Abstract
Background
Epidemiologic studies have shown associations between asthma outcomes and outdoor air pollutants such as nitrogen dioxide and particulate matter mass < 2.5 μm in diameter (PM2.5). Independent effects of specific pollutants have been difficult to detect because most studies have relied on highly correlated central-site measurements.
Objectives
This study was designed to evaluate the relationship of daily changes in percent-predicted forced expiratory volume in 1 sec (FEV1) with personal and ambient air pollutant exposures.
Methods
For 10 days each, we followed 53 subjects with asthma who were 9–18 years of age and living in the Los Angeles, California, air basin. Subjects self-administered home spirometry in themorning, afternoon, and evening. We measured personal hourly PM2.5 mass, 24-hr PM2.5 elemental and organic carbon (EC–OC), and 24-hr NO2, and the same 24-hr average outdoor central-site(ambient) exposures. We analyzed data with transitional mixed models controlling for personal temperature and humidity, and as-needed β2-agonist inhaler use.
Results
FEV1 decrements were significantly associated with increasing hourly peak and daily average personal PM2.5, but not ambient PM2.5. Personal NO2 was also inversely associated with FEV1. Ambient NO2 was more weakly associated. We found stronger associations among 37 subjects not taking controller bronchodilators as follows: Personal EC–OC was inversely associated with morning FEV1; for an interquartile increase of 71 μg/m3 1-hr maximum personal PM2.5, overall percent-predicted FEV1 decreased by 1.32% [95% confidence interval (CI), −2.00 to −0.65%]; and for an interquartile increase of 16.8 ppb 2-day average personal NO2, overall percent-predicted FEV1 decreased by 2.45% (95% CI, −3.57 to −1.33%). Associations of both personal PM2.5 and NO2 with FEV1 remained when co-regressed, and both confounded ambient NO2.
Conclusions
Independent pollutant associations with lung function might be missed using ambient data alone. Different sets of causal components are suggested by independence of FEV1 associations with personal PM2.5 mass from associations with personal NO2.
Keywords: asthma, epidemiology, forced expiratory flow rates, longitudinal data analysis, nitrogen dioxide, panel study, particulate air pollution
Acute adverse effects of air pollution on asthma outcomes in small cohorts of children have been reported in longitudinal studies using repeated daily measurements (panel studies). More recently, this includes positive associations between a biomarker of airway inflammation, exhaled nitric oxide, and both personal and outdoor ambient air pollutant exposures in children with asthma (Delfino et al. 2006; Koenig et al. 2005). Most panel studies of daily air pollution and acute changes in expiratory lung function reported before 2004 used measurements of peak expiratory flow (PEF). They generally showed consistent, albeit heterogeneous, inverse associations of PEF with ambient particulate matter (PM) < 2.5 μm in diameter (PM2.5), with somewhat weaker associations for PM < 10 μm in diameter (PM10) [reviewed by Ward and Ayers (2004)]. However, PEF is more effort dependent than another measure of lung function, forced expiratory volume in 1 sec (FEV1). PEF is also a poor surrogate of the more clinically relevant FEV1 (Giannini et al. 1997; Thiadens et al. 1999) because PEF measures only the first portion of expiration from larger proximal airways, whereas FEV1 reflects resistance in both proximal and distal airways. Lower FEV1 occurs when flow rate decreases because of airway obstruction, which is a key phenotype of asthma.
Most previous studies of the relationship between acute asthma in children and air pollution have relied on ambient central-site data (Sarnat and Holguin 2007; Trasande and Thurston 2005). Exposure error from using this data will likely diminish the accuracy of exposure–response estimates. High interpollutant correlations at ambient monitoring sites also make it difficult to identify independent associations from different regulated criteria air pollutants such as PM2.5 and nitrogen dioxide. Furthermore, criteria pollutants may be serving as markers for components not routinely monitored, such as combustion-related organic compounds. These component mixtures may lead to airway inflammation and bronchoconstriction. However, a range of individual responses for a given type of component exposure is likely for children with asthma. Children at greatest risk likely include those with persistent asthma, particularly if they are not taking controller medications. Personal exposure assessments (Jerrett et al. 2005) and assessments of clinical and biological differences in an individual’s asthma (Sarnat and Holguin 2007) have been proposed to clarify these issues regarding exposure and response.
We previously found that associations of asthma symptoms with ambient PM mass concentrations were completely explained by ambient elemental and organic carbon fractions of PM (EC and OC, respectively) (Delfino et al. 2003). Studies have shown much stronger correlations between traffic emission sources and EC (or a similar measure of black carbon reflectance) compared with PM mass (Cyrys et al. 2003), but OC is more difficult to apportion to emission sources (Fujita et al. 2007). Our earlier finding thus suggested that products of fossil fuel combustion were important in asthma outcomes that might otherwise be ascribed to uncharacterized PM mass. Many studies have also shown strong correlations between traffic emission sources and NO2 (Jerrett et al. 2005). In another panel study, we reported positive associations between repeated measures of exhaled NO and personal exposures to NO2 and EC that were largely independent of associations with personal PM2.5 mass (Delfino et al. 2006). These findings suggested that in addition to products of fossil fuel combustion, other particle components in personal air samples were proinflammatory. Here we aim to expand on these previous findings in the same cohort of children by evaluating the relationship of FEV1 to both personal and central-site NO2, PM2.5 mass, and EC–OC fractions of PM2.5.
Materials and Methods
Design and population
We followed a panel of 63 schoolchildren with asthma for daily repeated measures of personal exposure to air pollution in two regions of the Los Angeles air basin in Southern California: Riverside and Whittier. These regions are characterized by high levels of air pollution predominantly from mobile sources of fossil fuel combustion. Geographic areas of recruitment were delimited to a 10-mile radius around a central air monitoring site in Riverside (population density, 3,538/mi2), and a 5-mile radius around a central air monitoring site in Whittier (5,947/mi2) (RAND California 2007). The institutional review board of the University of California, Irvine, approved the study protocol. We obtained informed written consent from all subjects and one of their legal guardians.
We recruited subjects by referral to the study office by local school district nurses. Eligibility criteria included ages 9–18 years and parent-reported physician-diagnosed asthma, with a history of episodic symptoms including wheezing, cough, or dyspnea. For the cohort, we targeted children with evidence of mild to moderate persistent asthma, including a) a history in the previous 12 months of asthma exacerbations requiring the use of prescribed bronchodilator(s) on ≥ 2 days per week, regardless of anti-inflammatory medication use; b) current use of oral or inhaled anti-inflammatory medications, regardless of symptom frequency; or c) < 80% predicted normal FEV1 from office spirometry at the subject’s baseline visit to the General Clinical Research Center, University of California, Irvine. Subjects were ineligible if they smoked or if someone smoked in the subject’s home.
We followed subjects daily over a continuous 10-day period that involved wearing air samplers to measure personal exposure to air pollutants. There were sixteen 10-day periods of follow-up (a run) from July to December 2003 (Riverside) and 2004 (Whittier). Four subjects were followed daily at their home in each 10-day run (except one run with three subjects).
Lung function and diary data
We have presented spirometry methods and validation results for the present panel subjects in detail in our previous report (Thompson et al. 2006). Subjects self-administered spirometry at home using the hand-held ndd EasyOne Frontline Spirometer (ndd Technologies, Chelmsford, MA). Subjects were given detailed instructions and trained on its use in the home during a 5-day run-in period. Subjects were instructed to perform spirometry in the morning (up to 1100 hours), afternoon (1500–1800 hours), and evening (2000–2400 hours), referred to here as “session period.” Subjects were also instructed to complete a personal digital assistant (PDA) diary every 2 waking hours reporting asthma medication use. We mitigated missed PDA diary prompts with paper diaries and daily technician-administered questionnaires. Medications reported included daily preventive (controller) medications and as-needed (rescue) medications (inhaled β2-agonist bronchodilators). Near bedtime, the PDA diary prompted recall of rescue and controller medication use throughout the day. In the morning, it prompted recall of rescue inhaler use during the night. We also measured rescue inhaler use with a pressure-actuated recording device (Doser; Meditrack Products, Hudson, MA) that logged puffs in 24-hr intervals from midnight to midnight.
During each session, the spirometer stopped after three good spirometry maneuvers were obtained, and it gave each subject up to six chances to meet acceptability and repeatability criteria. Intermittent instructions to subjects were displayed on the spirometer’s display based on the success or type of error of each attempt. Subjects were instructed to perform sessions before the use of inhaled β2-agonist bronchodilator medications unless necessary, and to wait at least 4 hr after the use of them before performing a session. At the end of spirometry maneuvers, subjects answered a yes/no question on the spirometer screen: “Did you need to use your rescue medication in the last hour?”
Research technicians downloaded the spirometry data into laptops during daily home visits, and checked compliance and acceptability of maneuvers as generated by the ndd software (version 2.6). We retrained subjects as needed. Compliance was enhanced by monetary incentives, an on-screen point system, and audio alarms. We later evaluated each curve for acceptability and repeatability by selected criteria as previously described (Thompson et al. 2006). We then further evaluated these curves for visual acceptability. We found compliance was high (94%) and the number of sessions with acceptable and reproducible maneuvers by objective criteria as well as visually acceptable was moderately good (69%) (Thompson et al. 2006). To ensure a suitably complete time series of repeated measures, subjects included in the present analysis had to have at least a third of their 29 expected FEV1 maneuvers over the 10 days that were valid as such. We excluded 10 subjects who did not meet this compliance threshold, leaving 53 subjects who had 1,249 observed of 1,537 expected spirometry sessions (81%) with acceptable and reproducible maneuvers (individual subject range, 41–100%, median 86%).
The highest FEV1 (best effort) from the two acceptable and reproducible maneuvers was selected for analysis. We analyzed percent-predicted normal FEV1 based on a subject’s height, age, sex, and race/ethnicity (Hankinson et al. 1999). This standardizes measurements between subjects, provides overall estimates of association for the study population, and is clinically meaningful.
Exposures
The personal air monitors were active air samplers worn in a backpack daily over the 10 consecutive days. Personal measurements included continuous nephelometer mass measurements of PM2.5 (personal DataRAM model 1200; MIE Inc., Bedford, MA) and 24-hr EC and OC fractions of PM2.5, collected on quartz filters (Whatman Inc., Florham Park, NJ) using an attached filter cassette. A 2.5-μm sharp-cut cyclone was attached upstream of the nephelometer, and PM2.5 for EC and OC was collected down-stream at a flow rate of 4 L/min. We measured NO2 over 24-hr periods using a miniaturized diaphragm pump (VMP1625; Virtual Industry, Colorado Springs, CO) run at 0.1 L/min to sample air through tri-ethanolamine-treated molecular sieve sorbent tubes (SKC West Inc., Fullerton, CA). We measured NO2 based on National Institute for Occupational Safety and Health (1994) Method 6014. We collected personal temperature and relative humidity with attached loggers (Onset Computer Corp., Pocasset, MA). Elsewhere we provide data on the validation of both the personal PM2.5 sampler (Chakrabarti et al. 2004) and our personal NO2 active sampler (Staimer et al. 2005).
We measured a parallel set of exposures at our own outdoor central sites, one in Riverside and one in Whittier. PM2.5 and PM10 mass (Teflon filters), and PM2.5 EC and OC (quartz filters) were collected there using standard procedures with Harvard Impactors (Air Diagnostics and Engineering, Inc., Naples, ME). Sampling start and stop times occurred during the early evening of each day near the same time as personal samplers. For both personal and central-site sample collection on quartz filters, particulate carbon was speciated into OC and EC using the thermal manganese dioxide oxidation technique (Fung et al. 2002). Central-site gases included hourly ozone and NO2 measured by the South Coast Air Quality Management District. In Riverside, the district site was centrally located, and we sited Harvard Impactors there. In Whittier, we constructed a central site at a subject home elevated on a hill. However, data for O3 and NO2 came from two district sites at opposite ends of the Whittier study region. We averaged hourly concentrations of these gases for the two stations.
Analysis
We tested the relationship between percent-predicted FEV1 and each air pollutant using linear mixed-effects models, with each subject serving as his or her own control (Verbeke and Molenberghs 2001). Because correlation among outcomes was present for the within-individual repeated measures, and possibly for the exposure run, we assumed a two-stage hierarchical model with random effects at the subject level, nested within a run. We fit an autoregressive-1 correlation structure given the observed variability from empirical variograms. Air pollutant exposures were mean-centered by subject to yield comparability between subjects and across runs (Sheppard et al. 2005).
We investigated impacts of personal hourly PM2.5 mass exposures preceding the FEV1 measurement including the average of the preceding 24 hr (lag 0), the average of the 25th through 48th hr (lag 1), and a cumulative 2-day moving average. We retained PM2.5 data if at least 75% of the hours were nonmissing. The same approach was used for central-site hourly NO2. Given our previous findings (Delfino et al. 1998, 2002), we also examined 1-hr and 8-hr maximum moving average in personal PM2.5 during the 24 hr preceding the FEV1 measurement. We examined 8-hr peak central-site O3 given its well-known diurnal trend. For the filter-based measurements (personal and central-site EC and OC, and central-site PM2.5 mass) and for personal NO2, we defined lag 0 to be the same day and lag 1 was the preceding day’s 24-hr measurement. We did not extend the number of lags beyond that last 2 days to maintain a reasonable within-subject sample size, because a subject’s data were limited to a single 10-day consecutive monitoring period. We expressed results as percent change in predicted FEV1 per interquartile range (IQR) increase in each pollutant to standardize inter-pollutant comparisons.
We fit transitional models by adjusting for the previous FEV1 measurement to control for observed sinusoidal circadian rhythms. Transitional models condition the outcome in the current time on the previous outcome observation (e.g., afternoon FEV1 is regressed on the morning FEV1) (Diggle et al. 2002). We also tested for effect modification by session period (morning, afternoon, evening), and found several differences that we present below.
We decided a priori to adjust for use of rescue inhalers, including use last night, which was associated with a decrease of 3.5 percent-predicted FEV1 in the afternoon and evening [95% confidence interval (CI), −6.5 to −0.4]. We also included cumulative daily use of rescue inhalers during the previous day using Doser data [PDA diary data for 119 person-days were used where Doser data were missing (9.5%)]. Cumulative inhaler use was positively associated with an increase of 1.1 percent-predicted FEV1 in the morning per two-puff dose (95% CI, −0.2 to 2.5). In addition, we excluded observations where subjects reported use of rescue inhalers in either the ndd spirometer diary or PDA diary report covering the last 2–4 hr (57 FEV1 observations, 4.6% of total). Such use was associated with an increase of 2.2 percent-predicted FEV1 (95% CI, 0.07 to 4.3). Models also adjusted for personal temperature and relative humidity (both positively associated with FEV1).
We tested potential confounding by self-reported respiratory infections (22 person-days, 4.4% of total, p = 0.86 in relation to FEV1). We also tested confounding by the two regions of study, session period of day (morning, afternoon, or evening), and weekend. None of these variables influenced associations, with one exception discussed below for session period.
We conducted residual diagnostics to assess the presence of influential data points and subject clusters, as well as deviations from assumed functional form. One 10-year-old white female subject influenced personal PM models leading to a decrease in personal PM2.5 regression parameter estimates and increase in SE (Cook’s D, 0.38; restricted likelihood distance, 4.41). We present results with this subject and sensitivity analyses removing her data.
Given prior evidence (Becklake and Kauffman 1999; Delfino et al. 1998, 2002, 2006), we further tested models for effect modification by sex and by asthma controller medications using product terms with each air pollutant. We assumed product term interactions with a p-value < 0.1 suggested possible effect modification. We tested a binary (yes/no) indicator for use of anti-inflammatory medications, as well as separate indicators for inhaled corticosteroids with versus without leukotriene receptor antagonists. We also tested a binary indicator for prescribed daily use of short- or long-acting bronchodilators as controller medications. We anticipated both controller and rescue bronchodilators to have major impacts on temporal changes in FEV1.
We tested two-pollutant regression models to assess between-pollutant confounding after testing interaction between the pollutants in product term models. The aim here was to assess the extent to which associations with one pollutant was independent of another pollutant.
We retested selected regression models using generalized estimating equations with robust standard error estimates (Diggle et al. 2002) as a validity check to likelihood assumptions of the linear mixed-effects model. We found no qualitative differences in our study results.
Finally, we used a fifth-order polynomial distributed-lag mixed-effects model (Schwartz 2000) to investigate the relationship of FEV1 to lagged hourly personal PM2.5 exposures out to 48 hr. We found negligible difference in the response curves when models that are more flexible were considered. We fit distributed lag models via a linear mixed-effects model assuming an autoregressive-1 correlation structure.
Results
Descriptive data
Descriptive statistics for the 53 subjects in the present analysis are presented in Table 1. On average, FEV1 was lowest in the morning and gradually increased to its highest in the afternoon, then decreased toward the evening (Table 2). We found percent-predicted FEV1 was significantly higher among 28 subjects taking inhaled corticosteroids, and significantly lower among 13 subjects taking antileukotrienes compared with 20 subjects not taking controller medications (Table 2). There was no significant difference in FEV1 for 16 subjects taking controller bronchodilators versus those not taking them.
Table 1.
Study group characteristics.
| Characteristic | Data |
|---|---|
| Age [years, mean (range)] | 13.8 (9–18) |
| Sex [no. (%)] | |
| Female | 19 (35.9) |
| Male | 34 (64.1) |
| Race/ethnicity no. (%) | |
| Hispanica | 26 (49.1) |
| White | 12 (22.6) |
| Black | 13 (24.5) |
| Asian | 2 (3.8) |
| No. (%) with percent-predicted FEV1 < 80%b | 18 (34.0) |
Includes 20 Hispanic subjects who gave no race and 6 who gave their race as white; two blacks and 2 Asians also gave their ethnicity as Hispanic.
Predicted from the Third National Health and Nutrition Examination Survey (NHANES III) (Hankinson et al. 1999) from baseline spirometry.
Table 2.
Differences in subject FEV1 by time of day and medication use.
| Percent-predicted FEV1a | Mean ± SD | Median | Range |
|---|---|---|---|
| Overall (53 subjects) | 86.8 ± 15.9 | 89.4 | 30–126 |
| Morning | 84.7 ± 17.0 | 88.0 | 33–116 |
| Afternoon | 88.6 ± 15.0 | 90.5 | 40–123 |
| Evening | 87.5 ± 15.7 | 89.2 | 30–126 |
| Differences by medication use | |||
| No controller medications (20 subjects) | 86.3 ± 16.5 | 89.1 | 41–119 |
| Inhaled corticosteroids (27 subjects)b | 88.0 ± 14.4* | 89.0 | 44–126 |
| Antileukotrienes ± inhaled corticosteroids (13 subjects)c | 85.2 ± 16.8* | 89.2 | 30–126 |
| Controller bronchodilators (16 subjects)d | 86.1 ± 15.7 | 87.1 | 44–116 |
Predicted from NHANES III (Hankinson et al. 1999) and based on data from the panel follow-up used in the present analysis.
One subject was also using inhaled cromolyn.
Four subjects were using antileukotrienes only, and nine were using antileukotrienes plus inhaled corticosteroids.
Five subjects were using daily short-acting β2-agonist medications, two of whom were also using an anticholinergic medication (ipratropium bromide), 11 were using long-acting bronchodilator medications (sustained release theophylline and the long-acting β2-agonist, salmeterol xinafoate), and 14 were also using anti-inflammatory medications.
Random-effects model p < 0.05 for predicted FEV1 difference from subjects not on controller medications, adjusted for study region.
We collected 519 person-days of valid observations for the personal NO2 air monitor. It malfunctioned for only 3 person-days. The PM2.5 nephelometer malfunctioned for two subjects during most of their 10-day run and periodically for other subjects, leaving 416 person-days of observation. Table 3 presents descriptive statistics for the exposure data. Concentrations of peak hourly personal PM2.5 were high, averaging 90 μg/m3 with a maximum reaching 603 μg/m3. The U.S. Environmental Protection Agency National Ambient Air Quality Standard (NAAQS) for 8-hr ambient O3 (80 ppb) was never exceeded (U.S. Environmental Protection Agency 2008). However, the NAAQS for 24-hr average ambient PM2.5 (35 μg/m3) was exceeded on 28 of 170 days and NAAQS for 24-hr average ambient PM10 (150 μg/m3) was exceeded on only 1 day at the central sites.
Table 3.
Descriptive statistics of daily air pollutant measurements.
| Exposure | No. (missing) | Mean ± SD | Median | IQR | Min/max |
|---|---|---|---|---|---|
| Personal exposurea | |||||
| 1-hr max PM2.5 (μg/m3) | 416 (154) | 90.1 ± 79.8 | 66.2 | 70.6 | 14.1/603.4 |
| 8-hr max PM2.5 (μg/m3) | 416 (154) | 46.2 ± 33.4 | 36.8 | 33.6 | 7.5/240.8 |
| 24-hr PM2.5 (μg/m3) | 416 (154) | 31.2 ± 21.8 | 26.0 | 21.6 | 4.3/180.0 |
| 24-hr PM2.5 EC (μg/m3) | 481 (89) | 0.59 ± 1.11 | 0.33 | 0.54 | 0/17.2 |
| 24-hr PM2.5 OC (μg/m3) | 486 (84) | 6.0 ± 3.4 | 5.2 | 4.3 | 1.0/31.5 |
| 24-hr NO2 (ppb) | 519 (51) | 28.6 ± 13.2 | 26.7 | 16.8 | 2.8/105.7 |
| 24-hr temperature (°C) | 516 (54) | 24.8 ± 3.0 | 25.4 | 4.2 | 17.3/32.1 |
| Central site PM (μg/m3)b | |||||
| 24-hr PM2.5 | 170 (4) | 23.3 ± 17.7 | 17.1 | 15.6 | 2.8/87.2 |
| 24-hr PM10 | 170 (4) | 45.9 ± 26.3 | 39.1 | 23.7 | 5.9/154.0 |
| 24-hr PM2.5 EC | 167 (7) | 1.12 ± 0.77 | 0.97 | 0.90 | 0.14/5.04 |
| 24-hr PM2.5 OC | 167 (7) | 5.0 ± 2.4 | 4.7 | 2.8 | 1.5/19.7 |
| Central site gases (ppb)c | |||||
| 8-hr max O3 | 174 (0) | 50.7 ± 16.2 | 49.1 | 35.7 | 32.5/77.6 |
| 24-hr NO2 | 174 (0) | 25.0 ± 3.0 | 25.3 | 6.3 | 19.9/29.2 |
Abbreviations: min, minimum; max, maximum.
Person-days of observation, usually four personal exposure measurements per day.
Single days of observation, which would each be linked to all four subjects followed that day.
Around 4–5% of total hours on days with ≤ 5 contiguous hours missing were interpolated using a kernel smoother (running weighted average), including the daily calibration hour. In Riverside, 20 days with 6–24 hr of NO2 missing (15.3% of total days) and 1 day with 6 hr for O3 (0.8% of total days) were interpolated using prediction equations based on data from the nearby Rubidoux, California, station (8 km). In Whittier, 3 days with 7–24 hr of NO2 missing (2.4% of total days) and 1 day with 7 hr for O3 (0.8% of total days) were interpolated by linear regression equations based on data from the other nonmissing station data and used to estimate average regional exposure across the two stations.
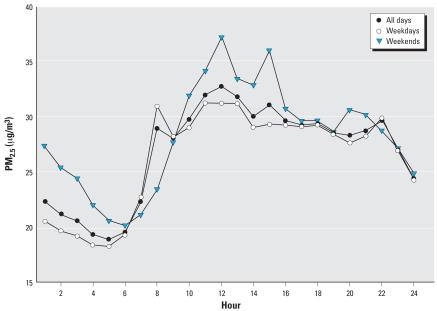
Figure 1 shows hourly average concentrations of personal PM2.5. Concentrations were lowest in the early morning, abruptly rising mid-morning with maximums around noon and sustained concentrations until late evening. The mid-morning peak occurred around 0800 hr during the school weekday but was delayed by several hours and was higher on weekends.
Figure 1.
Hourly average concentration of personal PM2.5 across 51 subjects for all days, weekdays, and weekends.
Table 4 shows the between-pollutant correlations. Small significant correlations of personal PM2.5 with personal EC, OC, and NO2 were found. Personal PM2.5 was moderately correlated with ambient PM2.5 (Spearman r = 0.60), and had small correlations with personal NO2 (r = 0.38) and ambient NO2 (r = 0.32). Personal NO2 showed low moderate correlation with ambient NO2 (Spearman r = 0.43). However, personal EC and OC were not correlated with ambient EC and OC but were weakly correlated with ambient NO2. Ambient exposures were moderately correlated with each other.
Table 4.
Exposure correlation matrix.
| Personal
|
Central site
|
|||||||
|---|---|---|---|---|---|---|---|---|
| PM2.5 | EC | OC | NO2 | PM2.5 | EC | OC | NO2 | |
| 24-hr personal PM2.5 | 1.00 | 0.22** | 0.26** | 0.38** | 0.60** | 0.14* | 0.24** | 0.32** |
| 24-hr personal EC | 1.00 | 0.44** | 0.22** | 0.02 | −0.01 | 0.00 | 0.20** | |
| 24-hr personal OC | 1.00 | 0.20** | −0.04 | −0.08 | 0.01 | 0.16** | ||
| 24-hr personal NO2 | 1.00 | 0.21** | 0.20** | 0.18** | 0.43** | |||
| 24-hr central PM2.5 | 1.00 | 0.51** | 0.62** | 0.36** | ||||
| 24-hr central EC | 1.00 | 0.84** | 0.61** | |||||
| 24-hr central OC | 1.00 | 0.56** | ||||||
| 24-hr central NO2 | 1.00 | |||||||
p < 0.05, and
p < 0.001, from Wald-based tests of Spearman correlation coefficients.
Regression analysis
Table 5 shows models for the relationship between percent-predicted FEV1 and air pollutants. We found significant inverse associations between FEV1 and 1-hr and 8-hr peak personal PM2.5 measured over the 24-hr periods preceding the lung function measurements (lag 0). The model for lag 0 24-hr average personal PM2.5 showed smaller associations for an interquartile increase in exposure, and was of borderline significance (p < 0.08). However, dropping the one influential subject discussed in “Methods” led to a stronger significant association with 24-hr personal PM2.5 (−0.69% predicted FEV1; 95% CI, −1.34 to −0.04%). Outdoor central-site 24-hr average PM2.5 (Table 5) and PM10 (not shown) were not associated with FEV1. Neither personal nor central-site EC or OC was associated with FEV1. Personal NO2 exposures were significantly inversely associated with FEV1, at lag 0 day and almost significant at lag 1 day (p = 0.06). This association was stronger with a 2-day moving average of lag 0 + 1 personal NO2 (not shown; −1.75%; 95% CI, −2.83 to −0.673%). Central-site NO2 was more weakly but significantly associated with FEV1 deficits at lag 0, but not at lag 1 day. Although regression coefficients were negative, central-site O3 was not significantly associated with FEV1.
Table 5.
Mixed-model estimates of the association between personal and central-site air pollutant exposures and percent-predicted FEV1 in 53 schoolchildren with asthma.
| Personal
|
Central site
|
|||
|---|---|---|---|---|
| Exposure | Coefficienta (95% CI) | p-Value | Coefficient (95% CI) | p-Value |
| PM2.5 1-hr maximum | ||||
| Lag 0 | −0.969 (−1.538 to −0.399) | 0.001 | NA | |
| Lag 1 | 0.073 (−0.595 to 0.740) | 0.831 | NA | |
| PM2.5 8-hr maximum | ||||
| Lag 0 | −0.801 (−1.465 to −0.137) | 0.018 | NA | |
| Lag 1 | 0.107 (−0.584 to 0.798) | 0.761 | NA | |
| PM2.5 24-hr average | ||||
| Lag 0 | −0.592 (−1.251 to 0.068) | 0.079 | −0.004 (−0.650 to 0.642) | 0.990 |
| Lag 1 | 0.049 (−0.613 to 0.711) | 0.885 | −0.142 (−0.775 to 0.491) | 0.660 |
| PM2.5 EC 24-hr average | ||||
| Lag 0 | −0.080 (−0.397 to 0.238) | 0.623 | −0.184 (−1.038 to 0.671) | 0.673 |
| Lag 1 | 0.067 (−0.467 to 0.602) | 0.805 | −0.129 (−0.970 to 0.712) | 0.763 |
| PM2.5 OC 24-hr average | ||||
| Lag 0 | −0.278 (−1.222 to 0.666) | 0.564 | −0.402 (−1.361 to 0.557) | 0.411 |
| Lag 1 | −0.368 (−1.548 to 0.812) | 0.540 | −0.188 (−1.169 to 0.793) | 0.707 |
| NO2 24-hr average | ||||
| Lag 0 | −1.217 (−1.958 to −0.476) | 0.001 | −0.408 (−0.768 to −0.047) | 0.027 |
| Lag 1 | −0.713 (−1.456 to 0.030) | 0.060 | −0.062 (−0.394 to 0.269) | 0.712 |
| O3 8-hr maximum | ||||
| Lag 0 | NA | −0.383 (−1.752 to 0.986) | 0.583 | |
| Lag 1 | NA | −0.904 (−2.314 to 0.506) | 0.209 | |
NA, not available. Lag 0: most recent 24-hr average measurement preceding the FEV1 measurement; lag 1: previous 24-hr average measurement preceding the FEV1 measurement.
Coefficients represent the expected change in FEV1 associated with one IQR change in each air pollutant level (see Table 2), adjusted for the previous FEV1 measurement, personal temperature, personal relative humidity, cumulative inhaler use on the previous day, and inhaler use during the last night, and excluding observations where there was use of inhaled as-needed bronchodilators in the preceding 4 hr.
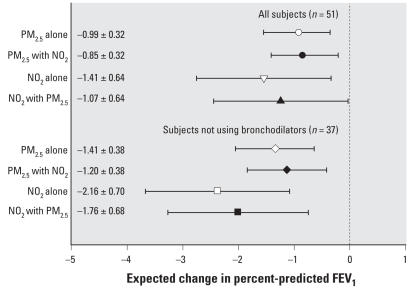
There was no difference in FEV1 associations between sexes in models including a product term of sex by air pollutant. There were also no significant interactions between use of anti-inflammatory medications and air pollutants. However, we did find significantly weaker associations among 16 children taking daily bronchodilator controller medications compared with those not taking these medications. Table 6 shows models for the relationship between percent-predicted FEV1 and lag 0 day air pollutants stratified by use of bronchodilator controller medications. Associations for personal NO2 and PM2.5 and ambient NO2 largely reflect those found among all subjects (Table 5), but are stronger in the 37 subjects not taking controller bronchodilators, including 24-hr average personal PM2.5. For an interquartile increase of 16.8 ppb 2-day average personal NO2, (not shown) percent-predicted FEV1 decreased by −2.45% (95% CI, −3.57 to −1.33%) in subjects not taking controller bronchodilators, but there was no association in subjects taking controller bronchodilators (p = 0.74).
Table 6.
Mixed-model estimates of associations between percent-predicted FEV1 and lag 0 air pollutant exposures stratified by preventive bronchodilator medication use.
| Not taking bronchodilator controller medications (37 subjects)
|
Taking bronchodilator controller medications (16 subjects)
|
|||
|---|---|---|---|---|
| Exposure | Coefficienta (95% CI) | p-Value | Coefficient (95% CI) | p-Value |
| Personal | ||||
| PM2.5 1-hr maximum | −1.324 (−2.001 to −0.648) | 0.0001 | −0.145 (−1.230 to 0.940) | 0.792 |
| PM2.5 24-hr average | −0.785 (−1.526 to −0.043) | 0.038 | 0.004 (−1.478 to 1.486) | 0.996 |
| PM2.5 EC | −0.249 (−1.022 to 0.524) | 0.527 | −0.075 (−0.442 to 0.293) | 0.689 |
| PM2.5 OC | −0.577 (−1.636 to 0.482) | 0.285 | 0.441 (−1.678 to 2.561) | 0.682 |
| NO2 | −1.443 (−2.257 to −0.629) | 0.001 | −0.587 (−2.432 to 1.257) | 0.531 |
| Central site | ||||
| PM2.5 | −0.003 (−0.719 to 0.712) | 0.992 | −0.101 (−1.745 to 1.544) | 0.904 |
| PM2.5 EC | −0.616 (−1.659 to 0.428) | 0.247 | 0.733 (−0.921 to 2.387) | 0.383 |
| PM2.5 OC | −0.503 (−1.666 to 0.660) | 0.396 | −0.329 (−2.198 to 1.540) | 0.729 |
| NO2 | −0.555 (−0.966 to −0.143) | 0.008 | −0.048 (−0.859 to 0.764) | 0.908 |
Lag 0: most recent 24-hr average measurement preceding the FEV1 measurement.
Coefficients represent the expected change in FEV1 associated with one IQR change in each air pollutant level (see Table 2), adjusted for the previous FEV1 measurement, personal temperature, personal relative humidity, cumulative inhaler use on the previous day, and inhaler use during the last night, and excluding observations where there was use of inhaled as-needed bronchodilators in the preceding 4 hr.
To assess the potential importance of indoor NO2 sources, we retested NO2 models by including the presence of gas stoves as a binary variable, and a trinomial variable to account for gas stoves with or without pilot lights. Concentrations of personal NO2 were significantly higher for 22 subjects with gas stoves having pilot lights than for 12 subjects without gas stoves (mean = 32.4 ppb vs. 25.0 ppb, respectively), and higher than for 19 subjects with gas stoves but no pilot lights (mean = 26.4 ppb). However, gas stove covariates in the mixed models did not affect the magnitude or statistical significance of associations of FEV1 with personal NO2. In addition, stratified analyses by gas stoves did not reveal significant differences in associations between FEV1 with NO2 (p > 0.6). These findings held when stratified by bronchodilator group.
Figure 2 shows single-pollutant compared with two-pollutant models including subjects with both personal PM2.5 and NO2 data, and excluding the influential subject. Significant associations for 2-day average personal NO2 and lag 0 1-hr maximum PM2.5 remained when regressed together in the same model, with small decreases in estimates of association. A two-pollutant model with lag 0 24-hr averages of both personal NO2 and PM2.5 was consistent with these findings (not shown). Models testing product terms between personal NO2 and PM2.5 on FEV1 showed no evidence of interaction.
Figure 2.
Adjusted single- and two-pollutant models (coefficient and 95% CIs) for change in FEV1 in relation to personal 1-hr maximum PM2.5 the last 24 hr, and 2-day average NO2 measurements. Expected change in FEV1 corresponds to an IQR change in the air pollutant (Table 2), and estimates are plotted by open symbols for single-pollutant models and solid symbols for models adjusting for the indicated co-pollutant. Single-pollutant models are for the subset of nonmissing observations for the other co-pollutant, and thus exclude two subjects who did not have personal PM2.5 data.
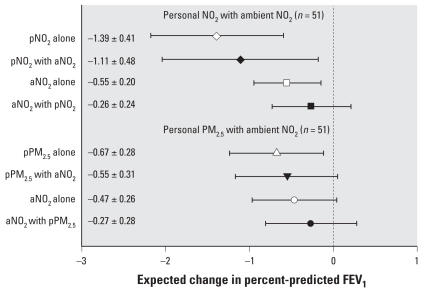
We also tested two-pollutant models for 24-hr average personal NO2 and ambient NO2, and for 24-hr average personal PM2.5 and ambient NO2, excluding the influential subject. Figure 3 shows that personal NO2 led to a halving of the estimated FEV1 regression coefficient for ambient NO2, whereas personal NO2 is reduced by 20% in the two-pollutant model. Similarly, personal PM2.5 led to a 43% reduction in the estimated regression coefficient for ambient NO2 whereas the personal PM2.5 coefficient is reduced by 18% in the two-pollutant model. Models with maximum personal PM2.5 were consistent with these findings (not shown). An enhancement of FEV1 deficits was observed with a product term of personal NO2 with ambient NO2 (p < 0.06).
Figure 3.
Adjusted single- and two-pollutant models (coefficient and 95% CIs) for change in FEV1 in relation to lag day 0 personal 24-hr average NO2 (pNO2) or PM2.5 (pPM2.5), with ambient 24-hr average NO2 (aNO2). Expected change in FEV1 corresponds to an IQR change in the air pollutant (Table 2), and estimates are plotted by open symbols for single-pollutant models and solid symbols for models adjusting for the indicated co-pollutant. Single-pollutant models are for the subset of nonmissing observations for the other co-pollutant in 51 subjects with pPM2.5 data.
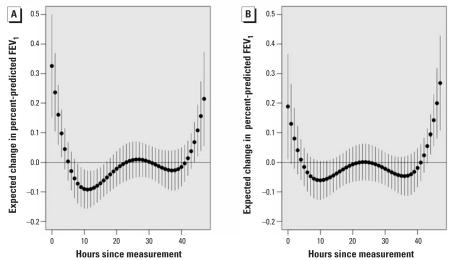
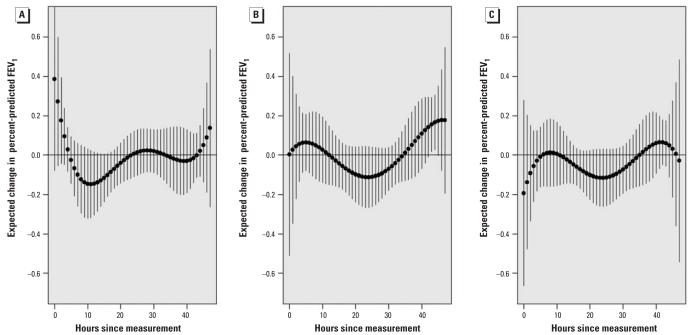
Figure 4A shows a distributed lag model across 48 hr of personal PM2.5 data including all 51 subjects with data. Inverse associations are shown between personal PM2.5 at the 9th through 18th hr preceding FEV1 measurements (FEV1 association with 9th through 18th-hr average, −0.73%; 95% CI, −1.25 to −0.22%). After 24 hr, CIs cross zero and there is evidence of a repeating 24-hr pattern across the 2 days. An unexpected positive association is shown in the 5 hr preceding FEV1 measurements (FEV1 association with 0- through 5th-hr average, 0.34%; 95% CI, −0.13 to 0.81). The 0-through 5th-hr average was confounded to −0.19% by adding an indicator for session period (morning, afternoon, or evening). This finding is attributable to morning FEV1 when both lung function (Table 2) and personal PM2.5 (Figure 1) were lowest, as expected. Thus, the positive association was temporally confounded. In contrast, the session period indicator did not confound the inverse association for the average of the 9th- through 18th-hr PM2.5 preceding FEV1. Figure 4B adjusts for session period. Figure 5A–C shows the distributed lag effects by session period in the group not taking bronchodilators (a similar but slightly less significant pattern was found using all subjects). Figure 5A shows that lags from the previous day (9th–18th hr) adversely affected morning FEV1 in particular. This lag effect then shifted back in time for the afternoon (Figure 5B) and evening FEV1 (Figure 5C) to approximately the same exposures on the previous day.
Figure 4.
Estimated lag effect of hourly personal PM2.5 on FEV1 in the full cohort of 51 subjects. (A) Not adjusted for maneuver; (B) adjusted for maneuver. Estimates are based on a 5th-degree linear mixed-effects polynomial distributed lag model with AR(1) correlation structure. Expected change in FEV1 for each hour corresponds to an IQR change (21.6 μg/m3) in 24-hr average PM2.5 and estimates are plotted by solid circles. Pointwise 95% CIs are plotted by error bars. All estimates are adjusted for the previous FEV1 measurement, personal temperature, personal relative humidity, cumulative inhaler use on the previous day, and inhaler use during the last night, and excluding observations where there was use of inhaled as-needed bronchodilators in the preceding 4 hr.
Figure 5.
Estimated lag effect of hourly personal PM2.5 on FEV1 by session period in 37 subjects with no controller bronchodilator use. (A) morning; (B) afternoon; and (C) evening. Estimates are based on a 5th-degree linear mixed-effects polynomial distributed lag model with AR(1) correlation structure. Expected change in FEV1 for each hour corresponds to an IQR change (21.6 μg/m3) in 24-hr average PM2.5, and estimates are plotted by solid circles. Pointwise 95% CIs are plotted by error bars. All estimates are adjusted for the previous FEV1 measurement, personal temperature, personal relative humidity, cumulative inhaler use on the previous day, and inhaler use during the last night, and excluding observations where there was use of inhaled as-needed bronchodilators in the preceding 4 hr.
These results suggested that effects might differ by session period. Therefore, we tested product term models for session period by each pollutant. Few meaningful product terms were found at p < 0.1. Personal 1-hr maximum PM2.5 associations were stronger for the afternoon (−1.91%, p < 0.0001) than for the morning (−0.85%, p < 0.08) or evening FEV1 (−0.42%, p < 0.29). In subjects not using bronchodilators, the coefficient for personal OC was significantly more negative for afternoon FEV1 (−1.47%, p < 0.05) and morning FEV1 (−1.53%, p < 0.1) than for evening FEV1 (1.46%, p < 0.1). The coefficient for personal EC was also significantly different for morning FEV1 (−1.11%, p < 0.08). In addition, the coefficient for ambient NO2 lag 0 was significantly more negative for the morning FEV1 (−1.16%, p < 0.0005) than other FEV1 (p = 0.5).
Discussion
We found that increased personal exposures to NO2 and PM2.5 were associated with lung function deficits in schoolchildren with persistent asthma. To our knowledge, this is the first report of associations between personal exposure to daily NO2 and FEV1 decrements in children with asthma. The largest magnitude of association was a 2.45% drop of percent-predicted FEV1 for a small interquartile increase of 16.8 ppb 2-day average NO2 in 37 subjects not taking controller bronchodilators. We found consistent but weaker associations for ambient NO2 measured at central regional sites. However, we found no associations of FEV1 with ambient PM, likely because of exposure error and the short sampling period of 10 days per subject.
In two-pollutant models for personal NO2 and PM2.5, we showed considerable independence of associations with FEV1 suggesting that personal PM2.5 mass represents different causal components than personal NO2. This may have at least partly resulted from the different averaging times for each of the pollutants because NO2 was sampled over fixed 24-hr intervals, whereas PM2.5 was measured continuously and linked to thrice daily FEV1 by real time. We previously reported consistent independent associations of exhaled NO (measured once daily) with personal PM2.5 and NO2 averaged across the same 24-hr intervals in 45 of the subjects in the present analysis (Delfino et al. 2006). In addition to products of fossil fuel combustion, personal PM2.5 mass may also represent a variety of other exposures, including bioaerosols such as endotoxin that can exacerbate asthma. Our data also suggest that personal PM2.5 reflects ambient PM2.5, given the moderate correlation between them (r = 0.60).
Because of the presumed superiority of personal exposures in assessments of exposure–response relationships, we anticipated that associations for personal exposures would confound associations for ambient exposures. Furthermore, Sarnat et al. (2005) found that ambient NO2 concentration was a good surrogate of personal PM2.5 exposure. This suggests that epidemiologic findings for ambient NO2 may be attributable to personal PM exposures. We confirmed and expanded these expectations by finding that both personal NO2 and personal PM2.5 confounded associations of FEV1 with ambient NO2. Because personal PM2.5 and personal NO2 had largely independent effects and both confounded ambient NO2, they may represent both similar and different information about causal components. The interaction between personal and ambient NO2 further support this. In a previous panel study of children with asthma in Southern California, we also found that FEV1 was inversely associated with ambient NO2, but this was completely confounded by personal PM (Delfino et al. 2004). These findings suggest that personal PM2.5 and NO2 represent some set of causal background air pollutants also represented by ambient NO2. What pollutant components and sources are driving associations, though?
Outdoor NO2 is strongly influenced by local traffic density (Jerrett et al. 2005). Although indoor sources such as gas stoves contribute to personal exposure as well (Levy et al. 1998), we found that presence of gas stoves did not explain the association of FEV1 with personal NO2. In a large study of 482 homes in Los Angeles, outdoor home NO2 was well correlated with personal NO2 (R2 = 0.52) because of indoor infiltration (Spengler et al. 1994). Traffic-related sources of NO2 contribute to high spatial variability of potentially important particulate and gaseous co-pollutants (Sioutas et al. 2005). There is considerable evidence that such variability is best captured by personal exposure measurements (Jerrett et al. 2005). This is important among children who may be exposed at home, at school, and at other locations including times in vehicles. The correlation between personal and ambient NO2 (r = 0.43) as well as the confounding of the ambient NO2 association by personal NO2 are consistent with the view that in addition to local traffic sources, some part of the association we found between personal NO2 and FEV1 was attributable to ambient background sources of NO2. The statistical interaction between personal NO2 with ambient NO2 may reflect this source difference.
Plausible mechanisms of NO2 toxicity have been well described (Persinger et al. 2002) and may contribute to part of our findings. However, in experimental exposure studies of adults with mild asthma, adverse pulmonary effects of NO2 have generally been demonstrated at levels of exposure a magnitude higher than reported here (Kraft et al. 2005). These experimental results contrast recent epidemiologic findings showing associations of asthma outcomes in children with low levels of indoor NO2 (Belanger et al. 2006), of weeklong personal NO2 (Chauhan et al. 2003), and of ambient NO2 (Kim et al. 2004; Schildcrout et al. 2006). We believe the low personal NO2 levels we found are more likely to have served as a surrogate for traffic-related air pollutants. These pollutants may be causally related to asthmatic responses through oxidative stress responses induced by pollutants highly correlated with NO2 (Li et al. 2003; Seaton and Dennekamp 2003).
Given this evidence and our findings for NO2, it is paradoxical that we did not find FEV1 to be associated with particulate EC or OC in either personal or ambient samples, except, in subjects not using bronchodilators, associations of personal OC with morning and afternoon FEV1 and personal EC with morning FEV1. The carbon fraction of PM is derived primarily from products of fossil fuel combustion, so EC and OC should be reasonably good surrogates for causal pollutant components derived from those sources. In our previous report using exhaled NO, we found associations with personal and ambient NO2 were largely independent of associations with personal and ambient EC and OC fractions of PM2.5 in two-pollutant models, thus suggesting different causal pollutant components (Delfino et al. 2006). It is conceivable that volatile and semivolatile organic compounds are behind these findings given their traffic-related sources and role in particle formation (Biswas et al. 2007; Schauer and Cass 2000).
Our results for personal PM2.5 are consistent with recent studies showing inverse associations of personal and/or ambient PM mass with FEV1 among schoolchildren with asthma (Aekplakorn et al. 2003; Delfino et al. 2004; Lewis et al. 2005; Trenga et al. 2006). Magnitudes of association could not be compared, though, because of differences in both the expression of lung function effect estimates and PM measurement methods. Investigators of a recent Denver panel study failed to show associations of ambient PM10 with FEV1 in schoolchildren with persistent asthma (Rabinovitch et al. 2004), but later showed that urinary leukotriene E4 and rescue inhaler use during school hours were positively associated with morning average and peak PM2.5 (Rabinovitch et al. 2006).
Few studies of lung function in children with asthma have used personal particulate air pollution measurements, and fewer still have used real-time personal measurements that allow the assessment of effects of peak particle exposures (Delfino et al. 2004, 2006). We previously followed for 2 weeks per subject a panel of 19 children, 9–17 years of age, with persistent asthma in San Diego County (Delfino et al. 2004). We found that FEV1 significantly decreased similarly in relation to both 24-hr personal PM and 1-hr maximum personal PM, but FEV1 was not associated with outdoor ambient PM2.5 In the present study, we found that personal hourly peak was a stronger and more significant predictor of FEV1 compared with 24-hr average personal PM2.5.
The present associations of FEV1 with hourly PM2.5 in the distributed lag models suggest that inverse associations were primarily from exposure ≥ 8 hr before the lung function measurement. PM2.5 concentrations peaked in mid-morning and they were sustained for several hours into the afternoon and evening (Figure 1). Particles from morning rush hour traffic and in-vehicle exposures followed by secondary photochemical particle formation would have occurred throughout the late morning and afternoon, including time in school. Although this was possibly important in our findings, the resolution of the hourly PM2.5 data is limited primarily by the fact that we used fine particle mass rather than composition or other particle size fractions.
We previously conducted distributed lag analyses of hourly personal PM2.5 using the present panel and showed that exhaled NO (collected in the late afternoon to early evening) was positively associated with PM2.5 in the 5 hr before measurement (Delfino et al. 2006).
Conclusions
The associations we found between personal NO2 and FEV1 deficits may be attributable to other more toxic pollutants from traffic-related sources. Largely independent associations between personal PM2.5 and FEV1 deficits suggest a subset of causal components different from personal NO2. We further conclude that associations of lung function with particulate air pollutants might be missed using ambient central-site data alone unless a large number of repeated observations per person are available. Our results may also not be generalizable to situations where central-site measurements are more representative of personal exposures in other geographic locations. Future work should focus on identifying causal pollutant components and their sources. This will require detailed assessments of exposure close to where children at risk live and attend school—a task not possible using available criteria air pollutant data.
Footnotes
We thank staff at the General Clinical Research Center University of California Irvine (National Institutes of Health grants MO1-RR00827), and the South Coast Air Quality Management District.
The project described was supported by grant ES11615 from the National Institute of Environmental Health Sciences (NIEHS) and grant HD048721 from the National Institute of Child Health and Human Development (NICHD), U.S. National Institutes of Health (NIH).
The contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIEHS, NICHD, or NIH.
References
- Aekplakorn W, Loomis D, Vichit-Vadakan N, Shy C, Wongtim S, Vitayanon P. Acute effect of sulphur dioxide from a power plant on pulmonary function of children, Thailand. Int J Epidemiol. 2003;32:854–861. doi: 10.1093/ije/dyg237. [DOI] [PubMed] [Google Scholar]
- Becklake MR, Kauffmann F. Gender differences in airway behaviour over the human life span. Thorax. 1999;54:1119–1138. doi: 10.1136/thx.54.12.1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belanger K, Gent JF, Triche EW, Bracken MB, Leaderer BP. Association of indoor nitrogen dioxide exposure with respiratory symptoms in children with asthma. Am J Respir Crit Care Med. 2006;173:297–303. doi: 10.1164/rccm.200408-1123OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biswas S, Ntziachristos L, Moore KF, Sioutas C. Particle volatility in the vicinity of a freeway with heavy-duty diesel traffic. Atmospheric Environment. 2007;41:3479–3493. doi: 10.1021/es062590s. [DOI] [PubMed] [Google Scholar]
- Chakrabarti B, Fine PM, Delfino RJ, Sioutas C. Performance evaluation of the active-flow personal DataRAM PM2.5 mass monitor (Thermo Anderson pDR-1200) designed for continuous personal exposure measurements. Atmos Environ. 2004;38:3329–3340. [Google Scholar]
- Chauhan AJ, Inskip HM, Linaker CH, Smith S, Schreiber J, Johnston SL, et al. Personal exposure to nitrogen dioxide (NO2) and the severity of virus-induced asthma in children. Lancet. 2003;361:1939–1944. doi: 10.1016/S0140-6736(03)13582-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cyrys J, Heinrich J, Hoek G, Meliefste K, Lewné M, Gehring U, et al. Comparison between different traffic-related particle indicators: elemental carbon (EC), PM2.5 mass, and absorbance. J Expo Anal Environ Epidemiol. 2003;13:134–143. doi: 10.1038/sj.jea.7500262. [DOI] [PubMed] [Google Scholar]
- Delfino RJ, Gong H, Jr, Linn WS, Hu Y, Pellizzari ED. Asthma symptoms in Hispanic children and daily ambient exposures to toxic and criteria air pollutants. Environ Health Perspect. 2003;111:647–656. doi: 10.1289/ehp.5992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delfino RJ, Quintana PJE, Floro J, Gastañaga VM, Samimi BS, Kleinman MT, et al. Association of FEV1 in asthmatic children with personal and microenvironmental exposure to airborne particulate matter. Environ Health Perspect. 2004;112:932–941. doi: 10.1289/ehp.6815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delfino RJ, Staimer N, Gillen D, Tjoa T, Sioutas C, Fung K, et al. Personal and ambient air pollution is associated with increased exhaled NO in children with asthma. Environ Health Perspect. 2006;114:1736–1743. doi: 10.1289/ehp.9141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delfino RJ, Zeiger RS, Seltzer JM, Street DH. Symptoms in pediatric asthmatics and air pollution: differences in effects by symptom severity, anti-inflammatory medication use, and particulate averaging time. Environ Health Perspect. 1998;106:751–761. doi: 10.1289/ehp.98106751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delfino RJ, Zeiger RS, Seltzer JM, Street DH, McLaren C. Effects of hourly particulate air pollution on asthma symptoms: interaction with use of anti-inflammatory medications. Environ Health Perspect. 2002;110:A607–A617. doi: 10.1289/ehp.021100607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diggle P, Heagerty P, Liang KY, Zeger S. Analysis of Longitudinal Data. 2. New York: Oxford University Press; 2002. [Google Scholar]
- Fujita EM, Campbell DE, Arnott WP, Chow JC, Zielinska B. Evaluations of the chemical mass balance method for determining contributions of gasoline and diesel exhaust to ambient carbonaceous aerosols. J Air Waste Manag Assoc. 2007;57:721–740. doi: 10.3155/1047-3289.57.6.721. [DOI] [PubMed] [Google Scholar]
- Fung K, Chow JC, Watson JG. Evaluation of OC/EC speciation by thermal manganese dioxide oxidation and the IMPROVE Method. J Air Waste Manag Assoc. 2002;52:1333–1341. doi: 10.1080/10473289.2002.10470867. [DOI] [PubMed] [Google Scholar]
- Giannini D, Paggiaro PL, Moscato G, Gherson G, Bacci E, Bancalari L, et al. Comparison between peak expiratory flow and forced expiratory volume in one second (FEV1) during bronchoconstriction induced by different stimuli. J Asthma. 1997;34:105–111. doi: 10.3109/02770909709075654. [DOI] [PubMed] [Google Scholar]
- Hankinson JL, Odencrantz JR, Fedan KB. Spirometric reference values from a sample of the general U.S. population. Am J Respir Crit Care Med. 1999;159:179–187. doi: 10.1164/ajrccm.159.1.9712108. [DOI] [PubMed] [Google Scholar]
- Jerrett M, Arain A, Kanaroglou P, Beckerman B, Potoglou D, Sahsuvaroglu T, et al. A review and evaluation of intraurban air pollution exposure models. J Expo Anal Environ Epidemiol. 2005;15:185–204. doi: 10.1038/sj.jea.7500388. [DOI] [PubMed] [Google Scholar]
- Kim JJ, Smorodinsky S, Lipsett M, Singer BC, Hodgson AT, Ostro B. Traffic-related air pollution near busy roads: the East Bay Children’s Respiratory Health Study. Am J Respir Crit Care Med. 2004;170:520–526. doi: 10.1164/rccm.200403-281OC. [DOI] [PubMed] [Google Scholar]
- Koenig JQ, Mar TF, Allen RW, Jansen K, Lumley T, Sullivan JH, et al. Pulmonary effects of indoor- and outdoor-generated particles in children with asthma. Environ Health Perspect. 2005;113:499–503. doi: 10.1289/ehp.7511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraft M, Eikmann T, Kappos A, Kunzli N, Rapp R, Schneider K, et al. The German view: effects of nitrogen dioxide on human health-derivation of health-related short-term and long-term values. Int J Hyg Environ Health. 2005;208:305–318. doi: 10.1016/j.ijheh.2005.04.002. [DOI] [PubMed] [Google Scholar]
- Levy JI, Lee K, Spengler JD, Yanagisawa Y. Impact of residential nitrogen dioxide exposure on personal exposure: an international study. J Air Waste Manag Assoc. 1998;48:553–560. doi: 10.1080/10473289.1998.10463704. [DOI] [PubMed] [Google Scholar]
- Lewis TC, Robins TG, Dvonch JT, Keeler GJ, Yip FY, Mentz GB, et al. Air pollution-associated changes in lung function among asthmatic children in Detroit. Environ Health Perspect. 2005;113:1068–1075. doi: 10.1289/ehp.7533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li N, Hao M, Phalen RF, Hinds WC, Nel AE. Particulate air pollutants and asthma. A paradigm for the role of oxidative stress in PM-induced adverse health effects. Clin Immunol. 2003;109:250–265. doi: 10.1016/j.clim.2003.08.006. [DOI] [PubMed] [Google Scholar]
- National Institute for Occupational Safety and Health. Nitric oxide and nitrogen dioxide: method 6014. In: Eller PM, Cassinelli ME, editors. NIOSH Manual of Analytical Methods (NMAM) 4. Cincinnati, OH: National Institute for Occupational Safety and Health; 1994. DHHS Publication No. 94–113. [Google Scholar]
- Persinger RL, Poynter ME, Ckless K, Janssen-Heininger YM. Molecular mechanisms of nitrogen dioxide induced epithelial injury in the lung. Mol Cell Biochem. 2002;234–235:71–80. [PubMed] [Google Scholar]
- Rabinovitch N, Matthew Strand M, Gelfand EW. Particulate levels are associated with early asthma worsening in children with persistent disease. Am J Respir Crit Care Med. 2006;173:1098–1105. doi: 10.1164/rccm.200509-1393OC. [DOI] [PubMed] [Google Scholar]
- Rabinovitch N, Zhang L, Murphy JR, Vedal S, Dutton SJ, Gelfand EW. Effects of wintertime ambient air pollutants on asthma exacerbations in urban minority children with moderate to severe disease. J Allergy Clin Immunol. 2004;114:1131–1137. doi: 10.1016/j.jaci.2004.08.026. [DOI] [PubMed] [Google Scholar]
- RAND California. RAND California Population Density Statistics in California Counties and Cities. 2007. [[accessed 24 August 2007]]. Available: http://ca.rand.org/stats/community/popdensity.html.
- Sarnat JA, Brown KW, Schwartz J, Coull BA, Koutrakis P. Ambient gas concentrations and personal particulate matter exposures: implications for studying the health effects of particles. Epidemiology. 2005;16:385–395. doi: 10.1097/01.ede.0000155505.04775.33. [DOI] [PubMed] [Google Scholar]
- Sarnat JA, Holguin F. Asthma and air quality. Curr Opin Pulm Med. 2007;13:63–66. doi: 10.1097/MCP.0b013e3280117d25. [DOI] [PubMed] [Google Scholar]
- Schauer JJ, Cass GR. Source apportionment of winter-time gas-phase and particle-phase air pollutants using organic compounds as tracers. Environ Sci Technol. 2000;34:1821–1832. [Google Scholar]
- Schildcrout JS, Sheppard L, Lumley T, Slaughter JC, Koenig JQ, Shapiro GG. Ambient air pollution and asthma exacerbations in children: an eight-city analysis. Am J Epidemiol. 2006;164:505–517. doi: 10.1093/aje/kwj225. [DOI] [PubMed] [Google Scholar]
- Schwartz J. The distributed lag between air pollution and daily death. Epidemiology. 2000;11:320–326. doi: 10.1097/00001648-200005000-00016. [DOI] [PubMed] [Google Scholar]
- Seaton A, Dennekamp M. Hypothesis: ill health associated with low concentrations of nitrogen dioxide—an effect of ultrafine particles? Thorax. 2003;58:1012–1015. doi: 10.1136/thorax.58.12.1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheppard L, Slaughter JC, Schildcrout J, Liu LJ, Lumley T. Exposure and measurement contributions to estimates of acute air pollution effects. J Expo Anal Environ Epidemiol. 2005;15:366–376. doi: 10.1038/sj.jea.7500413. [DOI] [PubMed] [Google Scholar]
- Sioutas C, Delfino RJ, Singh M. Exposure assessment for atmospheric ultrafine particles (UFP) and implications in epidemiological research. Environ Health Perspect. 2005;113:947–955. doi: 10.1289/ehp.7939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spengler J, Schwab M, Ryan PB, Colome S, Wilson AL, Billick I, et al. Personal exposure to nitrogen dioxide in the Los Angeles Basin. J Air Waste Manag Assoc. 1994;44:39–47. doi: 10.1080/1073161x.1994.10467236. [DOI] [PubMed] [Google Scholar]
- Staimer N, Delfino RJ, Sioutas C, Bufalino C, Fine PM, Meacher D, et al. Evaluation of an active personal exposure monitor for NO2. Anal Bioanal Chem. 2005;383:955–962. doi: 10.1007/s00216-005-0086-6. [DOI] [PubMed] [Google Scholar]
- Thiadens HA, De Bock GH, Van Houwelingen JC, Dekker FW, De Waal MW, Springer MP, et al. Can peak expiratory flow measurements reliably identify the presence of airway obstruction and bronchodilator response as assessed by FEV1 in primary care patients presenting with a persistent cough? Thorax. 1999;54:1055–1060. doi: 10.1136/thx.54.12.1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson R, Delfino RJ, Tjoa T, Nussbaum E, Cooper D. Evaluation of daily home spirometry for school children with asthma: new insights. Pediatr Pulmonol. 2006;41:819–828. doi: 10.1002/ppul.20449. [DOI] [PubMed] [Google Scholar]
- Trasande L, Thurston GD. The role of air pollution in asthma and other pediatric morbidities. J Allergy Clin Immunol. 2005;115:689–699. doi: 10.1016/j.jaci.2005.01.056. [DOI] [PubMed] [Google Scholar]
- Trenga CA, Sullivan JH, Schildcrout JS, Shepherd KP, Shapiro GG, Liu LJ, et al. Effect of particulate air pollution on lung function in adult and pediatric subjects in a Seattle panel study. Chest. 2006;129:1614–1622. doi: 10.1378/chest.129.6.1614. [DOI] [PubMed] [Google Scholar]
- U.S. Environmental Protection Agency. National Ambient Air Quality Standards. 2008. [[accessed 4 March 2008]]. Available: http://epa.gov/air/criteria.html.
- Verbeke G, Molenberghs G. Linear Mixed Models for Longitudinal Data. New York: Springer; 2001. [Google Scholar]
- Ward DJ, Ayres JG. Particulate air pollution and panel studies in children: a systematic review. Occup Environ Med. 2004;61:e13. doi: 10.1136/oem.2003.007088. [DOI] [PMC free article] [PubMed] [Google Scholar]