Abstract
In canonical Wnt signaling, Dishevelled (Dvl) is a critical cytoplasmic regulator that releases β-catenin from degradation. Here, we find that Dvl and c-Jun form a complex with β-catenin–T-cell factor 4 (TCF-4) on the promoter of Wnt target genes and regulate gene transcription. The complex forms via two interactions of nuclear Dvl with c-Jun and β-catenin, respectively, both of which bind to TCF. Disrupting the interaction of Dvl with either c-Jun or β-catenin suppresses canonical Wnt signaling–stimulated transcription, and the reduction of Dvl diminished β-catenin–TCF-4 association on Wnt target gene promoters in vivo. Expression of a TCF-Dvl fusion protein largely rescued the c-Jun knockdown Wnt signaling deficiency in mammalian cells and zebrafish. Thus, we confirm that c-Jun functions in canonical Wnt signaling and show that c-Jun functions as a scaffold in the β-catenin–TCFs transcription complex bridging Dvl to TCF. Our results reveal a mechanism by which nuclear Dvl cooperates with c-Jun to regulate gene transcription stimulated by the canonical Wnt signaling pathway.
Introduction
Wnt signaling plays pivotal roles in the regulation of body axis formation, cell proliferation, and organogenesis in many organisms and is important for homeostatic self-renewal in several adult tissues. Disorders in Wnt signaling cause human degenerative diseases as well as cancer (Nusse, 2005; Clevers, 2006). Wnt proteins initiate their signaling pathways by binding to their receptors, either Frizzled or a complex of Frizzled and LRP5/6. In cytoplasm, Wnt signaling branches into three distinct pathways via a cytoplasmic phosphoprotein Dishevelled (Dvl), namely the Wnt–β-catenin, Wnt–Ca2+, and Wnt–JNK (planar cell polarity or convergent extension) pathways (Sheldahl et al., 2003; Logan and Nusse, 2004; Clevers, 2006).
Previous studies have shown that β-catenin is an essential nuclear effector of canonical Wnt signaling. In the absence of Wnt signals, T-cell factor (TCF) family factors binding to the promoters leads to repression via association with Groucho/TLE proteins (Cavallo et al., 1998; Levanon et al., 1998; Roose et al., 1998), which tightly associate with chromatin and interact with histone H3 and the histone deacetylase Rpd3 (Palaparti et al., 1997; Chen et al., 1999). Under the activation of Wnt signaling, β-catenin forms a complex with TCFs that can displace Groucho/TLE and its partner Rpd3 from TCFs and recruit the coactivator CREB binding protein/p300 to the promoter (Hecht et al., 2000; Daniels and Weis, 2005). However, the formation of the β-catenin–TCFs transcriptional complex is subjected to many forms of regulation. A numerous of proteins, such as TATA binding protein (Hecht et al., 1999), Pontin52 (Bauer et al., 1998), Bcl-9/Legless, and Pygopus (Kramps et al., 2002; Townsley et al., 2004), are identified as the coactivators of the β-catenin–TCF complex. However, ICAT (Tago et al., 2000), Sox9 (Akiyama et al., 2004), Chibby (Takemaru et al., 2003), and adenomatous polyposis coli (APC; Sierra et al., 2006) have been identified as “destructors” of this complex. Therefore, it is believed that although β-catenin directly binds TCFs, this bilateral interaction is not sufficient, albeit required, for Wnt-mediated transcriptional activation.
Dvl is a critical cytoplasmic link between Frizzled and downstream components of the Wnt signaling pathway. Dvl has no known enzymatic activity. How Dvl transduces signals to distinct Wnt pathways has attracted considerable interest; it has been generally believed that Dvl functions as a scaffold protein bridging the receptors and downstream signaling components. However, recent findings that Dvl also exists in the nucleus and that its nuclear localization is required for the Wnt–β-catenin signaling pathway suggest that the involvement of Dvl in Wnt signaling may be more complex than previously thought (Torres and Nelson, 2000; Itoh et al., 2005).
In this study, we discovered two novel interactions between Dvl and c-Jun and between Dvl and β-catenin in the nucleus that mediate the formation of a Dvl–c-Jun–β-catenin–TCF functional complex. Our data revealed a novel nuclear function of Dvl in the β-catenin–TCFs–associated transcription complex besides its role in regulating the stability of β-catenin in the cytoplasm. In addition, c-Jun has been reported to cooperate with the β-catenin–TCFs transcriptional complex to regulate gene transcription (Nateri et al., 2005; Toualbi et al., 2007). Here, we demonstrate that c-Jun plays an important role in the canonical Wnt signaling pathway by acting primarily within the β-catenin–TCFs transcription complex as a scaffold protein to bridge Dvl to TCF.
Results
Dvl is recruited to the promoter of Wnt target genes
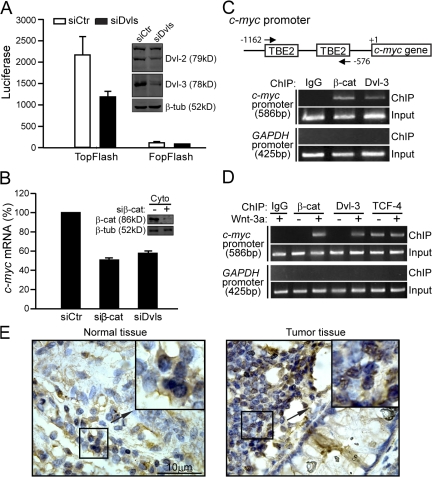
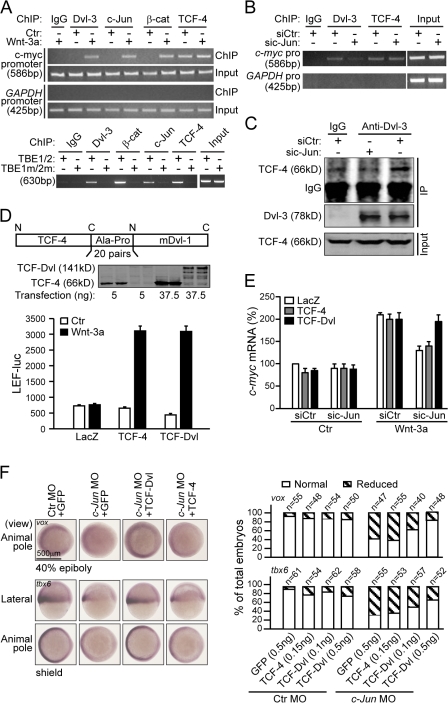
In the canonical Wnt pathway, Dvl has been established as a critical cytoplasmic regulator to release β-catenin from ubquitin-dependent degradation (Kimelman and Xu, 2006). Recent work of Itoh et al. (2005) suggests that Dvl's nuclear localization is required for the canonical Wnt signaling. To examine whether Dvl is required for Wnt signaling downstream of β-catenin, we knocked down endogenous Dvl in SW480 cells, which harbor a loss-of-function mutation in APC and thus exhibit “constitutive” β-catenin accumulation in the nucleus (Munemitsu et al., 1995; Korinek et al., 1997), and examined the effect on β-catenin–TCF–mediated transcriptional activity. Because there are three Dvl proteins in mammals, we had to cotransfect the cells with two siRNAs (named siDvls), one targeting human Dvl-1 and -3 and the other targeting human Dvl-2 (Fig. 1 A, inset). We found that the combination of these two siRNAs inhibited the activity of the TopFlash reporter (Fig. 1 A), which is known to specifically respond to Wnt–β-catenin signaling (Korinek et al., 1997). These siRNAs had no effect on the control reporter FopFlash (Fig. 1 A) nor did they affect soluble β-catenin levels or its distribution between cytosol and the nucleus (Fig. S1, available at http://www.jcb.org/cgi/content/full/jcb.200710050/DC1). Consistent with these observations, knockdown of Dvl in SW480 cells also led to reduction of endogenous c-myc expression, a well-characterized Wnt target gene (He et al., 1998), similar to knockdown of β-catenin (Fig. 1 B). Given that β-catenin is not subjected to APC-mediated degradation in SW480 cells, we reasoned that our results are best explained by the hypothesis that Dvl may participate in TopFlash reporter activation downstream of β-catenin stabilization.
Figure 1.
Dvl is recruited to the promoter of Wnt target genes. (A) Dvl siRNA suppresses TopFlash activity in SW480 cells. Cells in 6-well plates were transfected with 2 μg each of siDvl-1/3 and siDvl-2. After 48 h, cells were reseeded in 24-well plates and then transfected with 0.1 μg of TopFlash or FopFlash plasmid plus 0.5 μg each of siDvl-1/3 and siDvl-2. After 24 h, cells were lysed and luciferase activities were determined as described previously (Li et al., 1999a). Error bars indicate SD of three independent experiments. (inset) Western analysis of the Dvl-2 and Dvl-3 expression level. (B) Dvl siRNA suppresses c-myc expression in SW480 cells. SW480 cells in 6-well plates were transfected with 2 μg each of siDvl-1/3 and siDvl-2 or 4 μg of siβ-catenin for 72 h. The c-myc mRNA level was detected by qPCR using GAPDH as an inner control. Error bars indicate SD of three independent experiments. (C) Dvl-3 binds to the promoter of c-myc in SW480 cells. The ChIP assays in SW480 cells were performed as described in Materials and methods using anti–β-catenin and anti–Dvl-3 antibodies. For a negative control, mouse IgG was used. (D) Dvl-3 binds to the promoter of c-myc upon Wnt-3a stimulation in HEK293T cells. HEK293T cells were stimulated by Wnt-3a or control CM for 3 h and ChIP assays were performed as indicated. (E) Immunohistochemistry for Dvl-3 in colon cancer tissues. The subcellular distribution of Dvl-3 (brown) was detected using an anti–Dvl-3 antibody. The nucleus was stained by hematoxylin (blue).
Meanwhile, a surprising finding that Dvl is recruited to the promoter of Wnt target genes further supports this “downstream” role of Dvl in β-catenin–TCF–mediated gene transcription. As shown in Fig. 1 C, the promoter of the c-myc gene was efficiently pulled down by a specific Dvl-3 antibody, and, as a negative control, the promoter of the GAPDH gene could not be pulled down (Fig. 1 C). In addition, we also observed the binding of Dvl-3 to other Wnt targets' promoters, Axin2 and Fgf8 (unpublished data). We further confirmed this finding in HEK293T cells. Like β-catenin, Dvl-3 was recruited to the c-myc promoter in a Wnt-3a–dependent manner, whereas the binding of TCF-4 to the promoter was independent of Wnt stimulation (Fig. 1 D). These results suggest a novel role of Dvl in the canonical Wnt signaling pathway by mediating the transcriptional activity of the β-catenin–TCF complex at the promoter of Wnt target genes.
We also examined several colon cancer tissue sections by immunohistochemical staining with an anti–Dvl-3 monoclonal antibody. In contrast to the normal tissue sections, where Dvl-3 is more abundantly localized in the cytoplasm (Fig. 1 E, left), Dvl-3 appears to be accumulated at high levels in the nuclei of the cancer cells (Fig. 1 E, right). We examined 91 colon cancer tissue samples and found that 36% of the samples displayed marked nuclear accumulation of Dvl-3. These data are consistent with previous reports showing nuclear localization of Dvl and a critical role of such localization in Wnt–β-catenin signaling (Torres and Nelson, 2000; Itoh et al., 2005).
Dvl binds to c-Jun
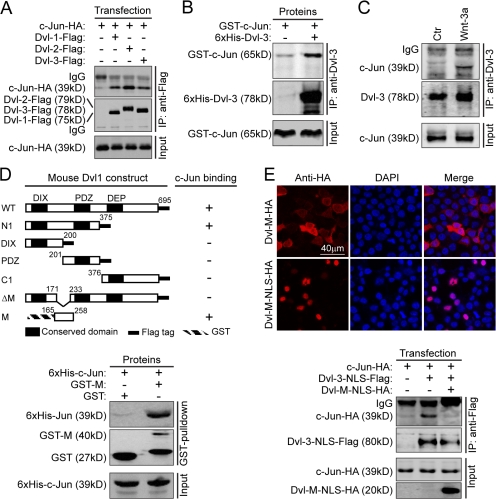
Although Itoh et al. (2005) found that nuclear localization of Dvl is required for canonical Wnt signaling and our results suggested that Dvl may function on the promoter of Wnt target genes (Fig. 1, C and D), the molecular context of nuclear Dvl function remains unknown. In the yeast two-hybrid screening using Dvl-1-N1 (residues 1–375) as the bait, we identified a positive clone that encodes a fragment of c-Jun (unpublished data). A recent study demonstrated that c-Jun can interact with TCF-4 (Nateri et al., 2005). These results suggested a clue as to how Dvl might function in the nucleus. The result shown in Fig. 2 A indicated that all three Dvl homologues could interact with c-Jun. The in vitro binding assay using recombinant proteins confirmed that the interaction is direct (Fig. 2 B). Because c-Jun is a nuclear protein, we examined the interaction between endogenous Dvl and c-Jun by immunoprecipitating Dvl-3 in the nuclear fraction and found that endogenous c-Jun was coprecipitated but only under Wnt-3a stimulation (Fig. 2 C), which suggests that the interaction of nuclear Dvl with c-Jun in vivo is regulated by Wnt signals.
Figure 2.
Dvl binds to c-Jun. (A) c-Jun interacts with three homologues of the Dvl family. HEK293T cells in 6-well plates were cotransfected with 250 ng of HA-tagged c-Jun plasmid and 250 ng of Flag-tagged Dvl-1, Dvl-2, or Dvl-3, respectively. After 24 h, immunoprecipitation was performed using an anti-Flag antibody and detected using an anti-HA antibody. (B) c-Jun directly interacts with Dvl in vitro. Recombinant His-Dvl-3 and GST–c-Jun were obtained from E. coli. The in vitro binding experiment was performed as described in Materials and methods. GST–c-Jun was pulled down with His–Dvl-3 by an anti–Dvl-3 antibody. (C) Endogenous interaction of Dvl with c-Jun. 5 × 107 of HEK293T cells were treated with Wnt-3a or control CM for 3 h and the nuclear extracts were then immunoprecipitated by an anti–Dvl-3 antibody as indicated in Materials and methods. c-Jun was detected by an anti–c-Jun antibody. (D) Schematic representation of mapping the c-Jun–interacting domain in Dvl-1. The binding of full-length Dvl or various Dvl fragments (as depicted) to c-Jun was examined by co-IP experiments with an anti-Flag antibody, except for Dvl-M. For detection of the interaction of Dvl-M with c-Jun, recombinant His–c-Jun and GST–Dvl-M were expressed in E. coli. His–c-Jun was coprecipitated with GST–Dvl-M pulled down by glutathione–Sepharose 4B. Numbers indicate sequence position. (E) Dvl-M-NLS can disrupt the interaction between Dvl and c-Jun. Subcellular distribution of Dvl-M and Dvl-M-NLS is shown on top. 500 ng of Dvl-M-HA or Dvl-M-NLS-HA was transfected into HEK293T cells in 6-well plates and detected using immunofluorescence (top). For the binding experiment, 250 ng c-Jun–HA was cotransfected with 250 ng Dvl-3-NLS–Flag plus 500 ng LacZ or Dvl-M-NLS–HA in HEK293T cells (bottom).
We further delineated the regions within Dvl responsible for the interaction with c-Jun and mapped the c-Jun–interacting domain to residues 165–258, which we designated as Dvl-M, by coimmunoprecipitation (co-IP) and in vitro binding experiments (Fig. 2 D). Expression of Dvl-M-NLS, which contains a nuclear localization sequence (NLS) that efficiently promoted Dvl-M nuclear translocation (Fig. 2 E, top), was able to disrupt the interaction between c-Jun and Dvl-3-NLS in the nucleus as shown in a co-IP experiment (Fig. 2 E, bottom). In addition, a truncated form of Dvl (named Dvl-ΔM) with a deletion of residues 172–232 could no longer bind to c-Jun (Figs. 2 D and S2, available at http://www.jcb.org/cgi/content/full/jcb.200710050/DC1).
c-Jun Is critically involved in the canonical Wnt pathway
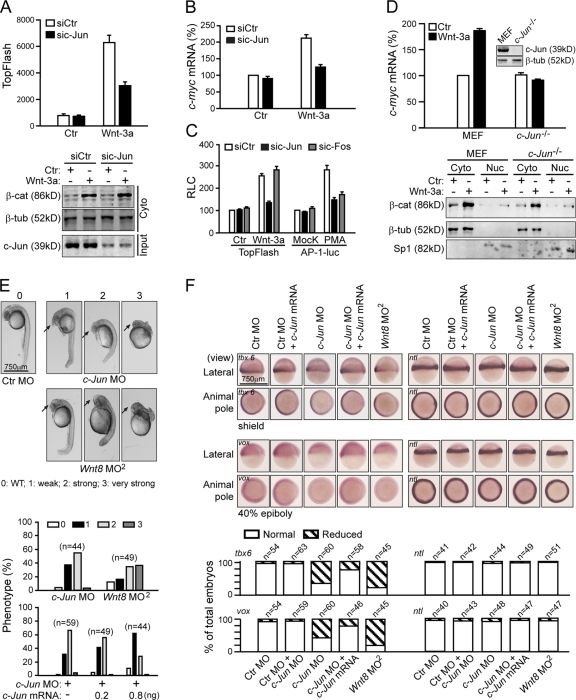
Our finding that canonical Wnt signals could induce nuclear Dvl and c-Jun interaction (Fig. 2 C) prompted us to investigate the role of c-Jun in Wnt-regulated gene transcription. We observed that knockdown of c-Jun in HEK293T cells by a c-Jun–specific siRNA resulted in a decrease in Wnt-3a–stimulated TopFlash activity (Fig. 3 A, top) and the expression of c-myc (Fig. 3 B). However, the accumulation of soluble β-catenin was not affected (Fig. 3 A, bottom). These results suggest that c-Jun might be involved in the canonical Wnt signaling pathway and act downstream of β-catenin stabilization. However, c-Fos siRNA did not affect Wnt-3a–induced TopFlash activity, whereas it could suppress AP-1 activity in a similar manner to c-Jun siRNA (Fig. 3 C), which suggests that the canonical Wnt signaling defect caused by c-Jun depletion is unlikely to be caused by AP-1 signaling deficiency in these cells. We further tested the canonical Wnt signaling activity in c-Jun knockout (KO) cells. As shown in Fig. 3 D, in c-Jun −/− cells, the level and the cellular distribution of free β-catenin were not different from that in wild-type mouse embryonic fibroblast (MEF) cells, but Wnt-3a–induced c-myc expression was suppressed.
Figure 3.
c-Jun is involved in canonical Wnt signaling. (A) Effects of c-Jun siRNA on Wnt signaling in HEK293T cells. Cells in 6-well plates were transfected with 3 μg of c-Jun siRNA. After 48 h, cells were reseeded in 24-well plates and transfected with 0.01 μg of TopFlash plasmid plus 1 μg sic-Jun. After 24 h, the cells were stimulated by Wnt-3a CM for 8 h for TopFlash assays. The free β-catenin level is shown (bottom). Error bars indicate SD of duplicate data in one experiment; the results were repeated three times. (B) c-Jun siRNA suppresses Wnt-3a–induced c-myc expression. c-myc mRNA level was detected by qPCR using GAPDH as an inner control. (C) c-Fos siRNA does not affect the activity of TopFlash. HEK293T cells in 6-well plate were transfected with 3 μg of sic-Jun, sic-Fos, or siCtr for 48 h and reseeded in a 24-well plate overnight. The reseeded cells were again transfected with 10 ng of TopFlash plasmid or 20 ng AP-1–luc plasmid plus 1 μg sic-Jun, sic-Fos, or siCtr for 24 h, then the cells were stimulated by Wnt-3a for 8 h for TopFlash or PMA (0.1 μM) for 24 h for the AP-1–luc assay. (D, top) Wnt-3a–induced c-myc expression was suppressed in c-Jun KO cells. (bottom) The cytoplasm β-catenin level and cell distribution under stimulation with Wnt-3a CM. Error bars in B, C, and D indicate SD of three independent experiments. (E) c-Jun morphants phenocopy Wnt8 morphants. Injections were done at the one-cell stage of zebrafish at the dose of 8 ng for c-Jun MO, and 1.25 ng for Wnt8 ORF1 and ORF2 MO. Phenotypes were scored at 24 h after fertilization on a 4-point scale as described previously, with 0 being wild-type and 3 being the most severe phenotype (top, arrows indicate the enlargement of the telencephalon; Waxman et al., 2004). Graph shows percentage of embryos with the respective phenotypes (bottom). Injection of human c-Jun mRNA into c-Jun morphants shifted the phenotypic distribution to less-severe dorsalized classes (bottom). (F) c-Jun MO reduces the expression of ventral markers during early zebrafish development. Embryos were examined by in situ hybridization for tbx6 expression at the shield stage and for vox expression at the 40% epiboly stage. As a control, no tail expression was examined at both stages (top). Percentage of the total embryos, in which the expression of markers was reduced, is indicated (bottom).
To further probe the physiological role of c-Jun in canonical Wnt signaling, we investigated the role of c-Jun in early embryonic development of zebrafish. In zebrafish, Wnt–β-catenin signaling is essential for the establishment of ventral and posterior fates (Erter et al., 2001; Lekven et al., 2001; Thorpe et al., 2005). In general, embryos injected with c-Jun morpholino oligonucleotides (MO) exhibited dorsalized phenotypes at 24 h after fertilization, with enlarged telencephalons and a reduced posterior (trunk and tail), that were quite similar to those of Wnt8 morphants (Fig. 3 E, top; Lekven et al., 2001; Waxman et al., 2004). Of the total 44 embryos injected with c-Jun MO, 38% showed a modest enlargement of their telencephalons (Fig. 3 E, phenotype 1), 57% exhibited a strong anteriorization phenotype with enlargement of their heads and reduction of their tails (phenotype 2), and 3% displayed a very strong anteriorization phenotype with over-enlargement of the telencephalons and lack of their trunks and tails (phenotype 3).
The fact that human c-Jun mRNA injection could rescue the embryonic defects induced by c-Jun MO in a dose-dependent manner (Fig. 3 E, bottom) suggests that the effect of c-Jun MO is specific, and the similarity in phenotypes of c-Jun and Wnt8 morphants supports the notion that c-Jun may be specifically involved in the Wnt-8–β-catenin signaling pathway during early zebrafish development. We also confirmed our observations using a second c-Jun MO, c-Jun MO2 (Fig. S3, available at http://www.jcb.org/cgi/content/full/jcb.200710050/DC1).
A previous study demonstrated that the ventrolateral mesoderm marker tbx6 is a direct target of Wnt–β-catenin signaling (Szeto and Kimelman, 2004). Consistent with the phenotype of c-Jun morphants, c-Jun MO injection caused a reduction of tbx6 expression. Unlike BMP signaling deficiency, which caused strong uniform reduction, c-Jun MO injection led to a graded reduction of tbx6 expression with the strongest effect in the lateral domains of the ventrolateral mesoderm, which is similar to that observed in Wnt8 morphants (Fig. 3 F; Szeto and Kimelman, 2004). Another ventral marker vox, whose expression is under the control of Wnt-8–β-catenin signaling at 40% epiboly (Ramel and Lekven, 2004), was also suppressed in the margin (Fig. 3 F). In addition, the expression of both marker genes could be similarly rescued by c-Jun mRNA in c-Jun morphants (Fig. 3 F). As a negative control, the expression of no tail was unaffected.
Putting all these results together, we conclude that c-Jun is a novel component critically involved in the canonical Wnt pathway.
Interaction of Dvl with c-Jun is crucial for Wnt–β-catenin signaling
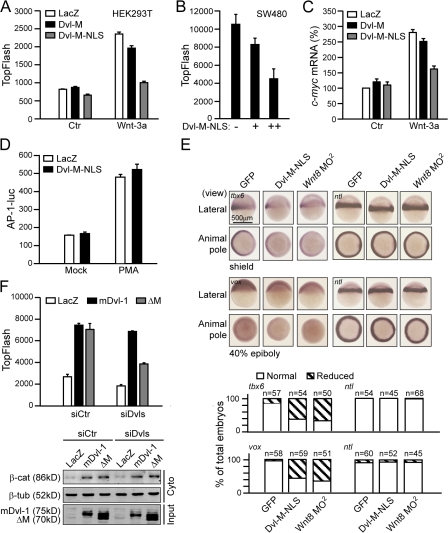
Next, we sought to determine whether the interaction between nuclear Dvl and c-Jun plays a role in canonical Wnt–β-catenin signaling. We examined the effect of Dvl-M, which is able to disrupt the Dvl–c-Jun interaction (Fig. 2 E), on Wnt-induced transcriptional activation. We cotransfected Dvl-M or Dvl-M-NLS with the TopFlash reporter plasmid into HEK293T cells and found that Dvl-M-NLS efficiently inhibited the Wnt-3a–induced increase in TopFlash activity (Fig. 4 A). This inhibitory effect of Dvl-M-NLS on TopFlash activity was also observed in SW480 cells (Fig. 4 B), which suggests that the interaction of Dvl with c-Jun plays a critical role downstream of β-catenin stabilization in canonical Wnt signaling. In agreement with the TopFlash assay, the expression of c-myc induced by Wnt-3a was also reduced by Dvl-M-NLS (Fig. 4 C). As c-Jun is a member of the transcription factor AP-1 family, we also tested whether the interaction of Dvl with c-Jun was involved in AP-1 signaling. The result shown in Fig. 4 D indicated that Dvl-M-NLS did not suppress PMA-induced AP-1 reporter activity. Therefore, we suggest that the interaction of Dvl with c-Jun is specifically involved in the canonical Wnt signaling pathway.
Figure 4.
Interaction of Dvl with c-Jun is crucial for Wnt–β-catenin signaling. (A) Disruption of the interaction of Dvl with c-Jun suppresses the TopFlash activity. HEK293T cells in 24-well plates were transfected with 10 ng of TopFlash plasmid plus 150 ng of Dvl-M, Dvl-M-NLS, or LacZ overnight and stimulated with Wnt-3a or control CM for an additional 8 h. (B) Dvl-M-NLS suppresses the TopFlash activity in SW480 cells. SW480 cells in 24-well plates were transfected with 100 ng TopFlash plus 100 or 300 ng of Dvl-M-NLS, respectively. Error bars in A and B indicate SD of duplicate data in one experiment; the results were repeated three times. (C) Dvl-M-NLS suppressed Wnt-3a–induced c-myc expression. HEK293T cells in 24-well plates were transfected with 75 ng of Dvl-M, Dvl-M-NLS, or LacZ overnight and stimulated by Wnt-3a or control CM. Error bars indicate SD of three independent experiments. (D) Disruption of the interaction of Dvl with c-Jun does not affect PMA-induced AP-1 activity. HEK293T cells in 24-well plates were transfected with 20 ng of AP-1–luc reporter plasmid plus 150 ng of Dvl-M-NLS or LacZ overnight; then PMA was added for an additional 24 h. (E) Dvl-M-NLS suppresses the expression of ventral markers in zebrafish. Embryos were injected with 2.5 ng mouse Dvl-M-NLS mRNA or GFP mRNA and 1.25 ng for Wnt8 ORF1 and ORF2 MO (Wnt8 MO2) at the one-cell stage. (F) Effects of Dvl-ΔM on TopFlash activity and β-catenin accumulation. HEK293T cells in 6-well plates were transfected with 2 μg each of siDvl-1/3 and siDvl-2. After 48 h, cells were reseeded in 24-well plates and transfected with 0.01 μg TopFlash plasmid plus 0.5 μg each of siDvl-1/3 and siDvl-2 and 50 ng of mouse Dvl-1 or Dvl-1-ΔM plasmids for 24 h. The free β-catenin in the cytoplasm induced by Dvl or Dvl-1-ΔM is shown (bottom). Error bars in D and F indicate SD of duplicate data in one experiment; the results were repeated three times.
We also probed the potential physiological role of the Dvl–c-Jun interaction during early zebrafish development. Zebrafish injected with Dvl-M-NLS mRNA displayed defects similar to c-Jun and Wnt8 morphants (not depicted) and showed an obvious reduction in the expression of canonical Wnt target genes tbx6 and vox (Fig. 4 E).
We further investigated the role of Dvl–c-Jun interaction in the canonical Wnt pathway using the Dvl-ΔM construct. Dvl-ΔM, which is unable to bind c-Jun (Fig. S2), still retained its ability to stabilize cytosolic β-catenin and stimulate TopFlash activity when overexpressed in HEK293T cells (Fig. 4 F). When the endogenous Dvls in HEK293T cells were knocked down with Dvls siRNAs, expression of mouse Dvl-1 largely rescued the TopFlash activity (Fig. 4 F). However, the rescue ability was significantly dampened for mouse Dvl-1-ΔM (Fig. 4 F), despite the fact that it could induce β-catenin accumulation in the cytoplasm just as well as the wild-type mDvl-1 (Fig. 4 F, bottom). Therefore, our results here strongly suggest that Dvl functions in the canonical Wnt pathway via two mechanisms: one is to stabilize β-catenin in the cytoplasm and the other is to directly regulate β-catenin–TCFs transcriptional activity dependent on its binding to c-Jun in the nucleus. In this respect, Dvl-ΔM could only substitute for the function of Dvl in the cytoplasm but not in the nucleus when endogenous Dvls are knocked down.
c-Jun mediates Dvl association with the functional TCF–β-catenin complex
Nateri et al. (2005) previously reported that c-Jun could associate with TCF-4. Our findings that Dvl is recruited to the promoter of c-myc (Fig. 1, C and D) and that the interaction of c-Jun with Dvl is critical for canonical Wnt signaling (Fig. 4) strongly suggest the possibility that c-Jun may mediate the association of Dvl with TCF-4 and recruit Dvl to the promoter. To test this possibility, we first examined whether c-Jun associates with the promoter of Wnt target genes. Chromatin immunoprecipitation (ChIP) assays indicated that c-Jun could be recruited to the c-myc promoter in a Wnt-3a–dependent manner (Fig. 5 A, top). Mutations in the TCF-binding sites eliminated the binding of TCF-4 and also abolished the association of Dvl and c-Jun as well as β-catenin to the promoter (Fig. 5 A, bottom), which suggests that the recruitment of the latter factors to c-myc promoter is dependent on TCF binding. These results, together with the observation that knockdown of c-Jun reduced the binding of Dvl-3 to the c-myc promoter (Fig. 5 B) and the amount of TCF-4 coprecipitated with nuclear Dvl-3 (Fig. 5 C), strongly suggest that the binding of Dvl to a native Wnt target gene promoter is mediated by c-Jun.
Figure 5.
c-Jun mediates Dvl association with the functional TCF–β-catenin complex. (A) c-Jun associates with the c-myc promoter in a TCFs-dependent and Wnt-induced manner. The ChIP assays were performed in HEK293T cells as indicated. To test whether the TCF-binding site is required for the binding of Dvl and c-Jun to the promoter, 100 ng of pBV-TBE1/2-luc (the wild type of the c-myc promoter) or pBV-TBE1m/2m-luc (a TCF binding site mutant of the c-myc promoter) plasmids (He et al., 1998) were transfected in HEK293T cells in a 100-mm plate. Before ChIP assays, the cells were stimulated with Wnt-3a CM for 3 h. The primer pairs used to detect exogenous c-myc promoter were derived from a pBV vector sequence: 5′-AGGTACGGGAGGTACTT-3′ and 5′-ACCAACAGTACCGGAAT-3′. (B) c-Jun depletion suppresses the binding of Dvl-3 to the c-myc promoter. HEK293T cells were transfected with c-Jun siRNA for 72 h and then treated with Wnt-3a CM for 3 h. The ChIP assays were performed as indicated. (C) c-Jun depletion disrupts the nuclear Dvl/TCF-4 association. HEK293T cells were treated with c-Jun siRNA for 72 h and then stimulated by Wnt-3a CM for 3 h. The nuclear extracts were immunoprecipitated by an anti–Dvl-3 antibody and then detected by an anti–TCF-4 antibody. (D) TCF-Dvl functions as well as TCF-4 in mediating Wnt-3a–stimulated transcription. HEK293T cells in 24-well plates were transfected with 20 ng of LEF-luc plasmid and either 5 ng of TCF-4 or 37.5 ng of TCF-Dvl. Western analysis of TCF-4 and TCF-Dvl expression is shown. Error bars indicate SD of duplicate data in one experiment; the results were repeated three times. (E) TCF-Dvl can relieve the suppression of c-myc expression caused by c-Jun siRNA. HEK293T cells in a 24-well plate were transfected with 0.5 μg of c-Jun siRNA or control siRNA. 5 ng TCF-4 or 20 ng TCF-Dvl was used to rescue the c-Jun depletion. Error bars indicate SD of three independent experiments. (F) TCF-Dvl can relieve the suppression of ventral marker expression caused by c-Jun MO in zebrafish. 8 ng of c-Jun MO were injected with 0.5 ng of GFP, 0.1 ng of TCF-Dvl, 0.5 ng of TCF-Dvl, or 0.15 ng TCF-4 mRNA, respectively, into one-cell embryos. Graph shows the penetrance of rescue.
To gain further insight into the mechanism by which c-Jun functions in the canonical Wnt signaling, we generated a fusion protein TCF-Dvl, in which TCF-4 and mouse Dvl-1 were linked by a linker consisting of 20 pairs of Ala-Pro (Fig. 5 D, top). The result shown in Fig. 5 D (bottom) indicated that TCF-Dvl efficiently mediated Wnt-3a–stimulated activation of the LEF-1–luc reporter, which does not respond to Wnt stimulation unless an additional LEF-1 or TCF plasmid was cotransfected (Li et al., 1999a) in a similar fashion to TCF-4. Interestingly, although c-Jun knockdown caused a reduction in Wnt-3a–induced c-myc expression, transfection of TCF-Dvl but not TCF-4 restored the c-myc expression level (Fig. 5 E), indicating that TCF-Dvl can rescue the defect in endogenous Wnt target gene expression caused by c-Jun depletion in mammalian cells. In zebrafish, the dorsalized phenotype induced by c-Jun MO was also rescued by expression of this TCF-Dvl fusion protein in a dose-dependent manner but not by TCF-4 (unpublished data). An in situ hybridization experiment further confirmed that the phenotypic rescue by TCF-Dvl was accompanied by the restoration of the expression of ventral Wnt target genes tbx6 and vox (Fig. 5 F). These results provide not only a direct proof for the mechanistic role of c-Jun, acting as an adaptor, in regulation of canonical Wnt signaling but also a strong piece of genetic evidence for the involvement of c-Jun in the canonical Wnt signaling pathway. Moreover, these results strongly suggest that the association of Dvl with TCFs mediated by c-Jun is a critical event for canonical Wnt signaling.
Previous work of Nateri et al. (2005) showed that the interaction between c-Jun and TCF-4 depends on c-Jun phosphorylation. We found that c-Jun AA (S63A and S73A) could still bind to Dvl (Fig. S4 A, available at http://www.jcb.org/cgi/content/full/jcb.200710050/DC1) and that overexpression of c-Jun AA significantly suppressed Wnt-3a–induced transcription (Fig. S4 B) and blocked the association of Dvl with the β-catenin–TCF complex on the promoter (Fig. S4 C). Putting these results together, we conclude that c-Jun N-terminal phosphorylation is likely required in canonical Wnt signaling to mediate Dvl association with TCFs in the nucleus.
Nuclear Dvl promotes functional β-catenin–TCF transcriptional complex formation
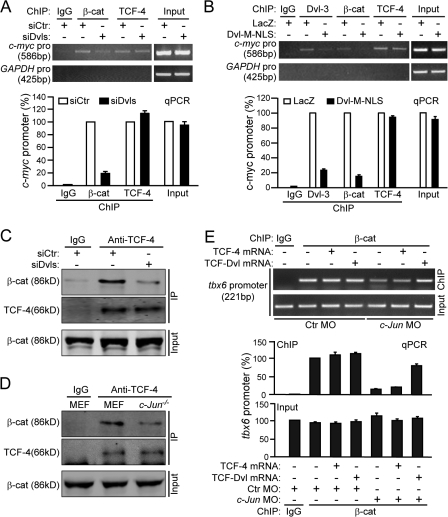
To better understand Dvl's role in the β-catenin–TCF transcriptional complex, we performed ChIP assays in SW480 cells under the conditions of depletion or reduction of endogenous Dvls using siRNA. We surprisingly observed that association of β-catenin with the c-myc promoter was suppressed under Dvls depletion, whereas TCF-4 remained bound to the promoter (Fig. 6 A). Consistent with our earlier observation that the association of Dvl-3 with TCF-4 on the promoter is mediated by c-Jun (Fig. 5 B), disruption of the interaction of Dvl-3 with c-Jun by Dvl-M-NLS not only removed Dvl-3 from the c-myc promoter but also suppressed the binding of β-catenin to the promoter (Fig. 6 B). Collectively, these results implied that the association of Dvl with TCF mediated by c-Jun plays a crucial role in promoting or stabilizing the formation of the β-catenin–TCFs complex at Wnt target gene promoters.
Figure 6.
Nuclear Dvl promotes functional TCF–β-catenin transcriptional complex formation. (A) Dvl depletion reduces the association of β-catenin with the c-myc promoter. SW480 cells in 100-mm plates were transfected with 12 μg each of siDvl-1/3 and siDvl-2 for 72 h and ChIP assays were performed as indicated. Corresponding qPCR data (bottom) show that the effect of siDvls represents as relative to that of control siRNA. Error bars indicate SD of two qPCR experiments. (B) Disruption of the interaction of Dvl with c-Jun suppresses the binding of Dvl and β-catenin to the c-myc promoter. Cells in 100-mm plates were transfected with 3 μg of Dvl-M-NLS for 24 h and stimulated by Wnt-3a CM for 3 h. (C) Dvl depletion reduces the β-catenin–TCF-4 complex formation. SW480 cells were treated with siDvls for 72 h and the nuclear extracts were immunoprecipitated by an anti–TCF-4 antibody. (D) The β-catenin–TCFs complex formation was impaired in c-Jun −/− cells. c-Jun −/− or wild-type MEF cells were stimulated with Wnt-3a CM for 3 h and the nuclear extract was precipitated by an anti–TCF-4 antibody. (E) ChIP analysis of endogenous β-catenin at the TCF-binding site of the zebrafish tbx6 promoter. The ChIP assays were performed as indicated in Materials and methods. The primer pairs used for the zebrafish tbx6 promoter were 5′-CACCTATATGTGCGTCTCT-3′ and 5′-TCTACCTTCTTCCATCACT-3′. Error bars indicate SD of two qPCR quantitative experiments.
To further confirm this finding, we used co-IP to examine the endogenous β-catenin–TCF complex upon Dvl depletion. As shown in Fig. 6 C, the association of β-catenin with TCF-4 was significantly reduced by Dvls siRNA in SW480 cells, indicating that β-catenin–TCF-4 complex formation or stabilization indeed requires nuclear Dvl. Consistent with this finding, β-catenin–TCF-4 complex formation or stability was also significantly impaired in c-Jun −/− cells (Fig. 6 D). Similarly, ChIP assays in zebrafish embryos indicated that c-Jun MO could reduce the binding of β-catenin to the tbx6 promoter (Fig. 6 E). More importantly, TCF-Dvl fusion but not TCF-4 rescued the binding of β-catenin to the tbx6 promoter (Fig. 6 E), further confirming the idea that Dvl can regulate the functional β-catenin–TCFs complex formation or its stabilization.
Dvl binds to β-catenin
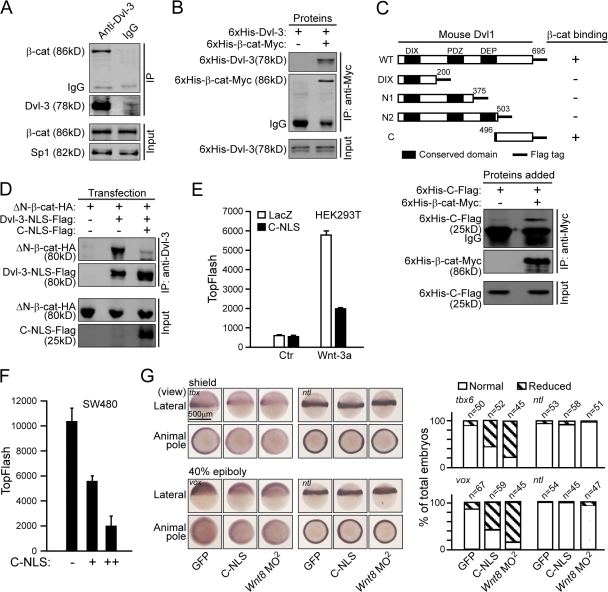
As nuclear Dvl can regulate the β-catenin–TCFs complex formation, it was not surprising that β-catenin was detected in the nuclear Dvl-3–associated complex (Fig. 7 A). Interestingly, an in vitro binding assay indicated that Dvl could directly bind to β-catenin (Fig. 7 B). We also examined the other two isoforms of Dvl, Dvl-1 and Dvl-2, and found that they both could bind to β-catenin (unpublished data). We further determined that the C-terminal 200 amino acids of Dvl-1 (designated as Dvl-C) were responsible for binding to β-catenin, and the in vitro binding assay confirmed the direct interaction between Dvl-C and β-catenin (Fig. 7 C). Importantly, this region could compete with full-length Dvl for β-catenin binding in the co-IP experiment (Fig. 7 D).
Figure 7.
Dvl binds to β-catenin. (A) β-catenin associates with Dvl in vivo. 5 × 107 of HEK293T cells were stimulated with Wnt-3a CM for 3 h and the nuclear extracts were then immunoprecipitated by an anti–Dvl-3 antibody or IgG as a control. (B) Dvl-3 directly interacts with β-catenin. Recombinant 6His-Dvl-3 and 6His-β-catenin-myc proteins were expressed in E. coli. The in vitro pull-down experiment was performed using an anti-Myc antibody. (C) Schematic representation of the wild-type mouse Dvl-1 and its mutants. The summary of the binding of various Dvl-1 constructs to β-catenin is tabulated at the right. (bottom) The interaction of Dvl-C with the β-catenin in vitro binding assay is shown. Numbers indicate sequence position. (D) Dvl-C-NLS can disrupt the interaction between Dvl-3-NLS and β-catenin. 250 ng ΔN-β-catenin-HA was cotransfected with 250 ng Dvl-3-NLS plus 500 ng of LacZ or Dvl-C-NLS-Flag into HEK293T cells in 6-well plates. Co-IP was performed using an anti-Dvl-3 antibody. (E) Dvl-C-NLS suppresses the TopFlash activity. HEK293T cells in 24-well plates were transfected with 10 ng of TopFlash plasmid plus 150 ng of Dvl-C-NLS or LacZ overnight and stimulated with Wnt-3a or control CM for an additional 8 h. (F) Dvl-C-NLS suppresses the TopFlash activity in SW480 cells. SW480 cells in a 24-well plate were transfected with 100 ng of TopFlash plasmid plus 100 or 300 ng Dvl-C-NLS for 24 h. Error bars indicate SD of duplicate data in one experiment; the results were repeated three times. (G) Dvl-C-NLS reduces the expression of ventral markers during early zebrafish development. Embryos were injected with 2.5 ng mouse Dvl-C-NLS mRNA or GFP mRNA and 1.25 ng for Wnt8 ORF1 and ORF2 MO (Wnt8 MO2).
Next, we examined whether the interaction of Dvl with β-catenin plays an important role in β-catenin–mediated gene transcription. We found that expression of Dvl-C-NLS markedly decreased the TopFlash reporter activity in HEK293T and SW480 cells (Fig. 7, E and F), which suggests that the interaction of Dvl with β-catenin acts downstream of β-catenin stabilization. We further confirmed the physiological role of Dvl–β-catenin interaction using the zebrafish model system. In agreement with the results in mammalian cells, zebrafish injected with Dvl-C-NLS mRNA showed reduced expression of canonical Wnt target genes tbx6 and vox (Fig. 7 G). Therefore, these results suggest a specific effect of the C terminus of Dvl on Wnt target gene expression and support a physiological role of Dvl–β-catenin interaction in the canonical Wnt signaling pathway.
In addition, ChIP assays showed that Dvl-C-NLS dramatically reduced the binding of β-catenin to the c-myc promoter (Fig. S5, available at http://www.jcb.org/cgi/content/full/jcb.200710050/DC1), further confirming the idea that the function of nuclear Dvl is to regulate the formation or stability of the β-catenin–TCFs complex.
Nuclear Dvl and c-Jun form a functional complex with β-catenin–TCF-4
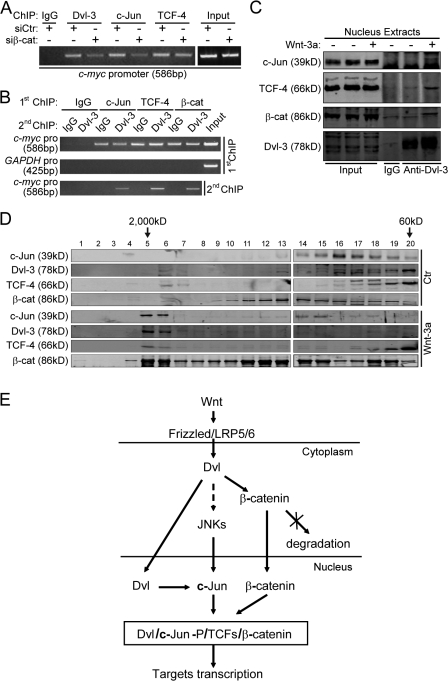
As the binding of Dvl and c-Jun to the c-myc promoter is Wnt- and TCF-dependent, our observation that knockdown of β-catenin reduced the binding of Dvl-3 and c-Jun to the c-myc promoter (Fig. 8 A), together with the findings that Dvl can directly interact with c-Jun and β-catenin (Figs. 2 and Figs.7), strongly suggests that these four proteins may form a complex. A re-ChIP assay confirmed the existence of this complex; Dvl-3 was found to be associated with the c-myc promoter sequences that had been preimmunoprecipitated by the anti–c-Jun, anti–TCF-4, or anti–β-catenin antibody (Fig. 8 B). To obtain further evidence for the presence of the functional Dvl-3–c-Jun–β-catenin–TCF-4 complex in the nucleus, nuclear extract of HEK293T cells pretreated with Wnt-3a conditional medium or the control medium was subjected to immunoprecipitation with the anti–Dvl-3 antibody. We could detect c-Jun, TCF-4, and β-catenin in the immunocomplexes from the Wnt-treated sample (Fig. 8 C). Moreover, we used fast-performance liquid chromatography to fractionate HEK293T nuclear extract with a Superose 6 10/300 GL column. All of these four proteins could be detected in the same peak at ∼2,000 kD in the Wnt-treated sample (Fig. 8 D). Therefore, we conclude that Dvl, c-Jun, β-catenin, and TCF-4 form a complex when the canonical Wnt pathway is activated.
Figure 8.
Dvl and c-Jun form a functional complex with β-catenin–TCF-4. (A) β-catenin siRNA suppresses the association of Dvl and c-Jun with the c-myc promoter in SW480 cells. Cells in 100-mm plates were transfected with 12 μg of β-catenin siRNA for 72 h and then ChIP experiments were performed. (B) Dvl-3 colocalizes with c-Jun, β-catenin, and TCF-4, respectively, on the c-myc promoter. The re-ChIP experiment is described in Materials and methods. HEK293T cells were treated with Wnt-3a CM for 3 h before the first ChIP experiment was performed. (C) c-Jun, β-catenin, and TCF-4 can be coimmunoprecipitated with Dvl-3. 5 × 107 of HEK293T cells were treated with Wnt-3a or control CM for 3 h and the nuclear extracts were immunoprecipitated by anti–Dvl-3 antibody or IgG as a control. (D) Fractionation of the nuclear extracts of HEK293T cells using fast protein liquid chromatography gel filtration. (E) A model for involvement of Dvl and c-Jun in the transcriptional complex in the canonical Wnt signaling pathway.
Discussion
In the nucleus, formation of the β-catenin–TCFs complex has been well established to be a prerequisite for the transcription of Wnt target genes. However, the formation of this complex is subjected to many forms of regulation. In this paper, we have demonstrated a novel role of Dvl and c-Jun in regulating the functional β-catenin–TCFs complex formation via two interactions of Dvl with c-Jun and β-catenin, respectively.
c-Jun functions as a key component of Wnt signaling in vivo
As an AP-1 family transcription factor, c-Jun regulates cell proliferation, survival, and death and, as one of the earliest responsive genes, is essential for embryonic development (Jochum et al., 2001). Recently, Nateri et al. (2005) demonstrated that c-Jun associates with TCF-4 and that this interaction mediates the cooperation of TCF and AP-1 promoter elements. Toualbi et al. (2007) reported that c-Jun as well as c-Fos interact with β-catenin and activate the pc-myc-luciferase reporter in a TCFs-dependent manner. In this study, we provide several lines of in vivo evidence demonstrating the involvement of c-Jun in canonical Wnt signaling: (a) knockdown of endogenous c-Jun with siRNA markedly suppressed Wnt-3a–induced transcriptional activity in HEK293T cells (Fig. 3, A and B); (b) the expression of c-myc failed to respond to canonical Wnt stimulation in c-Jun −/− cells (Fig. 3 D); and (c) c-Jun loss of function in zebrafish inhibited the induction of ventral mesoderm and reduced the expression of ventral marker genes as did the loss of function of Wnt-8 (Fig. 3, E and F).
Previous reports showed that mice lacking c-Jun died at midgestation (Hilberg et al., 1993; Johnson et al., 1993). c-Jun mutant fetuses displayed defects in liver and heart formation. In addition, these c-Jun −/− mice also exhibited cardiac neural crest cell defects with fewer connexin 43–positive staining cells in the outflow tract of the c-Jun −/− mice (Eferl et al., 1999). Connexin 43 is a target gene regulated by the Wnt–β-catenin pathway (van der Heyden et al., 1998; Ai et al., 2000). The fact that the Wnt-1/Wnt-3a double mutant and Dvl-2 mutant mice have the same cardiac neural crest defects (Ikeya et al., 1997; Hamblet et al., 2002) is consistent with the conclusion that c-Jun is a key component of the canonical Wnt signaling.
c-Jun functions as an adaptor protein that mediates the association of nuclear Dvl with TCF-4
Identification of the interaction between Dvl and c-Jun via the yeast two-hybrid screening and the previous finding of the interaction between c-Jun and TCFs led us to hypothesize that c-Jun may function to bridge Dvl and TCFs on the promoter of Wnt target genes. We have provided several lines of evidence to support this hypothesis. First, the interaction of c-Jun with Dvl is important for the canonical Wnt pathway in mammalian cells and during early zebrafish development (Fig. 4). Second, knockdown of c-Jun in mammalian cells significantly reduced the association between TCF-4 and nuclear Dvl (Fig. 5 C). Third, knockdown of c-Jun or disrupting the interaction between c-Jun and Dvl diminished the recruitment of Dvl-3 to the promoter of the native Wnt target c-myc (Figs. 5 B and 6 B). More importantly, a TCF-Dvl fusion protein can rescue the defect of Wnt target gene expression caused by c-Jun knockdown in mammalian cells (Fig. 5 E) and zebrafish (Fig. 5 F). Therefore, we propose that c-Jun mainly functions as a scaffold to bridge TCF and Dvl in the canonical Wnt signaling pathway.
It was previously reported that c-Jun and c-Fos directly interact with β-catenin and regulate pc-myc-luciferase reporter activity (Toualbi et al., 2007). Besides the work of Nateri et al. (2005), which could suggest that the association of c-Jun with β-catenin might be bridged by TCF-4, our finding here that a TCF-Dvl fusion protein can rescue the defect in canonical Wnt signaling caused by c-Jun knockdown (Fig. 5 E and F) demonstrated that c-Jun–β-catenin interaction, even if it exists, might be a less important contributor to c-Jun's function in the canonical Wnt signaling pathway. In addition, our experiment with the knockdown of endogenous c-Fos indicated that c-Fos did not participate in canonical Wnt signaling as did c-Jun (Fig. 3 C).
Role of nuclear Dvl in the canonical Wnt pathway
Two previous studies have elegantly demonstrated that Dvl, a pivotal regulator of the canonical Wnt pathway, is also localized in the nucleus (Torres and Nelson, 2000; Itoh et al., 2005), and its nuclear localization is required for canonical Wnt signaling (Itoh et al., 2005). However, it remained unclear how Dvl plays its role in the nucleus. In this paper, we found that Dvl can be recruited to the promoter of Wnt target genes (Fig. 1, C and D) and further identified the interactions of Dvl with c-Jun and β-catenin, respectively, in the nucleus (Figs. 2 and Figs.7) that mediate the association of Dvl with the β-catenin–TCFs transcriptional complex on the promoter of Wnt target genes. These findings provide an important clue for investigating the role of nuclear Dvl. Results shown in Fig. 6 clearly indicated that nuclear Dvl is crucial for the formation of a stable complex between β-catenin and TCFs in mammalian cells and zebrafish. In addition, the C-terminal fragment of Dvl, Dvl-C, which is responsible for the binding of Dvl to β-catenin and could act in a dominant-negative way to disrupt the binding between full-length Dvl and β-catenin (Fig. 7, C and D), had strong inhibitory effects on Wnt-regulated transcriptional activity (Fig. 7, E–G) and the binding of β-catenin to the c-myc promoter (Fig. S5). Similarly, disruption of the interaction of Dvl with c-Jun using the Dvl-M fragment also suppressed the binding of β-catenin to the c-myc promoter (Fig. 6 B). More importantly, covalently linking Dvl with TCF can rescue the canonical Wnt signaling deficiency and the diminished recruitment of β-catenin to the Wnt target gene promoter caused by c-Jun depletion (Fig. 5, E and F; and Fig. 6 E), which suggests that Dvl plays a novel role in regulating the functional β-catenin–TCF complex formation or its stability in the canonical Wnt signaling pathway. It is well known that β-catenin directly interacts with TCFs in vitro and in vivo. The fact that Dvl can affect β-catenin–TCFs association suggests that the binding of endogenous β-catenin with TCFs requires additional layers of regulation besides β-catenin accumulation in the nucleus. It would be interesting to determine how Dvl regulates the formation or stability of the β-catenin–TCFs complex.
A model for Wnt regulation of TCF-mediated transcription
Based on our and others' findings, we propose a model as depicted in Fig. 8 E to describe the action of canonical Wnt signaling in the nucleus. In this model, we propose that the Dvl proteins may have multiple roles in the canonical Wnt signaling pathway. First, cytoplasmic Dvl receives the canonical Wnt signals from the plasma membrane, resulting in accumulation of β-catenin in the nucleus. Second, the canonical Wnt signals promote Dvl nuclear accumulation (Torres and Nelson, 2000; Itoh et al., 2005). Third, Dvl may also activate JNK in some manner to promote c-Jun phosphorylation (Li et al., 1999a; Habas et al., 2003). In the nucleus, Dvl, via its interactions with phosphor–c-Jun and β-catenin, promotes the formation of a quaternary functional complex consisting of β-catenin, LEF-1/TCFs, c-Jun, and Dvl. This new mechanism revealed here extends our understanding of the roles of Dvl and c-Jun in the regulation of cell signaling. Given the well-established roles of Wnt signaling in various diseases, including cancer and degenerative diseases, the novel interactions described in this study may offer potential new therapeutic targets for treating some of these diseases.
Materials and methods
Materials:
The antibodies used were: β-catenin (14/β-catenin; BD Biosciences), TCF-4 (6H5-3; Millipore), Dvl-2 (10B5; Santa Cruz Biotechnology, Inc.), and c-Jun (H-79; Santa Cruz Biotechnology, Inc.). A mouse antibody to Dvl-3 was provided by D. Sussman (University of Maryland, Baltimore, MD). The plasmid of pBV-TBE1/2-luc and pBV-TBE1m/2m-luc were kindly provided by B. Vogelstein and K.W. Kinzler (Johns Hopkins University, Baltimore, MD; He et al., 1998). Wnt-3a conditioned medium (CM) and its control were described previously (Mao et al., 2001). The NLS sequence was derived from pHcRed1-Nuc vector (Clontech Laboratories, Inc.) and three copies of the NLS sequences were fused to the C terminus of Dvl.
Cell transfection and luciferase assay
HEK293T and SW480 cells were transfected with DNA using Lipofectamine Plus or with siRNA using Lipofectamine 2000 (Invitrogen) according to the manufacturer's instructions. The LacZ plasmid was added to make the total amount of DNA equal (0.5 μg/well in a 24-well plate for SW480 cells and 0.25 μg/well for HEK293T cells). Luciferase assays were performed and the luciferase activities presented were normalized against the levels of GFP expression as described previously (Li et al., 1999a). Luminescence intensity was normalized against fluorescence intensity of GFP.
siRNAs
Duplexed siRNAs targeting a human Dvl-2 mRNA sequence (TCCACAATGTCTCTCAATA), a human c-Jun mRNA sequence (GTCATGAACCACGTTAACA), and a human c-Fos mRNA sequence (GAATCCGAAGGGAAAGGAA) were synthesized by GenePharma. siDvl1/3 and siβ-catenin were synthesized as described previously (Li et al., 2002; Thompson et al., 2002).
Immunoprecipitation and an in vitro binding experiment
HEK293T cells were transiently transfected with the indicated constructs for 24 h. A Co-IP experiment was then performed as described previously (Li et al., 1999b). For the in vitro binding experiment, 6His-tagged and GST-fused proteins were expressed in Escherichia coli and partially purified by Ni-NTA and glutathione–Sepharose 4B beads, respectively. The proteins amounted to ∼1 μg each and antibodies were mixed for 4 h at 4°C. Then the protein A/G plus agarose (Santa Cruz Biotechnology, Inc.) was added for an additional 2 h. The beads were washed three times and resuspended in 40 μl SDS loading buffer.
Endogenous interaction and assays for protein complexes
The nucleus of 3–5 × 107 HEK293T or SW480 cells were isolated as described previously (Spector et al., 1998). Nuclear extracts were sonicated six times for 5 s in 1 ml of buffer containing 20 mM Tris-HCl, pH 7.4, 300 mM NaCl, 0.01% SDS, 1% Triton X-100, 2 mM EDTA, and a PI cocktail (Roche). For the endogenous interaction assay, the supernatants were incubated with a specific antibody for 4 h at 4°C and A/G plus agarose was added for an additional 2 h with mixture. The beads were washed three times and resuspended in 40 μl SDS loading buffer. For endogenous complexes assays, the supernatants were fractionated on a Superose 6 10/300 GL column using fast protein liquid chromatography (GE Healthcare).
RT- PCR and quantitative real-time PCR
Total RNAs were extracted from cultured cells with TRIzol and the reverse transcription of purified RNA was performed using oligo(dT) priming and superscript III reverse transcription according to the manufacturer's instructions (Invitrogen). The quantification of all gene transcripts was done by quantitative PCR (qPCR) using the Quantitect SYBR green PCR kit (QIAGEN) and a Rotor-Gene RG-3000A apparatus (Corbett Research). The primer pairs used for human c-myc gene were 5′-TGCTCCATGAGGAGACA-3′ and 5′-CCTCCAGCAGAAGGTGA-3′. The primer pairs used for the human GAPDH gene were 5′-GCACCACCAACTGCTTA-3′ and 5′-AGTAGAGGCAGGGATGAT-3′. The primer pairs used for the mouse c-myc gene were 5′-TAGTGCTGCATGAGGA-3′ and 5′-CTCGGGATGGAGATGA-3′. The primer pairs used for the mouse GAPDH gene were 5′-CTGTGGGCAAGGTCAT-3′ and 5′-AGATGCCTGCTTCACCA-3′. The primer pairs used for the c-myc promoter were 5′-ACAGGCAGACACATCTCA-3′ and 5′-GCCACGTATACTTGGAGA-3′.
ChIP and re-ChIP assays
107 HEK293T or SW480 cells were prepared for the ChIP assay as according to the manufacturor's instructions with the ChIP Immunoprecipitation Assay kit (Millipore). In the re-ChIP assay, the DNA complexes were first immunoprecipitated using a β-catenin, TCF-4, or c-Jun antibody and then eluted by incubation for 30 min at 37°C in 100 μl of 10 mM DTT. After centrifugation, the supernatant was diluted 50 times with re-ChIP buffer (20 mM Tris-HCl, pH 8.1, 150 mM NaCl, 2 mM EDTA, and 1% Triton X-100) and immunoprecipitated again by anti–Dvl-3 antibody as with the ChIP procedure. The primers used to detect the c-myc promoter were synthesized as described previously (He et al., 1998). The primer pairs used for the GAPDH promoter were 5′-TAGGCCTTTGCCTGAGCAGTCCGGTGT-3′ and 5′-TTGAGGCCTGAGCTACGTGCGCCCGTAA-3′.
Zebrafish strains and maintenance
Zebrafish were raised under standard conditions at 28.5°C. Wild-type embryos of the Tüebingen strain were used.
Morpholino antisense oligonucleuotides and mRNA synthesis and injection
The following antisense MO and a standard control MO were obtained from Gene Tools, LLC.: c-Jun MO, 5′-CTTGGTAGACATAGAAGGCAAAGCG-3′; and c-Jun MO 2, 5′-AGTCATCGTAGAAAGTAGTTTCCAT-3′. Wnt8 MO2 (Wnt8-ORF1 MO + Wnt8-ORF2 MO) has been described previously (Lekven et al., 2001). For sense RNA injections, capped mRNA was synthesized using the mMessage mMachine kit (Ambion). In all embryo microinjection experiments, a volume of ∼5 nl was injected into the yolk of one-cell stage embryos of zebrafish.
RNA probe synthesis and in situ hybridization
Antisense RNA probes were synthesized using the DIG RNA Labeling kit (Roche) according to the manufacturer's instructions. In situ hybridization was performed as described previously (Nüsslein-Volhard and Dahm, 2002) and the staining was performed with a DIG Nucleic Acid Detection kit (Roche).
ChIP assays in zebrafish
150 embryos injected with indicated morpholinos and mRNAs at the one-cell stage and harvested at the shield stage were cross-linked with 2% formaldehyde overnight. ChIP was performed as described in ChIP and Re-ChIP assays.
Immunohistochemistry
The colon cancer tissue slides were obtained from Shanghai Outdo Biotech. The slides were dewaxed in xylene and rehydrated according to a standard protocol and the procedure was performed as described previously (Nateri et al., 2005). Slides were stained with DAB and then counterstained with hematoxylin, dehydrated, and mounted.
Microscopy
All images were captured at room temperature. Immunohistochemistry images were captured using a microscope (BX50; Olympus) with a UPlan APO 100× 1.35 NA oil iris objective (Olympus). Immunofluorescence images were captured using a microscope (TCS SP2 AOBS; Leica) with a HCX PL APO lbd.BL 63× 1.4 NA oil objective (Leica). Embryo images were captured using a camera (DP71; Olympus) on a microscope (SZX9 1x; Olympus). The acquiring software was Spot32 (BX50; Olympus), TCS (Leica), or Image-Pro Express 5.1 (DP71; Olympus).
Online supplemental material
Fig. S1 demonstrates that knockdown of Dvls does not affect the stability and distribution of β-catenin in SW480 cells. Fig. S2 shows that Dvl-ΔM does not bind c-Jun in a co-IP experiment. Fig. S3 shows the effect of c-Jun MO2 on the phenotype and the expression of ventral markers in zebrafish. Fig. S4 shows that canonical Wnt signaling requires c-Jun N-terminal phosphorylation. Fig. S5 shows that Dvl-C-NLS suppressed the association of β-catenin with the c-myc promoter. Online supplemental material is available at http://www.jcb.org/cgi/content/full/jcb.200710050/DC1.
Supplementary Material
Acknowledgments
We thank Q-.S. Zhao and J.-L. Du for providing zebrafish; A.-M. Meng for providing the tbx6 plasmid and technical help in the zebrafish experiment; Z.-G. Liu for providing c-Jun KO cells; and Y.-H. Wang, Q.-Y. Liu, W. Bian, Y. Ding, and W. Wang for technical support. We are grateful to D. Wu and D. Li for critical reading and commenting on this paper.
This work is supported by the Ministry of Science and Technology of China (grants 2002CB513000 and 2007CB914500 to L. Li and grant 2007CB947100 to Y. Li), the Science and Technology Commission of Shanghai Municipality, the National Natural Science Foundation of China (grant 30521005 to L. Li and grant 30600305 to J. Wang), and the Chinese Academy of Science.
X.-q. Gan and J.-y. Wang contributed equally to this paper.
Abbreviations used in this paper: APC, adenomatous polyposis coli; ChIP, chromatin immunoprecipitation; co-IP, coimmunoprecipitation; CM, conditioned medium; Dvl, Dishevelled; KO, knockout; MEF, mouse embryonic fibroblast; MO, morpholino oligonucleotides; NLS, nuclear localization sequence; qPCR, quantitative PCR; TCF, T-cell factor.
References
- Ai, Z., A. Fischer, D.C. Spray, A.M. Brown, and G.I. Fishman. 2000. Wnt-1 regulation of connexin43 in cardiac myocytes. J. Clin. Invest. 105:161–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akiyama, H., J.P. Lyons, Y. Mori-Akiyama, X. Yang, R. Zhang, Z. Zhang, J.M. Deng, M.M. Taketo, T. Nakamura, R.R. Behringer, et al. 2004. Interactions between Sox9 and β-catenin control chondrocyte differentiation. Genes Dev. 18:1072–1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer, A., O. Huber, and R. Kemler. 1998. Pontin52, an interaction partner of β-catenin, binds to the TATA box binding protein. Proc. Natl. Acad. Sci. USA. 95:14787–14792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavallo, R.A., R.T. Cox, M.M. Moline, J. Roose, G.A. Polevoy, H. Clevers, M. Peifer, and A. Bejsovec. 1998. Drosophila Tcf and Groucho interact to repress Wingless signalling activity. Nature. 395:604–608. [DOI] [PubMed] [Google Scholar]
- Chen, G., J. Fernandez, S. Mische, and A.J. Courey. 1999. A functional interaction between the histone deacetylase Rpd3 and the corepressor Groucho in Drosophila development. Genes Dev. 13:2218–2230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clevers, H. 2006. Wnt/beta-catenin signaling in development and disease. Cell. 127:469–480. [DOI] [PubMed] [Google Scholar]
- Daniels, D.L., and W.I. Weis. 2005. Beta-catenin directly displaces Groucho/TLE repressors from Tcf/Lef in Wnt-mediated transcription activation. Nat. Struct. Mol. Biol. 12:364–371. [DOI] [PubMed] [Google Scholar]
- Eferl, R., M. Sibilia, F. Hilberg, A. Fuchsbichler, I. Kufferath, B. Guertl, R. Zenz, E.F. Wagner, and K. Zatloukal. 1999. Functions of c-Jun in liver and heart development. J. Cell Biol. 145:1049–1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erter, C.E., T.P. Wilm, N. Basler, C.V.E. Wright, and L. Solnica-Krezel. 2001. Wnt8 is required in lateral mesendodermal precursors for neural posteriorization in vivo. Development. 128:3571–3583. [DOI] [PubMed] [Google Scholar]
- Habas, R., I.B. Dawid, and X. He. 2003. Coactivation of Rac and Rho by Wnt/Frizzled signaling is required for vertebrate gastrulation. Genes Dev. 17:295–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamblet, N.S., N. Lijam, P. Ruiz-Lozano, J. Wang, Y. Yang, Z. Luo, L. Mei, K.R. Chien, D.J. Sussman, and A. Wynshaw-Boris. 2002. Dishevelled 2 is essential for cardiac outflow tract development, somite segmentation and neural tube closure. Development. 129:5827–5838. [DOI] [PubMed] [Google Scholar]
- He, T.C., A.B. Sparks, C. Rago, H. Hermeking, L. Zawel, L.T. da Costa, P.J. Morin, B. Vogelstein, and K.W. Kinzler. 1998. Identification of c-MYC as a target of the APC pathway. Science. 281:1509–1512. [DOI] [PubMed] [Google Scholar]
- Hecht, A., C.M. Litterst, O. Huber, and R. Kemler. 1999. Functional characterization of multiple transactivating elements in beta-catenin, some of which interact with the TATA-binding protein in vitro. J. Biol. Chem. 274:18017–18025. [DOI] [PubMed] [Google Scholar]
- Hecht, A., K. Vleminckx, M.P. Stemmler, F. van Roy, and R. Kemler. 2000. The p300/CBP acetyltransferases function as transcriptional coactivators of beta-catenin in vertebrates. EMBO J. 19:1839–1850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilberg, F., A. Aguzzi, N. Howells, and E.F. Wagner. 1993. c-jun is essential for normal mouse development and hepatogenesis. Nature. 365:179–181. [DOI] [PubMed] [Google Scholar]
- Ikeya, M., S.M. Lee, J.E. Johnson, A.P. McMahon, and S. Takada. 1997. Wnt signalling required for expansion of neural crest and CNS progenitors. Nature. 389:966–970. [DOI] [PubMed] [Google Scholar]
- Itoh, K., B.K. Brott, G.U. Bae, M.J. Ratcliffe, and S.Y. Sokol. 2005. Nuclear localization is required for Dishevelled function in Wnt/beta-catenin signaling. J. Biol. 4:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jochum, W., E. Passegue, and E.F. Wagner. 2001. AP-1 in mouse development and tumorigenesis. Oncogene. 20:2401–2412. [DOI] [PubMed] [Google Scholar]
- Johnson, R.S., B. van Lingen, V.E. Papaioannou, and B.M. Spiegelman. 1993. A null mutation at the c-jun locus causes embryonic lethality and retarded cell growth in culture. Genes Dev. 7:1309–1317. [DOI] [PubMed] [Google Scholar]
- Kimelman, D., and W. Xu. 2006. beta-catenin destruction complex: insights and questions from a structural perspective. Oncogene. 25:7482–7491. [DOI] [PubMed] [Google Scholar]
- Korinek, V., N. Barker, P.J. Morin, D. van Wichen, R. de Weger, K.W. Kinzler, B. Vogelstein, and H. Clevers. 1997. Constitutive transcriptional activation by a beta-catenin-Tcf complex in APC−/− colon carcinoma. Science. 275:1784–1787. [DOI] [PubMed] [Google Scholar]
- Kramps, T., O. Peter, E. Brunner, D. Nellen, B. Froesch, S. Chatterjee, M. Murone, S. Zullig, and K. Basler. 2002. Wnt/wingless signaling requires BCL9/legless-mediated recruitment of pygopus to the nuclear beta-catenin-TCF complex. Cell. 109:47–60. [DOI] [PubMed] [Google Scholar]
- Lekven, A.C., C.J. Thorpe, J.S. Waxman, and R.T. Moon. 2001. Zebrafish wnt8 encodes two Wnt8 proteins on a bicistronic transcript and is required for mesoderm and neurectoderm patterning. Dev. Cell. 1:103–114. [DOI] [PubMed] [Google Scholar]
- Levanon, D., R. Goldstein, Y. Bernstein, H. Tang, S.S. Goldenberg, Z. Paroush, and Y. Groner. 1998. Transcriptional repression by AML1 and LEF-1 is mediated by the TLE/Groucho corepressors. Proc. Natl. Acad. Sci. USA. 95:11590–11595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, L., H. Yuan, W. Xie, J. Mao, A.M. Caruso, A. McMahon, D.J. Sussman, and D. Wu. 1999. a. Dishevelled proteins lead to two signaling pathways. Regulation of LEF-1 and c-Jun N-terminal kinase in mammalian cells. J. Biol. Chem. 274:129–134. [DOI] [PubMed] [Google Scholar]
- Li, L., H. Yuan, C.D. Weaver, J. Mao, G.H. Farr III, D.J. Sussman, J. Jonkers, D. Kimelman, and D. Wu. 1999. b. Axin and Frat1 interact with dvl and GSK, bridging Dvl to GSK in Wnt-mediated regulation of LEF-1. EMBO J. 18:4233–4240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, L., J. Mao, L. Sun, W. Liu, and D. Wu. 2002. Second cysteine-rich domain of Dickkopf-2 activates canonical Wnt signaling pathway via LRP-6 independently of Dishevelled. J. Biol. Chem. 277:5977–5981. [DOI] [PubMed] [Google Scholar]
- Logan, C.Y., and R. Nusse. 2004. The Wnt signaling pathway in development and disease. Annu. Rev. Cell Dev. Biol. 20:781–810. [DOI] [PubMed] [Google Scholar]
- Mao, J., J. Wang, B. Liu, W. Pan, G.H. Farr III, C. Flynn, H. Yuan, S. Takada, D. Kimelman, L. Li, and D. Wu. 2001. Low-density lipoprotein receptor-related protein-5 binds to Axin and regulates the canonical Wnt signaling pathway. Mol. Cell. 7:801–809. [DOI] [PubMed] [Google Scholar]
- Munemitsu, S., I. Albert, B. Souza, B. Rubinfeld, and P. Polakis. 1995. Regulation of intracellular beta-catenin levels by the adenomatous polyposis coli (APC) tumor-suppressor protein. Proc. Natl. Acad. Sci. USA. 92:3046–3050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nateri, A.S., B. Spencer-Dene, and A. Behrens. 2005. Interaction of phosphorylated c-Jun with TCF4 regulates intestinal cancer development. Nature. 437:281–285. [DOI] [PubMed] [Google Scholar]
- Nusse, R. 2005. Wnt signaling in disease and in development. Cell Res. 15:28–32. [DOI] [PubMed] [Google Scholar]
- Nüsslein-Volhard, C., and R. Dahm, editors. 2002. Zebrafish: a Practical Approach. Oxford University Press, Oxford. 303 pp.
- Palaparti, A., A. Baratz, and S. Stifani. 1997. The Groucho/transducin-like enhancer of split transcriptional repressors interact with the genetically defined amino-terminal silencing domain of histone H3. J. Biol. Chem. 272:26604–26610. [DOI] [PubMed] [Google Scholar]
- Ramel, M.-C., and A.C. Lekven. 2004. Repression of the vertebrate organizer by Wnt8 is mediated by Vent and Vox. Development. 131:3991–4000. [DOI] [PubMed] [Google Scholar]
- Roose, J., M. Molenaar, J. Peterson, J. Hurenkamp, H. Brantjes, P. Moerer, M. van de Wetering, O. Destrée, and H. Clevers. 1998. The Xenopus Wnt effector XTcf-3 interacts with Groucho-related transcriptional repressors. Nature. 395:608–612. [DOI] [PubMed] [Google Scholar]
- Sheldahl, L.C., D.C. Slusarski, P. Pandur, J.R. Miller, M. Kuhl, and R.T. Moon. 2003. Dishevelled activates Ca2+ flux, PKC, and CamKII in vertebrate embryos. J. Cell Biol. 161:769–777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sierra, J., T. Yoshida, C.A. Joazeiro, and K.A. Jones. 2006. The APC tumor suppressor counteracts beta-catenin activation and H3K4 methylation at Wnt target genes. Genes Dev. 20:586–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spector, D.L., R.D. Goldman, and L.A. Leinwand, editors. 1998. Cells: a Laboratory Manual. Cold Spring Harbor Laboratory Press, New York. 2136 pp.
- Szeto, D.P., and D. Kimelman. 2004. Combinatorial gene regulation by Bmp and Wnt in zebrafish posterior mesoderm formation. Development. 131:3751–3760. [DOI] [PubMed] [Google Scholar]
- Tago, K., T. Nakamura, M. Nishita, J. Hyodo, S. Nagai, Y. Murata, S. Adachi, S. Ohwada, Y. Morishita, H. Shibuya, and T. Akiyama. 2000. Inhibition of Wnt signaling by ICAT, a novel beta-catenin-interacting protein. Genes Dev. 14:1741–1749. [PMC free article] [PubMed] [Google Scholar]
- Takemaru, K., S. Yamaguchi, Y.S. Lee, Y. Zhang, R.W. Carthew, and R.T. Moon. 2003. Chibby, a nuclear β-catenin associated antagonist of the Wnt/Wingless pathway. Nature. 422:905–909. [DOI] [PubMed] [Google Scholar]
- Thompson, B., F. Townsley, R. Rosin-Arbesfeld, H. Musisi, and M. Bienz. 2002. A new nuclear component of the Wnt signalling pathway. Nat. Cell Biol. 4:367–373. [DOI] [PubMed] [Google Scholar]
- Thorpe, C.J., G. Weidinger, and R.T. Moon. 2005. Wnt/beta-catenin regulation of the Sp1-related transcription factor sp5l promotes tail development in zebrafish. Development. 132:1763–1772. [DOI] [PubMed] [Google Scholar]
- Torres, M.A., and W.J. Nelson. 2000. Colocalization and redistribution of dishevelled and actin during Wnt-induced mesenchymal morphogenesis. J. Cell Biol. 149:1433–1442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toualbi, K., M.C. Guller, J.L. Mauriz, C. Labalette, M.A. Buendia, A. Mauviel, and D. Bernuau. 2007. Physical and functional cooperation between AP-1 and beta-catenin for the regulation of TCF-dependent genes. Oncogene. 26:3492–3502. [DOI] [PubMed] [Google Scholar]
- Townsley, F.M., A. Cliffe, and M. Bienz. 2004. Pygopus and Legless target Armadillo/β-catenin to the nucleus to enable its transcriptional co-activator function. Nat. Cell Biol. 6:626–633. [DOI] [PubMed] [Google Scholar]
- van der Heyden, M.A., M.B. Rook, M.M. Hermans, G. Rijksen, J. Boonstra, L.H. Defize, and O.H. Destree. 1998. Identification of connexin43 as a functional target for Wnt signalling. J. Cell Sci. 111:1741–1749. [DOI] [PubMed] [Google Scholar]
- Waxman, J.S., A.M. Hocking, C.L. Stoick, and R.T. Moon. 2004. Zebrafish Dapper1 and Dapper2 play distinct roles in Wnt-mediated developmental processes. Development. 131:5909–5921. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.