Abstract
Objective
Phytoestrogens display an array of pharmacologic properties, and in recent years investigation of their potential as anticancer agents has increased dramatically. In this article we review the published literature related to phytoestrogens and breast cancer as well as suggest the possible mechanisms that may underlie the relationship between phytoestrogens and breast cancer.
Data sources
Electronic searches on phytoestrogens and breast cancer were performed on MEDLINE and EMBASE in June 2007. No date restriction was placed on the electronic search.
Data extraction
We focused on experimental data from published studies that examined the characteristics of phytoestrogens using in vivo or in vitro models. We also include human intervention studies in this review.
Data synthesis
We evaluated evidence regarding the possible mechanisms of phytoestrogen action. Discussions of these mechanisms were organized into those activities related to the estrogen receptor, cell growth and proliferation, tumor development, signaling pathways, and estrogen-metabolizing enzymes.
Conclusions
We suggest that despite numerous investigations, the mechanisms of phytoestrogen action in breast cancer have yet to be elucidated. It remains uncertain whether these plant compounds are chemoprotective or whether they may produce adverse outcomes related to breast carcinogenesis.
Keywords: breast cancer, catechol estrogen, catechol-O-methyltransferase, chemoprevention, cytochrome P450, estrogen, estrogen receptor, phytoestrogen
Breast cancer is an important public health problem worldwide. In the United States, breast cancer represents the most common neoplasm and the second most frequent cause of cancer death in women (American Cancer Society 2006). Steroidal estrogens have been implicated in the etiology of breast cancer and have been added to the list of known human carcinogens [International Agency for Research on Cancer (IARC) 1999, 1987; National Toxicology Program (NTP) 2002]. Estrogens are suggested to cause breast cancer by stimulating cell growth and proliferation through receptor-mediated processes and via their genotoxic metabolites (Cavalieri et al. 2006; Yager and Davidson 2006). Phytoestrogens are a class of plant-derived compounds that are structurally similar to mammalian estrogens (Sirtori et al. 2005). Ecologic observations indicate that the incidence of breast cancer is much lower in Asian women, who consume significantly higher amounts of phytoestrogens than Western women (Adlercreutz 2002). Second- and third-generation descendants of women who migrated to Western countries from Asia have breast cancer risks similar to those of women in the host country, suggesting that lifestyle and not genetic factors explain the low breast cancer risk observed in Asian women (Probst-Hensch et al. 2000; Usui 2006). However, despite recent attention related to the putative chemoprotective properties of phytoestrogens, epidemiologic studies have produced inconsistent results, and the relationship between phytoestrogens and breast cancer remains enigmatic (Gikas and Mokbel 2005; Messina et al. 2006; Peeters et al. 2003; Trock et al. 2006). Moreover, the possible mechanisms of phytoestrogen action in breast cancer have yet to be resolved.
Phytoestrogen Classification
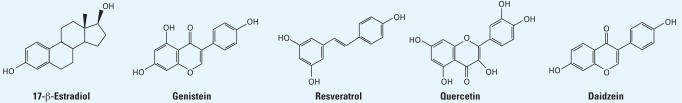
Phytoestrogens are biologically active phenolic compounds of plant origin that structurally mimic the principal mammalian estrogen 17β-estradiol (E2; Figure 1) (Sirtori et al. 2005). Shared structures include a pair of hydroxyl groups and a phenolic ring, which is required for binding to estrogen receptors (ER)-α and ER-β, and the position of these hydroxyl groups appears to be an important factor in determining their abilities to bind the ERs and activate transcription (Le Bail et al. 2000). Four main classes of compounds are currently recognized as phytoestrogens—the isoflavones, stilbenes, coumestans, and lignans (Moon et al. 2006; Sirtori et al. 2005). These types of phytochemicals are some of the most prevalent compounds found in fruits, vegetables, legumes, and tea and are generally concentrated in the fruit skin, bark, and flowers of plants (Moon et al. 2006). Resveratrol, daidzein, quercetin, and genistein represent four of the most commonly ingested and most intensely studied phytoestrogens (Figure 1).
Figure 1.
Chemical structures of E2 and the phytoestrogens resveratrol, genistein, quercetin, and daidzein.
In East and Southeast Asia, the average daily intake of phytoestrogens is estimated to be between 20 and 50 mg (Adlercreutz 1998; Sirtori et al. 2005). In contrast, the typical diet of an adult in the United States contains only 0.15–3 mg phytoestrogens per day, and in Europe the average daily phytoestrogen consumption is estimated to be even lower, falling between 0.49 and 1 mg (Adlercreutz 1998; Sirtori et al. 2005). According to various epidemiologic studies, plasma isoflavone concentrations range from 2 μM (Japanese men) to 5 nM (Finnish study subjects); however, local tissue phytoestrogen concentrations are suggested to be 2–3 times higher than plasma levels (Adlercreutz et al. 1993; Arai et al. 2000; Morton et al. 2002; Uehar et al. 2000).
Phytoestrogens and Breast Cancer Risk
The importance of estrogens in the etiology of breast cancer is widely recognized (Bhat et al. 2003; Cavalieri et al. 2006; Yager and Davidson 2006). Estrogens have been implicated in the initiation and promotion stages of breast cancer, and lifetime estrogen exposure is a major risk factor for breast cancer development (Yager and Davidson 2006). Estrogens exert their carcinogenic effects via ER-dependent mechanisms as well as their genotoxic metabolites (Bhat et al. 2003; Cavalieri et al. 2006; Yager and Davidson 2006).
Epidemiologic evidence suggests that diet and nutrition can influence cancer development, and women living in Asia, where diets have traditionally included soybean products, report fewer postmenopausal symptoms and experience fewer breast cancers than women in Western countries (Adlercreutz 2002; Nichenametla et al. 2006; Usui 2006). More specifically, Asian women have a 3-fold lower breast cancer risk than women in the United States, independent of body weight (Ursin et al. 1994). Furthermore, serum concentrations of E2 are 40% lower in Asian women compared with their Caucasian counterparts (Peeters et al. 2003). Thus, environmental and dietary factors may explain at least some of the discrepancy in breast cancer risk between populations (Adlercreutz 2002; Nichenametla et al. 2006). The assertion that dietary and lifestyle factors may be partially responsible for the low breast cancer risks detected in Asian women is supported by observations in Asian women who immigrate to Western countries. The second- and third-generation descendants of women who migrated from Asia to Western countries have breast cancer risks similar to those of women in the host country, suggesting that lifestyle and not genetic factors explain the low breast cancer risk of women in Asia (Probst-Hensch et al. 2000; Usui 2006).
Phytoestrogens exhibit a wide array of pharmacologic properties, and recently, interest in the potential benefits of diets high in phytoestrogens has intensified, especially those related to chemoprevention. The link between phytoestrogens and breast cancer prevention has been the subject of numerous studies, and the epidemiology of breast cancer in relation to phytoestrogen consumption has recently been extensively reviewed (Adlercreutz 2002; Messina et al. 2006; Ziegler 2004). Generally, epidemiologic studies have been inconclusive, and the relationship between phytoestrogens and breast cancer prevention remains uncertain (Messina et al. 2006; Trock et al. 2006; Ziegler 2004). Some studies have revealed the modest protective effects of phytoestrogens; others have detected no association between phytoestrogen intake and breast cancer risk; and a few have reported marked protective effects (Hirohata et al. 1985; Hirose et al. 1995; Key et al. 1999; Nomura et al. 1978, 1985; Trock et al. 2006; Wu et al. 1996; Yuan et al. 1995). A recent review of 21 case–control and 15 prospective studies concluded that there is no clear evidence that phytoestrogen intake influences the risk of developing breast cancer (Gikas and Mokbel 2005). Nevertheless, some evidence suggests that soy intake must be high during certain windows of development, specifically prepubescence, in order to gain the protective effect of phytoestrogens (Hirayama 1990a, 1990b; Key et al. 1999; Lamartiniere 2000; Shu et al. 2001; Wakai et al. 1999; Wu et al. 1996). Despite intense investigation, it remains unclear whether phytoestrogens are actually chemoprotective agents or whether their presence is simply a biomarker indicative of a healthy diet.
Phytoestrogens and Estrogen Biosynthesis
Various dietary intervention studies in humans have examined the effects of phytoestrogens on estrogen biosynthesis and estrogen biosynthetic enzymes. Neither a 2-year study in which 220 premenopausal women consumed 100 mg isoflavones per day nor a short-term study in which premenopausal women consumed 38 mg isoflavones per day revealed significant alterations in steroid hormone levels or menstrual cycle length (Hargreaves et al. 1999; Maskarinec et al. 2002). In contrast, other researchers have reported decreased plasma concentrations of follicle-stimulating hormone (FSH), luteinizing hormone (LH), E2, and progesterone as well as decreased serum concentrations of E2 and estrone following increased phytoestrogen consumption (Duncan et al. 1999; Kumar et al. 2002; Lu et al. 2000a; Nagata et al. 1998). Another study, which evaluated pre-menopausal women after consumption of isoflavone-supplemented diets for three menstrual cycles, reported that isoflavone intake decreased urinary excretion of E2, estrone, estriol, and total estrogens (Xu et al. 1998). Moreover, the isoflavone diet increased the ratio of 2-hydroxyestrone to 16α-hydroxyestrone and decreased in the ratio of genotoxic estrogens to total estrogens (Xu et al. 1998). A separate study identified a 27% increase in the ratio of 2-hydroxyestrone to 16α-hydroxyestrone in women given an isoflavone-rich diet compared with women on an isoflavone-free diet (Lu et al. 2000b). Furthermore, in women consuming 40 mg isoflavones each day for 3 months, the average menstrual cycle length was increased 3.52 days, and the follicular phase of the cycle was increased 1.46 days on average (Kumar et al. 2002). The implication of increased menstrual cycle length is a decrease in the total lifetime number of cycles, thereby minimizing the exposure of breast epithelial cells to estrogens.
The decrease in circulating estrogen concentrations after phytoestrogen consumption may be a result of interference with estrogen biosynthetic enzymes, namely cytochrome P450 19 aromatase (Cyp19) and 17β-hydroxysteroid dehydrogenase (HSD) (Rice and Whitehead 2006). Cyp19 catalyzes the conversion of androstenedione and testosterone to estrone (E1) and E2, respectively (Thompson and Siiteri 1974). HSD catalyzes the inter-conversion of the relatively inactive 17β-keto steroids, such as estrone and androstenedione, to active 17β-hydroxyl steroids such as E2 and testosterone (Gunnarsson et al. 2003). In the breast tissue of postmenopausal women, HSD and Cyp19 are responsible for the local production of estrogens, and overexpression or increased activity of these enzymes is associated with breast cancer (Li et al. 1998; Pasqualini et al. 1996; Yue et al. 2001).
Among the phytoestrogens, flavones, and flavonones are the most potent inhibitors of Cyp19 aromatase, whereas the isoflavones are relatively weaker aromatase inhibitors. Several phytoestrogens, including 7-hydroxyflavone, apigenin, chrysin, and hesperetin were found to be effective aromatase inhibitors in human placental microsomes, with IC50 (concentration of phytoestrogens that reduces enzyme activity by 50%) values ranging from 0.3 to 3.0 μM (Jeong et al. 1999; Le Bail et al. 2000). In H295R human adrenocortical carcinoma cells, both flavones and flavonones exerted inhibitory effects on aromatase (Sanderson et al. 2004). Similarly, quercetin, genistein, and daidzein suppressed the transcription of Cyp19 mRNA in human granulosa luteal cells (Rice et al. 2006). Although isoflavones are generally weak aromatase inhibitors, isoflavone mixtures displayed an increased ability to inhibit aromatase activity and transcription compared with any of the isoflavones alone (Rice et al. 2006). A mixture of genistein, daidzein, and biochanin A almost completely eliminated transcription of Cyp19 mRNA and significantly reduced Cyp19 enzyme activity (Rice et al. 2006). Resveratrol exerted both competitive and noncompetitive inhibitory effects on aromatase activity in MCF7 cells stably transfected with Cyp19, with an IC50 value of approximately 25 μM (Wang et al. 2006). Similarly, resveratrol suppressed transcription of Cyp19 mRNA in SK-BR-3 breast cancer cells. In contrast, genistein increased aromatase activity in H295R cells and in isolated rat follicles (Myllymaki et al. 2005; Sanderson et al. 2004). In addition to their interactions with Cyp19, phytoestrogens have been shown to inhibit HSD (Le Bail et al. 1998). For example, genistein decreased HSD activity in human placental microsomes, genital skin fibroblasts, granulosa luteal cells, MCF7 breast cancer cells and T47D breast cancer cells (Brooks and Thompson 2005; Evans et al. 1995; Le Bail et al. 2000; Whitehead et al. 2002). In MCF7 cells, genistein inhibited HSD-catalyzed E2 production by 59% (Brooks and Thompson 2005).
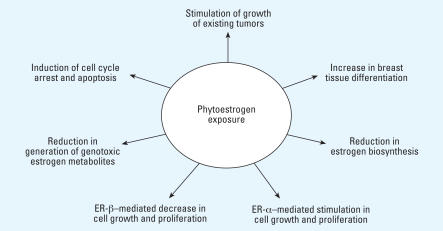
Given the carcinogenic properties of endogenous estrogens, reducing their levels in the body by inhibition of steroidogenic enzymes such as Cyp19 and HSD would protect against breast cancer development (Figure 2; Appendix 1). Thus, although studies have not detected consistent changes in hormone levels after phytoestrogen intake and the overall health effects of phytoestrogen exposure remain unclear, these plant compounds may decrease lifetime exposure to estrogens, via two mechanisms, namely by decreasing estrogen biosynthesis and by increasing menstrual cycle length.
Figure 2.
Summary of potential actions of phytoestrogens. Arrows indicate possible functions of phytoestrogens.
Phytoestrogens and the Estrogen Receptors
The estrogen receptors ER-α and ER-β function as ligand-activated transcription factors that initiate transcription by translocating to the nucleus and binding to estrogen response elements (ERE) in the promoter regions of target genes (McDonnell 2004). The actions of ER-α and ER-β on gene transcription can be opposite, depending on cell context (Koehler et al. 2005). It is thought that ER-βmay impact estrogen action by directly modulating gene transcription or by modulating ER-α activity in tissues that express both ER subtypes (Hall and McDonnell 1999). ER-β can function as a transcriptional inhibitor or activator, depending on the agonist concentration, such that different patterns of gene expression are produced at different agonist concentrations (Hall and McDonnell 1999). Studies in MCF7 cells suggest that ER-β is not necessary for proliferation and that ER-β opposes the proliferative effects exerted by ER-α (Koehler et al. 2005; Omoto et al. 2003; Strom et al. 2004). The interactions between the two main ERs and their specific cofactors provide a mechanistic basis for the tissue-selective actions of estrogens (McDonnell 2004). The ratio of ER-α to ER-β is a prognostic marker in breast tumors, such that ER-β expression is indicative of more benign tumors, whereas ER-α indicates malignant, aggressive tumors (Balfe et al. 2004; Shaaban et al. 2003).
Many phytoestrogens, including resveratrol, genistein, daidzein, and quercetin, have been shown to bind both ER-α and ER-β and to induce the transcription of estrogen-responsive target genes in a dose-dependent manner (Bowers et al. 2000; Kuiper et al. 1997, 1998; Maggiolini et al. 2001). However, phytoestrogens bind the ER with much lower affinity compared with E 2 (McCarty 2006; van der Woude et al. 2005). The affinity of quercetin for ER-α and ER-β was shown to be 105- to 106-fold lower than the affinity of E2 for ER-α and ER-β (van der Woude et al. 2005). Similarly, the affinity of daidzein for ER-α and ER-β was found to be approximately 20,000- and 500-fold lower than that of E2 (Lehmann et al. 2005)
Unlike E2, which binds both ER-α and ER-β with similar affinity, many phytoestrogens display a substantially higher affinity for ER-β. For example, the binding affinities of genistein and daidzein for ER-β were shown to be significantly higher than for ER-α (Kuiper et al. 1997, 1998; Lehmann et al. 2005). Moreover, phytoestrogens induce the transcription of estrogen-responsive target genes to much greater levels when bound to ER-β than when bound to ER-α. In MCF7 cells co-transfected with either ER-α or ER-β, genistein was shown to induce a 100-fold greater induction of gene expression when bound to ER-β than when bound to ER-α (Harris et al. 2005). This result is in agreement with other evidence that genistein preferentially binds ER-β and induces greater DNA-binding and transcriptional activity when bound to ER-β (An et al. 2001; Barkhem et al. 1998; Kostelac et al. 2003; Liu et al. 2003; Morito et al. 2001; Mueller et al. 2004; Routledge et al. 2000). Like genistein, resveratrol induced higher transcriptional activity in estrogen-responsive genes when bound to ER-β compared with ER-α (Bowers et al. 2000).
Despite, the significantly lower affinity of phytoestrogens for the ER compared with E2,some phytoestrogens reportedly induce ER-mediated gene transcription from both ER-α and ER-β to higher levels than E2 (Harris et al. 2005; van der Woude et al. 2005). The reported maximal inductions of gene transcription from ER-α by genistein and quercetin were 1.4- and 1.7-fold greater than those produced by E2 and 2.4- and 4.5-fold greater than those produced by E2 for ER-β (van der Woude et al. 2005). Similarly, the maximum induction of ER-mediated genes by quercetin for ER-α and ER-β were 1.7 and 4.5 times greater than those reached by E2 (van der Woude et al. 2005). In addition, the presence of endogenous estrogens has been shown to influence the effect of phytoestrogens on gene transcription. For example, both genistein and resveratrol were found to act synergistically with E2 to activate ER-α– and ER-β–induced gene transcription in MCF7 breast cancer cells (Gehm et al. 1997; Harris et al. 2005).
Because phytoestrogens have significantly different affinities for ER-α and ER-β, the net effect of exposure to a particular phytoestrogen may depend on the distinctive patterns of ER-α and ER-β expression in different cell types (McDonnell 2004). The differential affinities of phytoestrogens for ER-α and ER-β suggest that physiologic concentrations of phytoestrogens may be enough to activate ER-β but not ER-α, implying that rather than acting via the classical ER-α pathway, phytoestrogens may activate ER-β and induce its antiproliferative effects (Figure 2; Appendix 1) (McCarty 2006). Moreover, the presence of type II sites in the breast and uterus adds another dimension of complexity to phytoestrogen action. Type II sites, low-affinity nuclear binding sites for E2 in the breast and uterus, are suggested to be involved in regulating the growth and proliferation of both normal and malignant cells (Markaverich et al. 2001; Shoulars et al. 2002, 2005). Type II sites have not been fully characterized, although histone H4 binds type II ligands and is thought to be the type II site (Markaverich et al. 2001; Shoulars et al. 2002, 2005). Phytoestrogens, specifically flavonoids such as quercetin, bind type II sites with high affinity and antagonize growth in a number of cell types, suggesting another mechanism by which phytoestrogens may modulate cell proliferation (Griffiths and Smith 1972; Markaverich et al. 2001; Shoulars et al. 2002, 2005). Although many studies have been performed, a more detailed understanding of how phytoestrogens interact with the estrogen receptor is critical to fully evaluate their toxicologic and pharmacologic properties.
Phytoestrogens: Cellular Growth and Proliferation
Evidence that phytoestrogens can activate the estrogen receptor and may mimic endogenous estrogens has raised concerns regarding their effects on cell growth and proliferation. If phytoestrogens, like estrogens, promote cell growth, they may stimulate the expansion of pre-existing tumors (Figure 2; Appendix 1). However, the distinctive activities of the ER isoforms as well as the differential affinities of low concentrations of phytoestrogens for ER-β over ER-α suggest that the net effect of phytoestrogen exposure on cell growth may be quite different from those of estrogen on the classic ER system (McCarty 2006).
Many phytoestrogens appear to have a biphasic effect on cell proliferation, stimulating growth at low concentrations and suppressing growth at high concentrations. At low concentrations, resveratrol and quercetin dose dependently promoted growth in ER-positive MCF7 cells but inhibited proliferation and induced cell death at high concentrations (Ashby et al. 1999; Bowers et al. 2000; Gehm et al. 1997; Lu and Serrero 1999; Maggiolini et al. 2001; Schmitt et al. 2002; van der Woude et al. 2005). Similarly, genistein was shown to increase growth in estrogen-sensitive cells at low concentrations but decreased cell growth, suppressed DNA synthesis, and induced cell death at high concentrations (Maggiolini et al. 2001; Seo et al. 2006). A recent study revealed that the proliferation observed in daidzeintreated MCF7 cells was blocked by the pure antiestrogen ICI 182,780, indicating that the stimulatory effect exerted by daidzein was ER-mediated (Ju et al. 2006b). While phytoestrogens promote cell growth in ER-positive cells, evidence suggests that ER-negative cells may have different responses to phytoestrogen exposure. In the ER-negative breast cancer cell line MDA-MB-468, resveratrol inhibited cell proliferation at all concentrations lower than 10 nM (Ashby et al. 1999; Bowers et al. 2000; Gehm et al. 1997; Lu and Serrero 1999; Schmitt et al. 2002). Similarly, low concentrations of quercetin and genistein reduced proliferation or had no stimulatory effect on ER-negative MDA-MB-231, HCC-38, and HeLa cells (Balabhadrapathruni et al. 2000; van der Woude et al. 2005).
The effects of phytoestrogens on the growth and proliferation of tumor cells have also been evaluated in vivo, using animal models of breast cancer and breast cancer cell xenografts. Genistein and soy protein stimulated the growth of MCF7 breast cancer cell xenografts implanted in mice (Allred et al. 2001; Hsieh et al. 1998). Similarly, genistein, in the presence of low levels of E2, acted in an additive manner to stimulate the growth of MCF7 tumors in mice (Ju et al. 2006a). However, other studies have generated conflicting results. Genistein inhibited the growth of both ER-positive and ER-negative breast cancer xenografts and induced apoptosis in tumor cells (Shao et al. 1998). Similarly, resveratrol reduced tumor growth and increased apoptosis in ER-α–negative/ER-β–positive MDA-MB-231 tumor xeno-grafts (Garvin et al. 2006).
The effects of phytoestrogens on cell growth and proliferation may be explained by their ability to alter the expression of a number of proteins that control cell cycle and induce cell cycle arrest and apoptosis. In MCF7 and MDA-MB-231 cells, resveratrol caused cells to accumulate in the S-phase and down-regulated Bcl-2, resulting in apoptosis (Pozo-Guisado et al. 2002, 2005). The effect of resveratrol on the cell cycle is suggested to be mediated by its opposing effects on cell cycle regulators. Resveratrol increased the expression and activity of positive G1/S and G2/M cell cycle regulators, while simultaneously increasing protein levels of p21, p53, and p27 (Pozo-Guisado et al. 2002). Similarly, both genistein and quercetin caused G2/M arrest and apoptosis in MDA-MB-231 cells (Balabhadrapathruni et al. 2000). Increased cyclin B1 protein levels were observed in MDA-MB-231 cells following exposure to low doses of genistein, but MDA-MB-231 cells exposed to high concentrations of genistein displayed decreased levels of cyclin B1 and phosphorylated Cdc2 (Balabhadrapathruni et al. 2000). Further, daidzein was shown to alter cell cycle distribution and induce apoptosis in HeLa cells (Guo et al. 2004).
To resolve the dilemma regarding the potential beneficial or harmful effects of phytoestrogens in breast cancer development, numerous studies have attempted to characterize the estrogenic and growth-stimulatory actions of phytoestrogens. Most of these studies have been carried out in transformed breast cancer cell lines. To further our understanding of the proliferative effects of phytoestrogens, studies need to be performed in both nontumorigenic and tumorigenic breast cells with varying ER status and in environments with varying estrogen concentrations.
Phytoestrogens and Tumor Development
Various phytoestrogens have been evaluated for their ability to prevent chemically induced mammary carcinogenesis. Resveratrol blocked the formation of preneoplastic lesions, suppressed mammary carcinogenesis, reduced tumor incidence, and increased tumor latency in Sprague-Dawley rats treated with dimethylbenz[a]anthracene (DMBA) (Whitsett et al. 2006). Both resveratrol and quercetin have been shown to inhibit N-methyl-N-nitrosourea (NMU) and DMBA-induced mammary carcinogenesis in rats (Banerjee et al. 2002; Bhat et al. 2001; Verma et al. 1988). Resveratrol decreased NMU- and DMBA-induced tumor incidence and multiplicity by 50% in Sprague-Dawley rats (Banerjee et al. 2002; Bhat et al. 2001). Daidzein inhibited DMBA-induced mammary tumors in rats and significantly increased tumor latency in mouse mammary tumor virus-neu mice (Constantinou et al. 2001; Jin and MacDonald 2002). Similarly, several studies have demonstrated that rats exposed to genistein early in life have a decreased incidence of DMBA-induced mammary tumors in adulthood (Fritz et al. 1998; Hilakivi-Clarke et al. 1999; Lamartiniere et al. 1998; Murrill et al. 1996). However, others have reported that genistein increased tumor cross-sectional area, increased tumor multiplicity, elevated the percentage of proliferative cells in tumors and increased the weight of estrogen-dependent mammary adenocarcinomas in rat models of mammary cancer (Allred et al. 2004; Kijkuokool et al. 2006).
In addition, phytoestrogen exposure has been shown to alter breast development (Figure 2; Appendix 1). Resveratrol-exposed female rats displayed more differentiated lobular structures and decreased proliferation in the mammary terminal ductal structures, making them less vulnerable to damage by carcinogens (Whitsett et al. 2006). Exposure to genistein during breast development altered breast morphology and resulted in decreased terminal ductal formation (Hilakivi-Clarke et al. 1999). However, the relationship between phytoestrogens and tumor development does not always appear to be protective and may rely on age at exposure and the hormonal environment. Quercetin potentiated the severity of E2-induced kidney tumorigenesis in male Syrian hamsters, and prepubescent rats treated with resveratrol showed accelerated NMU-induced mammary carcinogenesis and elevated tumor incidence and multiplicity (Sato et al. 2003; Zhu and Liehr 1994).
Taken together, these studies indicate that certain phytoestrogens might reduce the risk of chemically induced mammary cancers in animal models, particularly if exposure is early in life. However, very few studies have focused on the effect of phytoestrogens on estrogen-induced breast cancers, which would be the most relevant model for gaining insight into the relationship between phytoestrogens and human mammary carcinogenesis.
Phytoestrogens and Signaling Pathways
The influence of ERs on the transcription of estrogen-sensitive genes is not limited to ERE binding. An increasing body of evidence suggests that both ER-α and ER-β participate in some of the signaling cascades responsible for controlling gene expression, cell cycle, cell proliferation and apoptosis (Levin 1999; Marquez and Pietras 2001; Pietras et al. 2005a, 2005b). Several phytoestrogens have been shown to modulate the activity of ER-associated signaling cascades and transcription factors. In human breast cells, resveratrol inhibited ER-α–associated PI3K activity, thereby exerting an inhibitory effect on cell proliferation and survival (Pozo-Guisado et al. 2005). Genistein and daidzein activated Akt in the ER-α–positive T47D breast cancer cell line, whereas resveratrol inhibited Akt phosphorylation (Brownson et al. 2002). In the ER-α–negative MDA-MB-231 breast cancer cell line, resveratrol and daidzein activated Akt but genistein did not (Brownson et al. 2002). Resveratrol has been shown to modulate nuclear factor kappa B (NFκB) and AP-1 activation in various cancer cell lines, leading to the speculation that NFκB and AP-1 are potential targets of resveratrol (Banerjee et al. 2002; Kundu and Surh 2004). In MCF7 cells and chemically induced rat mammary tumors, resveratrol inhibited the DNA-binding activity of NFκB (Banerjee et al. 2002). Resveratrol inhibited extracellular signal–regulated kinase (ERK) and p38 mitogen-activated protein kinase (MAPK) activation in mouse skin cells, suggesting that resveratrol may inhibit the activation of NFκB and AP-1 at the level of their upstream kinases, ERK and p38 MAPK (Yu et al. 2001). Similarly, genistein blocked the NFκB signaling pathway via an Akt-dependent mechanism in both MDA-MB-231 breast cancer cells and PC3 prostate cancer cells (Gong et al. 2003; Li and Sarkar 2002). Genistein and daidzein suppressed NFκB activation in TNFα-stimulated mouse fibroblasts and in ER-negative breast cancer cells by a mechanism that involved abrogation of MEK1 and ERK activity (Vanden Berghe et al. 2006). Treatment of mouse fibroblasts with the antiestrogen ICI 182780 failed to reverse the effects of daidzein and genistein on NFκB-dependent gene expression, indicating that suppression of NFκB is independent of the estrogenic activity of phytoestrogens (Vanden Berghe et al. 2006).
Recently, microarray technologies have been exploited in order to clarify the estrogenic effects of phytoestrogen exposure on gene expression and signaling pathways (Chen et al. 2003; Ise et al. 2005; Naciff et al. 2002). One such study compared the effects of genistein and E2 exposure on the reproductive tissues of the developing rat fetus, specifically the ovaries and uterus (Naciff et al. 2002). Expression patterns of genes whose products were involved in cell growth, differentiation, stress response and apoptosis were modulated by both genistein and E2. However, genistein exposure altered the expression patterns of a number of genes in a manner distinct from E2 (Naciff et al. 2002). Genistein increased the expression of MAPK and topisomerase II, whereas the expression of phospholipase A2 was down-regulated. In contrast, the expression of these genes was not affected by E2 exposure (Naciff et al. 2002). Another study, in which MCF7 breast cancer cells were treated with genistein, showed down-regulation of genes whose products are associated with cell growth, DNA replication, and growth factor response (Chen et al. 2003). In a separate study, gene expression profiles in MCF7 breast cancer cells were evaluated after exposure to either phytoestrogens or E2 (Iseet al. 2005). The authors reported similar but distinct expression patterns for each of the phytoestrogens tested and analyses revealed that the phytoestrogen exposure induced expression profiles with differing degrees of similarity to E2 (Ise et al. 2005).
Phytoestrogens and Estrogen-Metabolizing Enzymes
Once ingested, phytoestrogens interact with many of the same enzymes as endogenous estrogens and have been shown to interfere with the process of estrogen metabolism (Moon et al. 2006). Several phytoestrogens are known to modify the CYP450 enzyme system by either inducing or suppressing the transcription of CYP450 enzymes or by inhibiting or enhancing enzyme activity (Figure 2; Appendix 1) (Moon et al. 2006). The expression of two main estrogen-metabolizing enzymes, Cyp1A1 and Cyp1B1, is under the control of the aryl hydrocarbon receptor (AhR), a ligand-activated transcription factor, which binds a specific DNA sequence designated xenobiotic response element (XRE) in the promoter regions of its target genes (Nebert et al. 2000).
Resveratrol, genistein and quercetin have been shown to decrease both xenobiotic-induced transcription and activity of Cyp1A1 and Cyp1B1 in numerous cell types (Berge et al. 2004; Chan et al. 2003; Chang et al. 2001; Chun et al. 1999; Ciolino and Yeh 1999; Han et al. 2006; Lee and Safe 2001; Ramadass et al. 2003; Roberts et al. 2004; Shertzer et al. 1999). Resveratrol is an AhR antagonist and is suggested to exert its inhibitory effects on Cyp1A1 and Cyp1B1 expression either by suppressing AhR DNA-binding activity or by preventing the interaction between AhR and the transcriptional complex, thereby blocking induction of AhR-mediated genes (Casper et al. 1999; Chen et al. 2004). Similarly, genistein and quercetin are suspected to decrease xenobiotic-induced Cyp1A1 and Cyp1B1 mRNA expression by interfering with activation of the XRE by AhR (Chan et al. 2003; Ramadass et al. 2003). In contrast, quercetin has been shown to both increase and decrease Cyp1A1 enzyme activity (Ciolino et al. 1999; Ramadass et al. 2003).
Not only do phytoestrogens interact with the Cyp450 enzyme system, quercetin inhibits the O-methylation of endogenous estrogens by catechol-O-methyltransferase (COMT) by a combination of three mechanisms—direct competition for COMT, noncompetitive inhibition via an increase in S-adenosyl-L-homocysteine (SAH) concentrations and by reducing the availability of the methyl donor S-adenosyl methionine (SAM) (Zhu 2002). Various studies have established that quercetin is an excellent substrate for COMT, having a metabolic rate up to 30 times higher than catechol estrogens (Zhu 2002). In Syrian hamsters treated with E2, quercetin increased the concentrations of 2-hydroxyestradiol (2-OHE2) and 4-hydroxyestradiol (4-OHE2) in kidney and decreased urinary excretion of 2-methoxyestradiol (2-MeOHE2) and 4-methoxyestradiol (4-MeOHE2) (Zhu and Liehr 1996).
The evidence that phytoestrogens alter estrogen-metabolizing enzymes is not limited to in vitro data. Data from nonhuman primate studies suggest that exposure to phytoestrogens alters the pathways of estrogen metabolism by Cyp1A1 and Cyp1B1 in vivo such that it is shifted toward the production of fewer genotoxic metabolites (Lu et al. 2000b; Wood et al. 2006). However, inhibition of COMT by phytoestrogens would not only lead to elevated tissue levels of the procarcinogenic estrogen metabolite 4-OHE2, but also to decreased levels of the anti-carcinogenic estrogen metabolite 2-MeOHE2 (Badawi et al. 2001; Zhu 2002). Thus, it is
Conclusion
Epidemiologic evidence has been inconclusive regarding the effects of phytoestrogen consumption on breast cancer risk. Nevertheless, extensive research has been performed to provide a detailed description of the possible mechanisms of action of phytoestrogens. Taken together, the research on phytoestrogens, particularly studies relevant to genistein, daidzein, resveratrol, and quercetin, suggests that these compounds do not act by a single mechanism to achieve their effects. Instead, these plant substances exert their effects by way of various mechanisms, including effects on estrogen-metabolizing enzymes, cell cycle, cell differentiation, proliferation, apoptosis, the inflammatory response and various cell signaling pathways.
While there is some evidence supporting a chemoprotective role for phytoestrogens in breast cancer, there is also evidence suggesting the possible adverse effects of phytoestrogen consumption. More research is needed in order to fully evaluate the activities of phytoestrogens and the biological relevance of experimental findings. Future studies may focus elucidating the mechanisms underlying phytoestrogen action and to characterizing the actions of phytoestrogens in different hormonal environments.
Appendix 1. Overview of possible mechanisms of phytoestrogen action
Putative anticancer actions
Inhibition of HSD and Cyp19 to decrease endogenous estrogen levels
Modulation of Cyp450s to reduce ratio of genotoxic estrogens to total estrogens
Activation of ER-β to exert antiproliferative and prodifferentiative effects
Reduction of mammary sensitivity to carcinogens by altering breast development/morphology
Putative procancer actions
Stimulation of growth and proliferation of breast epithelial cells via ER-α activation
Stimulation of growth and proliferation of existing tumors via ER-α activation important for future studies to further elucidate the abilities of phytoestrogens to alter the metabolism of endogenous estrogens, as these processes are critical to understanding the relationship between phytoestrogens and breast cancer development.
Footnotes
This work was supported by National Institute of Health grants CA 109551 and P30 ES 09089CA (H.K.B..).
References
- Adlercreutz H. Epidemiology of phytoestrogens. Baillieres Clin Endocrinol Metab. 1998;12(4):605–623. doi: 10.1016/s0950-351x(98)80007-4. [DOI] [PubMed] [Google Scholar]
- Adlercreutz H. Phytooestrogens and cancer. Lancet Oncol. 2002;3(6):364–373. doi: 10.1016/s1470-2045(02)00777-5. [DOI] [PubMed] [Google Scholar]
- Adlercreutz H, Markkanen H, Watanabe S. Plasma concentrations of phyto-oestrogens in Japanese men. Lancet. 1993;342(8881):1209–1210. doi: 10.1016/0140-6736(93)92188-y. [DOI] [PubMed] [Google Scholar]
- Allred CD, Allred KF, Ju YH, Clausen LM, Doerge DR, Schantz SL, et al. Dietary genistein results in larger MNU-induced, estrogen-dependent mammary tumors following ovariectomy of Sprague-Dawley rats. Carcinogenesis. 2004;25(2):211–218. doi: 10.1093/carcin/bgg198. [DOI] [PubMed] [Google Scholar]
- Allred CD, Allred KF, Ju YH, Virant SM, Helferich WG. Soy diets containing varying amounts of genistein stimulate growth of estrogen-dependent (MCF-7) tumors in a dose-dependent manner. Cancer Res. 2001;61(13):5045–5050. [PubMed] [Google Scholar]
- American Cancer Society. Cancer Statistics 2006. 2006. [[accessed 11 November 2007]]. Available: http://www.cancer.org/docroot/PRO/content/PRO_1_1_Cancer_Statistics_2006_presentation.asp.
- An J, Tzagarakis-Foster C, Scharschmidt TC, Lomri N, Leitman DC. Estrogen receptor beta-selective transcriptional activity and recruitment of coregulators by phytoestrogens. J Biol Chem. 2001;276(21):17808–17814. doi: 10.1074/jbc.M100953200. [DOI] [PubMed] [Google Scholar]
- Arai Y, Uehara M, Sato Y, Kimira M, Eboshida A, Adlercreutz H, et al. Comparison of isoflavones among dietary intake, plasma concentration and urinary excretion for accurate estimation of phytoestrogen intake. J Epidemiol. 2000;10(2):127–135. doi: 10.2188/jea.10.127. [DOI] [PubMed] [Google Scholar]
- Ashby J, Tinwell H, Pennie W, Brooks AN, Lefevre PA, Beresford N, et al. Partial and weak oestrogenicity of the red wine constituent resveratrol: consideration of its superagonist activity in MCF-7 cells and its suggested cardiovascular protective effects. J Appl Toxicol. 1999;19(1):39–45. doi: 10.1002/(sici)1099-1263(199901/02)19:1<39::aid-jat534>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- Badawi AF, Cavalieri EL, Rogan EG. Role of human cytochrome P450 1A1, 1A2, 1B1, and 3A4 in the 2-, 4-, and 16alpha-hydroxylation of 17beta-estradiol. Metabolism. 2001;50(9):1001–1003. doi: 10.1053/meta.2001.25592. [DOI] [PubMed] [Google Scholar]
- Balabhadrapathruni S, Thomas TJ, Yurkow EJ, Amenta PS, Thomas T. Effects of genistein and structurally related phytoestrogens on cell cycle kinetics and apoptosis in MDA-MB-468 human breast cancer cells. Oncol Rep. 2000;7(1):3–12. [PubMed] [Google Scholar]
- Balfe P, McCann A, McGoldrick A, McAllister K, Kennedy M, Dervan P, et al. Estrogen receptor alpha and beta profiling in human breast cancer. Eur J Surg Oncol. 2004;30(5):469–474. doi: 10.1016/j.ejso.2004.02.010. [DOI] [PubMed] [Google Scholar]
- Banerjee S, Bueso-Ramos C, Aggarwal BB. Suppression of 7,12-dimethylbenz(a)anthracene-induced mammary carcinogenesis in rats by resveratrol: role of nuclear factor-kappaB, cyclooxygenase 2, and matrix metalloprotease 9. Cancer Res. 2002;62(17):4945–4954. [PubMed] [Google Scholar]
- Barkhem T, Carlsson B, Nilsson Y, Enmark E, Gustafsson J, Nilsson S. Differential response of estrogen receptor alpha and estrogen receptor beta to partial estrogen agonists/antagonists. Mol Pharmacol. 1998;54(1):105–112. doi: 10.1124/mol.54.1.105. [DOI] [PubMed] [Google Scholar]
- Berge G, Ovrebo S, Botnen IV, Hewer A, Phillips DH, Haugen A, et al. Resveratrol inhibits benzo[a]pyrene-DNA adduct formation in human bronchial epithelial cells. Br J Cancer. 2004;91(2):333–338. doi: 10.1038/sj.bjc.6601898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhat HK, Calaf G, Hei TK, Loya T, Vadgama JV. Critical role of oxidative stress in estrogen-induced carcinogenesis. Proc Natl Acad Sci USA. 2003;100(7):3913–3918. doi: 10.1073/pnas.0437929100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhat KP, Lantvit D, Christov K, Mehta RG, Moon RC, Pezzuto JM. Estrogenic and antiestrogenic properties of resveratrol in mammary tumor models. Cancer Res. 2001;61(20):7456–7463. [PubMed] [Google Scholar]
- Bowers JL, Tyulmenkov VV, Jernigan SC, Klinge CM. Resveratrol acts as a mixed agonist/antagonist for estrogen receptors alpha and beta. Endocrinology. 2000;141(10):3657–3667. doi: 10.1210/endo.141.10.7721. [DOI] [PubMed] [Google Scholar]
- Brooks JD, Thompson LU. Mammalian lignans and genistein decrease the activities of aromatase and 17beta-hydroxysteroid dehydrogenase in MCF-7 cells. J Steroid Biochem Mol Biol. 2005;94(5):461–467. doi: 10.1016/j.jsbmb.2005.02.002. [DOI] [PubMed] [Google Scholar]
- Brownson DM, Azios NG, Fuqua BK, Dharmawardhane SF, Mabry TJ. Flavonoid effects relevant to cancer. J Nutr. 2002;132(suppl 11 ):3482S–3489S. doi: 10.1093/jn/132.11.3482S. [DOI] [PubMed] [Google Scholar]
- Casper RF, Quesne M, Rogers IM, Shirota T, Jolivet A, Milgrom E, et al. Resveratrol has antagonist activity on the aryl hydrocarbon receptor: implications for prevention of dioxin toxicity. Mol Pharmacol. 1999;56(4):784–790. [PubMed] [Google Scholar]
- Cavalieri E, Chakravarti D, Guttenplan J, Hart E, Ingle J, Jankowiak R, et al. Catechol estrogen quinones as initiators of breast and other human cancers: implications for biomarkers of susceptibility and cancer prevention. Biochim Biophys Acta. 2006;1766(1):63–78. doi: 10.1016/j.bbcan.2006.03.001. [DOI] [PubMed] [Google Scholar]
- Chan HY, Wang H, Leung LK. The red clover (Trifolium pratense) isoflavone biochanin A modulates the biotrans-formation pathways of 7,12-dimethylbenz[a]anthracene. Br J Nutr. 2003;90(1):87–92. doi: 10.1079/bjn2003868. [DOI] [PubMed] [Google Scholar]
- Chang TK, Chen J, Lee WB. Differential inhibition and inactivation of human CYP1 enzymes by trans-resveratrol: evidence for mechanism-based inactivation of CYP1A2. J Pharmacol Exp Ther. 2001;299(3):874–882. [PubMed] [Google Scholar]
- Chen WF, Huang MH, Tzang CH, Yang M, Wong MS. Inhibitory actions of genistein in human breast cancer (MCF-7) cells. Biochim Biophys Acta. 2003;1638(2):187–196. doi: 10.1016/s0925-4439(03)00082-6. [DOI] [PubMed] [Google Scholar]
- Chen ZH, Hurh YJ, Na HK, Kim JH, Chun YJ, Kim DH, et al. Resveratrol inhibits TCDD-induced expression of CYP1A1 and CYP1B1 and catechol estrogen-mediated oxidative DNA damage in cultured human mammary epithelial cells. Carcinogenesis. 2004;25(10):2005–2013. doi: 10.1093/carcin/bgh183. [DOI] [PubMed] [Google Scholar]
- Chun YJ, Kim MY, Guengerich FP. Resveratrol is a selective human cytochrome P450 1A1 inhibitor. Biochem Biophys Res Commun. 1999;262(1):20–24. doi: 10.1006/bbrc.1999.1152. [DOI] [PubMed] [Google Scholar]
- Ciolino HP, Daschner PJ, Yeh GC. Dietary flavonols quercetin and kaempferol are ligands of the aryl hydrocarbon receptor that affect CYP1A1 transcription differentially. Biochem J. 1999;340 (pt 3):715–722. [PMC free article] [PubMed] [Google Scholar]
- Ciolino HP, Yeh GC. Inhibition of aryl hydrocarbon-induced cytochrome P-450 1A1 enzyme activity and CYP1A1 expression by resveratrol. Mol Pharmacol. 1999;56(4):760–767. [PubMed] [Google Scholar]
- Constantinou AI, Lantvit D, Hawthorne M, Xu X, van Breemen RB, Pezzuto JM. Chemopreventive effects of soy protein and purified soy isoflavones on DMBA-induced mammary tumors in female Sprague-Dawley rats. Nutr Cancer. 2001;41(1–2):75–81. doi: 10.1080/01635581.2001.9680615. [DOI] [PubMed] [Google Scholar]
- Duncan AM, Underhill KE, Xu X, Lavalleur J, Phipps WR, Kurzer MS. Modest hormonal effects of soy isoflavones in postmenopausal women. J Clin Endocrinol Metab. 1999;84(10):3479–3484. doi: 10.1210/jcem.84.10.6067. [DOI] [PubMed] [Google Scholar]
- Evans BA, Griffiths K, Morton MS. Inhibition of 5 alpha-reductase in genital skin fibroblasts and prostate tissue by dietary lignans and isoflavonoids. J Endocrinol. 1995;147(2):295–302. doi: 10.1677/joe.0.1470295. [DOI] [PubMed] [Google Scholar]
- Fritz WA, Coward L, Wang J, Lamartiniere CA. Dietary genistein: perinatal mammary cancer prevention, bioavailability and toxicity testing in the rat. Carcinogenesis. 1998;19(12):2151–2158. doi: 10.1093/carcin/19.12.2151. [DOI] [PubMed] [Google Scholar]
- Garvin S, Ollinger K, Dabrosin C. Resveratrol induces apoptosis and inhibits angiogenesis in human breast cancer xenografts in vivo. Cancer Lett. 2006;231(1):113–122. doi: 10.1016/j.canlet.2005.01.031. [DOI] [PubMed] [Google Scholar]
- Gehm BD, McAndrews JM, Chien PY, Jameson JL. Resveratrol, a polyphenolic compound found in grapes and wine, is an agonist for the estrogen receptor. Proc Natl Acad Sci USA. 1997;94(25):14138–14143. doi: 10.1073/pnas.94.25.14138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gikas PD, Mokbel K. Phytoestrogens and the risk of breast cancer: a review of the literature. Int J Fertil Womens Med. 2005;50(6):250–258. [PubMed] [Google Scholar]
- Gong L, Li Y, Nedeljkovic-Kurepa A, Sarkar FH. Inactivation of NF-kappaB by genistein is mediated via Akt signaling pathway in breast cancer cells. Oncogene. 2003;22(30):4702–4709. doi: 10.1038/sj.onc.1206583. [DOI] [PubMed] [Google Scholar]
- Griffiths LA, Smith GE. Metabolism of apigenin and related compounds in the rat. Metabolite formation in vivo and by the intestinal microflora in vitro. Biochem J. 1972;128(4):901–911. doi: 10.1042/bj1280901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunnarsson C, Ahnstrom M, Kirschner K, Olsson B, Nordenskjold B, Rutqvist LE, et al. Amplification of HSD17B1 and ERBB2 in primary breast cancer. Oncogene. 2003;22(1):34–40. doi: 10.1038/sj.onc.1206078. [DOI] [PubMed] [Google Scholar]
- Guo JM, Kang GZ, Xiao BX, Liu DH, Zhang S. Effect of daidzein on cell growth, cell cycle, and telomerase activity of human cervical cancer in vitro. Int J Gynecol Cancer. 2004;14(5):882–888. doi: 10.1111/j.1048-891X.2004.14525.x. [DOI] [PubMed] [Google Scholar]
- Hall JM, McDonnell DP. The estrogen receptor betaisoform (ERbeta) of the human estrogen receptor modulates ERalpha transcriptional activity and is a key regulator of the cellular response to estrogens and antiestrogens. Endocrinology. 1999;140(12):5566–5578. doi: 10.1210/endo.140.12.7179. [DOI] [PubMed] [Google Scholar]
- Han EH, Kim JY, Jeong HG. Effect of biochanin A on the aryl hydrocarbon receptor and cytochrome P450 1A1 in MCF-7 human breast carcinoma cells. Arch Pharm Res. 2006;29(7):570–576. doi: 10.1007/BF02969267. [DOI] [PubMed] [Google Scholar]
- Hargreaves DF, Potten CS, Harding C, Shaw LE, Morton MS, Roberts SA, et al. Two-week dietary soy supplementation has an estrogenic effect on normal premenopausal breast. J Clin Endocrinol Metab. 1999;84(11):4017–4024. doi: 10.1210/jcem.84.11.6152. [DOI] [PubMed] [Google Scholar]
- Harris DM, Besselink E, Henning SM, Go VL, Heber D. Phytoestrogens induce differential estrogen receptor alpha- or beta-mediated responses in transfected breast cancer cells. Exp Biol Med (Maywood) 2005;230(8):558–568. doi: 10.1177/153537020523000807. [DOI] [PubMed] [Google Scholar]
- Hilakivi-Clarke L, Onojafe I, Raygada M, Cho E, Skaar T, Russo I, et al. Prepubertal exposure to zearalenone or genistein reduces mammary tumorigenesis. Br J Cancer. 1999;80(11):1682–1688. doi: 10.1038/sj.bjc.6690584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirayama T. A large scale cohort study on the effect of life styles on the risk of cancer by each site [in Japanese] Gan No Rinsho. 1990a Spec No:233–242. [PubMed] [Google Scholar]
- Hirayama T. Contribution of a long-term prospective cohort study to the issue of nutrition and cancer with special reference to the role of alcohol drinking. Prog Clin Biol Res. 1990b;346:179–187. [PubMed] [Google Scholar]
- Hirohata T, Shigematsu T, Nomura AM, Nomura Y, Horie A, Hirohata I. Occurrence of breast cancer in relation to diet and reproductive history: a case-control study in Fukuoka, Japan. Natl Cancer Inst Monogr. 1985;69:187–190. [PubMed] [Google Scholar]
- Hirose K, Tajima K, Hamajima N, Inoue M, Takezaki T, Kuroishi T, et al. A large-scale, hospital-based case-control study of risk factors of breast cancer according to menopausal status. Jpn J Cancer Res. 1995;86(2):146–154. doi: 10.1111/j.1349-7006.1995.tb03032.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsieh CY, Santell RC, Haslam SZ, Helferich WG. Estrogenic effects of genistein on the growth of estrogen receptor-positive human breast cancer (MCF-7) cells in vitro and in vivo. Cancer Res. 1998;58(17):3833–3838. [PubMed] [Google Scholar]
- IARC (International Agency for Research on Cancer) Overall Evaluations of Carcinogenicity: An Updating of IARC Monographs Volumes 1 to 42. IARC Monogr Eval Carcinog Risks Hum Suppl. 1987;7:272–310. [PubMed] [Google Scholar]
- IARC (International Agency for Research on Cancer) Hormonal contraception and postmenopausal hormone therapy. IARC Monogr Eval Carcinog Risks Hum. 1999;72:474–530. [Google Scholar]
- IIse R, Han D, Takahashi Y, Terasaka S, Inoue A, Tanji M, et al. Expression profiling of the estrogen responsive genes in response to phytoestrogens using a customized DNA microarray. FEBS Lett. 2005;579(7):1732–1740. doi: 10.1016/j.febslet.2005.02.033. [DOI] [PubMed] [Google Scholar]
- Jeong HJ, Shin YG, Kim IH, Pezzuto JM. Inhibition of aromatase activity by flavonoids. Arch Pharm Res. 1999;22(3):309–312. doi: 10.1007/BF02976369. [DOI] [PubMed] [Google Scholar]
- Jin Z, MacDonald RS. Soy isoflavones increase latency of spontaneous mammary tumors in mice. J Nutr. 2002;132(10):3186–3190. doi: 10.1093/jn/131.10.3186. [DOI] [PubMed] [Google Scholar]
- Ju YH, Allred KF, Allred CD, Helferich WG. Genistein stimulates growth of human breast cancer cells in a novel, postmenopausal animal model, with low plasma estradiol concentrations. Carcinogenesis. 2006a;27(6):1292–1299. doi: 10.1093/carcin/bgi370. [DOI] [PubMed] [Google Scholar]
- Ju YH, Fultz J, Allred KF, Doerge DR, Helferich WG. Effects of dietary daidzein and its metabolite, equol, at physiological concentrations on the growth of estrogen-dependent human breast cancer (MCF-7) tumors implanted in ovariectomized athymic mice. Carcinogenesis. 2006b;27(4):856–863. doi: 10.1093/carcin/bgi320. [DOI] [PubMed] [Google Scholar]
- Key TJ, Sharp GB, Appleby PN, Beral V, Goodman MT, Soda M, et al. Soya foods and breast cancer risk: a prospective study in Hiroshima and Nagasaki, Japan. Br J Cancer. 1999;81(7):1248–1256. doi: 10.1038/sj.bjc.6690837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kijkuokool P, Parhar IS, Malaivijitnond S. Genistein enhances N-nitrosomethylurea-induced rat mammary tumorigenesis. Cancer Lett. 2006;242(1):53–59. doi: 10.1016/j.canlet.2005.10.033. [DOI] [PubMed] [Google Scholar]
- Koehler KF, Helguero LA, Haldosen LA, Warner M, Gustafsson JA. Reflections on the discovery and significance of estrogen receptor beta. Endocr Rev. 2005;26(3):465–478. doi: 10.1210/er.2004-0027. [DOI] [PubMed] [Google Scholar]
- Kostelac D, Rechkemmer G, Briviba K. Phytoestrogens modulate binding response of estrogen receptors alpha and beta to the estrogen response element. J Agric Food Chem. 2003;51(26):7632–7635. doi: 10.1021/jf034427b. [DOI] [PubMed] [Google Scholar]
- Kuiper GG, Carlsson B, Grandien K, Enmark E, Haggblad J, Nilsson S, et al. Comparison of the ligand binding specificity and transcript tissue distribution of estrogen receptors alpha and beta. Endocrinology. 1997;138(3):863–870. doi: 10.1210/endo.138.3.4979. [DOI] [PubMed] [Google Scholar]
- Kuiper GG, Lemmen JG, Carlsson B, Corton JC, Safe SH, van der Saag PT, et al. Interaction of estrogenic chemicals and phytoestrogens with estrogen receptor beta. Endocrinology. 1998;139(10):4252–4263. doi: 10.1210/endo.139.10.6216. [DOI] [PubMed] [Google Scholar]
- Kumar NB, Cantor A, Allen K, Riccardi D, Cox CE. The specific role of isoflavones on estrogen metabolism in pre-menopausal women. Cancer. 2002;94(4):1166–1174. doi: 10.1002/cncr.10320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kundu JK, Surh YJ. Molecular basis of chemoprevention by resveratrol: NF-kappaB and AP-1 as potential targets. Mutat Res. 2004;555(1–2):65–80. doi: 10.1016/j.mrfmmm.2004.05.019. [DOI] [PubMed] [Google Scholar]
- Lamartiniere CA. Protection against breast cancer with genistein: a component of soy. Am J Clin Nutr. 2000;71(suppl 6):1705S–1707S. 1708S–1709S. doi: 10.1093/ajcn/71.6.1705S. [DOI] [PubMed] [Google Scholar]
- Lamartiniere CA, Zhang JX, Cotroneo MS. Genistein studies in rats: potential for breast cancer prevention and reproductive and developmental toxicity. Am J Clin Nutr. 1998;68(suppl 6):1400S–1405S. doi: 10.1093/ajcn/68.6.1400S. [DOI] [PubMed] [Google Scholar]
- Le Bail JC, Champavier Y, Chulia AJ, Habrioux G. Effects of phytoestrogens on aromatase, 3beta and 17beta-hydroxy-steroid dehydrogenase activities and human breast cancer cells. Life Sci. 2000;66(14):1281–1291. doi: 10.1016/s0024-3205(00)00435-5. [DOI] [PubMed] [Google Scholar]
- Le Bail JC, Laroche T, Marre-Fournier F, Habrioux G. Aromatase and 17beta-hydroxysteroid dehydrogenase inhibition by flavonoids. Cancer Lett. 1998;133(1):101–106. doi: 10.1016/s0304-3835(98)00211-0. [DOI] [PubMed] [Google Scholar]
- Lee JE, Safe S. Involvement of a post-transcriptional mechanism in the inhibition of CYP1A1 expression by resveratrol in breast cancer cells. Biochem Pharmacol. 2001;62(8):1113–1124. doi: 10.1016/s0006-2952(01)00763-8. [DOI] [PubMed] [Google Scholar]
- Lehmann L, Esch HL, Wagner J, Rohnstock L, Metzler M. Estrogenic and genotoxic potential of equol and two hydroxylated metabolites of daidzein in cultured human Ishikawa cells. Toxicol Lett. 2005;158(1):72–86. doi: 10.1016/j.toxlet.2005.02.011. [DOI] [PubMed] [Google Scholar]
- Levin ER. Cellular functions of the plasma membrane estrogen receptor. Trends Endocrinol Metab. 1999;10(9):374–377. doi: 10.1016/s1043-2760(99)00192-7. [DOI] [PubMed] [Google Scholar]
- Li KX, Smith RE, Krozowski ZS. Cloning and expression of a novel tissue specific 17beta-hydroxysteroid dehydrogenase. Endocr Res. 1998;24(3–4):663–667. doi: 10.3109/07435809809032667. [DOI] [PubMed] [Google Scholar]
- Li Y, Sarkar FH. Inhibition of nuclear factor kappaB activation in PC3 cells by genistein is mediated via Akt signaling pathway. Clin Cancer Res. 2002;8(7):2369–2377. [PubMed] [Google Scholar]
- Liu J, Knappenberger KS, Kack H, Andersson G, Nilsson E, Dartsch C, et al. A homogeneous in vitro functional assay for estrogen receptors: coactivator recruitment. Mol Endocrinol. 2003;17(3):346–355. doi: 10.1210/me.2002-0331. [DOI] [PubMed] [Google Scholar]
- Lu LJ, Anderson KE, Grady JJ, Kohen F, Nagamani M. Decreased ovarian hormones during a soya diet: implications for breast cancer prevention. Cancer Res. 2000a;60(15):4112–4121. [PubMed] [Google Scholar]
- Lu LJ, Cree M, Josyula S, Nagamani M, Grady JJ, Anderson KE. Increased urinary excretion of 2-hydroxyestrone but not 16alpha-hydroxyestrone in premenopausal women during a soya diet containing isoflavones. Cancer Res. 2000b;60(5):1299–1305. [PubMed] [Google Scholar]
- Lu R, Serrero G. Resveratrol, a natural product derived from grape, exhibits antiestrogenic activity and inhibits the growth of human breast cancer cells. J Cell Physiol. 1999;179(3):297–304. doi: 10.1002/(SICI)1097-4652(199906)179:3<297::AID-JCP7>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- Maggiolini M, Bonofiglio D, Marsico S, Panno ML, Cenni B, Picard D, et al. Estrogen receptor alpha mediates the proliferative but not the cytotoxic dose-dependent effects of two major phytoestrogens on human breast cancer cells. Mol Pharmacol. 2001;60(3):595–602. [PubMed] [Google Scholar]
- Markaverich BM, Shoulars K, Brown MA. Purification and characterization of nuclear type II [(3)H]estradiol binding sites from the rat uterus: covalent labeling with [( 3)H]luteolin. Steroids. 2001;66(9):707–719. doi: 10.1016/s0039-128x(01)00099-x. [DOI] [PubMed] [Google Scholar]
- Marquez DC, Pietras RJ. Membrane-associated binding sites for estrogen contribute to growth regulation of human breast cancer cells. Oncogene. 2001;20(39):5420–5430. doi: 10.1038/sj.onc.1204729. [DOI] [PubMed] [Google Scholar]
- Maskarinec G, Williams AE, Inouye JS, Stanczyk FZ, Franke AA. A randomized isoflavone intervention among premenopausal women. Cancer Epidemiol Biomarkers Prev. 2002;11(2):195–201. [PubMed] [Google Scholar]
- McCarty MF. Isoflavones made simple–genistein’s agonist activity for the beta-type estrogen receptor mediates their health benefits. Med Hypotheses. 2006;66(6):1093–1114. doi: 10.1016/j.mehy.2004.11.046. [DOI] [PubMed] [Google Scholar]
- McDonnell DP. The molecular determinants of estrogen receptor pharmacology. Maturitas. 2004;48 (suppl 1):S7–S12. doi: 10.1016/j.maturitas.2004.03.006. [DOI] [PubMed] [Google Scholar]
- Messina M, McCaskill-Stevens W, Lampe JW. Addressing the soy and breast cancer relationship: review, commentary, and workshop proceedings. J Natl Cancer Inst. 2006;98(18):1275–1284. doi: 10.1093/jnci/djj356. [DOI] [PubMed] [Google Scholar]
- Moon YJ, Wang X, Morris ME. Dietary flavonoids: effects on xenobiotic and carcinogen metabolism. Toxicol In Vitro. 2006;20(2):187–210. doi: 10.1016/j.tiv.2005.06.048. [DOI] [PubMed] [Google Scholar]
- Morito K, Hirose T, Kinjo J, Hirakawa T, Okawa M, Nohara T, et al. Interaction of phytoestrogens with estrogen receptors alpha and beta. Biol Pharm Bull. 2001;24(4):351–356. doi: 10.1248/bpb.24.351. [DOI] [PubMed] [Google Scholar]
- Morton MS, Arisaka O, Miyake N, Morgan LD, Evans BA. Phytoestrogen concentrations in serum from Japanese men and women over forty years of age. J Nutr. 2002;132(10):3168–3171. doi: 10.1093/jn/131.10.3168. [DOI] [PubMed] [Google Scholar]
- Mueller SO, Simon S, Chae K, Metzler M, Korach KS. Phytoestrogens and their human metabolites show distinct agonistic and antagonistic properties on estrogen receptor alpha (ERalpha) and ERbeta in human cells. Toxicol Sci. 2004;80(1):14–25. doi: 10.1093/toxsci/kfh147. [DOI] [PubMed] [Google Scholar]
- Murrill WB, Brown NM, Zhang JX, Manzolillo PA, Barnes S, Lamartiniere CA. Prepubertal genistein exposure suppresses mammary cancer and enhances gland differentiation in rats. Carcinogenesis. 1996;17(7):1451–1457. doi: 10.1093/carcin/17.7.1451. [DOI] [PubMed] [Google Scholar]
- Myllymaki S, Haavisto T, Vainio M, Toppari J, Paranko J. In vitro effects of diethylstilbestrol, genistein, 4-tert-butylphenol, and 4-tert-octylphenol on steroidogenic activity of isolated immature rat ovarian follicles. Toxicol Appl Pharmacol. 2005;204(1):69–80. doi: 10.1016/j.taap.2004.08.009. [DOI] [PubMed] [Google Scholar]
- Naciff JM, Jump ML, Torontali SM, Carr GJ, Tiesman JP, Overmann GJ, et al. Gene expression profile induced by 17alpha-ethynyl estradiol, bisphenol A, and genistein in the developing female reproductive system of the rat. Toxicol Sci. 2002;68(1):184–199. doi: 10.1093/toxsci/68.1.184. [DOI] [PubMed] [Google Scholar]
- Nagata C, Takatsuka N, Inaba S, Kawakami N, Shimizu H. Effect of soymilk consumption on serum estrogen concentrations in premenopausal Japanese women. J Natl Cancer Inst. 1998;90(23):1830–1835. doi: 10.1093/jnci/90.23.1830. [DOI] [PubMed] [Google Scholar]
- Nebert DW, Roe AL, Dieter MZ, Solis WA, Yang Y, Dalton TP. Role of the aromatic hydrocarbon receptor and [Ah] gene battery in the oxidative stress response, cell cycle control, and apoptosis. Biochem Pharmacol. 2000;59(1):65–85. doi: 10.1016/s0006-2952(99)00310-x. [DOI] [PubMed] [Google Scholar]
- Nichenametla SN, Taruscio TG, Barney DL, Exon JH. A review of the effects and mechanisms of polyphenolics in cancer. Crit Rev Food Sci Nutr. 2006;46(2):161–183. doi: 10.1080/10408390591000541. [DOI] [PubMed] [Google Scholar]
- Nomura A, Henderson BE, Lee J. Breast cancer and diet among the Japanese in Hawaii. Am J Clin Nutr. 1978;31(11):2020–2025. doi: 10.1093/ajcn/31.11.2020. [DOI] [PubMed] [Google Scholar]
- Nomura AM, Hirohata T, Kolonel LN, Hankin JH, Lee J, Stemmermann G. Breast cancer in Caucasian and Japanese women in Hawaii. Natl Cancer Inst Monogr. 1985;69:191–196. [PubMed] [Google Scholar]
- NTP. 10th Report on Carcinogens. Research Triangle Park, NC: National Toxicology Program; 2002. [Google Scholar]
- Omoto Y, Eguchi H, Yamamoto-Yamaguchi Y, Hayashi S. Estrogen receptor (ER) beta1 and ERbetacx/beta2 inhibit ERalpha function differently in breast cancer cell line MCF7. Oncogene. 2003;22(32):5011–5020. doi: 10.1038/sj.onc.1206787. [DOI] [PubMed] [Google Scholar]
- Pasqualini JR, Chetrite G, Blacker C, Feinstein MC, Delalonde L, Talbi M, et al. Concentrations of estrone, estradiol, and estrone sulfate and evaluation of sulfatase and aromatase activities in pre- and postmenopausal breast cancer patients. J Clin Endocrinol Metab. 1996;81(4):1460–1464. doi: 10.1210/jcem.81.4.8636351. [DOI] [PubMed] [Google Scholar]
- Peeters PH, Keinan-Boker L, van der Schouw YT, Grobbee DE. Phytoestrogens and breast cancer risk. Review of the epidemiological evidence. Breast Cancer Res Treat. 2003;77(2):171–183. doi: 10.1023/a:1021381101632. [DOI] [PubMed] [Google Scholar]
- Pietras RJ, Levin ER, Szego CM. Estrogen receptors and cell signaling [Letter] Science. 2005a;310(5745):51–53. doi: 10.1126/science.310.5745.51. author reply 51–53. [DOI] [PubMed] [Google Scholar]
- Pietras RJ, Marquez DC, Chen HW, Tsai E, Weinberg O, Fishbein M. Estrogen and growth factor receptor interactions in human breast and non-small cell lung cancer cells. Steroids. 2005b;70(5–7):372–381. doi: 10.1016/j.steroids.2005.02.017. [DOI] [PubMed] [Google Scholar]
- Pozo-Guisado E, Alvarez-Barrientos A, Mulero-Navarro S, Santiago-Josefat B, Fernandez-Salguero PM. The antiproliferative activity of resveratrol results in apoptosis in MCF-7 but not in MDA-MB-231 human breast cancer cells: cell-specific alteration of the cell cycle. Biochem Pharmacol. 2002;64(9):1375–1386. doi: 10.1016/s0006-2952(02)01296-0. [DOI] [PubMed] [Google Scholar]
- Pozo-Guisado E, Merino JM, Mulero-Navarro S, Lorenzo-Benayas MJ, Centeno F, Alvarez-Barrientos A, et al. Resveratrol-induced apoptosis in MCF-7 human breast cancer cells involves a caspase-independent mechanism with downregulation of Bcl-2 and NF-kappaB. Int J Cancer. 2005;115(1):74–84. doi: 10.1002/ijc.20856. [DOI] [PubMed] [Google Scholar]
- Probst-Hensch NM, Pike MC, McKean-Cowdin R, Stanczyk FZ, Kolonel LN, Henderson BE. Ethnic differences in post-menopausal plasma oestrogen levels: high oestrone levels in Japanese-American women despite low weight. Br J Cancer. 2000;82(11):1867–1870. doi: 10.1054/bjoc.1999.1082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramadass P, Meerarani P, Toborek M, Robertson LW, Hennig B. Dietary flavonoids modulate PCB-induced oxidative stress, CYP1A1 induction, and AhR-DNA binding activity in vascular endothelial cells. Toxicol Sci. 2003;76(1):212–219. doi: 10.1093/toxsci/kfg227. [DOI] [PubMed] [Google Scholar]
- Rice S, Mason HD, Whitehead SA. Phytoestrogens and their low dose combinations inhibit mRNA expression and activity of aromatase in human granulosaluteal cells. J Steroid Biochem Mol Biol. 2006;101(4–5):216–225. doi: 10.1016/j.jsbmb.2006.06.021. [DOI] [PubMed] [Google Scholar]
- Rice S, Whitehead SA. Phytoestrogens and breast cancer—promoters or protectors? Endocr Relat Cancer. 2006;13(4):995–1015. doi: 10.1677/erc.1.01159. [DOI] [PubMed] [Google Scholar]
- Roberts DW, Doerge DR, Churchwell MI, Gamboa da Costa G, Marques MM, Tolleson WH. Inhibition of extrahepatic human cytochromes P450 1A1 and 1B1 by metabolism of isoflavones found in Trifolium pratense (red clover) J Agric Food Chem. 2004;52(21):6623–6632. doi: 10.1021/jf049418x. [DOI] [PubMed] [Google Scholar]
- Routledge EJ, White R, Parker MG, Sumpter JP. Differential effects of xenoestrogens on coactivator recruitment by estrogen receptor (ER) alpha and ERbeta. J Biol Chem. 2000;275(46):35986–35993. doi: 10.1074/jbc.M006777200. [DOI] [PubMed] [Google Scholar]
- Sanderson JT, Hordijk J, Denison MS, Springsteel MF, Nantz MH, van den Berg M. Induction and inhibition of aromatase (CYP19) activity by natural and synthetic flavonoid compounds in H295R human adrenocortical carcinoma cells. Toxicol Sci. 2004;82(1):70–79. doi: 10.1093/toxsci/kfh257. [DOI] [PubMed] [Google Scholar]
- Sato M, Pei RJ, Yuri T, Danbara N, Nakane Y, Tsubura A. Prepubertal resveratrol exposure accelerates N-methyl-N-nitrosourea-induced mammary carcinoma in female Sprague-Dawley rats. Cancer Lett. 2003;202(2):137–145. doi: 10.1016/j.canlet.2003.08.016. [DOI] [PubMed] [Google Scholar]
- Schmitt E, Lehmann L, Metzler M, Stopper H. Hormonal and genotoxic activity of resveratrol. Toxicol Lett. 2002;136(2):133–142. doi: 10.1016/s0378-4274(02)00290-4. [DOI] [PubMed] [Google Scholar]
- Seo HS, Denardo DG, Jacquot Y, Laios I, Vidal DS, Zambrana CR, et al. Stimulatory effect of genistein and apigenin on the growth of breast cancer cells correlates with their ability to activate ER alpha. Breast Cancer Res Treat. 2006;99(2):121–134. doi: 10.1007/s10549-006-9191-2. [DOI] [PubMed] [Google Scholar]
- Shaaban AM, O’Neill PA, Davies MP, Sibson R, West CR, Smith PH, et al. Declining estrogen receptor-beta expression defines malignant progression of human breast neoplasia. Am J Surg Pathol. 2003;27(12):1502–1512. doi: 10.1097/00000478-200312000-00002. [DOI] [PubMed] [Google Scholar]
- Shao ZM, Wu J, Shen ZZ, Barsky SH. Genistein exerts multiple suppressive effects on human breast carcinoma cells. Cancer Res. 1998;58(21):4851–4857. [PubMed] [Google Scholar]
- Shertzer HG, Puga A, Chang C, Smith P, Nebert DW, Setchell KD, et al. Inhibition of CYP1A1 enzyme activity in mouse hepatoma cell culture by soybean isoflavones. Chem Biol Interact. 1999;123(1):31–49. doi: 10.1016/s0009-2797(99)00121-0. [DOI] [PubMed] [Google Scholar]
- Shoulars K, Brown T, Alejandro MA, Crowley J, Markaverich BM. Identification of nuclear type II [(3)H]estradiol binding sites as histone H4. Biochem Biophys Res Commun. 2002;296(5):1083–1090. doi: 10.1016/s0006-291x(02)02042-9. [DOI] [PubMed] [Google Scholar]
- Shoulars K, Rodrigues MA, Crowley JR, Turk J, Thompson T, Markaverich BM. Nuclear type II [3H]estradiol binding sites: a histone H3-H4 complex. J Steroid Biochem Mol Biol. 2005;96(1):19–30. doi: 10.1016/j.jsbmb.2004.12.047. [DOI] [PubMed] [Google Scholar]
- Shu XO, Jin F, Dai Q, Wen W, Potter JD, Kushi LH, et al. Soyfood intake during adolescence and subsequent risk of breast cancer among Chinese women. Cancer Epidemiol Biomarkers Prev. 2001;10(5):483–488. [PubMed] [Google Scholar]
- Sirtori CR, Arnoldi A, Johnson SK. Phytoestrogens: end of a tale? Ann Med. 2005;37(6):423–438. doi: 10.1080/07853890510044586. [DOI] [PubMed] [Google Scholar]
- Strom A, Hartman J, Foster JS, Kietz S, Wimalasena J, Gustafsson JA. Estrogen receptor beta inhibits 17beta-estradiol-stimulated proliferation of the breast cancer cell line T47D. Proc Natl Acad Sci USA. 2004;101(6):1566–1571. doi: 10.1073/pnas.0308319100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson EA, Jr, Siiteri PK. Utilization of oxygen and reduced nicotinamide adenine dinucleotide phosphate by human placental microsomes during aromatization of androstenedione. J Biol Chem. 1974;249(17):5364–5372. [PubMed] [Google Scholar]
- Trock BJ, Hilakivi-Clarke L, Clarke R. Meta-analysis of soy intake and breast cancer risk. J Natl Cancer Inst. 2006;98(7):459–471. doi: 10.1093/jnci/djj102. [DOI] [PubMed] [Google Scholar]
- Uehar M, Arai Y, Watanabe S, Adlercreutz H. Comparison of plasma and urinary phytoestrogens in Japanese and Finnish women by time-resolved fluoroimmunoassay. Biofactors. 2000;12(1–4):217–225. doi: 10.1002/biof.5520120134. [DOI] [PubMed] [Google Scholar]
- Ursin G, Bernstein L, Pike MC. Breast cancer Cancer Surv. 1994;19–20:241–264. [PubMed] [Google Scholar]
- Usui T. Pharmaceutical prospects of phytoestrogens. Endocr J. 2006;53(1):7–20. doi: 10.1507/endocrj.53.7. [DOI] [PubMed] [Google Scholar]
- Vanden Berghe W, Dijsselbloem N, Vermeulen L, Ndlovu N, Boone E, Haegeman G. Attenuation of mitogen-and stress-activated protein kinase-1-driven nuclear factor-kappaB gene expression by soy isoflavones does not require estrogenic activity. Cancer Res. 2006;66(9):4852–4862. doi: 10.1158/0008-5472.CAN-05-2957. [DOI] [PubMed] [Google Scholar]
- van der Woude H, Ter Veld MG, Jacobs N, van der Saag PT, Murk AJ, Rietjens IM. The stimulation of cell proliferation by quercetin is mediated by the estrogen receptor. Mol Nutr Food Res. 2005;49(8):763–771. doi: 10.1002/mnfr.200500036. [DOI] [PubMed] [Google Scholar]
- Verma AK, Johnson JA, Gould MN, Tanner MA. Inhibition of 7,12-dimethylbenz(a)anthracene- and N-nitrosomethylurea-induced rat mammary cancer by dietary flavonol quercetin. Cancer Res. 1988;48(20):5754–5758. [PubMed] [Google Scholar]
- Wakai K, Egami I, Kato K, Kawamura T, Tamakoshi A, Lin Y, et al. Dietary intake and sources of isoflavones among Japanese. Nutr Cancer. 1999;33(2):139–145. doi: 10.1207/S15327914NC330204. [DOI] [PubMed] [Google Scholar]
- Wang Y, Lee KW, Chan FL, Chen S, Leung LK. The red wine polyphenol resveratrol displays bilevel inhibition on aromatase in breast cancer cells. Toxicol Sci. 2006;92(1):71–77. doi: 10.1093/toxsci/kfj190. [DOI] [PubMed] [Google Scholar]
- Whitehead SA, Cross JE, Burden C, Lacey M. Acute and chronic effects of genistein, tyrphostin and lavendustin A on steroid synthesis in luteinized human granulosa cells. Hum Reprod. 2002;17(3):589–594. doi: 10.1093/humrep/17.3.589. [DOI] [PubMed] [Google Scholar]
- Whitsett T, Carpenter M, Lamartiniere CA. Resveratrol, but not EGCG, in the diet suppresses DMBA-induced mammary cancer in rats. J Carcinog. 2006;5:15–26. doi: 10.1186/1477-3163-5-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood CE, Register TC, Cline JM. Soy isoflavonoid effects on endogenous estrogen metabolism in postmenopausal female monkeys. Carcinogenesis. 2007;28(4):801–808. doi: 10.1093/carcin/bgl163. [DOI] [PubMed] [Google Scholar]
- Wu AH, Ziegler RG, Horn-Ross PL, Nomura AM, West DW, Kolonel LN, et al. Tofu and risk of breast cancer in Asian-Americans. Cancer Epidemiol Biomarkers Prev. 1996;5(11):901–906. [PubMed] [Google Scholar]
- Xu X, Duncan AM, Merz BE, Kurzer MS. Effects of soy isoflavones on estrogen and phytoestrogen metabolism in premenopausal women. Cancer Epidemiol Biomarkers Prev. 1998;7(12):1101–1108. [PubMed] [Google Scholar]
- Yager JD, Davidson NE. Estrogen carcinogenesis in breast cancer. N Engl J Med. 2006;354(3):270–282. doi: 10.1056/NEJMra050776. [DOI] [PubMed] [Google Scholar]
- Yu R, Hebbar V, Kim DW, Mandlekar S, Pezzuto JM, Kong AN. Resveratrol inhibits phorbol ester and UV-induced activator protein 1 activation by interfering with mitogen-activated protein kinase pathways. Mol Pharmacol. 2001;60(1):217–224. doi: 10.1124/mol.60.1.217. [DOI] [PubMed] [Google Scholar]
- Yuan JM, Wang QS, Ross RK, Henderson BE, Yu MC. Diet and breast cancer in Shanghai and Tianjin, China. Br J Cancer. 1995;71(6):1353–1358. doi: 10.1038/bjc.1995.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yue W, Berstein LM, Wang JP, Clark GM, Hamilton CJ, Demers LM, et al. The potential role of estrogen in aromatase regulation in the breast. J Steroid Biochem Mol Biol. 2001;79(1–5):157–164. doi: 10.1016/s0960-0760(01)00154-6. [DOI] [PubMed] [Google Scholar]
- Zhu BT. Catechol-O-methyltransferase (COMT)-mediated methylation metabolism of endogenous bioactive catechols and modulation by endobiotics and xenobiotics: importance in pathophysiology and pathogenesis. Curr Drug Metab. 2002;3(3):321–349. doi: 10.2174/1389200023337586. [DOI] [PubMed] [Google Scholar]
- Zhu BT, Liehr JG. Quercetin increases the severity of estradiol-induced tumorigenesis in hamster kidney. Toxicol Appl Pharmacol. 1994;125(1):149–158. doi: 10.1006/taap.1994.1059. [DOI] [PubMed] [Google Scholar]
- Zhu BT, Liehr JG. Inhibition of catechol O-methyltransferase-catalyzed O-methylation of 2- and 4-hydroxyestradiol by quercetin. Possible role in estradiol-induced tumorigenesis. J Biol Chem. 1996;271(3):1357–1363. doi: 10.1074/jbc.271.3.1357. [DOI] [PubMed] [Google Scholar]
- Ziegler RG. Phytoestrogens and breast cancer. Am J Clin Nutr. 2004;79(2):183–184. doi: 10.1093/ajcn/79.2.183. [DOI] [PubMed] [Google Scholar]