Abstract
Both tyrosine-phosphorylated caveolin-1 (pY14Cav1) and GlcNAc-transferase V (Mgat5) are linked with focal adhesions (FAs); however, their function in this context is unknown. Here, we show that galectin-3 binding to Mgat5-modified N-glycans functions together with pY14Cav1 to stabilize focal adhesion kinase (FAK) within FAs, and thereby promotes FA disassembly and turnover. Expression of the Mgat5/galectin lattice alone induces FAs and cell spreading. However, FAK stabilization in FAs also requires expression of pY14Cav1. In cells lacking the Mgat5/galectin lattice, pY14Cav1 is not sufficient to promote FAK stabilization, FA disassembly, and turnover. In human MDA-435 cancer cells, Cav1 expression, but not mutant Y14FCav1, stabilizes FAK exchange and stimulates de novo FA formation in protrusive cellular regions. Thus, transmembrane crosstalk between the galectin lattice and pY14Cav1 promotes FA turnover by stabilizing FAK within FAs defining previously unknown, interdependent roles for galectin-3 and pY14Cav1 in tumor cell migration.
Introduction
Optimal cell migration requires spatiotemporal feedback between actomyosin contraction, actin polymerization, and continuous formation and disassembly of adhesions (Gupton and Waterman-Storer, 2006). Focal adhesions (FAs), via integrin aggregation, mediate interaction between the ECM and cytoskeletal proteins (Burridge and Fath, 1989). Tyrosine kinase signaling and local proteolysis are critical regulators of the dynamic recruitment of FA components and regulate leading edge activity (Franco et al., 2004; Webb et al., 2004). Integrin binding to ECM and clustering of integrins induces autophosphorylation (Tyr397) of focal adhesion kinase (FAK), the major kinase implicated in FA signaling, generating a high-affinity binding site for SH2-containing proteins such as Src family kinases, PI3K, Grb7, and phospholipase Cγ (Mitra and Schlaepfer, 2006).
Caveolins are integral membrane proteins involved in formation of caveolae, omega-shaped invaginations involved in signal transduction and vesicular transport (Parton and Simons, 2007). Caveolin-1 (Cav1; the caveolin family also includes Cav2 and Cav3) was initially identified as a tyrosine-phosphorylated Src kinase substrate (Glenney and Zokas, 1989). Less than 1% of total cellular Cav1 is phosphorylated on tyrosine-14 (Y14) and it is virtually absent from caveolae (del Pozo et al., 2005). Cav1 and caveolae locate to the rear of migrating cells and Y14 of Cav1 is required for it to be localized to the leading edge of cells (Isshiki et al., 2002; Parat et al., 2003). A phosphospecific monoclonal antibody against Y14-phosphorylated Cav1 (pY14Cav1) localizes it mainly to the leading edge, where it associates with FAK, β1-integrin, and phospho-paxillin (Beardsley et al., 2005). However, the anti-pY14Cav1 monoclonal antibody has recently been reported to crossreact with phospho-paxillin, questioning the extent of pY14Cav1 localization to FAs (Hill et al., 2007). Cav1 depletion results in loss of FA sites and adhesion as well as of cell polarization and directional cell movement (Wei et al., 1999; Beardsley et al., 2005; Grande-Garcia et al., 2007). Conversely, Cav1 depletion can result in increased migration and Cav1 overexpression has been associated with decreased migration, including EGF-stimulated migration of tumor cells (Zhang et al., 2000; Gonzalez et al., 2004; Brouet et al., 2005). Although the role of Cav1 in the biogenesis of caveolae is well established, the role of pY14Cav1 in the regulation of FA expression and dynamics remains elusive.
Cav1 functions as a membrane adaptor that, upon integrin ligation in FAs, promotes integrin signaling through Src kinase and FAK and links Fyn kinase to Shc/Grb2/SOC/Rac activation and progression through G1 phase of the cell cycle (Wary et al., 1998; Wei et al., 1999; Mettouchi et al., 2001). pY14Cav1 generates a docking site for SH2 domain–containing proteins such as Grb7 (Lee et al., 2000) and the C-terminal Src kinase Csk that down-regulates Src activity via phosphorylation (Cao et al., 2002). Integrin activation appears to be essential for Cav1 phosphorylation, as β1-integrin blocking antibodies inhibit shear stress-induced Cav1 phosphorylation and actin reorganization in bovine aortic endothelial cells (Radel and Rizzo, 2005). Relocalization of pY14Cav1 from FAs induces the raft-dependent internalization of Erk, PI3K, and Rac from the plasma membrane upon cellular detachment (del Pozo et al., 2004, 2005). FAs have recently been shown to express highly ordered pY14Cav1-dependent membrane organization (Gaus et al., 2006). Defects in cytoskeletal organization, FA architecture, polarization, and directional cell migration of Cav1-deficient fibroblasts are rescued by reintroducing a wild-type form of Cav1, but not its Y14F mutant form (Grande-Garcia et al., 2007). However, it has yet to be demonstrated that pY14Cav1 recruitment impacts on FA behavior and dynamics.
The galectin lattice, a cell surface microdomain, recruits glycoproteins based on the extent of N-glycan number and branching, the latter determined in large part by modification by the Golgi enzyme GlcNAc-transferase V (Mgat5) (Lau et al., 2007). Studies with Mgat5−/− mice and derived cell lines have shown that recruitment to the lattice regulates receptor signaling by lowering activation thresholds and protecting receptors from loss due to constitutive endocytosis (Demetriou et al., 2001; Partridge et al., 2004). Using Mgat5−/− mammary epithelial tumor cells, we have shown that galectin binding to Mgat5-modified N-glycans regulates fibronectin (FN) matrix remodelling and cell spreading and motility (Lagana et al., 2006). Galectin-3 (Gal-3) stimulated FAK and PI3K activation, increased F-actin turnover, and enhanced integrin activation and recruitment to elongated fibrillar adhesions. Mgat5−/− cells are deficient for FAs and actin stress fibers (Granovsky et al., 2000; Lagana et al., 2006) and they also express reduced levels of Cav1 (Lajoie et al., 2007). This led us to study the role of the Mgat5/Gal-3 lattice and Cav1 phosphorylation in FA dynamics in these cells. Using mammary carcinoma cell lines differentially expressing Mgat5 and Cav1, we show that Cav1 tyrosine phosphorylation, in concert with the Mgat5/Gal-3 lattice, stabilizes FAK, paxillin, and α5-integrin in FAs, thereby promoting FA turnover. This suggests that Gal-3–mediated activation of integrins recruits tyrosine-phosphorylated Cav1, thereby stabilizing FAK in FA domains, promoting FA disassembly and formation and stimulating cellular displacement and motility.
Results
Deficient FA maturation in Mgat5−/− cells
In contrast to the actin stress fibers and well-developed FAs of Mgat5+/+ cells or of Mgat5−/− cells rescued by infection with an Mgat5 retroviral vector (Rescue cells), Mgat5−/− cells present a predominantly cytosolic distribution of vinculin and cortical actin (Fig. 1 A). Immunofluorescence and Western blot analysis with anti-pFAK(Y397) antibodies revealed that FAK shows reduced phosphorylation in Mgat5−/− cells compared with Mgat5+/+ and Rescue cells (Fig. 1, A and B).
Figure 1.
Tyrosine phosphorylation of FAK and expression of Cav1 are deficient in Mgat5−/− cells. (A) Mgat5+/+, Mgat5−/−, and Rescue cells were immunofluorescently labeled with Hoechst (blue), Alexa568-phalloidin (red), and anti-vinculin (green) antibodies (top) or Hoechst (blue) and anti-pFAK(Y397) (red) antibodies (bottom). Bar, 20 μm. (B) Cell lysates of Mgat5+/+, Mgat5−/−, and Rescue cells were probed by Western blotting for pFAK(Y397), FAK, and β-actin (top); Cav1, pY14Cav1, and β-actin (middle); or Gal-3 and β-actin (bottom). (C). Mgat5+/+ cells were treated with 1 μg/ml Gal-3 for 48 h in serum-free media and cell lysates probed for pY14Cav1, Cav1, and β-actin by Western blot. Intensity of the pY14Cav1 band relative to Cav1 was quantified by densitometry (P < 0.05, n = 4). Molecular mass markers (in kD) are indicated.
Reduced Cav1 expression in Mgat5−/− cells (Lajoie et al., 2007) is associated with a reduction of total cellular pY14Cav1 expression to very low, indeed undetectable, levels that are restored in Rescue cells (Fig. 1, A and B). Gal-3 expression is similar between the three cell lines with a slight increase in Rescue cells (Fig. 1 B). In serum-free conditions, binding of Gal-3 stimulates motility, FN fibrillogenesis, and fibrillar adhesions in Mgat5+/+ cells in a concentration-dependent manner (Lagana et al., 2006). Under similar conditions, Gal-3 also stimulates Cav1 Y14 phosphorylation in Mgat5+/+ cells (Fig. 1 C).
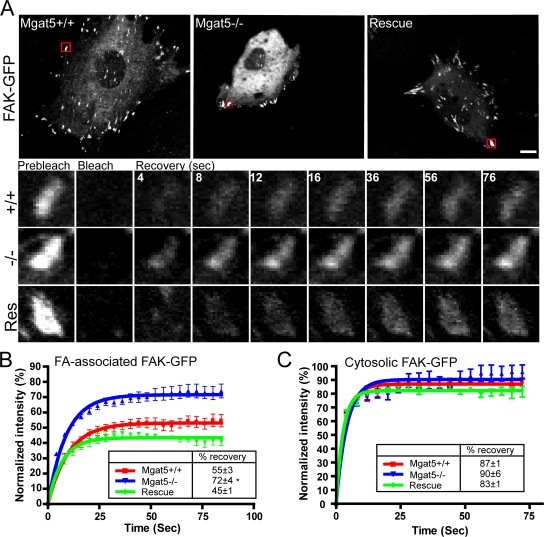
FAK lacking its kinase domain (FRNK) or its Y397 autophosphorylation site shows increased exchange between FAs and cytosol that is associated with reduced FA dynamics and cell migration (Giannone et al., 2004; Hamadi et al., 2005). To determine whether FA defects observed in Mgat5−/− cells were related to altered exchange of FAK between FAs and cytosol, we performed FRAP analysis on FAK-GFP transfected cells (Fig. 2). Due to the molecular and functional heterogeneity of integrin-mediated adhesion sites, we studied only peripheral FAs. Although half-time of recovery of FAK-GFP was not significantly affected between the three cell lines, a significant increase in the mobile fraction (percentage of recovery) was noted in Mgat5−/− cells compared with Mgat5+/+ and Rescue cells (Fig. 2, A and B). To ensure that differences in FAK-GFP mobile fraction in FAs were not due to differences in overall diffusion of FAK-GFP in the three cell lines, we performed FRAP analysis on non-FA associated, cytosolic FAK-GFP. The three cell lines all displayed a quasi-instantaneous recovery of FAK-GFP reaching close to prebleach intensity levels (around 90%), indicative of rapid diffusion of cytosolic FAK-GFP (Fig. 2 C).
Figure 2.
Increased FAK exchange in FAs of Mgat5−/− cells. (A) Mgat5+/+, Mgat5−/−, and Rescue cells transfected with FAK-GFP were imaged before laser bleaching of one FA-containing region of interest (ROI; red square). A time-lapse sequence (in seconds) shows the corresponding ROI before photobleaching (prebleach), immediately after photobleaching (bleach), and during recovery (recovery). Quantification of FAK-GFP fluorescence over time is presented for Mgat5+/+, Mgat5−/−, and Rescue cells in FA (B) or non-FA (C) ROIs. Percentage of recovery (boxes) shows the extent of FAK-GFP mobile fraction. *, P < 0.05. Bar, 20 μm.
We subsequently performed FRAP analysis on cells expressing α5-integrin-GFP and paxillin-GFP (Fig. 3). α5-integrin-GFP is distributed to smaller, less organized FAs in live Mgat5−/− cells, compared with the more elongated fibrillar adhesions present in Mgat5+/+ and Rescue cells (Fig. 3 A), as previously observed for anti-β1-integrin immunofluorescent labeling (Lagana et al., 2006). α5-integrin recovery by FRAP analysis was slower than FAK-GFP and showed a significant increase in the mobile fraction (percentage of recovery) in Mgat5−/− cells compared with Mgat5+/+ and Rescue cells (Fig. 3 B). No significant variation was noted when bleaching was performed on non-FA localized integrin (Fig. 3 C). Similarly, FRAP analysis of paxillin-GFP revealed an increased mobile fraction in Mgat5−/− cells compared with Mgat5+/+ and Rescue cells (Fig. 3 D). Paxillin-GFP recovery also showed significant differences in half-time of recovery that corresponded to the changes in mobile fraction. Interestingly, Rescue cells, which show enhanced FN fibrillogenesis relative to Mgat5+/+ cells (Lagana et al., 2006), also showed significantly increased stability of FA-associated paxillin but not α5-integrin (Fig. 3 B) or FAK (Fig. 2 B).
Figure 3.
Increased exchange of α5-integrin and paxillin in FAs of Mgat5−/− cells. (A) Representative images of live Mgat5+/+, Mgat5−/−, and Rescue cells transfected with α5-integrin-GFP are presented. Quantification of α5-integrin-GFP fluorescence recovery after bleaching in FA (B) and non-FA (C) ROIs over time is presented for Mgat5+/+, Mgat5−/−, and Rescue cells. (D) Quantification of FA-associated paxillin-GFP fluorescence recovery over time is presented for Mgat5+/+, Mgat5−/−, and Rescue cells. The boxes show the percentage of recovery and, for paxillin, the half-time of recovery (*, P < 0.05).
Expression of the Mgat5/galectin lattice and pY14Cav1 regulates FAK exchange in FAs and cell migration
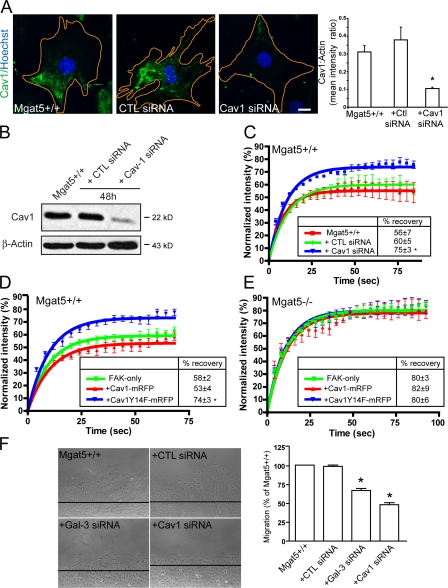
Treatment of Mgat5+/+ cells with β-lactose, a competitive inhibitor of galectin binding, but not control sucrose, or with swainsonine (SW), an α-mannosidase II inhibitor that blocks N-glycan branching, increased the mobile fraction of FAK-GFP compared with untreated Mgat5+/+ cells (Fig. 4, A and B). Expression of the Mgat5/galectin lattice therefore recruits FAK to immobile FA domains. Gal-3 stimulation (1 μg/ml) of Mgat5+/+ promotes integrin activation, F-actin turnover, fibrillar adhesion expression, and FN fibrillogenesis (Lagana et al., 2006). However, addition of Gal-3 did not affect FAK exchange in FAs (Fig. 4 B), suggesting that endogenous expression levels of Gal-3 are sufficient to recruit FAK-GFP to immobile FA domains. FACS analysis showed that lactose, SW, and Gal-3 siRNA specifically reduced cell surface Gal-3 expression (Fig. 4 C). Gal-3 siRNA significantly knocked down endogenous Gal-3 levels in Mgat5+/+ cells after 48 h of transfection, as assessed by both Western blot and immunofluorescence but affected neither Cav1 tyrosine phosphorylation nor Cav1 expression levels (Fig. 4, D and E). Upon Gal-3 knockdown, we observed a significant increase in FAK-GFP mobile fraction (Fig. 4 F), equivalent to that observed in SW- and β-lactose–treated Mgat5+/+ cells (Fig. 4 A,B). Galectin-mediated cross-linking of glycosylated FA components is therefore associated with FAK stability within FA domains.
Figure 4.
Expression of the Mgat5/galectin lattice regulates FAK exchange in FAs. Mgat5+/+ cells were transfected with FAK-GFP and treated with either β-lactose (20 mM) and sucrose (20 mM) (A) or Gal-3 (1 μg/ml) and swainsonine (+SW, 1 μg/ml) (B) and subjected to FRAP analysis of FA localized FAK-GFP. Percent intensity (±SEM) in the bleached zone of FAK-GFP during recovery and quantification of percentage of recovery (box) are shown (±SEM). (C) Mgat5+/+ cells were either treated with 20 mM lactose, 20 mM sucrose, or 1 μg/ml SW, or transfected with Gal-3, control (CTL), or Cav1 siRNA and then surface labeled at 4°C with anti-Gal-3 mAb and analyzed by FACS. The percentage of positive cells is shown (±SEM; n = 4; *, P < 0.01; **, P < 0.001). (D) Mgat5+/+ cells not transfected or transfected for 2 d with either control (CTL) siRNA or Gal-3 siRNA were immunofluorescently labeled with Hoechst (blue) and mouse anti-Gal-3 (green) antibodies. Quantification of Gal-3 mean intensity is shown as a bar graph for untreated (white), CTL siRNA (gray), and Gal-3 siRNA transfected (black) Mgat5+/+ cells (n = 3; ±SEM; *, P < 0.05). (E) Mgat5+/+ cells were not transfected or transfected for 2 d with either control (CTL) siRNA or Gal-3 siRNA, lysed, and subjected to Western blot analysis for Gal-3, pY14Cav1, Cav1, and β-actin expression. (F) Percent intensity (±SEM) in the bleached zone of FAK-GFP during recovery and percent recovery (box) are shown (n = 3; ±SEM; *, P < 0.05).
Cav1 depletion has been shown to reduce FA sites and cellular adhesion (Wei et al., 1999; Grande-Garcia et al., 2007). In Mgat5+/+ cells, transfection of Cav1 siRNA reduced Cav1 expression by >70% as monitored by quantitative immunofluorescence and Western blot (Fig. 5, A and B). FRAP analysis of Cav1 siRNA-transfected Mgat5+/+ cells revealed that Cav1 depletion significantly increased the mobile fraction of FAK-GFP compared with CTL siRNA transfected and nontransfected cells (Fig. 5 C). Similarly, cotransfection of Mgat5+/+ cells with FAK-GFP and Cav1Y14F-mRFP significantly increased the mobile fraction of FAK-GFP compared with cells cotransfected with FAK-GFP and Cav1-mRFP (Fig. 5 D). This suggests that Cav1Y14F-mRFP acts as a dominant-negative and implicates Cav1 Y14 phosphorylation in the regulation of FAK-GFP exchange in FAs of Mgat5+/+ cells.
Figure 5.
Cav1 tyrosine 14 regulates FAK exchange. (A) Mgat5+/+ cells were not transfected or transfected for 2 d with either control (CTL) siRNA or Cav1 siRNA and labeled with Hoechst and anti-Cav1 antibodies. Quantification of Cav1 mean intensity relative to Alexa568-phalloidin labeled F-actin (not depicted) is shown as a bar graph (n = 3; ±SEM; *, P < 0.05). (B) Cell lysates from untransfected and CTL and Cav1 siRNA transfected Mgat5+/+ cells were subjected to Western blot analysis for Cav1 and β-actin. (C) Percent intensity (±SEM) in the bleached zone of FAK-GFP during recovery and quantification of percentage of recovery (box) are shown for Mgat5+/+ cells transfected with FAK-GFP alone or with FAK-GFP and either CTL or Cav1 siRNA transfected Mgat5+/+ cells (n = 3, ±SEM; *, P < 0.05). Mgat5+/+ (D) and Mgat5−/− (E) cells were cotransfected with FAK-GFP and either Cav1-mRFP or Cav1Y14F-mRFP and subjected to FRAP analysis of FAK-GFP. Percent intensity (±SEM) in the bleached zone of FAK-GFP during recovery and quantification of percentage of recovery (box) are shown (±SEM; *, P < 0.05). (F) Migration of untransfected Mgat5+/+ and control, Gal-3, and Cav1 siRNA transfected Mgat5+/+ cells was determined over a 24-h period in serum-free medium on an FN substrate (10 μg/ml) using a wound-healing assay (n = 3; ±SEM; *, P < 0.05).
The extent of FAK-GFP recovery in Mgat5+/+ cells transfected with either Cav1 siRNA or Cav1Y14F was similar to that observed in Mgat5−/− cells (Fig. 2 B). This suggested that Cav1 depletion may be responsible for the defects in FAK dynamics observed in Mgat5−/− cells. However, cotransfection with either Cav1-mRFP or Cav1Y14F-mRFP did not affect the FAK-GFP mobile fraction in Mgat5−/− cells. In Mgat5−/− cells, β1-integrin is poorly localized to FAs and distributed over the plasma membrane (Lagana et al., 2006). Overexpression of Cav1 did not impact on β1-integrin distribution in these cells suggesting that Cav1 deficiency is not solely responsible for the loss of FA organization in Mgat5−/− cells (Fig. S1, available at http://www.jcb.org/cgi/content/full/jcb.200709019/DC1). In support of the essential role of both Gal-3 and Cav1 in FA turnover, knockdown of either protein by siRNA was associated with reduced migration of Mgat5+/+ cells (Fig. 5 F).
Reduced FAK exchange requires expression of both the Mgat5/Gal-3 lattice and pY14Cav1
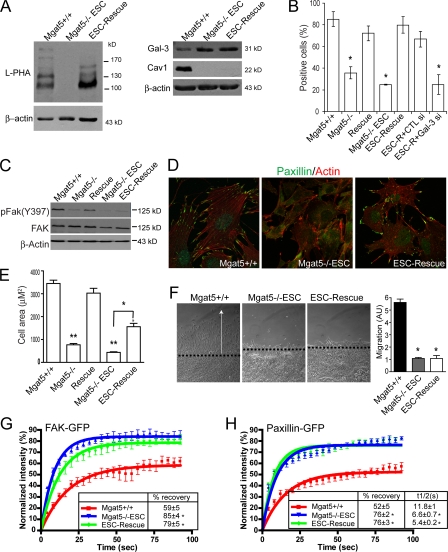
Cell lines established from larger PyMT Mgat5−/− tumors that escape the constraints imposed by Mgat5 deficiency, called Mgat5−/−ESC, present reduced Cav1 expression and restored EGF signaling. Stable infection of Mgat5−/−ESC cells with an Mgat5 retroviral expression vector generated the ESC-Rescue cell line, that presents restored β1-6-branched N-glycan expression but not Cav1 expression, indicative of disruption of Cav1 gene expression in Mgat5−/−ESC cells (Lajoie et al., 2007). These cells express L-PHA reactive β1-6 branched glycoproteins, maintain cellular expression of Gal-3 and, importantly, show restored cell surface Gal-3 expression relative to Mgat5−/−ESC cells (Fig. 6, A and B), thereby providing a model for the study of the role of the Mgat5/galectin lattice independently of Cav1 expression.
Figure 6.
Regulation of FA exchange requires both the Mgat5/Gal-3 lattice and Cav1. (A) Equal protein amounts of cell lysates from Mgat5+/+, Mgat5−/−ESC, and ESC-Rescue cells were blotted with L-PHA-HRP or with antibodies to Gal-3, Cav1, and β-actin and HRP-conjugated secondary antibodies. (B) Mgat5+/+, Mgat5−/−, Rescue, Mgat5−/−ESC, and ESC-Rescue cells as well as ESC-Rescue (ESC-R) cells transfected with control (CTL) or Gal-3 siRNA were surface labeled at 4°C with anti-Gal-3 mAb and analyzed by FACS. The percentage of positive cells is shown (±SEM; n = 4; *, P < 0.001). (C) Cell lysates of Mgat5+/+, Mgat5−/−, Rescue, Mgat5−/−ESC, and ESC-Rescue cells were probed by Western blotting for pFAK(Y397), FAK, and β-actin and HRP-conjugated secondary antibodies. (D) Mgat5+/+, Mgat5−/−ESC, and ESC-Rescue cells were grown in serum-free conditions on fibronectin-coated coverslips (10 μg/ml) for 48 h and stained with Alexa568-Phalloidin (red) and antibodies to paxillin (green). (E) Mgat5+/+, Mgat5−/−, Rescue, Mgat5−/−ESC, and ESC-Rescue cells were grown in serum-free conditions on fibronectin-coated coverslips (10 μg/ml) for 48 h, labeled with Alexa568-Phalloidin, and cell area determined (±SEM; n = 3; *, P < 0.05; **, P < 0.005). (F) Migration of Mgat5+/+ (black), Mgat5−/−ESC (gray), and ESC-Rescue cells (white) was determined over a 24-h period in serum-free medium on an FN substrate (10 μg/ml) using a wound-healing assay (±SEM; *, P < 0.01). FRAP analysis was performed on Mgat5+/+, Mgat5−/−ESC, and ESC-Rescue cells transfected with FAK-GFP (G) or paxillin-GFP (H). Percentage of recovery (box) shows the extent of the FAK-GFP (G) and paxillin-GFP (H) mobile fraction. Half-time for recovery of fluorescence toward the asymptote of paxillin-GFP is also presented (Box) (H) (*, P < 0.05).
As in Mgat5−/− cells, Mgat5−/−ESC cells show a reduction of pFAK expression levels that remain low in ESC-Rescue cells relative to Mgat5+/+ or Rescue cells (Fig. 6 C). In serum-free conditions, ESC-Rescue cells present denser peripheral FAs than Mgat5−/−ESC cells and also show increased cell spreading (Fig. 6, D and E; Fig. S3, available at http://www.jcb.org/cgi/content/full/jcb.200709019/DC1). In contrast to the restored motility of Cav1-expressing Rescue cells (Granovsky et al., 2000; Lagana et al., 2006), both Mgat5−/−ESC and ESC-Rescue cells present a dramatic inhibition of motile ability (Fig. 6 F). FRAP analysis of FAK-GFP and paxillin-GFP in FAs revealed that Mgat5−/−ESC and ESC-Rescue cells exhibited a similar elevated mobile fraction for these two FA markers relative to Mgat5+/+ cells (Fig. 6, G and H). Half-time of recovery of paxillin-GFP in Mgat5−/−ESC and ESC-Rescue cells was also significantly faster than in Mgat5+/+ cells (Fig. 6 H).
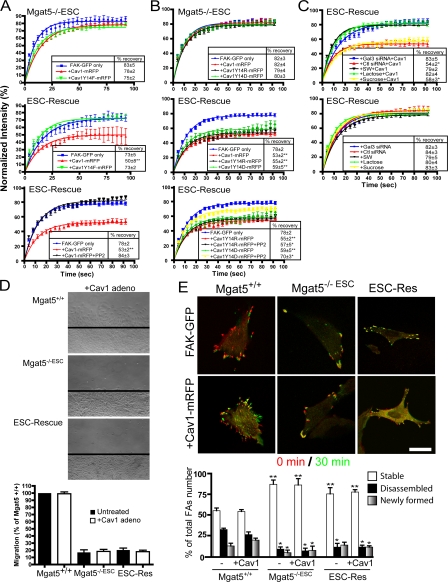
We then cotransfected Mgat5−/−ESC and ESC-Rescue cells with FAK-GFP and either Cav1-mRFP or Cav1Y14F-mRFP and performed FRAP analysis of FAK-GFP. Cav1-mRFP, but not Cav1Y14F-mRFP, stabilized FAK in FAs of ESC-Rescue cells (Fig. 7 A). As for Mgat5−/− cells (Fig. 5 E), neither Cav1-mRFP nor Cav1Y14F-mRFP affected FAK exchange in FAs of Mgat5−/−ESC cells (Fig. 7 A). To assess whether Src-dependent phosphorylation of Cav1 was required for stabilization of FAK exchange in FAs, ESC-Rescue cells cotransfected with FAK-GFP and Cav1-mRFP were treated with PP2, a well-characterized inhibitor of Src family kinases. PP2 completely prevented Cav1-mRFP stabilization of FAK-GFP exchange in ESC-Rescue cells (Fig. 7 A). PP2 did not affect FAK-GFP exchange in ESC-Rescue cells transfected with FAK-GFP alone (Fig. S2, available at http://www.jcb.org/cgi/content/full/jcb.200709019/DC1). To determine whether tyrosine phosphorylation of Cav1 is required for Cav1 regulation of FAK exchange in FAs, we introduced a phosphomimetic glutamate (D) residue or a positively charged arginine (R) residue in place of Y14 on Cav1. Expression of Cav1Y14D-mRFP or Cav1Y14R-mRFP did not affect FAK exchange in Mgat5−/−ESC cells, but both stabilized FAK-GFP in FAs of ESC-Rescue cells to a similar extent as Cav1-mRFP (Fig. 7 B). PP2 only partially enhanced FAK-GFP stabilization in Cav1Y14D-mRFP transfected cells and did not affect FAK-GFP stabilization in Cav1Y14R-mRFP transfected cells (Fig. 7 B). The inability of Src inhibition to completely prevent Cav1Y14D-mRFP or Cav1Y14R-mRFP stabilization of FAK-GFP in FAs is indicative of a role for Src-dependent phosphorylation of Cav1Y14 in FA turnover.
Figure 7.
pY14Cav1 regulates FAK exchange in Mgat5-expressing cells. FRAP analysis was performed on Mgat5−/−ESC and ESC-Rescue cells transfected with FAK-GFP and either Cav1-mRFP or Cav1Y14F-mRFP (A) or with Cav1-mRFP, Cav1Y14R-mRFP, or Cav1Y14D-mRFP (B) and treated (or not) for 1 h with PP2, as indicated. (C) ESC-Rescue cells were either treated with 20 mM lactose, 20 mM sucrose, or 1 μg/ml SW, or transfected with Gal-3, control (CTL), or Cav1 siRNA for 48 h and then transfected with FAK-GFP (top) or cotransfected with FAK-GFP and Cav1-mRFP (bottom). FRAP analysis of FAK-GFP in FAs was then performed. Percent intensity (±SEM) in the bleached zone of FAK-GFP during recovery and quantification of percentage of recovery (box) are shown (n = 3; ± SEM; *, P < 0.05; **, P < 0.01). (D) Migration of Mgat5+/+, Mgat5−/−ESC, and ESC-Rescue cells untreated (black bars) or infected with a Cav1-expressing adenovirus (white bars) was determined over a 24-h period in serum-free medium on an FN substrate (10 μg/ml) using a wound-healing assay. Images show migration of Cav1 adenovirus-infected cells. (E) Mgat5+/+, Mgat5−/−ESC, and ESC-Rescue cells were transfected with FAK-GFP alone (left) or FAK-GFP and Cav1-mRFP (right) and FAK-GFP imaged every 30 s for 30 min (see Videos 1–3, available at http://www.jcb.org/cgi/content/full/jcb.200709019/DC1). Overlay of FAK-GFP images at time 0 (red) and 30 min (green) shows disassembled (red), stable (yellow), and newly formed (green) FAs.
To verify that pY14Cav1 regulation of FAK stabilization in FAs in ESC-Rescue cells was indeed due to the presence of the galectin lattice, we disrupted the galectin lattice with lactose competition of galectin binding, SW inhibition of N-glycan terminal processing, and Gal-3 siRNA (Fig. 7 C). These treatments did not affect FAK recovery in FAs of ESC-Rescue cells; however, lactose, SW, and Gal-3 siRNA, which disrupt cell surface Gal-3 binding (Figs. 4 C and 6 B) but not sucrose or control siRNA, all prevented Cav1-dependent stabilization of FAK in FAs. However, Cav1 expression did not promote cell migration in Mgat5−/−ESC or ESC-Rescue cells (Fig. 7 D). Furthermore, relative to Mgat5+/+ cells, Mgat5−/−ESC and ESC-Rescue cells showed increased stability and reduced turnover of FAs in 30-min time-lapse videos, even upon overexpression of Cav1 (Fig. 7 E; Videos 1–6, available at http://www.jcb.org/cgi/content/full/jcb.200709019/DC1). The Mgat5/galectin lattice and pY14Cav1 therefore work in concert to regulate FAK stabilization in FAs, but their coordinate action is not sufficient to promote FA disassembly and turnover.
pY14Cav1 regulates FA turnover in MDA-435 breast carcinoma cells
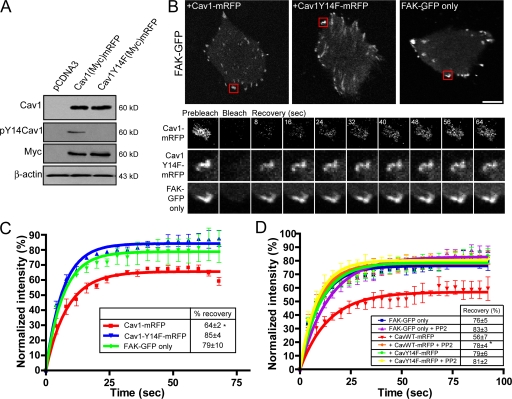
MDA-435 human breast cancer cells express low levels of Cav1 and few caveolae (Kojic et al., 2007). Wild-type myc-tagged Cav1-mRFP, but not mutant myc-tagged Cav1Y14F-mRFP, transfected in MDA-435 cells was tyrosine phosphorylated (Fig. 8 A). In FAK-GFP transfected MDA-435 cells, the mobile fraction of FA localized FAK-GFP was high (Fig. 8, B and C). Cotransfection of MDA-435 with FAK-GFP and Cav1-mRFP, but not Cav1Y14F-mRFP, significantly decreased the mobile fraction of FAK within FAs (Fig. 8, B and C). PP2 treatment of MDA-435 cells transfected with Cav1-mRFP, but not of untransfected cells or Cav1Y14F-mRFP transfected cells, significantly increased FAK-GFP mobile fraction levels (Fig. 8 D). Tyrosine-phosphorylated Cav1 therefore mediates FAK stabilization in FAs in MDA-435 cells.
Figure 8.
pY14Cav1 regulates FAK exchange in MDA-435 human breast cancer cells. (A) MDA-435 cells transfected with vector control (pcDNA), myc-tagged Cav1-mRFP, or Cav1Y14F-mRFP were Western blotted with antibodies to Cav1, pY14Cav1, the myc epitope tag, and β-actin. (B) FRAP analysis of FAK-GFP was performed in MDA-435 cells transfected with FAK-GFP (FAK-GFP only) or cotransfected with FAK-GFP and either Cav1-mRFP or Cav1Y14F-mRFP. A time-lapse sequence (in seconds) shows the corresponding ROI (boxed in red) before photobleaching (prebleach), immediately after photobleaching (bleach), and during recovery (recovery). (C) For MDA-435 cells transfected with FAK-GFP (FAK-GFP only) or cotransfected with FAK-GFP and either Cav1-mRFP or Cav1Y14F-mRFP, percent intensity (±SEM) in the bleached zone of FAK-GFP during recovery and percentage of recovery (box) are shown (n = 3; ±SEM). (D) MDA-435 cells were cotransfected with FAK-GFP and either Cav1-mRFP or CavY14F-mRFP, treated 1 h with PP2, and subjected to FRAP analysis of FAK-GFP. Percent intensity (±SEM) in the bleached zone of FAK-GFP during recovery and quantification of percentage of recovery (box) are shown (n = 3; ±SEM; *, P < 0.05). Bar, 20 μm.
To determine whether the extent of FAK exchange between FA and cytosolic pools, as measured by FRAP analysis, impacts on FA disassembly and formation, we tracked FAs in MDA-435 cells transfected with FAK-GFP alone or cotransfected with FAK-GFP and either Cav1-mRFP or Cav1Y14F-mRFP (Fig. 9 A). Time-lapse images were acquired every 30 s for half an hour. In Cav1-mRFP transfected cells, multiple FAs underwent disassembly in retractile areas of the cell (Fig. 9 B, red arrows; Video 7, available at http://www.jcb.org/cgi/content/full/jcb.200709019/DC1) and protrusive areas contained newly formed FAs (Fig. 9 B, green arrows; Video 4). In contrast, nontransfected and Cav1Y14F-mRFP transfected cells exhibit reduced retractile areas and only a few disassembled FAs (Fig. 9 B), corresponding to the limited displacement of the cells (Videos 8 and 9). Quantification revealed that the percentage of disassembled and newly formed FAs over 30 min is significantly higher in cells transfected with Cav1-mRFP compared with the mostly stable FAs of nontransfected and Cav1Y14F-mRFP transfected cells (Fig. 9 C).
Figure 9.
pY14Cav1 regulates FA turnover and cellular displacement in MDA-435 human breast cancer cells. (A) MDA-435 cells were transfected with FAK-GFP alone or FAK-GFP with either Cav1-mRFP or CavY14F-mRFP and FAK-GFP imaged every 30 s for 30 min. Representative images at time 0, 6, 12, 18, 24, and 30 min are shown (see Videos 1–3, available at http://www.jcb.org/cgi/content/full/jcb.200709019/DC1). (B) Overlay of FAK-GFP images at time 0 (red) and 30 min (green) shows FA displacement over time (top) and cell outlines show cellular displacement over time (bottom). Protrusive regions are indicated by green arrows and retractile areas by red arrows. (C) Stable, disassembled, and newly formed FAs were quantified as a percentage of total FAs during the 30-min interval of acquisition. (D) Retractile and protrusive cell areas over the 30-min interval of acquisition and total cell area at 30 min were normalized to cell area at time 0 and displayed as a percentage (mean ± SEM; n = 4; *, P < 0.05; **, P < 0.01 relative to FAK-GFP only). Bar, 20 μm.
Increased FA turnover in Cav1-mRFP transfected cells corresponded to increased cellular retraction and protrusion (Fig. 9, B and D). Although cell area did not significantly change over the 30-min time period, MDA-435 cells transfected with Cav1-mRFP presented an approximate 3-fold increase in new protrusive areas and a 2.5-fold increase in retractile areas relative to untransfected and CavY14F-mRFP transfected cells (Fig. 9 D). Together, those observations suggest that the increased FA disassembly and formation observed in Cav1-mRFP transfected MDA-435 cells are associated with increased tumor cell motility.
Discussion
Tyrosine-phosphorylated Cav1 regulates FA dynamics
Gal-3 binding to Mgat5-modified N-glycans induces FN fibrillogenesis and cell motility via activation of α5β1-integrin (Lagana et al., 2006). Here, we show that the Mgat5/galectin lattice regulates FA dynamics and that this activity is dependent on expression of tyrosine-phosphorylated Cav1. Gal-3 and pY14Cav1 act in concert to increase the immobile fraction of FAK, paxillin, and α5-integrin in FAs, consistent with the formation of a stable membrane domain within FAs (Gaus et al., 2006). Gal-3–mediated integrin activation therefore acts through pY14Cav1 to induce FA membrane organization that restricts exchange of FAK and other FA components and enables FA turnover.
FAKY397 phosphorylation is a downstream response to integrin clustering and activation (Mitra and Schlaepfer, 2006) and is essential for FA assembly and disassembly (Giannone et al., 2004; Webb et al., 2004; Hamadi et al., 2005; Owen et al., 2007). It represents a high-affinity binding site for the SH2 domain of Src, leading to formation of a transient FAK-Src signaling complex that further phosphorylates FAK (Y576, Y577, Y861, Y925) recruiting adaptor proteins such as p130Cas, paxillin, and Crk promoting FA dynamics and tumor cell motility (Mitra and Schlaepfer, 2006). pY14Cav1 has been shown to recruit Csk (Cao et al., 2002; Radel and Rizzo, 2005), inhibiting Src activity and leading to a p190RhoGAP-dependent increase in RhoGTP levels (Grande-Garcia et al., 2007). Cav1−/− MEFs present fewer newly formed adhesions in cellular protrusions as well as defects in polarized spreading and reduced mobility (Grande-Garcia et al., 2007). We report that pY14Cav1 promotes the FA stabilization that is required for substrate adherence of cellular protrusions and directional motility. Indeed, Cav1, but not Cav1Y14F, promotes both FA turnover and protrusive activity in MDA-435 tumor cells, implicating pY14Cav1 expression in tumor cell migration.
Cav1 is a well-recognized scaffolding protein that interacts with Shp2 and other phosphatases including PTP-1B (Caselli et al., 2001, 2002; Lee et al., 2006). Similarly to the pY14Cav1-dependent FA turnover that we describe here, absence of Shp2 phosphatase activity results in a spreading defect due to hyperphosphorylation of FAK and decreased adhesion site dynamics (von Wichert et al., 2003). PTP-1B interacts closely with pY14Cav1, and promotes integrin-mediated responses by activating Src kinase, thereby contributing to maturation of cell-matrix adhesions (Liang et al., 2005; Hernandez et al., 2006; Lee et al., 2006). By sequestering Csk and recruiting Shp2 and PTP-1B, pY14Cav1 recruitment to FAs may promote the Rho GTPase activation and FA maturation that is essential for cell spreading and motility.
Using the fluorescent probe Laurdan that labels ordered membrane domains, the FA membrane has been shown to be highly ordered and in fact better ordered than caveolae and cholesterol-dependent raft domains (Gaus et al., 2006). FA membrane order was reduced in Cav1−/− MEFs and more efficiently restored in cells transfected with wild-type Cav1 than in cells transfected with Cav1Y14F. Together with the reduced sensitivity to Src inhibition of FAK stabilization in FAs by Cav1Y14D compared with wild-type Cav1, this indicates that pY14Cav1 recruitment to FAs regulates the order and structure of these cellular domains. The ability of Cav1Y14R, in which a positively charged arginine residue replaces the negatively charged phosphotyrosine, to functionally mimic pY14Cav1 in a Src-independent manner suggests that conformational changes due to Y14 phosphorylation may be involved in pY14Cav1 function. The demonstration here that pY14Cav1 reduces the availability of FAK within FAs for dynamic exchange is consistent with its ability to promote membrane order within FAs (Gaus et al., 2006). It further argues that regulation of membrane order within FAs by pY14Cav1 is a determinant of the dynamics and stabilization of FA components, including FAK.
Interdependence of the galectin lattice and pY14Cav1 on FA dynamics in tumor cells
Expression of the Mgat5/Gal-3 lattice is required for pY14Cav1 to impact on FA dynamics. Gal-3 forms pentamers, shows close molecular interaction on the cell surface (Ahmad et al., 2004; Nieminen et al., 2007), and exhibits affinity for N-glycans in proportion to GlcNAc branching (Hirabayashi et al., 2002). Recruitment to the galectin lattice regulates receptor signaling (Demetriou et al., 2001; Partridge et al., 2004). Gal-3–mediated clustering of Mgat5-modified N-glycans stimulates integrin activation and phosphorylation of FAKY397 (Lagana et al., 2006). Disruption of the Mgat5/Gal-3 lattice by siRNA knockdown of Mgat5 attenuated EGF-induced FAK dephosphorylation and activation of Shp2 and inhibited tumor cell motility and invasiveness (Guo et al., 2007). Interestingly, cell surface clustering of GalT, which exhibits a similar galactose specificity to Gal-3, has also been shown to promote FAKY397 phosphorylation and loss of FAs (Wassler and Shur, 2000). FRAP analysis shows here that it also plays a critical role in regulating FAK molecular dynamics.
Lateral association of integrins into clusters generates FA precursors and is a result of a combination of various extracellular and intracellular stimuli including ligand binding, integrin activation, and actin polymerization (DeMali et al., 2003; Ginsberg et al., 2005). Introduction of an N-glycosylation site at the interface of the I-like and the hybrid domain of β3 integrin forces the integrin to adopt an extended conformation that results in integrin activation and clustering and slows integrin exchange (Cluzel et al., 2005). Expression of the Mgat5/Gal-3 lattice slows α5-integrin exchange (Fig. 3) and promotes integrin organization into focal and fibrillar adhesions (Lagana et al., 2006). Gal-3–dependent integrin activation was enhanced by addition of RGD ligand, suggesting that integrin clustering promotes but may not be sufficient for activation (Lagana et al., 2006). Consistent with a role for Gal-3 in integrin clustering, Mgat5 rescue of Mgat5−/−ESC cells induced FA formation and cell spreading in the absence of serum but not FAK stabilization in FAs. Indeed, clustering is not sufficient for integrin αIIbβ3-mediated signaling, and also requires integrin conformational changes that involve separation of integrin transmembrane domains (Zhu et al., 2007).
Regulation of FA dynamics by the galectin lattice is also dependent on expression of pY14Cav1. Interdependence of FA turnover on both extracellular Gal-3 and intracellular pY14Cav1 is an elegant example of the outside-in signaling that regulates integrin activation and cell adhesion (Luo et al., 2007). Cav1 binds directly to integrin, recruits Src-kinase, regulates FAK phosphorylation, and couples integrin activation to Ras-ERK signaling (Wary et al., 1998; Chapman et al., 1999; Wei et al., 1999). Cav1 phosphorylation is a consequence of integrin-mediated mechanotransduction after shear stress is applied to endothelial cells (Radel and Rizzo, 2005) and is lost upon integrin dissociation from ligand (del Pozo et al., 2005). Both Gal-3 and pY14Cav1 are therefore required, but not necessarily sufficient, for activation of Src kinases resulting in the stabilization of FA components and promotion of FA signaling, disassembly, and translocation.
Our data supports a model in which Gal-3 binding promotes integrin clustering and formation of focal contacts (Fig. 10). Subsequent recruitment of pY14Cav1 will result in the formation of a stabilized domain within the FA that promotes the stabilized recruitment of FAK, paxillin, and other effectors of FA turnover. This domain is proposed to be equivalent to the ordered lipid domains in FAs previously described to be pY14Cav1 dependent (Gaus et al., 2006). Together with the established interaction of pY14Cav1 with integrins (Wary et al., 1998; Wei et al., 1999; Mettouchi et al., 2001; del Pozo et al., 2005; Radel and Rizzo, 2005), these data conclusively describe a role for pY14Cav1 in FA domain organization. However, it is important to recognize that evidence for a stable association of pY14Cav1 within FAs is based on the use of a single monoclonal antibody that crossreacts with phospho-paxillin (Hill et al., 2007). These data do not exclude a localization of pY14Cav1 to FAs, but do suggest that the exclusive FA distribution of pY14Cav1 based on antibody labeling may be exaggerated. In addition, the role for pY14Cav1 as an inhibitor of Src leading to Rho activation (Grande-Garcia et al., 2007) contrasts the critical role of Src kinase activity in phosphorylation of Cav1 and other FA components, such as FAK, leading to FA disassembly. As such, the formation of a stable pY14Cav1 containing domain within the FA, as proposed in Fig. 10, remains hypothetical and pY14Cav1-dependent regulation of FA domains may invoke the temporal and spatial recruitment of pY14Cav1 to FAs and mechanistic details that remain to be defined.
Figure 10.
Gal-3 and Cav1 work in concert to generate intra-FA domains. (A) In Mgat5−/− cells lacking the Mgat5/galectin lattice, FAs are deficient and FAK is mostly cytosolic. (B) Gal-3-dependent integrin clustering promotes integrin activation as well as ligand-induced integrin activation (Lagana et al., 2006). Under serum-free conditions, expression of the Mgat5/galectin lattice alone can induce the formation of FAs and cell spreading (i.e., ESC-Rescue cells) but not FAK stabilization in FA domains. (C) Expression of pY14Cav1 results in the formation of an ordered membrane domain (Gaus et al., 2006) that results in the stabilization within the FA of integrin, FAK, and paxillin as well as, potentially, other FA components. Src-dependent FAK phosphorylation and its stable association with FAs lead to focal disassembly and turnover (Hamadi et al., 2005) and pY14Cav1 promotes FA disassembly such as observed in Cav1 transfected MDA-435 cells (Fig. 9). However, Gal-3/pY14Cav1-mediated stabilization of FAK in FAs is not sufficient to induce FA disassembly (Fig. 7). How domain organization occurs within the FA remains speculative and the concentration of integrin and FAK within an intra-FA liquid ordered domain is hypothetical. Indeed, the temporal and spatial nature of pY14Cav1 association with FAs remains to be determined.
FA translocation and fibronectin remodeling are complex multistep processes (Geiger et al., 2001). Both Gal-3 and Cav1 are multifunctional proteins with a multitude of binding proteins. Gal-3 stimulation of pY14Cav1 may not be limited to FAs, and it is also possible that Gal-3 and pY14Cav1 play multiple roles in FA turnover and maturation. Indeed, Cav1 and raft membrane domains have been shown to be involved in FN reorganization (Hocking and Kowalski, 2002; Sottile and Chandler, 2005).
The galectin lattice, Cav1, and tumor progression
Crosstalk between integrins and growth factor receptors regulates epithelial–mesenchymal transition, angiogenesis, survival, and tumor cell invasion by disrupting cell–cell adhesion, inducing FA maturation and disassembly, and enabling matrix remodelling (Guo and Giancotti, 2004). Affinity for the galectin lattice will vary for different proteins depending on the number of N-glycan chains (Lau et al., 2007), and the lattice is likely a highly heterogeneous domain composed of multiple and varied glycoproteins. It is therefore tempting to speculate that recruitment to the galectin lattice promotes interaction between growth factor receptors and integrins.
Our results show that extracellular cross-linking of glycoproteins by the Mgat5-dependent galectin lattice and intracellular Cav1 binding to FA proteins via the pY14 motif cooperate to slow the exchange of FAK, paxillin, and α5-integrin in FAs. Increasing the stability of these proteins in FA domains promotes FA maturation, due to increased FA signaling intensity as well as phospho-peptide turnover rates. Shallow external gradients of growth factors can amplify internal gradients of PtdIns(3,4,5)P3 at the leading edge due to positive feedback through PI3K and Rac/Cdc42, resulting in membrane ruffling and persistent cell protrusion and motility (Wang et al., 2002). Mgat5 opposes PTEN phosphatase to regulate cellular sensitivity to extracellular cues and cell polarity (Cheung and Dennis, 2007). Coordinate recruitment via the galectin lattice of cytokine receptors, such as EGFR, and integrins to nascent FAs at the leading edge may selectively promote the FA domain stabilization and signaling enhanced by pY14Cav1 that is associated with dynamic adhesion turnover and directional cell migration.
Interestingly, recruitment of EGFR to oligomerized Cav1 microdomains, via the scaffolding domain and not the pY14 motif, inhibits lateral mobility of EGFR and EGF-dependent activation in lattice-deficient Mgat5−/− cells. Conversely, restricting EGFR diffusion by recruitment to the galectin lattice limits interaction with negative regulatory Cav1 microdomains, promoting EGFR signaling and tumor growth (Lajoie et al., 2007). This suggests that Cav1 is a conditional tumor suppressor, acting to restrict cytokine growth signaling only in the absence of Mgat5 and the galectin lattice. However, as shown here, and consistent with the poor prognosis associated with elevated Cav1 expression in a number of tumor types (Yang et al., 1999; Suzuoki et al., 2002; Savage et al., 2007), coexpression of the Mgat5/galectin lattice and Cav1 in tumor cells will not only result in domain competition to limit negative regulation of cytokine receptor signaling by Cav1, but also result in their concerted action via pY14Cav1 to promote FA turnover and tumor cell migration.
Materials and methods
Antibodies and reagents
Bovine plasmatic FN, bovine serum albumin solution (BSA, 30%), swainsonine, β-lactose, mouse anti-talin, and mouse anti-β-actin antibodies were purchased from Sigma-Aldrich. L-PHA-HRP was purchased from EY Laboratories. Rabbit anti-Cav1, mouse anti-myc, and mouse anti-Gal-3 antibodies were purchased from Santa Cruz Biotechnology, Inc.; mouse anti-pY14Cav1 antibodies from Transduction Laboratories; rat anti–mouse β1-integrin (MAB1997) antibody from Chemicon; rabbit anti-FAK-P397 and anti-FAK antibodies from Invitrogen; mouse anti-paxillin antibodies from EMD; and mouse anti-ILK antibodies from Millipore. Monoclonal rat anti-Gal-3 antibody (TIB166) was purchased from the American Type Culture Collection (ATCC). HRP-conjugated rat, mouse, and rabbit secondary antibodies were purchased from Jackson ImmunoResearch Laboratories. Phalloidin and secondary antibodies conjugated to Alexa 488, 568, or 647 were purchased from Invitrogen. Human recombinant Gal-3 was produced as previously described (Gong et al., 1999).
Cell culture
Mgat5+/+ (Mgat5+/+(2.6)), Mgat5−/− (Mgat5−/−(22.9)), and Mgat5−/−ESC (Mgat5−/−(22.10)) murine mammary tumor cells were obtained from PyMT mammary tumors (Granovsky et al., 2000). Mgat5−/− and Mgat5−/−ESC cells were genetically rescued by infection with a pMX-PIE retroviral vector for expression of murine Mgat5 selected by growth in medium containing 1 μg/ml puromycin (Partridge et al., 2004) and designated Rescue and ESC-Rescue, respectively. PyMT murine mammary epithelial cells were grown in DMEM and MDA-435 human breast carcinoma cells (ATCC) in RPMI supplemented with nonessential amino acids, vitamins, glutamine, penicillin-streptomycin (Invitrogen), and 10% fetal bovine serum (Immunocorp) at 37°C in a humidified 5% CO2/95% air incubator. Where indicated, cells grown under serum-free conditions were plated for 24 h in complete medium and then rinsed and grown for 24 h in serum-free media.
Adenoviral infection, constructs, and siRNA transfection
Human Cav1-myc-mRFP was cloned into mammalian expressing pRFP-N1 placing under CMV promoter. To prepare phosphomimetic Cav1Y14D-mRFP, Cav1Y14D cDNA was PCR amplified from GFP-Cav1Y14D (provided by Rich Minshall, University of Illinois at Chicago, Chicago, IL) using Cav1-BamHI-Rev (5′ GGG GAT CCC TAT TTC TTT CTG CA AGT TGA TGC GGA C 3′), Cav1-HindIII-For (5′ GGA AGC TTA GCA TGT CTG GGG GCA AAT AC 3′) primers. Amplified product and pRFP-N1 vector were restriction digested with HindIII–BamHI, gel purified, cloned, and verified by sequencing. Tyrosine (Y)14 in human Cav1-mRFP was mutated to either phenylalanine (F) or arginine (R) by PCR-based overlapping extension technique with the altered codon indicated in bold (Forward primers: 5′ G GGA ATT CTA GCA TGT CTG GGG GCA AAT ACG TAG ACT CGG AGG GAC ATC TCT TCA ML 3′, 5′ AAG CTT AGC ATG TCT GGG GGC AAA TAC GTA GAC TCG GAG GGA CAT CTC CGC ACC 3′; Reverse primer: 5′ GGG ATC CCC AGA TCC TCT TCT GAG ATG AG 3′).
Transient transfections were performed using Effectene (QIAGEN). Cells infected for 48 h with adenovirus expressing myc-tagged Cav1 under the control of the tetracycline-regulated promoter were visualized with anti-myc antibodies (Santa Cruz Biotechnology, Inc.).
To knockdown Cav1, Mgat5+/+ cells were cultured in complete medium for 24 h before the transfection with specific mouse Cav1 siRNA or control siRNA using Dharmafect 3 transfection reagent (Dharmacon). For the Gal-3 knockdown, we used the previously described siRNA oligonucleotide sequences and protocol (Henderson et al., 2006). In brief, Mgat5+/+ cells were transfected with the specific pool of four mouse Gal-3 siRNA oligonucleotides (custom synthesized) or control siRNA (siCONTROL nontargeting siRNA no. 1; Dharmacon) using Lipofectamine 2000 transfection reagent following the protocol described by the supplier (Invitrogen).
Immunofluorescence labeling and FACS analysis
Cells plated on glass coverslips coated with 10 μg/ml of FN were fixed with 3% paraformaldehyde and permeabilized with 0.1% Triton X-100 before washing and incubation with phalloidin and primary and fluorescent secondary antibodies (Lagana et al., 2006). After labeling, coverslips were mounted in CelVol (Celanese, Ltd.) and imaged with the 60 or 100x planApochromat objectives (NA 1.35) of a confocal microscope (FV1000; Olympus). Cell surface Gal-3 expression was determined by FACS analysis of surface-labeled cells at 4°C with the TIB-466 anti-Gal-3 mAb (ATCC). Dead cells were excluded using propidium iodide and flow cytometry measurements of at least 50,000 viable cells were performed using CellQuest software on a FACSCalibur (BD Biosciences).
Western blotting
Cell pellets from 80% confluent cultures were washed with cold PBS and resuspended in lysis buffer (20 mM Tris-HCl, pH 7.6, 0.5% NP-40, 250 mM NaCl, 3 mM EDTA, 3 mM EGTA containing freshly added 2 mM DTT, 0.5 mM PMSF, 1 mM sodium vanadate, 2.5 mM sodium fluoride, and 1 μM leupeptin) for 30 min at 4°C, pelleted at 13,000 rpm at 4°C, and the supernatant collected and stored at −80°C. Equal amounts of proteins were separated on 12% SDS-PAGE, electroblotted onto nitrocellulose, probed with L-PHA-HRP or the indicated antibodies and HRP-conjugated secondary antibodies, and revealed by ECL.
FRAP measurements
FRAP was performed on a confocal microscope (FV1000; Olympus) equipped with a 60x planApochromat objective (NA 1.35; oil) and SIM scanner. Cells were plated at low density on FN (10 μg/ml) for 24 h in an 8-well IDIBI chamber, transfected with FAK-GFP, Paxillin-GFP, or α5-integrin-GFP, and experiments were performed 24 h later at 37°C. Two prebleach events were acquired followed by a single bleach event using the simultaneous and independent stimulation of the 405 line of the SIM scanner. Fluorescence recovery was followed at 4-s time intervals until the intensity reached a plateau. Fluorescence during recovery was normalized to the prebleach intensity. Relative recovery rates for FAK-GFP at FAs were compared using the half-time for recovery of fluorescence toward the asymptote. Intensity ratios in the bleached area were compared before bleaching and after recovery to calculate mobile and immobile fractions using Prism 4 (GraphPad). Graphs are representative of a minimum of three independent experiments in which between 6 and 15 FAs were bleached.
Wound-healing motility assay and cell spreading
Motility of PyMT mammary tumor cells plated on 35-mm plastic dishes coated with 10 μg/ml of FN was assessed in serum-free medium by wound healing over 24 h as previously described (Lagana et al., 2006). Cell motility was quantified with ImagePro analysis software by measuring the distance from the scrape of the 10 most motile cells for 4 fields of each condition. Cell area was measured from confocal images taken with a 40x objective of Alexa568-phalloidin labeled cells using ImagePro. Cells not fully in the frame and overlapping cells were excluded and the areas of at least 15 cells per cell line per experiment were measured.
FA turnover and live cell imaging
Cells were plated at low density on FN (10 μg/ml) for 24 h in an 8-well IDIBI chamber, transfected with FAK-GFP and either Cav1-mRFP or CavY14F-mRFP. After 24 h, transfected cells were identified and images of FAK-GFP acquired every 30 s for 30 min at 37°C. FA disassembly and formation were visualized and quantified manually as the loss or appearance of fluorescence in a selected region of interest. Quantification of the percentage of stable (immobile), disassembled, or newly formed FAs is presented as mean (± SEM) of four different experiments (n = 4, 362 FAs for Cav1-mRFP, 178 for CavY14F-mRFP, and 237 for FAK-GFP only). Cell outlines were manually drawn and areas of cellular protrusion were quantified by counting nonoverlapping pixels at time 30 compared with time 0 min and retraction by counting nonoverlapping pixels at time 0 compared with time 30 min. Image analysis and quantification was performed using either ImageJ (NIH Image software, http://rsb.info.nih.gov/ij/) or Image Pro.
Online supplemental material
Supplemental figures show that Cav1 expression does not impact on β1-integrin distribution in Mgat5−/− cells (Fig. S1) and that PP2 does not affect FAK-GFP dynamics in focal adhesions of ESC-Rescue cells using FRAP (Fig. S2), and provide representative images of phalloidin- and Hoechst-labeled cells that were used for the cell spreading analysis in Fig. 6 E (Fig. S3). Videos 1–6 are 30-min movies of FAK-GFP distribution in Mgat5+/+, Mgat5−/−ESC, and ESC-Rescue cells transfected with FAK-GFP and where indicated, Cav1-mRFP, as presented in Fig. 7 E. Videos 7–9 are 30-min movies of FAK-GFP distribution in MDA-435 cells transfected with FAK-GFP and either Cav1-mRFP or Cav1Y14F-mRFP, as indicated, as presented in Fig. 9 B. Online supplemental material is available at http://www.jcb.org/cgi/content/full/jcb.200709019/DC1.
Supplemental Material
Acknowledgments
We thank Jim Dennis for stimulating interaction, support, and helpful comments, and Avraham Raz for providing the TIB-466 anti-Gal-3 antibody. We thank Michael Gold and Spencer Freeman for providing the FAK-GFP construct; Alan Horwitz for α5-integrin-GFP; Rich Minshall for GFP-Cav1Y14D; and Konstantin Birukov for paxillin-GFP.
This study was supported by grant MOP-43988 from the Canadian Institutes of Health Research. J.G. Goetz is the recipient of doctoral fellowships from the Ministère de la Recherche et des Technologies (France) and the Association pour la Recherche sur le Cancer (ARC) for his doctoral studies to be submitted jointly to the Université de Montréal and the Université Louis Pasteur de Strasbourg (UMR CNRS 7175). P. Lajoie is a research student of the Terry Fox Foundation through an award from the National Cancer Institute of Canada.
J.G. Goetz's present address is Integrin Signaling Laboratory, Department of Vascular Biology and Inflammation, Centro Nacional de Investigaciones Cardiovasculares (CNIC), 28029 Madrid, Spain.
Abbreviations used in this paper: Cav1, caveolin-1; FA, focal adhesion; FAK, focal adhesion kinase; FN, fibronectin; Gal-3; galectin-3; Mgat5, GlcNAc-transferase V; p, phospho; SW, swainsonine; Y14, tyrosine-14.
References
- Ahmad, N., H.J. Gabius, S. Andre, H. Kaltner, S. Sabesan, R. Roy, B. Liu, F. Macaluso, and C.F. Brewer. 2004. Galectin-3 precipitates as a pentamer with synthetic multivalent carbohydrates and forms heterogeneous cross-linked complexes. J. Biol. Chem. 279:10841–10847. [DOI] [PubMed] [Google Scholar]
- Beardsley, A., K. Fang, H. Mertz, V. Castranova, S. Friend, and J. Liu. 2005. Loss of caveolin-1 polarity impedes endothelial cell polarization and directional movement. J. Biol. Chem. 280:3541–3547. [DOI] [PubMed] [Google Scholar]
- Brouet, A., J. DeWever, P. Martinive, X. Havaux, C. Bouzin, P. Sonveaux, and O. Feron. 2005. Antitumor effects of in vivo caveolin gene delivery are associated with the inhibition of the proangiogenic and vasodilatory effects of nitric oxide. FASEB J. 19:602–604. [DOI] [PubMed] [Google Scholar]
- Burridge, K., and K. Fath. 1989. Focal contacts: transmembrane links between the extracellular matrix and the cytoskeleton. Bioessays. 10:104–108. [DOI] [PubMed] [Google Scholar]
- Cao, H., W.E. Courchesne, and C.C. Mastick. 2002. A phosphotyrosine-dependent protein interaction screen reveals a role for phosphorylation of caveolin-1 on tyrosine 14: recruitment of C-terminal Src kinase. J. Biol. Chem. 277:8771–8774. [DOI] [PubMed] [Google Scholar]
- Caselli, A., M.L. Taddei, G. Manao, G. Camici, and G. Ramponi. 2001. Tyrosine-phosphorylated caveolin is a physiological substrate of the low M(r) protein-tyrosine phosphatase. J. Biol. Chem. 276:18849–18854. [DOI] [PubMed] [Google Scholar]
- Caselli, A., B. Mazzinghi, G. Camici, G. Manao, and G. Ramponi. 2002. Some protein tyrosine phosphatases target in part to lipid rafts and interact with caveolin-1. Biochem. Biophys. Res. Commun. 296:692–697. [DOI] [PubMed] [Google Scholar]
- Chapman, H.A., Y. Wei, D.I. Simon, and D.A. Waltz. 1999. Role of urokinase receptor and caveolin in regulation of integrin signaling. Thromb. Haemost. 82:291–297. [PubMed] [Google Scholar]
- Cheung, P., and J.W. Dennis. 2007. Mgat5 and Pten interact to regulate cell growth and polarity. Glycobiology. 17:767–773. [DOI] [PubMed] [Google Scholar]
- Cluzel, C., F. Saltel, J. Lussi, F. Paulhe, B.A. Imhof, and B. Wehrle-Haller. 2005. The mechanisms and dynamics of {alpha }v{beta }3 integrin clustering in living cells. J. Cell Biol. 171:383–392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- del Pozo, M.A., N.B. Alderson, W.B. Kiosses, H.H. Chiang, R.G. Anderson, and M.A. Schwartz. 2004. Integrins regulate Rac targeting by internalization of membrane domains. Science. 303:839–842. [DOI] [PubMed] [Google Scholar]
- del Pozo, M.A., N. Balasubramanian, N.B. Alderson, W.B. Kiosses, A. Grande-Garcia, R.G. Anderson, and M.A. Schwartz. 2005. Phospho-caveolin-1 mediates integrin-regulated membrane domain internalization. Nat. Cell Biol. 7:901–908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeMali, K.A., K. Wennerberg, and K. Burridge. 2003. Integrin signaling to the actin cytoskeleton. Curr. Opin. Cell Biol. 15:572–582. [DOI] [PubMed] [Google Scholar]
- Demetriou, M., M. Granovsky, S. Quaggin, and J.W. Dennis. 2001. Negative regulation of T-cell activation and autoimmunity by Mgat5 N-glycosylation. Nature. 409:733–739. [DOI] [PubMed] [Google Scholar]
- Franco, S.J., M.A. Rodgers, B.J. Perrin, J. Han, D.A. Bennin, D.R. Critchley, and A. Huttenlocher. 2004. Calpain-mediated proteolysis of talin regulates adhesion dynamics. Nat. Cell Biol. 6:977–983. [DOI] [PubMed] [Google Scholar]
- Gaus, K., S. Le Lay, N. Balasubramanian, and M.A. Schwartz. 2006. Integrin-mediated adhesion regulates membrane order. J. Cell Biol. 174:725–734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geiger, B., A. Bershadsky, R. Pankov, and K.M. Yamada. 2001. Transmembrane crosstalk between the extracellular matrix and the cytoskeleton. Nat. Rev. Mol. Cell Biol. 2:793–805. [DOI] [PubMed] [Google Scholar]
- Giannone, G., P. Ronde, M. Gaire, J. Beaudouin, J. Haiech, J. Ellenberg, and K. Takeda. 2004. Calcium rises locally trigger focal adhesion disassembly and enhance residency of focal adhesion kinase at focal adhesions. J. Biol. Chem. 279:28715–28723. [DOI] [PubMed] [Google Scholar]
- Ginsberg, M.H., A. Partridge, and S.J. Shattil. 2005. Integrin regulation. Curr. Opin. Cell Biol. 17:509–516. [DOI] [PubMed] [Google Scholar]
- Glenney, J.R. Jr., and L. Zokas. 1989. Novel tyrosine kinase substrates from Rous sarcoma virus-transformed cells are present in the membrane skeleton. J. Cell Biol. 108:2401–2408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong, H.C., Y. Honjo, P. Nangia-Makker, V. Hogan, N. Mazurak, R.S. Bresalier, and A. Raz. 1999. The NH2 terminus of galectin-3 governs cellular compartmentalization and functions in cancer cells. Cancer Res. 59:6239–6245. [PubMed] [Google Scholar]
- Gonzalez, E., A. Nagiel, A.J. Lin, D.E. Golan, and T. Michel. 2004. Small interfering RNA-mediated down-regulation of caveolin-1 differentially modulates signaling pathways in endothelial cells. J. Biol. Chem. 279:40659–40669. [DOI] [PubMed] [Google Scholar]
- Grande-Garcia, A., A. Echarri, J. de Rooij, N.B. Alderson, C.M. Waterman-Storer, J.M. Valdivielso, and M.A. del Pozo. 2007. Caveolin-1 regulates cell polarization and directional migration through Src kinase and Rho GTPases. J. Cell Biol. 177:683–694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granovsky, M., J. Fata, J. Pawling, W.J. Muller, R. Khokha, and J.W. Dennis. 2000. Suppression of tumor growth and metastasis in Mgat5-deficient mice. Nat. Med. 6:306–312. [DOI] [PubMed] [Google Scholar]
- Guo, H.-B., M. Randolph, and M. Pierce. 2007. Inhibition of a specific N-glycosylation activity results in attenuation of breast carcinoma cell invasiveness-related phenotypes: inhibition of epidermal growth factor-induced dephosphorylation of focal adhesion kinase. J. Biol. Chem. 282:22150–22162. [DOI] [PubMed] [Google Scholar]
- Guo, W., and F.G. Giancotti. 2004. Integrin signalling during tumour progression. Nat. Rev. Mol. Cell Biol. 5:816–826. [DOI] [PubMed] [Google Scholar]
- Gupton, S.L., and C.M. Waterman-Storer. 2006. Spatiotemporal feedback between actomyosin and focal-adhesion systems optimizes rapid cell migration. Cell. 125:1361–1374. [DOI] [PubMed] [Google Scholar]
- Hamadi, A., M. Bouali, M. Dontenwill, H. Stoeckel, K. Takeda, and P. Ronde. 2005. Regulation of focal adhesion dynamics and disassembly by phosphorylation of FAK at tyrosine 397. J. Cell Sci. 118:4415–4425. [DOI] [PubMed] [Google Scholar]
- Henderson, N.C., A.C. Mackinnon, S.L. Farnworth, F. Poirier, F.P. Russo, J.P. Iredale, C. Haslett, K.J. Simpson, and T. Sethi. 2006. Galectin-3 regulates myofibroblast activation and hepatic fibrosis. Proc. Natl. Acad. Sci. USA. 103:5060–5065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez, M.V., M.G. Sala, J. Balsamo, J. Lilien, and C.O. Arregui. 2006. ER-bound PTP1B is targeted to newly forming cell-matrix adhesions. J. Cell Sci. 119:1233–1243. [DOI] [PubMed] [Google Scholar]
- Hill, M.M., N. Scherbakov, N. Schiefermeier, J. Baran, J.F. Hancock, L.A. Huber, R.G. Parton, and M.O. Parat. 2007. Reassessing the Role of phosphocaveolin-1 in cell adhesion and migration. Traffic. 8:1695–1705. [DOI] [PubMed] [Google Scholar]
- Hirabayashi, J., T. Hashidate, Y. Arata, N. Nishi, T. Nakamura, M. Hirashima, T. Urashima, T. Oka, M. Futai, W.E. Muller, et al. 2002. Oligosaccharide specificity of galectins: a search by frontal affinity chromatography. Biochim. Biophys. Acta. 1572:232–254. [DOI] [PubMed] [Google Scholar]
- Hocking, D.C., and K. Kowalski. 2002. A cryptic fragment from fibronectin's III1 module localizes to lipid rafts and stimulates cell growth and contractility. J. Cell Biol. 158:175–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isshiki, M., J. Ando, K. Yamamoto, T. Fujita, Y. Ying, and R.G. Anderson. 2002. Sites of Ca(2+) wave initiation move with caveolae to the trailing edge of migrating cells. J. Cell Sci. 115:475–484. [DOI] [PubMed] [Google Scholar]
- Kojic, L.D., B. Joshi, P. Lajoie, P.U. Le, S. Leung, M.E. Cox, D.A. Turbin, S.A. Wiseman, and I.R. Nabi. 2007. Raft-dependent endocytosis of autocrine motility factor is phosphatidylinositol-3-kinase-dependent in breast carcinoma cells. J. Biol. Chem. 282:29305–29313. [DOI] [PubMed] [Google Scholar]
- Lagana, A., J.G. Goetz, P. Cheung, A. Raz, J.W. Dennis, and I.R. Nabi. 2006. Galectin binding to Mgat5-modified N-glycans regulates fibronectin matrix remodeling in tumor cells. Mol. Cell. Biol. 26:3181–3193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lajoie, P., E. Partridge, G. Guay, J.G. Goetz, J. Pawling, A. Lagana, B. Joshi, J.W. Dennis, and I.R. Nabi. 2007. Plasma membrane domain organization regulates EGFR signaling in tumor cells. J. Cell Biol. 179:341–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau, K.S., E.A. Partridge, A. Grigorian, C.I. Silvescu, V.N. Reinhold, M. Demetriou, and J.W. Dennis. 2007. Complex N-glycan number and degree of branching cooperate to regulate cell proliferation and differentiation. Cell. 129:123–134. [DOI] [PubMed] [Google Scholar]
- Lee, H., D. Volonte, F. Galbiati, P. Iyengar, D.M. Lublin, D.B. Bregman, M.T. Wilson, R. Campos-Gonzalez, B. Bouzahzah, R.G. Pestell, et al. 2000. Constitutive and growth factor-regulated phosphorylation of caveolin-1 occurs at the same site (Tyr-14) in vivo: identification of a c-Src/Cav-1/Grb7 signaling cassette. Mol. Endocrinol. 14:1750–1775. [DOI] [PubMed] [Google Scholar]
- Lee, H., L. Xie, Y. Luo, S.Y. Lee, D.S. Lawrence, X.B. Wang, F. Sotgia, M.P. Lisanti, and Z.Y. Zhang. 2006. Identification of phosphocaveolin-1 as a novel protein tyrosine phosphatase 1B substrate. Biochemistry. 45:234–240. [DOI] [PubMed] [Google Scholar]
- Liang, F., S.-Y. Lee, J. Liang, D.S. Lawrence, and Z.-Y. Zhang. 2005. The role of protein-tyrosine phosphatase 1B in integrin signaling. J. Biol. Chem. 280:24857–24863. [DOI] [PubMed] [Google Scholar]
- Luo, B.H., C.V. Carman, and T.A. Springer. 2007. Structural basis of integrin regulation and signaling. Annu. Rev. Immunol. 25:619–647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mettouchi, A., S. Klein, W. Guo, M. Lopez-Lago, E. Lemichez, J.K. Westwick, and F.G. Giancotti. 2001. Integrin-specific activation of Rac controls progression through the G(1) phase of the cell cycle. Mol. Cell. 8:115–127. [DOI] [PubMed] [Google Scholar]
- Mitra, S.K., and D.D. Schlaepfer. 2006. Integrin-regulated FAK-Src signaling in normal and cancer cells. Curr. Opin. Cell Biol. 18:516–523. [DOI] [PubMed] [Google Scholar]
- Nieminen, J., A. Kuno, J. Hirabayashi, and S. Sato. 2007. Visualization of galectin-3 oligomerization on the surface of neutrophils and endothelial cells using fluorescence resonance energy transfer. J. Biol. Chem. 282:1374–1383. [DOI] [PubMed] [Google Scholar]
- Owen, K.A., F.J. Pixley, K.S. Thomas, M. Vicente-Manzanares, B.J. Ray, A.F. Horwitz, J.T. Parsons, H.E. Beggs, E.R. Stanley, and A.H. Bouton. 2007. Regulation of lamellipodial persistence, adhesion turnover, and motility in macrophages by focal adhesion kinase. J. Cell Biol. 179:1275–1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parat, M.O., B. Anand-Apte, and P.L. Fox. 2003. Differential caveolin-1 polarization in endothelial cells during migration in two and three dimensions. Mol. Biol. Cell. 14:3156–3168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parton, R.G., and K. Simons. 2007. The multiple faces of caveolae. Nat. Rev. Mol. Cell Biol. 8:185–194. [DOI] [PubMed] [Google Scholar]
- Partridge, E.A., C. Le Roy, G.M. Di Guglielmo, J. Pawling, P. Cheung, M. Granovsky, I.R. Nabi, J.L. Wrana, and J.W. Dennis. 2004. Regulation of cytokine receptors by Golgi N-glycan processing and endocytosis. Science. 306:120–124. [DOI] [PubMed] [Google Scholar]
- Radel, C., and V. Rizzo. 2005. Integrin mechanotransduction stimulates caveolin-1 phosphorylation and recruitment of Csk to mediate actin reorganization. Am. J. Physiol. Heart Circ. Physiol. 288:H936–H945. [DOI] [PubMed] [Google Scholar]
- Savage, K., M.B.K. Lambros, D. Robertson, R.L. Jones, C. Jones, A. Mackay, M. James, J.L. Hornick, E.M. Pereira, F. Milanezi, et al. 2007. Caveolin 1 is overexpressed and amplified in a subset of basal-like and metaplastic breast carcinomas: a morphologic, ultrastructural, immunohistochemical, and in situ hybridization analysis. Clin. Cancer Res. 13:90–101. [DOI] [PubMed] [Google Scholar]
- Sottile, J., and J. Chandler. 2005. Fibronectin matrix turnover occurs through a caveolin-1-dependent process. Mol. Biol. Cell. 16:757–768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuoki, M., M. Miyamoto, K. Kato, K. Hiraoka, T. Oshikiri, Y. Nakakubo, A. Fukunaga, T. Shichinohe, T. Shinohara, T. Itoh, et al. 2002. Impact of caveolin-1 expression on prognosis of pancreatic ductal adenocarcinoma. Br. J. Cancer. 87:1140–1144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Wichert, G., B. Haimovich, G.S. Feng, and M.P. Sheetz. 2003. Force-dependent integrin-cytoskeleton linkage formation requires downregulation of focal complex dynamics by Shp2. EMBO J. 22:5023–5035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, F., P. Herzmark, O.D. Weiner, S. Srinivasan, G. Servant, and H.R. Bourne. 2002. Lipid products of PI(3)Ks maintain persistent cell polarity and directed motility in neutrophils. Nat. Cell Biol. 4:513–518. [DOI] [PubMed] [Google Scholar]
- Wary, K.K., A. Mariotti, C. Zurzolo, and F.G. Giancotti. 1998. A requirement for caveolin-1 and associated kinase Fyn in integrin signaling and anchorage-dependent cell growth. Cell. 94:625–634. [DOI] [PubMed] [Google Scholar]
- Wassler, M.J., and B.D. Shur. 2000. Clustering of cell surface (beta)1,4-galactosyltransferase I induces transient tyrosine phosphorylation of focal adhesion kinase and loss of stress fibers. J. Cell Sci. 113:237–245. [DOI] [PubMed] [Google Scholar]
- Webb, D.J., K. Donais, L.A. Whitmore, S.M. Thomas, C.E. Turner, J.T. Parsons, and A.F. Horwitz. 2004. FAK-Src signalling through paxillin, ERK and MLCK regulates adhesion disassembly. Nat. Cell Biol. 6:154–161. [DOI] [PubMed] [Google Scholar]
- Wei, Y., X. Yang, Q. Liu, J.A. Wilkins, and H.A. Chapman. 1999. A role for caveolin and the urokinase receptor in integrin-mediated adhesion and signaling. J. Cell Biol. 144:1285–1294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang, G., L.D. Truong, T.M. Wheeler, and T.C. Thompson. 1999. Caveolin-1 expression in clinically confined human prostate cancer: a novel prognostic marker. Cancer Res. 59:5719–5723. [PubMed] [Google Scholar]
- Zhang, W., B. Razani, Y. Altschuler, B. Bouzahzah, K.E. Mostov, R.G. Pestell, and M.P. Lisanti. 2000. Caveolin-1 inhibits epidermal growth factor-stimulated lamellipod extension and cell migration in metastatic mammary adenocarcinoma cells (MTLn3). Transformation suppressor effects of adenovirus-mediated gene delivery of caveolin-1. J. Biol. Chem. 275:20717–20725. [DOI] [PubMed] [Google Scholar]
- Zhu, J., C.V. Carman, M. Kim, M. Shimaoka, T.A. Springer, and B.H. Luo. 2007. Requirement of alpha and beta subunit transmembrane helix separation for integrin outside-in signaling. Blood. 110:2475–2483. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.