Abstract
The formation of thick filaments in striated muscle involves the chaperones Hsp90a and Unc45. We show that Unc45b and Hsp90a, two zebrafish orthologues, colocalize with myosin during myofibrillogenesis and associate with the Z line when myofibril assembly is completed. In response to stress or damage to the myofiber, Unc45b and Hsp90a dissociate from the Z line and transiently associate with myosin. Although chaperone activity of Unc45b requires the full-length protein, only the central and Unc45-Cro1p-She4p domains are required to anchor it to the Z line, and multiple subdomains mediate association with nascent myosin. We propose that the Z line serves as a reservoir for chaperones, allowing a rapid mobilization in response to muscle damage. Our data are consistent with a differential affinity model as an explanation for the shuttling of the chaperones between the Z line and myosin.
Introduction
Molecular chaperones are essential components of quality-control machinery, which can either aid in the folding and maintenance of newly translated proteins or lead to the degradation of misfolded and destabilized proteins (Zhao and Houry, 2005). The most prominent muscle proteins, myosin and actin, are assembled in the highly repetitive contractile structures, the sarcomeres of the striated vertebrate muscles. The folding of the helical rod of myosin occurs autonomously, whereas the assembly of the globular head domains requires the assistance of several chaperone proteins (Atkinson and Stewart, 1991; Srikakulam and Winkelmann, 2004). The Hsp90 and 70 chaperones were suggested to fold de novo synthesized myosin (Barral et al., 2002; Srikakulam and Winkelmann, 2004).
Hsp90 is a dimeric protein consisting of two major domains: an N-terminal ATP binding domain and a C-terminal domain involved in homodimerization and interactions with cochaperones (Prodromou et al., 2000; Zhao and Houry, 2007). During myofibrillogenesis, Hsp90 proteins interact with UCS proteins. These proteins, which are conserved from yeast to man, form a family that is characterized by a carboxy-terminal Unc45-Cro1p-She4p (UCS) domain, which binds to myosin heads. The Caenorhabditis elegans UCS protein UNC-45 and its vertebrate homologues have two additional domains, an N-terminal tetratricopeptide repeat (TPR) domain binding the C-terminal domain of Hsp90 (Barral et al., 2002) and a central domain of unknown function. In contrast to flies and worms, there are two isoforms of UNC-45 in vertebrates (Hutagalung et al., 2002). The two related genes have distinct chromosomal locations and are differentially expressed in both embryonic and adult tissues. The general cell isoform (GC UNC-45 in mammals and Unc45a in zebrafish) appears to be ubiquitously expressed, whereas the striated muscle isoform (SM UNC-45 in mammals or Unc45b in zebrafish) was found only in cardiac and skeletal muscle (Price et al., 2002; Etard et al., 2007; Wohlgemuth et al., 2007).
From biochemical studies, it has been suggested that C. elegans UNC-45 exerts chaperone activity in two different ways (Barral et al., 2002). First, it can prevent protein aggregation in a citrate synthase assay like a chaperone. Second, it was proposed to act as a cochaperone by recruiting the chaperones Hsp90 and 70 to the myosin client (Barral et al., 2002). Genetic analysis in Saccharomyces cerevisiae and Schizosaccharomyces pombe similarly showed an interaction between Hsp90-related chaperones and the UCS proteins She4p and Rng3p, respectively (Toi et al., 2003; Mishra et al., 2005). Furthermore, several lines of evidence suggest that Hsp90a interacts with Unc45b in the myofibril assembly pathway in the zebrafish (Etard et al., 2007). Hsp90a is coexpressed with Unc45b at high levels in the skeletal muscles of the zebrafish (Etard et al., 2007). Hsp90a interacts with Unc45b in pulldown assays in vitro, and knockdown of hsp90a translation causes a similar phenotype in skeletal muscle as mutation or knockdown of unc45b (Etard et al., 2007; Wohlgemuth et al., 2007). In addition to hsp90a, two other related hsp90 genes named hsp90a2 and b were identified in zebrafish (Krone and Sass, 1994; Murtha and Keller, 2003; Etard et al., 2007). However, knockdown of these related proteins does not affect myofibrillogenesis (Etard et al., 2007).
In zebrafish, the hsp90a and unc45b genes are coregulated because embryos with myosin assembly defects express high levels of either mRNA (Etard et al., 2007). Transgenic worms overexpressing UNC-45 display defects in myosin assembly with decreased myosin content and mild paralysis (Landsverk et al., 2007). In C. elegans, the E3–E4 complex, which is formed by CHN-1, the orthologue of mammalian CHIP, and UFD-2, a ubiquitin-conjugating enzyme, were shown to multiubiquitylate UNC-45, leading to its degradation (Hoppe et al., 2004). Mutations in the human ubiquitin-selective chaperone CDC48/p97/VCP abrogate UNC-45 degradation, resulting in disorganized myofibrils (Janiesch et al., 2007). Moreover, mutations in CDC48/p97/VCP can cause late onset hereditary inclusion body myopathy in humans (Hubbers et al., 2007; Janiesch et al., 2007).
Muscle is damaged under normal workload. The most common type of damage of human muscle arises from small lesions in the membranes of muscle cells (McNeil and Khakee, 1992; Clarke et al., 1995), suggesting a continuous requirement for repair enzymes and chaperones. In mature C. elegans muscle, UNC-45 is associated with the myosin heavy chain B (Ao and Pilgrim, 2000), indicating a role for UNC-45 not only in development but also in maintenance of the myofibrils.
In this paper, we show that Unc45b and Hsp90a are located at the Z line in mature myofibrils in the zebrafish skeletal muscle. The two chaperones are only associated with nascent myosin at high levels during development of the myofibril. Stress or membrane damage can induce a transient relocation of both chaperones to the myosin-containing A bands of the myofibril. We propose that the Z lines form a reservoir of myosin chaperones, from which they can be recruited to their myosin clients upon damage. FRAP showed that the turnover of Unc45b and Hsp90a molecules at the Z line is very high, suggesting a constant dynamic exchange between the Z line and the surrounding cytosol. Chaperone activity requires an intact Unc45b molecule, whereas several subdomains mediate myosin association. Both the central and UCS domains of Unc45b are required to anchor Unc45b to the Z line. We discuss a differential affinity model, in which the location of the chaperones is determined by differential affinities to nascent myosin, mature myosin, and the Z line.
Results
Unc45b and Hsp90a localize to the Z line in mature muscle
To study the intracellular location of Unc45b, we constructed an unc45b-gfp chimeric gene by fusing the unc45b cDNA to gfp at the C terminus. We injected the construct into fertilized zebrafish eggs and followed the location of the fusion protein by confocal microscopy. The Unc45b-GFP protein accumulated at high concentrations at reiterative transverse bands along the myofibril in 72-h postfertilization (hpf) embryos, which correspond to the Z lines (Fig. 1 A, arrowhead). Although the nucleus was free of Unc45b-GFP, the perinuclear area had frequently increased GFP fluorescence relative to the rest of the myofibril-free cytoplasm and the nucleus (Fig. 1 A, arrow; Fig. 2 C).
Figure 1.
The chaperones Unc45b and Hsp90a are localized at the Z line of the myofibril. (A) Live embryos expressing unc45b-gfp show an enrichment of Unc45b-GFP at the Z line (A), as revealed by costaining with α-actinin antibody (F, A/PFA-fixed embryo). (B) A/PFA-fixed embryo expressing unc45b-myc. Immunohistochemistry with the 9E10 antibody indicates Z-line localization of the Unc45b-Myc protein. (C) Live embryo expressing pCS2+gfp shows ubiquitous GFP distribution. (D) A/PFA-fixed embryo injected with the pCS2+myc plasmid shows ubiquitous anti-Myc 9E10 antibody staining. (E) Live embryo expressing hsp90a-gfp also has an enrichment of Hsp90a-GFP at the Z line. Graphs represent distribution of fluorescence over two sarcomeres. High levels are evident at the Z lines (green arrows) of Unc45b-GFP–, Unc45b-Myc–, and Hsp90a-GFP–expressing cells (A, B, and E), whereas a diffuse nonspecific distribution of fluorescence was observed in cells expressing GFP or Myc tags alone (C and D). (F) A/PFA-fixed embryo expressing Unc45b-GFP, costained with α-actinin antibody. (G–I) A/PFA-fixed embryo expressing Hsp90a-GFP (G), showing colocalization with α-actinin (H). I, merge. All pictures were taken from 72-hpf embryos in an area corresponding to fast muscles. Arrowheads indicate the Z line, the white arrow indicates the perinuclear area, and stars indicate the nucleus. Bars, 4 μm.
Figure 2.
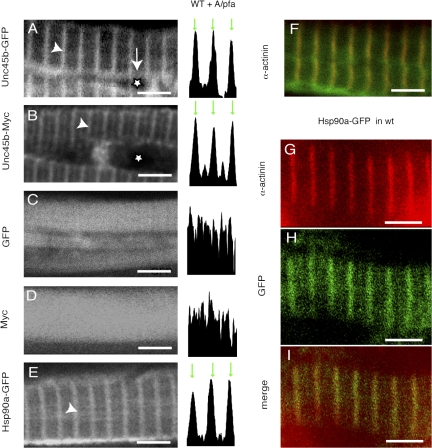
Unc45b-GFP associates with nascent myosin. (A–C) Wild-type embryo expressing Unc45b-GFP. At 24 hpf, Unc45b-GFP was ubiquitously localized in the cytoplasm (A). In later developmental stages, Unc45b-GFP localized to the Z line (B, 48 hpf; and C, 72 hpf). (D–G) unc45b mutant rescued to varying degrees by expression of Unc45b-GFP. At 24 hpf, Unc45b-GFP was distributed ubiquitously (D). From 30 hpf, it started to aggregate in patches (E) and to form A-band–like structures (F). From 72 hpf, Unc45b-GFP localized to the Z line in rescued embryos (G). (H–J) Wild-type embryos were coinjected with unc45b-gfp plasmid and morpholinos against hsp90a mRNA (hsp90a-Mo). At 30 hpf, Unc45b-GFP aggregated in patches and a striation started to appear (H). At 48 hpf, Unc45b-GFP was localized in A-band–like structures (I) and remained associated with those at 96 hpf (J). (K and L) unc45b mutant expressing Unc45b-GFP (K) in A-band–like patterns. Embryos were fixed with A/PFA, and stained with anti–slow muscle myosin antibody F59 (L). (M) Merge. Unc45b-GFP colocalizes with myosin. Bars, 4 μm. Arrowheads indicate the Z line and white boxes outline an A-band–like pattern. Stars indicate the nucleus and arrows indicate the perinuclear area. (N) Unc45b-GST protein interacts with myosin from rabbit muscle in an in vitro pulldown assay (2). GST protein alone used as a control did not bind myosin (1). Anti-myosin MF20 antibody was used to develop the Western blot (250 kD).
To verify the association with the Z line, we fixed Unc45b-GFP–expressing embryos with acetone/PFA (A/PFA) and stained them with an antibody directed against α-actinin, a major Z-line protein. GFP fluorescence and α-actinin immunofluorescence colocalized, confirming that Unc45b-GFP is enriched at the Z line (Fig. 1 F). In addition, a different construct encoding Unc45b tagged with five Myc epitopes at the N terminus was expressed in muscle. Immunostaining of A/PFA-fixed 72-hpf embryos with the anti-Myc antibody 9E10 revealed a localization identical to that of the Unc45b-GFP protein (Fig. 1 B). Injection of plasmids encoding only GFP or the Myc tags resulted in diffuse protein localization throughout the muscle cell, including the nucleus (Fig. 1, C and D). Finally, expression of CFP-tagged Unc45b engineered into a Bac clone under control of the endogenous unc45b promotor also resulted in accumulation of Unc45b-CFP at the Z line (unpublished data). Collectively, these results suggest that Unc45b is enriched in 72-hpf embryos at the Z lines of the myofibrils.
We demonstrated previously that Unc45b interacts with Hsp90a during myofibrillogenesis (Etard et al., 2007). To assess whether this protein has the same subcellular distribution as Unc45b-GFP, an Hsp90a-GFP fusion construct was expressed in 72-hpf embryos. Like Unc45b-GFP, Hsp90a-GFP resided at the Z line (Fig. 1, E and G–I). The Z-line association of the two proteins was observed both in superficial slow fibers and deep fast muscle fibers of the somites (Fig. 1 and not depicted). This subcellular location appears to be contradictory to the role of these proteins in myosin folding (Etard et al., 2007) and to the location reported for C. elegans UNC-45 that is associated with myosin B in mature muscle (Ao and Pilgrim, 2000).
Unc45b colocalizes with myosin during myofibrillogenesis
At 72 hpf, the overall assembly of the embryonic myofibrils appears to have been completed. To assess whether Unc45b interacts with myosin during assembly of the myofibril, the localization of Unc45b-GFP was investigated at earlier developmental stages. At 24 hpf, Unc45b-GFP was distributed ubiquitously in the cytoplasm of the muscle cells with exclusion from the nucleus (n = 30 embryos; Fig. 2 A). At 48 hpf, Unc45b-GFP is enriched at the Z line (n = 100 embryos each; Fig. 2, B and C). We could not find a time window where Unc45b-GFP was localized at the myosin-containing A band.
In the presence of endogenous Unc45b, the association with the developing A band may have been so transient that it escaped detection. Thus, we slowed down myofibril assembly by lowering levels of Unc45b. To this end, we injected a low amount of unc45b-gfp–encoding plasmid into unc45b mutants (Etard et al., 2007) to trigger slow rescue. At 24 hpf, the protein is distributed uniformly in the cytoplasm as seen in wild-type embryos injected with the plasmid (Fig. 2 D). At 30 hpf, Unc45b-GFP aggregates in patches and a striation start to appear (Fig. 2 E). At 48 hpf, Unc45b-GFP is associated with double bands characteristic of the myosin-containing A bands (Fig. 2 F). Labeling with antibodies directed against slow muscle myosin, α-actinin, and troponin T revealed that these double bands indeed contain myosin (Fig. 2, K–M, and data not shown). Thus, at limiting concentrations, Unc45b-GFP colocalizes with myosin. This suggests binding of Unc45b-GFP to myosin. This potential direct interaction of Unc45b with myosin was verified in vitro in pulldown assays (Fig. 2 N).
Collectively, these data are in agreement with the association of UNC-45 with myosin B in C. elegans muscle (Ao and Pilgrim, 2000). However, in zebrafish muscle, this association is transient. By 72 hpf, Unc45b-GFP has translocated to the Z line in rescued and in wild-type embryos (Fig. 2 G). When examined under differential interference contrast optics, the location at the Z line is accompanied by the appearance of the anisotropic/isotropic striation. This suggests that the prior association of Unc45-GFP with myosin marks a precursor state containing either unfolded myosin or partially assembled filaments in immature A-band–like structures.
We hypothesized that under conditions in which myosin assembly or folding could not be completed, Unc45b would remain associated with myosin. To investigate this, we took advantage of the improper myosin assembly in hsp90a morphants (Etard et al., 2007). Morpholinos directed against hsp90a mRNA were injected together with unc45b-gfp plasmid, and the localization of GFP was followed during development of the embryos. Under these circumstances, Unc45b-GFP appeared in filamentous structures distributed throughout the cytoplasm at 30 hpf (Fig. 2 H). As the assembly of the myofibril proceeded, presumably because of gradual loss of the morpholinos, some A-band–like striations of GFP distribution appeared (Fig. 2 I). However, under differential interference contrast optics, these fibrils did not show the isotropic/anisotropic banding patterns that are characteristic of mature fibers. Unc45b-GFP remained arranged in the reiterated doublet bands characteristic of the myosin distribution in the A bands at least until 4 d in these knockdown embryos (Fig. 2 J). Colocalization of myosin and Unc45-GFP was confirmed by immunohistochemistry with antibodies directed against slow muscle myosin and troponin T (unpublished data). At very high concentrations of coinjected Unc45b-GFP, high GFP fluorescence was also noted at the Z line (unpublished data), suggesting that when in excess, association can occur with the Z line even though the assembly of the myofibril has not been completed.
Hsp90a also colocalizes with unfolded myosin
Because Hsp90a cooperates with Unc45b in myofibril assembly (Etard et al., 2007), we also analyzed the distribution of Hsp90a-GFP in unc45b mutant embryos. We found that Hsp90a-GFP associated in patches at 72 hpf (Fig. 3 A), and immunohistochemistry with antibody F59 revealed colocalization with myosin (not depicted). To investigate whether Hsp90a is also associated with myosin during formation of the myofibril, we initiated slow myofibril formation in unc45b mutants by injecting low doses (0.1 μg/μl) of rescuing unc45b-myc mRNA. Under these conditions, the Hsp90a-GFP fusion protein arranged in reiterated doublets characteristic of the myosin in the A bands at 48 hpf (Fig. 3, B and F-F″). By 72 hpf, Hsp90a-GFP had shifted to the Z line (Fig. 3 C). Similar results were obtained when we reduced Unc45b activity in wild-type embryos by injection of a low concentration (0.1 mM) of unc45b morpholinos. As in the rescue experiments, Hsp90a-GFP was distributed in the double-banded pattern characteristic of the A bands (Fig. 3 D).
Figure 3.
Hsp90a-GFP associates with nascent myosin independently of Unc45b. (A) unc45b mutant expressing Hsp90a-GFP shows cytoplasmic aggregates of Hsp90a at 72 hpf. (B and C) unc45b mutant coinjected with hsp90a-gfp plasmid and unc45b-myc mRNA to induce slow rescue. Hsp90a-GFP first colocalized with myosin in A-band–like structures at 48 hpf (B) and then appeared at the Z line at 72 hpf (C). (D and E) Wild-type 72-hpf embryo coinjected with unc45b morpholinos (unc45b-Mo) and hsp90a-gfp plasmid (D) or with unc45b-Mo and hsp90aΔ-gfp plasmid (lacking the C-terminal MEEVD motif; E). Hsp90a-GFP and Hsp90aΔ-GFP colocalized with unfolded myosin at the forming A band. (F–F″) unc45b mutant injected with a low concentration (50 ng/μl) of unc45b-myc mRNA and hsp90a-gfp plasmid. Hsp90a-GFP colocalized with slow muscle myosin at the forming A band. F, Hsp90a-GFP; F′, slow muscle myosin revealed with F59 antibody; F″, merge. White box indicates A-band–like structure. (G–G″) Hsp90a-GFP colocalized with patches of nascent slow muscle myosin in unc45b mutants coinjected with unc45b morpholinos. G, Hsp90a-GFP; G′, slow muscle myosin stained with F59 antibody; G″, merge. Bars, 2 μm. Arrowhead indicates the Z line and white boxes outline the A-band–like striation. (H) Hsp90a-GST interacts with myosin. Pulldown of rabbit myosin (250 kD) by Hsp90a-GST (1) was revealed by anti-myosin antibody MF20 in Western blots. GST alone does not bind myosin (2). 3, protein ladder; 4, myosin protein alone.
To investigate whether the colocalization of Hsp90a with nascent myosin is Unc45b dependent, we expressed Hsp90a-GFP in unc45b mutants. Unc45b mutants express a truncated protein that appears to lack chaperone activity completely (Etard et al., 2007). To exclude the possibility that the truncated mutant protein could nevertheless anchor Hsp90a to myosin, we blocked the translation of the mutant unc45b allele with unc45b morpholinos. Labeling with myosin antibodies showed colocalization of Hsp90-GFP and myosin, indicating that Hsp90a can interact with myosin in the absence of Unc45b (Fig. 3, D and G–G″). A direct interaction between Hsp90a-GST and myosin was confirmed by in vitro pulldown assays (Fig. 3 H). GST-tagged Hsp90a pulled down myosin (Fig. 3 H, 1), whereas GST was ineffective to retain myosin (Fig. 3 H, 2).
To verify these findings by an independent experimental approach, we expressed the deletion construct hsp90aΔ, which lacks the MEEVD motif and was proposed to interact with a TPR domain such as that present in Unc45b (Barral et al., 2002). Hsp90aΔ behaved like Hsp90a. In the complete absence of Unc45b (unc45b mutant embryos injected with unc45b morpholinos) it colocalized with myosin (unpublished data). Moreover, in wild-type embryos injected with low doses of unc45b morpholinos, the MEEVD deletion variant of Hsp90 associated with the developing A band (Fig. 3 E), again indicating that it can associate with myosin. Thus, Hsp90a can colocalize with myosin independently of Unc45b.
Stress can induce relocalization of Unc45b from the Z line to the A band
Strikingly, when 72-hpf embryos were fixed with PFA, Unc45b-GFP accumulated in the duplet pattern characteristic of the A bands (Fig. 4, A, B, G–G″, H–H″, and I–I″). This differs from the data obtained in live and A/PFA-fixed embryos, showing localization at the Z line (Fig. 4, E and F). Similar results were obtained when we fixed Unc45b-Myc–expressing embryos with PFA (unpublished data). Moreover, the GFP fusion protein, Capza1-GFP, which is known to be localized at the Z line (Schafer et al., 1993; Papa et al., 1999; Wang et al., 2005), remained associated with the Z line after PFA fixation (Fig. 4, C and D), showing that the relocation of Unc45b from the Z line to the A band is a specific feature of Unc45b and not an artifact of fixation. These results suggest that Unc45b can relocate from the Z lines to the A bands under certain conditions.
Figure 4.
Stress triggers the relocalization of Unc45b-GFP from the Z line to the A band. (A and B) Unc45b-GFP localized preferentially from the Z line (A) to the A band after PFA fixation (B) in a 72-hpf embryo. (C and D) In contrast, Capza1-GFP stayed at the Z line after PFA fixation. C, control; D, fixed embryo. (E and F) Unc45b-GFP remained at the Z line after fixation with the fast-acting A/PFA fixative. E, unfixed control; F, A/PFA-fixed embryo. (G–G″) Wild-type embryo injected with unc45b-gfp plasmid, fixed with PFA, and stained with an antibody directed against α-actinin. (H–H″) Slow muscle myosin stained with antibody F59 in a PFA-fixed embryo injected with unc45b-gfp plasmid. (I–I″) Immunohistochemistry with anti–troponin T antibody on a PFA-fixed embryo injected with unc45b-gfp plasmid. Unc45b-GFP colocalized with myosin (H″) but not with α-actinin (G″), marking the Z line, and only partially with troponin T, labeling the I-band (I″), in a PFA-fixed embryo. White boxes outline an A-band–like pattern, white stars indicate the nucleus, and arrowheads indicate the Z line. (J–L) Unc45b-GFP dissociates from the Z line after cold treatment. J, before cold shock at 22°C; K, after 20 min at 4°C. 10 min after recovery at 22°C, Unc45b-GFP was relocalized at the Z lines (L). Graphs represent distribution of fluorescence over two sarcomeres. Peaks of GFP fluorescence at the Z lines (green arrows) were reduced after cold treatment and had shifted to the A band (red stars). After recovery at room temperature, Z-line peaks were as prominent as before the experiment and fluorescence over the A bands returned to its low baseline levels (L, stars). (M and N) Capza1-GFP (M, before cold shock) did not change location after a 20-min cold shock (N) and remained associated with the Z line. Bars, 2 μm.
We tested next whether cold or heat stress affects the localization of Unc45b-GFP protein. Cooling the embryos to 4°C for 20 min led to dissociation of Unc45b-GFP from the Z line. GFP fluorescence localized transiently to the A bands and was also diffusely distributed over the myofibrils. Within 5 min after transfer back to normal temperature (22°C), the fusion protein returned to the Z line (Fig. 4, J–L). Heat shock at 37°C similarly affected the subcellular distribution of the chimeric protein (unpublished data). In contrast, the Capza1-GFP fusion protein, which is also localized at the Z line, remained associated with the Z line after cold shock (Fig. 4, M and N), underscoring that this relocalization is a specific property of Unc45b. Thus, association of Unc45b-GFP with the Z line is temperature sensitive. Collectively, our results suggest that Unc45b can move back to the A bands under stress conditions.
Fibril damage induces Unc45 and Hsp90a to relocate from the Z line to the A band
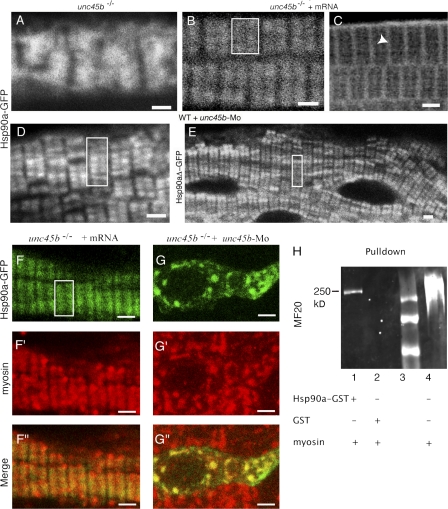
Because chemical and physical stress appears to trigger relocation of Unc45b from the Z lines to the A bands, we wished to assess next whether more physiological damage and stress to mature myofibrils such as membrane lesions can also affect the location of Unc45b and Hsp90a. We generated membrane lesions (2–14 μm in diameter) with the laser of a two-photon confocal microscope. A fusion of Unc45b with the bright YFP was used for these experiments. Unc45b-YFP maintained its location at the Z line immediately after wounding (Fig. 5, A, A′, B, and B′). However, after 5–10 min, YFP emission started to accumulate in the middle of the sarcomere (Fig. 5, C and C′). By 15 min, YFP emission was evident in the characteristic double peak representing the A band in the center of the sarcomere (Fig. 5 D; and D′, arrows). The accumulation at the A band was more obvious in some experiments, depending on the injury inflicted to the fibers (Fig. 5 D, inset). In the next 30 min, the Unc45b-YFP was barely detectable over the A bands but was prominently enriched at the Z lines again (Fig. 5 E and E′, dashed lines).
Figure 5.
Relocation of Unc45b and Hsp90a in response to plasma membrane damage. (A–E and A′–E′) Unc45b-YFP; (F–J and F′–J′) Hsp90a-YFP. (K–O and K′–O′) Capza1-GFP fusion proteins were expressed in muscle cells and distribution of chimeric proteins was analyzed during cell wounding and recovery by confocal microscopy. The Unc45b and Hsp90a fusion proteins were initially located at the Z line (A, A′, F, and F′) where they were maintained immediately after wounding (B, B′, G, and G′). They started to diffuse out in the next 5–10 min (C, C′, H, and H′). Subsequently, the YFP-tagged chaperones often accumulated in A-band–like structures (D and D′; D, inset; I and I′; and I, inset). Relocation to the Z lines was observed within the next 30–60 min (E, E′, J, and J′). In contrast, the Capza1-YFP fusion protein remained associated with the Z line after membrane damage (K–O). Asterisks indicate the lesion site. Graphs represent the distribution of fluorescence over two consecutive sarcomeres. Peaks of YFP fluorescence at the Z line (dashed lines) or in between in the putative A bands (arrows) are indicated. The sensitivity of the camera was slightly increased from time point to time point to compensate the bleaching. The camera setting was ∼20% more sensitive in the last frames taken. Bars, 6 μm.
The distribution of Hsp90a-YFP (Fig. 5, F–J) followed a similar sequence of relocation as the Unc45b-YFP. Within 10 min, the pattern became diffuse and YFP emission was enriched in the center of the sarcomere in A-band–like structures (Fig. 5, H, H′, and I; and I′, arrows). By 90 min, high levels of Hsp90-YFP had relocated to the Z line (Fig. 5, J and J′). The timing of the individual stages in the sequence of events varied from experiment to experiment. The kinetics of relocation appeared to correlate with the size of the lesion. To exclude differences in the behavior of Hsp90a and Unc45b in these experiments, we coexpressed an Hsp90a-Cherry with the Unc45b-GFP fusion protein in the same myofibril. Both proteins moved simultaneously to the A band upon inflicting a membrane lesion (unpublished data).
The Capza1-GFP fusion construct was used as a control. Indeed, this Z-line–associated protein did not change its localization. Hence, the relocation after membrane damage was specific to both Unc45b and Hsp90a, and the laser treatment did not damage the organization of the Z line because this structure remained intact in most regions of the fibril (Fig. 5, K–O).
Collectively, cell membrane wounding caused a similar redistribution of Unc45b and Hsp90a as cold or heat shock, suggesting that this reshifting of Unc45b and Hsp90a has a physiological role in response to and possible repair of myofibrillar damage. Once cellular homeostasis appeared to be reestablished, the chaperones translocated back to the Z line.
The Z-line association of Unc45b and Hsp90a is highly dynamic
To assess the dynamics of association of Unc45b and Hsp90a with the Z line, FRAP was used. YFP-tagged Unc45b and Hsp90a were expressed in 72-hpf embryos. Using the laser of a confocal microscope, one Z line was bleached at a time and the recovery was recorded (see Materials and methods for a detailed description). Unc45b-YFP (Fig. 6, A–D) and Hsp90a-YFP (Fig. 6, E–H) showed a very fast redistribution in the muscle cell (Fig. 6, M and N). Fluorescence intensity recovered within t 1/2 = 0.266 s for Unc45b and t 1/2 = 0.275 s for Hsp90a. This rapid recovery suggests fast shuttling of chaperones between the Z line and the surrounding cytosol. As a control, we used the Z-line–associated protein Capza1-YFP. The results demonstrated that this protein needed a much longer period. To reach a recovery level of 70%, Capza1 required 6 min, whereas Unc45b-YFP and Hsp90a-YFP achieved 90% recovery within 3 s (Fig. 6, I–L and O). This slow recovery of Capza1 is in agreement with previously published data on other Z-line–associated proteins (Wang et al., 2005) and underscores that in contrast, the two chaperone, Unc45b and Hsp90a associate with the Z line in a highly dynamic fashion.
Figure 6.
FRAP indicates rapid shuffling between Z line and cytoplasm. (A–D) Unc45b-YFP; (E–H) Hsp90a-YFP; (I–L) Capza1-YFP–expressing embryos (72 hpf). One Z line was bleached and the fluorescence recovery was monitored at 295-ms intervals for Unc45b-YFP and Hsp90a-YFP and at 20-s intervals for capza1-YFP. Prebleach (A, E, and I), bleach (B, F, and J), and two recovery phase captures, one initially after bleaching (C, G, and K) and the other as the fluorescence intensity has reached a stable plateau (D, H, and L), are shown. (M–O) FRAP curves represent all 35 time points and were averaged over 20 independent experiments (Unc45b-YFP and Hsp90a-YFP) or 10 experiments (Capza1). Bar, 1 μm. Arrows indicate the bleached Z line. Error bars represent standard errors calculated over 20 independent experiments.
The UCS and the central domain mediate Z-line location
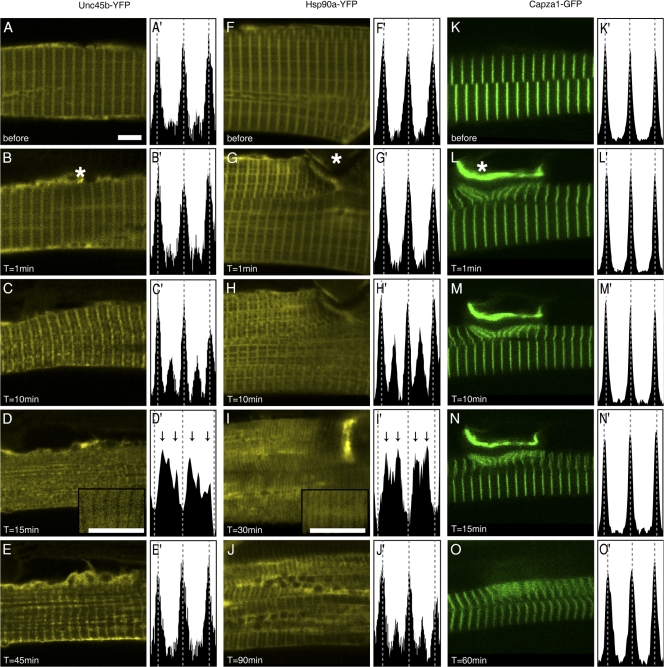
To identify which domain of Unc45b is required for Z-line association, we tested several mutant GFP chimeras deleted for either the TPR (aa 119–934), or the UCS domain (aa 1–509) or containing the TPR (aa 1–118), central (aa 119–509), or UCS (aa 510–934) domain alone. With the exception of TPR-GFP, all the proteins were able to localize to the Z line in a similar manner as the wild-type Unc45-GFP in 72-hpf embryos (n = 20 embryos for each construct; Fig. 7, A–F). In many cases, the TPR-GFP chimera showed a diffuse localization throughout the cytoplasm. In some experiments (Fig. 7 F), a weak enrichment over the Z line was visible against the haze of cytoplasmic staining, suggesting that TPR can mediate weak interaction with the Z line. Collectively, our data suggest that the UCS and the central domain can mediate strong Z-line attachment.
Figure 7.
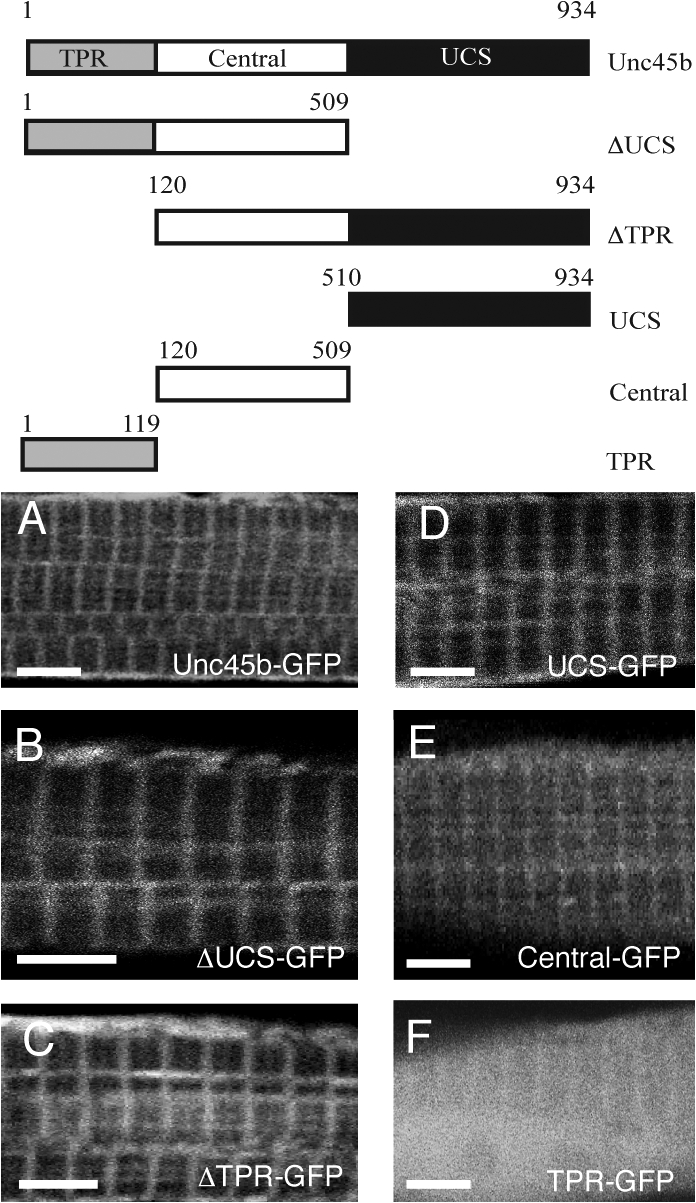
The UCS and the central domains can anchor GFP at the Z line. The scheme (top) outlines the different deletion constructs with the number of amino acids. (A–F) Unc45b-GFP (A), ΔUCS-GFP (B), ΔTPR-GFP (C), UCS-GFP (D), central-GFP (E), and TPR-GFP (F) proteins were expressed from injected plasmids. With the exception of TPR-GFP, all chimeric proteins localized at the Z line in 72-hpf wild-type embryos. In contrast, TPR-GFP was diffusely distributed throughout the cytoplasm. Bars, 4 μm.
Multiple domains mediate association with the A band
Next, we wished to establish which domains of Unc45b are required for the association with nascent myosin. First, we tested whether any of the Unc45b-GFP mutant proteins retained activity. In contrast to the wild-type Unc45b-GFP protein (Fig. 8, A and F; and Fig. 2, K–M), none of the mutant proteins are able to rescue the phenotype when injected into unc45b mutant embryos (20 embryos analyzed for each construct; Fig. 8, B–E; and not depicted).
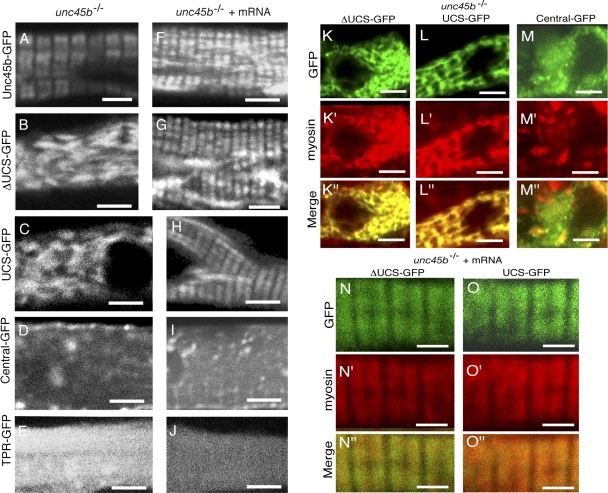
Figure 8.
Multiple sub-regions of Unc45b mediate association with nascent myosin. (A–E) unc45b mutants injected with plasmids encoding unc45b deletion constructs. Only intact Unc45b-GFP rescued the phenotype and colocalized with myosin in the nascent A band of the recovering myofibril (A). ΔUCS-GFP (B) and UCS-GFP (C) aggregated in patches corresponding to myosin localization, whereas central-GFP (D) and TPR-GFP (E) were uniformly distributed throughout the cytoplasm. (F–J) unc45b mutants coinjected with unc45b-myc mRNA and plasmids encoding different deletion constructs fused to GFP. GFP fusion protein for Unc45b (F), ΔUCS (G), and UCS (H) colocalized with myosin at the forming A band, whereas central (I) and TPR (J) were diffusely expressed in the cytoplasm. (K–K″, L–L″, and M–M″) ΔUCS-GFP (K–K″), UCS-GFP (L–L″), and central-GFP (M–M″) expressed in unc45b mutants. ΔUCS-GFP and UCS-GFP colocalized with unfolded patches of myosin, whereas central-GFP did not colocalize specifically with myosin. (N–N″ and O–O″) ΔUCS-GFP (N–N″) and UCS-GFP (O–O″) expressed in unc45b mutants coinjected with unc45b-myc mRNA. Both deletion constructs show a colocalization with nascent slow muscle myosin already arranged in A-band–like structures. (K–O) GFP; (K′–O′) immunohistochemistry with anti–slow muscle myosin antibody F59; (K″–O″) merge. All embryos were fixed with A/PFA at 72 hpf. Bars: (A–J) 4 μm; (K–O, K′–O′, and K″–O″) 2 μm.
Expression of the central-GFP and the TPR-GFP chimeras (10 embryos analyzed for each construct; Fig. 8, D and E) harboring the central and the TPR domain, respectively, resulted in diffuse cytoplasmic staining. In contrast, irregular cytoplasmic clusters of GFP fluorescence were detected in myofibers expressing UCS-deleted ΔUCS-GFP and the UCS-GFP proteins (n = 10 embryos for each construct; Fig. 8, B and C). This latter pattern is reminiscent of the distribution of myosin in the unc45b mutant (Etard et al., 2007). To test this notion directly, we performed immunohistochemistry with the slow muscle myosin antibody F59. Myosin and GFP signals overlapped in the case of ΔUCS-GFP and the UCS-GFP–expressing myofibrils (Five embryos analyzed for each construct; Fig. 8, K–K″ and L–L″). The central-GFP and the TPR-GFP proteins did not overlap as perfectly with myosin (Five embryos analyzed for each construct; Fig. 8, M–M″; and not depicted), indicating that these two chimeric proteins were not associated tightly with myosin. In summary, not only UCS but also the UCS-deleted protein colocalized with myosin, suggesting that the UCS domain and the central-TPR domains can associate with myosin. These results suggest that the interaction with myosin and the role in myofibril assembly are independent processes.
Next, we monitored whether the mutant proteins can transiently associate with the developing A bands in unc45b mutant embryos, in which myofibrillogenesis was rescued by injection of low levels of unc45b-myc mRNA (0.1 μg/μl). Like Unc45b-GFP (Fig. 8 F), the ΔUCS-GFP (Fig. 8 G) and the UCS-GFP (Fig. 8 H) proteins are able to anchor GFP in an A-band–like pattern along the myofibril. The association with myosin was confirmed by immunohistochemistry with anti–slow muscle myosin antibody (Fig. 8, N–N″ and O–O″). Neither central nor TPR domains alone are sufficient to associate with the developing A band (Fig. 8, I and J). Collectively these data suggest that association with myosin is mediated by two regions of Unc45b, the UCS domain alone, and the combination of the TPR with the central domains.
Discussion
The myosin chaperones Unc45b and Hsp90a play crucial roles in the formation of myofibrils in the zebrafish embryo (Etard et al., 2007, Wohlgemuth et al., 2007). In this paper, we show that these proteins associate with nascent myosin. Chaperone activity of Unc45b requires an intact protein. Upon maturation of the myofibril, both proteins accumulate at the Z line. Z-line association is highly dynamic and sensitive to stress or damage to the muscle fiber. Based on our data, we propose that the Z line serves as a reservoir for these chaperones from which they can be mobilized in a reversible manner by damage to the myofibrils.
Multiple domains of unc45b contribute to association with nascent myosin
The assembly of myosin in striated muscles entails three steps: the folding of the myosin molecules, the assembly of myosin filaments, and the incorporation of the filaments into myofibrils (Srikakulam and Winkelmann, 2004). The first step requires the participation of chaperone proteins.
A common function of all UCS proteins is their interaction with myosins (Barral et al., 2002; Toi et al., 2003; Mishra et al., 2005; Etard et al., 2007; Landsverk et al., 2007). Studies of the yeast proteins She4p and Rng3p showed that the UCS domain mediates the interaction with myosins (Toi et al., 2003; Mishra et al., 2005). The importance of the UCS domain for myofibrillogenesis is further underscored by the complete loss-of-function phenotype caused by the UCS-truncated Steifsb60 protein in zebrafish (Etard et al., 2007). In this paper, we showed that the UCS domain by itself can interact with nascent myosin. However, chaperone activity of Unc45b requires an intact protein. We found that the TPR in combination with the central domain can also interact with nascent myosin but, as in the case of the isolated UCS domain, this subfragment of Unc45b is unable to assemble myofibrils. Thus, myosin binding involves multiple domains, and myofibril assembly and myosin binding of Unc45b are separable activities.
Unc45 interacts with Hsp90 proteins in vitro (Barral et al., 2002; Etard et al., 2007). Moreover, Hsp90 proteins colocalize with myosin in differentiating C2C12 myoblasts (Srikakulam and Winkelmann, 2004), and knockdown of hsp90a in zebrafish results in a phenotype very similar to that of the unc45b mutant (Etard et al., 2007). The TPR domain, such as that in Unc45b, is known to interact with the MEEVD domain of Hsp90 (Barral et al., 2002). Our data suggest that Hsp90a can associate independently of Unc45b with nascent myosin, an interaction that is presumably further stabilized by the binding of the TPR domain of myosin-bound Unc45b to the MEEVD domain of Hsp90a.
Unc45b and Hsp90a are localized at the Z line in zebrafish
The subcellular localization of Unc45 and Hsp90a is dependent on developmental stage. Unc45b-GFP and Hsp90a-GFP are ubiquitously distributed throughout the cytoplasm of early developing myocytes at 24 hpf. Then, as myosin folding proceeds, the chaperones colocalize with foci of presumptive immature myosin, labeling the myofibril in an A-band–like pattern. As soon as the folding of myosin is completed, the chaperones accumulate at the Z line. Change of localization of sarcomeric proteins during development of the myofibril has also been observed in other instances. For example, in nascent myofibrils of chick cardiomyocytes, the muscle LIM protein, MLP, is diffusely distributed in the cytoplasm. As the myofibril matures, the expression of MLP becomes restricted to two bands flanking α-actinin. Later on, MLP expression coincides with α-actinin at the Z line (Henderson et al., 2003).
The assembly of properly folded myosin into myofibrils is a very rapid process that cannot be separated into individual steps in wild-type embryos. We could only observe a transient A-band–like association of Unc45b-GFP in animals with artificially lowered chaperone activity. In wild-type animals, a Z-line pattern of fluorescence was instantly detected after the phase of diffuse cytoplasmic distribution.
A similar developmental sequence of events was observed in C. elegans, in which UNC-45 also initially forms cytosolic foci and then associates with the folding myosin in the A band of the myofibril (Ao and Pilgrim, 2000). In the mature fiber, however, it decorates the peripheral zones of the thick filaments composed of myosin B (Ao and Pilgrim, 2000). This is in contrast to the situation in the zebrafish, where Unc45b interacts only with nascent myosin and is then present at high levels at the Z line. The S. cerevisiae UCS protein She4p is also only transiently associated with the myo3/4/5p myosin (Toi et al., 2003). Similarly, S. pombe Rng3p is mostly located in the cytoplasm, suggesting also a transient interaction with the myosins in the cytokinetic ring (Wong et al., 2000).
The central and UCS domains of Unc45b can mediate Z-line association. The functional importance of the central domain was previously indicated by the isolation of a temperature-sensitive missense mutation localized in the central region of C. elegans unc-45 (Barral et al., 1998). However, a specific function was not attributed to the central domain in these studies. Whether the central domain has a similar docking function in C. elegans UNC-45 remains to be clarified.
Genetic and biochemical data suggest that Hsp90a and Unc45b interact in myofibrillogenesis (Barral et al., 2002; Etard et al., 2007). Hsp90a and Unc45b not only cooperate in myofibrillogenesis, they are also colocalized at the Z line, suggesting that Hsp90a and Unc45b can also form a complex at this location.
The Z-line location is reversible
Upon stressing, the myofibers Unc45b and Hsp90a can detach from the Z line to associate transiently with the myosin-containing A bands. Heat or cold shock, as well as membrane damage, leads to the redistribution of the chaperones. It is tempting to speculate that the slow-acting fixative PFA generates chemical stress, which would damage the myofibril and cause detachment of the Unc45 proteins from the Z lines and accumulation at the A bands where the proteins become eventually terminally fixed.
One of the previously established functions of chaperones is to prevent aggregation of partially unfolded proteins (Hartl and Hayer-Hartl, 2002). Accordingly, Unc45b and Hsp90a could relocate to the A band to avoid misfolding and aggregation of myosin in situations of stress and damage. For example, B-crystallin, a member of the family of small heat shock proteins, shows a rapid ischemia-induced redistribution from a cytosolic pool to intercalated disks and Z lines of the myofibrils (Golenhofen et al., 1998). B-crystallin has been proposed to protect titin from denaturation (Bullard et al., 2004).
The association of Unc45b-GFP and Hsp90a-GFP with the Z line appears highly dynamic as recovery from photobleaching occurs in <1 s. This fast rate is in striking contrast to the mobility of PKCε, which becomes Z-line associated in response to ischemic damage and has a half-maximal FRAP rate in the 10–20-min range (Robia et al., 2005). Similarly, Capza1 and other Z-line–associated proteins, like myotilin or α-actinin, show FRAP rates in a minute time scale (Wang et al., 2005; our data) rather than in a fraction of a second as observed for Hsp90a and Unc45b.
The fast recovery of fluorescence of Unc45b-GFP and Hsp90-GFP at the Z line suggests that these Z-line–bound proteins are in constant exchange with cytosolic molecules. PFA fixation, heat or cold shock, or membrane damage causes dissociation of Unc45b and Hsp90a from the Z line. In particular, membrane damage appears to be a cause of myofibril stress under normal physiological load (McNeil and Khakee, 1992). It has been estimated that as much as 5–30% of striated muscle cells get small membrane injuries during contraction (McNeil, 2002). Our data suggest that the Z line serves as a reservoir for Unc45b and Hsp90a, from which they can be released in response to damage or stress to maintain the integrity of the myofibrils. The fission yeast Rng3p is likewise required to maintain a contractile ring, as the structure collapses when temperature-sensitive rng3-65 mutants are shifted to the restrictive temperature (Mishra et al., 2005).
Transgenic worms overexpressing UNC-45 were shown to display defects in myosin assembly with decreased myosin levels and mild paralysis (Landsverk et al., 2007). The E4 ubiquitin ligases Ufd-2 and CHIP regulate the levels of UNC-45 in C. elegans (Hoppe et al., 2004). Reduced myosin levels are the result of degradation through the ubiquitin–proteasome system, suggesting a mechanism in which UNC-45–related proteins may contribute to the degradation of myosin in conditions such as cardiac and skeletal myopathies, cancer, and starvation (Landsverk et al., 2007). We did not observe adverse effects of overexpression in the zebrafish, even after varying the concentration of expressed Unc45b-GFP over a fivefold range or high expression of Unc45b-CFP from a BAC clone (Etard et al., 2007). These animals did not show defective myofibrils or an impairment of movement (unpublished data). The association of Unc45b with the Z line in zebrafish may have the function to buffer against high levels of Unc45b activity and at the same time maintain an efficient reservoir of chaperones that can be rapidly mobilized to repair myofibrils.
The Z lines define the lateral boundaries of the sarcomeres and are responsible for force transmission by anchoring actin, titin, and nebulin filaments (Knöll et al., 2002). In addition to these structural roles, the Z line may also act as a signaling node (Frey et al., 2000). A central issue is how the chaperones get mobilized to leave the Z line. Impaired motility may be a signal to mobilize Unc45b and Hsp90a from the Z line. However, paralyzed animals carrying a mutation in the δ-subunit of the acetylcholine receptor (Etard et al., 2007) show normal Z-line association of the chaperones (unpublished data). The rapid recovery after photobleaching suggests that the molecules shuttle between the Z line and the surrounding cytosol. In this way, the chaperones could constantly probe the folding state of myosin. In case of damage, the affinity to myosin could become higher than to the Z line, thereby resulting in a shift away from the Z line. Interestingly, although the central-GFP fusion protein is attached to the Z line, it is not mobilized by membrane damage or PFA fixation (unpublished data), suggesting that movement to the A band requires an intact Unc45b protein. This would be consistent with the differential affinity model. It does not, however, rule out other possibilities such as, e.g., active translocation in response to a signal that communicates damage to myosin.
Materials and methods
Fish stocks
Fish were bred and raised as previously described (Westerfield, 1993). For a description of the steifsb60 mutants, see Etard et al. (2007).
Cloning
Full-length unc45b-gfp and unc45b-cfp was described in Etard et al. (2007). For construction of unc45b-myc and unc45b-yfp, the unc45b PCR-amplified fragment was inserted into pCS2+myc and pCS2+yfp, respectively (Rupp et al., 1994). All truncated unc45b variants were obtained by PCR with the primers described in Table I, and cloned into pCS2+gfp. All constructs were sequenced.
Table I. Cloning details of unc45b, hsp90a, and Capza1 expression constructs.
| Constructs | Cloned with | Amino acids | Artificial ATG | Utilized primers |
|---|---|---|---|---|
| unc45-gfp | E/X | 1–934 | no | 1 + 6 |
| ΔUCS-gfp | E/X | 1–509 | no | 1 + 4 |
| ΔTPR-gfp | E/X | 120–509 | yes | 3 + 6 |
| UCS-gfp | E/X | 510–934 | yes | 5 + 6 |
| TPR-gfp | E/X | 1–119 | no | 1 + 2 |
| central-gfp | E/X | 120–509 | yes | 3 + 4 |
| Hsp90a-gfp | B/X | 1–725 | no | 7 + 8 |
| Hsp90aΔ−gfp | B/X | 1–720 | no | 7 + 9 |
| CapZa1 | E/X | 1–292 | no | 10 + 11 |
Primer 1, catcgaattcagtc atgacgatgggagaaattgg; primer 2, tagctcgagagtccagaaaggtcttgttttttggctc; primer 3, catcgaattcagtcatggagaccctcaggagacttggagctgaa; primer 4, tagctcgagagtcctcgttggaaaagggctttataag; primer 5, catcgaattcagtcatggagaccctcaggagacttggagctgaa; primer 6, tagctcgagagtcctcgttggaaaagggctttataag; primer 7, ctgggatcctcgatgcctgagaagtcggcccag; primer 8, gtaggaattcaaggtagtcgacttcctccattctc; primer 9, gtaggaattcagctctgaagtgtcgtcgtctccctc; primer 10, cgacgaattcaccgatgcagtttgacagggcaggtccag; primer 11, gagctcgagtgtgctgagcgttctgcatctctttgccg. E, EcoRI; B, BamHI; X, XhoI.
hsp90a, hsp90aΔ, and capza1 were amplified from cDNA obtained from 72-hpf embryos with the primers described in Table I and cloned into pCS2+gfp plasmid. All constructs were sequenced.
Histology, immunohistochemistry, and microscopy
Whole-mount immunohistochemistry was performed as described previously (Crow and Stockdale, 1986; Costa et al., 2002), with monoclonal antibodies directed against troponin T (1:200; Sigma-Aldrich), sarcomeric α-actinin (1:200; Sigma-Aldrich), slow muscle myosin F59 (Crow and Stockdale, 1986), and anti–Myc epitope antibody 9E10 (1:25; Developmental Studies Hybridoma Bank). Anti–mouse Cy3 conjugate secondary antibody was used for fluorescent detection (1:1,000; Sigma-Aldrich).
GST pulldown
unc-45b and Hsp90a full-length cDNA were cloned into pGEX-4T-3 vector. Expression of recombinant proteins in BL21D3 cells was induced by adding 1 mM IPTG at OD600 = 0.8. After incubation for 4 h at room temperature, proteins were extracted by sonication in NOP buffer (PBS, 0.1% Triton X-100, 1 mM DTT, and 0.5 mM PMSF). For pulldown experiments, the bacterial protein lysates were incubated with immobilized fusion proteins according to previously described protocols (Bauer et al., 2000) and washed three times with NOP buffer. Rabbit myosin from rabbit muscle (Sigma-Aldrich) was incubated at room temperature for 1 h with glutathione-sepharose beads/GST protein complex. After three successive washes, bound proteins were detached from the bead by boiling them for 10 min at 95°C. Proteins were separated by 10% SDS PAGE, transferred onto nitrocellulose, and incubated with primary antibody MF20 overnight at 4°C. After washing three times, a secondary antibody (goat anti–mouse Alexa Fluor 680; Invitrogen) was applied for 1 h at room temperature. Visualization was performed with the Odyssey infrared imaging system (BD Biosciences).
Confocal microscopy, FRAP, and membrane wounding
Optical sections were taken with a confocal microscope (TCS SP2; Leica) and analyzed with the interactive LCS software (Leica). Live fish were immersed in a water drop and analyzed with a dip-in 63× objective (NA: 0.90; HCX apo water; Leica). The observations were performed at 22°C.
FRAP experiments were performed with a confocal microscope (TCS SP2). Embryos were embedded in 0.5% low melting point agarose and analyzed with a dip-in 63× objective (HCX apo water). Muscle fibers expressing YFP-tagged proteins were analyzed at 256 × 256 pixel dimension at 1,400 MHz, using beam expander three and pinhole setting 66. This made it possible to capture one frame in 295 ms and not significantly bleach the tissue during the recording phase. For bleaching the lasers were set at 100% intensity. An area of 0.3–0.5×6 μm was bleached. The experiment was recorded with Flymode (Leica) and consisted of 3 prebleaching frames, 2 bleaching frames, and 30 postbleaching frames. Image series were analyzed in ImageJ (National Institutes of Health), where fluorescence intensities were calculated. The values were corrected for fluorescence loss during postbleaching, and the presented graph represents the mean over 20 independent experiments. Approximate time needed to reach half of the final intensity after bleaching was estimated by linear extrapolation.
For Capza1-GFP recovery experiments, we used conventional FRAP because the Flymode FRAP program bleached the cell too much over the 20-min recording period. The first image was captured 0.336 s after bleaching and the rest of the images were taken at 20-s intervals. The final calculations are based on 10 independent experiments.
Plasma membrane wounds were generated with a two-photon confocal microscope (TCS SP2) targeting an area of 2–14 μm. 840-nm laser power was set to 85% and gain to 65%. Embryos were embedded into 0.5% low-melting agarose.
Microinjections
Injections were performed as previously described (Müller et al., 1999). Morpholinos (Gene Tools, LLC) were injected at 0.3 mM to obtain a maximal knockdown effect and at 0.1 mM to obtain a reduction of protein synthesis. For unc45b-Mo, counc45b-Mo, hsp90a-Mo, and cohsp90a-Mo, see Etard et al., (2007). For synthesis of capped unc45b-myc mRNA, pCS2+unc45b-myc plasmid was cut with NotI and translated with the Sp6 Message Machine kit (Ambion). Plasmids were injected at 0.3 μg/μl. For slow recovery, unc45b-myc mRNA was injected at 50 ng/μl.
Acknowledgments
We thank M. Rastegar for help with picture processing, N. Borel and C. Villani for fish care, N. Gretz for technical assistance and S. Paul and S. Vinothkumar for critical reading of the manuscript.
This work was supported by Forschungszentrum Karlsruhe and European commission IP ZF-MODELS. U. Roostalu was supported by a Boehringer Ingelheim PhD Scholarship.
Abbreviations used in this paper: A/PFA, acetone/PFA; hpf, hours postfertilization; Hsp, heat shock protein; TPR, tetratricopeptide repeat; UCS, Unc45-Cro1p-She4p.
References
- Ao, W., and D. Pilgrim. 2000. Caenorhabditis elegans UNC-45 is a component of muscle thick filaments and colocalizes with myosin heavy chain B, but not myosin heavy chain A. J. Cell Biol. 148:375–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atkinson, S.J., and M. Stewart. 1991. Molecular basis of myosin assembly: coiled-coil interactions and the role of charge periodicities. J. Cell Sci. Suppl. 14:7–10. [DOI] [PubMed] [Google Scholar]
- Barral, J.M., C.C. Bauer, I. Ortiz, and H.F. Epstein. 1998. Unc-45 mutations in Caenorhabditis elegans implicate a CRO1/She4p-like domain in myosin assembly. J. Cell Biol. 143:1215–1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barral, J.M., A.H. Hutagalung, A. Brinker, F.U. Hartl, and H.F. Epstein. 2002. Role of the myosin assembly protein UNC-45 as a molecular chaperone for myosin. Science. 295:669–671. [DOI] [PubMed] [Google Scholar]
- Bauer, A., S. Chauvet, O. Huber, F. Usseglio, U. Rothbacher, D. Aragnol, R. Kemler, and J. Pradel. 2000. Pontin52 and reptin52 function as antagonistic regulators of beta-catenin signalling activity. EMBO J. 19:6121–6130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bullard, B., C. Ferguson, A. Minajeva, M.C. Leake, M. Gautel, D. Labeit, L. Ding, S. Labeit, J. Horwitz, K.R. Leonard, and W.A. Linke. 2004. Association of the chaperone alphaB-crystallin with titin in heart muscle. J. Biol. Chem. 279:7917–7924. [DOI] [PubMed] [Google Scholar]
- Clarke, M.S., R.W. Caldwell, H. Chiao, K. Miyake, and P.L. McNeil. 1995. Contraction-induced cell wounding and release of fibroblast growth factor in heart. Circ. Res. 76:927–934. [DOI] [PubMed] [Google Scholar]
- Costa, M.L., R.C. Escaleira, V.B. Rodrigues, M. Manasfi, and C.S. Mermelstein. 2002. Some distinctive features of zebrafish myogenesis based on unexpected distributions of the muscle cytoskeletal proteins actin, myosin, desmin, alpha-actinin, troponin and titin. Mech. Dev. 116:95–104. [DOI] [PubMed] [Google Scholar]
- Crow, M.T., and F.E. Stockdale. 1986. Myosin expression and specialization among the earliest muscle fibers of the developing avian limb. Dev. Biol. 113:238–254. [DOI] [PubMed] [Google Scholar]
- Etard, C., M. Behra, N. Fischer, D. Hutcheson, R. Geisler, and U. Strahle. 2007. The UCS factor Steif/Unc-45b interacts with the heat shock protein Hsp90a during myofibrillogenesis. Dev. Biol. 308:133–143. [DOI] [PubMed] [Google Scholar]
- Frey, N., J.A. Richardson, and E.N. Olson. 2000. Calsarcins, a novel family of sarcomeric calcineurin-binding proteins. Proc. Natl. Acad. Sci. USA. 97:14632–14637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golenhofen, N., W. Ness, R. Koob, P. Htun, W. Schaper, and D. Drenckhahn. 1998. Ischemia-induced phosphorylation and translocation of stress protein alpha B-crystallin to Z lines of myocardium. Am. J. Physiol. 274:H1457–H1464. [DOI] [PubMed] [Google Scholar]
- Hartl, F.U., and M. Hayer-Hartl. 2002. Molecular chaperones in the cytosol: from nascent chain to folded protein. Science. 295:1852–1858. [DOI] [PubMed] [Google Scholar]
- Henderson, J.R., P. Pomies, C. Auffray, and M.C. Beckerle. 2003. ALP and MLP distribution during myofibrillogenesis in cultured cardiomyocytes. Cell Motil. Cytoskeleton. 54:254–265. [DOI] [PubMed] [Google Scholar]
- Hoppe, T., G. Cassata, J.M. Barral, W. Springer, A.H. Hutagalung, H.F. Epstein, and R. Baumeister. 2004. Regulation of the myosin-directed chaperone UNC-45 by a novel E3/E4-multiubiquitylation complex in C. elegans. Cell. 118:337–349. [DOI] [PubMed] [Google Scholar]
- Hubbers, C.U., C.S. Clemen, K. Kesper, A. Boddrich, A. Hofmann, O. Kamarainen, K. Tolksdorf, M. Stumpf, J. Reichelt, U. Roth, et al. 2007. Pathological consequences of VCP mutations on human striated muscle. Brain. 130:381–393. [DOI] [PubMed] [Google Scholar]
- Hutagalung, A.H., M.L. Landsverk, M.G. Price, and H.F. Epstein. 2002. The UCS family of myosin chaperones. J. Cell Sci. 115:3983–3990. [DOI] [PubMed] [Google Scholar]
- Janiesch, P.C., J. Kim, J. Mouysset, R. Barikbin, H. Lochmuller, G. Cassata, S. Krause, and T. Hoppe. 2007. The ubiquitin-selective chaperone CDC-48/p97 links myosin assembly to human myopathy. Nat. Cell Biol. 9:379–390. [DOI] [PubMed] [Google Scholar]
- Knöll, R., M. Hoshijima, and K.R. Chien. 2002. Z-line proteins: implications for additional functions. Eur. Heart J. Suppl. 4:I13–I17. [Google Scholar]
- Krone, P.H., and J.B. Sass. 1994. HSP 90 alpha and HSP 90 beta genes are present in the zebrafish and are differentially regulated in developing embryos. Biochem. Biophys. Res. Commun. 204:746–752. [DOI] [PubMed] [Google Scholar]
- Landsverk, M.L., S. Li, A.H. Hutagalung, A. Najafov, T. Hoppe, J.M. Barral, and H.F. Epstein. 2007. The UNC-45 chaperone mediates sarcomere assembly through myosin degradation in Caenorhabditis elegans. J. Cell Biol. 177:205–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNeil, P.L. 2002. Repairing a torn cell surface: make way, lysosomes to the rescue. J. Cell Sci. 115:873–879. [DOI] [PubMed] [Google Scholar]
- McNeil, P.L., and R. Khakee. 1992. Disruptions of muscle fiber plasma membranes. Role in exercise-induced damage. Am. J. Pathol. 140:1097–1109. [PMC free article] [PubMed] [Google Scholar]
- Mishra, M., V.M. D'Souza, K.C. Chang, Y. Huang, and M.K. Balasubramanian. 2005. Hsp90 protein in fission yeast Swo1p and UCS protein Rng3p facilitate myosin II assembly and function. Eukaryot. Cell. 4:567–576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller, F., B. Chang, S. Albert, N. Fischer, L. Tora, and U. Strahle. 1999. Intronic enhancers control expression of zebrafish sonic hedgehog in floor plate and notochord. Development. 126:2103–2116. [DOI] [PubMed] [Google Scholar]
- Murtha, J.M., and E.T. Keller. 2003. Characterization of the heat shock response in mature zebrafish (Danio rerio). Exp. Gerontol. 38:683–691. [DOI] [PubMed] [Google Scholar]
- Papa, I., C. Astier, O. Kwiatek, F. Raynaud, C. Bonnal, M.C. Lebart, C. Roustan, and Y. Benyamin. 1999. Alpha actinin-CapZ, an anchoring complex for thin filaments in Z-line. J. Muscle Res. Cell Motil. 20:187–197. [DOI] [PubMed] [Google Scholar]
- Price, M.G., M.L. Landsverk, J.M. Barral, and H.F. Epstein. 2002. Two mammalian UNC-45 isoforms are related to distinct cytoskeletal and muscle-specific functions. J. Cell Sci. 115:4013–4023. [DOI] [PubMed] [Google Scholar]
- Prodromou, C., B. Panaretou, S. Chohan, G. Siligardi, R. O'Brien, J.E. Ladbury, S.M. Roe, P.W. Piper, and L.H. Pearl. 2000. The ATPase cycle of Hsp90 drives a molecular ‘clamp’ via transient dimerization of the N-terminal domains. EMBO J. 19:4383–4392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robia, S.L., M. Kang, and J.W. Walker. 2005. Novel determinant of PKC-epsilon anchoring at cardiac Z-lines. Am. J. Physiol. Heart Circ. Physiol. 289:H1941–H1950. [DOI] [PubMed] [Google Scholar]
- Rupp, R.A., L. Snider, and H. Weintraub. 1994. Xenopus embryos regulate the nuclear localization of XMyoD. Genes Dev. 8:1311–1323. [DOI] [PubMed] [Google Scholar]
- Schafer, D.A., J.A. Waddle, and J.A. Cooper. 1993. Localization of CapZ during myofibrillogenesis in cultured chicken muscle. Cell Motil. Cytoskeleton. 25:317–335. [DOI] [PubMed] [Google Scholar]
- Srikakulam, R., and D.A. Winkelmann. 2004. Chaperone-mediated folding and assembly of myosin in striated muscle. J. Cell Sci. 117:641–652. [DOI] [PubMed] [Google Scholar]
- Toi, H., K. Fujimura-Kamada, K. Irie, Y. Takai, S. Todo, and K. Tanaka. 2003. She4p/Dim1p interacts with the motor domain of unconventional myosins in the budding yeast, Saccharomyces cerevisiae. Mol. Biol. Cell. 14:2237–2249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, J., N. Shaner, B. Mittal, Q. Zhou, J. Chen, J.M. Sanger, and J.W. Sanger. 2005. Dynamics of Z-band based proteins in developing skeletal muscle cells. Cell Motil. Cytoskeleton. 61:34–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westerfield, M. 1993. The Zebrafish Book: A Guide for the Laboratory Use of Zebrafish. University of Oregon Press, Eugene, OR.
- Wohlgemuth, S.L., B.D. Crawford, and D.B. Pilgrim. 2007. The myosin co-chaperone UNC-45 is required for skeletal and cardiac muscle function in zebrafish. Dev. Biol. 303:483–492. [DOI] [PubMed] [Google Scholar]
- Wong, K.C., N.I. Naqvi, Y. Iino, M. Yamamoto, and M.K. Balasubramanian. 2000. Fission yeast Rng3p: an UCS-domain protein that mediates myosin II assembly during cytokinesis. J. Cell Sci. 113:2421–2432. [DOI] [PubMed] [Google Scholar]
- Zhao, R., and W.A. Houry. 2005. Hsp90: a chaperone for protein folding and gene regulation. Biochem. Cell Biol. 83:703–710. [DOI] [PubMed] [Google Scholar]
- Zhao, R., and W.A. Houry. 2007. Molecular interaction network of the Hsp90 chaperone system. Adv. Exp. Med. Biol. 594:27–36. [DOI] [PubMed] [Google Scholar]