;14:333–367. doi: 10.1146/annurev.immunol.14.1.333. [DOI] [PubMed] [Google Scholar]
- 6.Manz RA, Radbruch A. Plasma cells for a lifetime? Eur J Immunol. 2002;32:923–927. doi: 10.1002/1521-4141(200204)32:4<923::AID-IMMU923>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 7.Slifka MK, Ahmed R. Long-lived plasma cells: A mechanism for maintaining persistent antibody production. Curr Opin Immunol. 1998;10:252–258. doi: 10.1016/s0952-7915(98)80162-3. [DOI] [PubMed] [Google Scholar]
- 8.Slifka MK, Matloubian M, Ahmed R. Bone marrow is a major site of long-term antibody production after acute viral infection. J Virol. 1995;69:1895–1902. doi: 10.1128/jvi.69.3.1895-1902.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Slifka MK, Antia R, Whitmire JK, Ahmed R. Humoral immunity due to long-lived plasma cells. Immunity. 1998;8:363–372. doi: 10.1016/s1074-7613(00)80541-5. [DOI] [PubMed] [Google Scholar]
- 10.Bernasconi NL, Traggiai E, Lanzavecchia A. Maintenance of serological memory by polyclonal activation of human memory B cells. Science. 2002;298:2199–2202. doi: 10.1126/science.1076071. [DOI] [PubMed] [Google Scholar]
- 11.Traggiai E, Puzone R, Lanzavecchia A. Antigen-dependent and -independent mechanisms that sustain serum antibody levels. Vaccine. 2003;21(Suppl 2):S35–S37. doi: 10.1016/s0264-410x(03)00198-1. [DOI] [PubMed] [Google Scholar]
- 12.Bernasconi NL, Onai N, Lanzavecchia A. A role for Toll-like receptors in acquired immunity: Up-regulation of TLR9 by BCR triggering in naive B cells and constitutive expression in memory B cells. Blood. 2003;101:4500–4504. doi: 10.1182/blood-2002-11-3569. [DOI] [PubMed] [Google Scholar]
- 13.Raman VS, Lind EF, Benson MJ, Noelle RJ. Strategies for selective priming of memory B cells. Immunol Lett. 2007;109:93–100. doi: 10.1016/j.imlet.2007.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sanz I, Anolik JH, Looney RJ. B cell depletion therapy in autoimmune diseases. Front Biosci. 2007;12:2546–2567. doi: 10.2741/2254. [DOI] [PubMed] [Google Scholar]
- 15.Grillo-Lopez AJ. Monoclonal antibody therapy for B cell lymphoma. Int J Hematol. 2002;76:385–393. doi: 10.1007/BF02982803. [DOI] [PubMed] [Google Scholar]
- 16.Klein U, Rajewsky K, Kuppers R. Human immunoglobulin (Ig)M+IgD+ peripheral blood B cells expressing the CD27 cell surface antigen carry somatically mutated variable region genes: CD27 as a general marker for somatically mutated (memory) B cells. J Exp Med. 1998;188:1679–1689. doi: 10.1084/jem.188.9.1679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Leandro MJ, Cambridge G, Ehrenstein MR, Edwards JC. Reconstitution of peripheral blood B cells after depletion with rituximab in patients with rheumatoid arthritis. Arthritis Rheum. 2006;54:613–620. doi: 10.1002/art.21617. [DOI] [PubMed] [Google Scholar]
- 18.van der Kolk LE, Baars JW, Prins MH, van Oers MH. Rituximab treatment results in impaired secondary humoral immune responsiveness. Blood. 2002;100:2257–2259. [PubMed] [Google Scholar]
- 19.Bearden CM, et al. Rituximab inhibits the in vivo primary and secondary antibody response to a neoantigen, bacteriophage φX174. Am J Transplant. 2005;5:50–57. doi: 10.1111/j.1600-6143.2003.00646.x. [DOI] [PubMed] [Google Scholar]
- 20.Gonzalez-Stawinski GV, Yu PB, Love SD, Parker W, Davis RD., Jr Hapten-induced primary and memory humoral responses are inhibited by the infusion of anti-CD20 monoclonal antibody (IDEC-C2B8, rituximab) Clin Immunol. 2001;98:175–179. doi: 10.1006/clim.2000.4980. [DOI] [PubMed] [Google Scholar]
- 21.Sfikakis PP, Boletis JN, Tsokos GC. Rituximab anti-B-cell therapy in systemic lupus erythematosus: Pointing to the future. Curr Opin Rheumatol. 2005;17:550–557. doi: 10.1097/01.bor.0000172798.26249.fc. [DOI] [PubMed] [Google Scholar]
- 22.Ahuja A, et al. Depletion of B cells in murine lupus: Efficacy and resistance. J Immunol. 2007;179:3351–3361. doi: 10.4049/jimmunol.179.5.3351. [DOI] [PubMed] [Google Scholar]
- 23.Anolik JH, et al. Rituximab improves peripheral B cell abnormalities in human systemic lupus erythematosus. Arthritis Rheum. 2004;50:3580–3590. doi: 10.1002/art.20592. [DOI] [PubMed] [Google Scholar]
- 24.Uchida J, et al. The innate mononuclear phagocyte network depletes B lymphocytes through Fc receptor-dependent mechanisms during anti-CD20 antibody immunotherapy. J Exp Med. 2004;199:1659–1669. doi: 10.1084/jem.20040119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gong Q, et al. Importance of cellular microenvironment and circulatory dynamics in B cell immunotherapy. J Immunol. 2005;174:817–826. doi: 10.4049/jimmunol.174.2.817. [DOI] [PubMed] [Google Scholar]
- 26.Taki S, Meiering M, Rajewsky K. Targeted insertion of a variable region gene into the immunoglobulin heavy chain locus. Science. 1993;262:1268–1271. doi: 10.1126/science.8235657. [DOI] [PubMed] [Google Scholar]
- 27.Uchida J, et al. Mouse CD20 expression and function. Int Immunol. 2004;16:119–129. doi: 10.1093/intimm/dxh009. [DOI] [PubMed] [Google Scholar]
- 28.Takahashi Y, Ohta H, Takemori T. Fas is required for clonal selection in germinal centers and the subsequent establishment of the memory B cell repertoire. Immunity. 2001;14:181–192. doi: 10.1016/s1074-7613(01)00100-5. [DOI] [PubMed] [Google Scholar]
- 29.McHeyzer-Williams MG, Nossal GJV, Lalor PA. Molecular characterization of single memory B cells. Nature. 1991;350:502–505. doi: 10.1038/350502a0. [DOI] [PubMed] [Google Scholar]
- 30.Smith KG, Nossal GJ, Tarlinton DM. FAS is highly expressed in the germinal center but is not required for regulation of the B cell response to antigen. Proc Natl Acad Sci USA. 1995;92:11628–11632. doi: 10.1073/pnas.92.25.11628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.White H, Gray D. Analysis of immunoglobulin (Ig) isotype diversity and IgM/D memory in the response to phenyl-oxazolone. J Exp Med. 2000;191:2209–2220. doi: 10.1084/jem.191.12.2209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cambridge G, et al. Circulating levels of B lymphocyte stimulator in patients with rheumatoid arthritis after rituximab treatment: Relationships with B cell depletion, circulating antibodies, and clinical relapse. Arthritis Rheum. 2006;54:723–732. doi: 10.1002/art.21650. [DOI] [PubMed] [Google Scholar]
- 33.Vallerskog T, et al. Differential effects on BAFF and APRIL levels in rituximab-treated patients with systemic lupus erythematosus and rheumatoid arthritis. Arthritis Res Ther. 2006;8:R167. doi: 10.1186/ar2076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mackay F, Schneider P, Rennert P, Browning J. BAFF and APRIL: A tutorial on B cell survival. Annu Rev Immunol. 2003;21:231–264. doi: 10.1146/annurev.immunol.21.120601.141152. [DOI] [PubMed] [Google Scholar]
- 35.O'Connor BP, Cascalho M, Noelle RJ. Short-lived and long-lived bone marrow plasma cells are derived from a novel precursor population. J Exp Med. 2002;195:737–745. doi: 10.1084/jem.20011626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Macallan DC, et al. B cell kinetics in humans: Rapid turnover of peripheral blood memory cells. Blood. 2005;105:3633–3640. doi: 10.1182/blood-2004-09-3740. [DOI] [PubMed] [Google Scholar]
- 37.Xiang Z, et al. FcγRIIb controls bone marrow plasma cell persistence and apoptosis. Nat Immunol. 2007;8:419–429. doi: 10.1038/ni1440. [DOI] [PubMed] [Google Scholar]
- 38.Hofer T, et al. Adaptation of humoral memory. Immunol Rev. 2006;211:295–302. doi: 10.1111/j.0105-2896.2006.00380.x. [DOI] [PubMed] [Google Scholar]
- 39.Radbruch A, et al. Competence and competition: The challenge of becoming a long-lived plasma cell. Nat Rev Immunol. 2006;6:741–750. doi: 10.1038/nri1886. [DOI] [PubMed] [Google Scholar]
- 40.Hannum LG, Ni D, Haberman AM, Weigert MG, Shlomchik MJ. A disease-related rheumatoid factor autoantibody is not tolerized in a normal mouse: Implications for the origins of autoantibodies in autoimmune disease. J Exp Med. 1996;184:1269–1278. doi: 10.1084/jem.184.4.1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Allman DM, Ferguson SE, Lentz VM, Cancro MP. Peripheral B cell maturation. II. Heat-stable antigen(hi) splenic B cells are an immature developmental intermediate in the production of long-lived marrow-derived B cells. J Immunol. 1993;151:4431–4444. [PubMed] [Google Scholar]