Abstract
Serotonin (5-HT) derived from bulbo-spinal projection is released by nociceptive input into the spinal dorsal horn. Here we report that formalin injection in the paw produced pain behavior (flinching) and phosphorylation of spinal ERK1/2 (P-ERK1/2, indicating activation) in rats. Depletion of spinal 5-HT by intrathecal (IT) 5,7-DHT, a serotonergic neurotoxin, profoundly reduced formalin evoked flinching and the increase in P-ERK1/2. Ondansetron (a 5-HT3 receptor antagonist) at IT doses that inhibited flinching also attenuated spinal ERK activation. These findings reveal that primary afferent-evoked activation of spinal ERK requires the input from an excitatory 5-HT descending pathway.
Keywords: Serotonin, Descending facilitation, ERK, Spinal cord, Primary afferent, Nociception
1. Introduction
Several reports indicate that extracellular signal-regulated kinases 1 and 2 (ERK1/2) expressed in the spinal cord are involved in pain signal processing. ERK1/2 are activated in the spinal dorsal horn following peripheral inflammation and tissue/nerve injury. Pharmacologically blocking this activation reduces the hypersensitivity otherwise observed in these experimental models [1–5]. Current thinking has been that this activation of ERK is directly mediated by a local afferent evoked excitation of the dorsal horn neurons. This assertion, while reasonable, ignores the possible contribution of other dorsal horn inputs that are believed to regulate dorsal horn excitability. The excitatory serotonergic pathway, which arises from the brainstem medullary 5-HT containing nuclei of the caudal midline raphe [6–8], plays a significant role in controlling spinal excitability through activation of spinal 5-HT3 receptors [6,9–11]. These observations led us to advance the hypothesis that spinal ERK1/2 activation observed in the face of noxious afferent stimulation is in fact mediated by this descending pathway.
2. Materials and methods
2.1. Animals
All experiments were carried out according to protocols approved by the Institutional Animal Care Committee of University of California, San Diego. Male Holzman Sprague-Dawley rats (300–350 g) were used. Chronic lumbar intrathecal (IT) catheters (PE-5, 8.5 cm) were implanted through a cisternal exposure under isoflurane anesthesia [12]. PD98059 (Calbiochem, San Diego, CA), 5,7-dihydroxy-tryptamine (5,7-DHT, Sigma), and Ondansetron hydrochloride (ZofranR, GlaxoSmithKline, UK) were prepared for IT delivery in 10 µl followed by 10 µl saline. PD98059 was dissolved in 25% DMSO, 0.5% Tween-80 in saline, and 5,7-DHT in 45% cyclodextrin.
2.2. Western blot
Spinal cords harvested 5, 30 and 60 minutes after hind paw formalin (50 µl, 2.5%) injection were homogenized by sonication in extraction buffer (50 mM Tris buffer, containing 0.5% Triton X-100, 150 mM NaCl, 1 mM EDTA, Protease inhibitor cocktail (P-8340, Sigma 1:100), phosphatase inhibitor cocktail I and II (Sigma 1:100). The samples were subjected to 4–12% Bis-Tris gel electrophoresis (Invitrogen, Carlsbad, CA) and transferred to nitrocellulose membranes (Invitrogen). After 1 hour incubation in 5% low-fat milk the membranes were probed with antibodies overnight at 4°C followed by HRP-conjugated secondary antibody for 1 hour at room temperature. The signal was detected with chemiluminescent reagents (SuperSignal, Pierce, Rockford, IL). The nitrocellulose membranes were then stripped with Re-Blot Western blot recycling kit (Chemicon, Temecula, CA) and incubated with different antibodies. Antibodies used in this study: phosphorylated ERK1/2 (1:1000), total ERK1/2 (1:1000) (Cell Signaling Technology, Beverly, MA) and β-actin (1:25,000) (Sigma). Intensity of immunoreactive bands was quantified using ImageQuant software (Molecular Dynamics, Sunnyvale, CA). ERK1/2 immuno-positive bands were normalized relative to β-actin.
2.3. Nociceptive models
To quantify formalin induced flinching, an automated sensing system was employed [13]. Briefly, a soft metal band was placed on the hind paw of the animal being tested, and 2.5% formalin (50 µl) was injected into the dorsal side of the banded paw. Nociceptive flinching behavior was counted for 1-min periods for 60 minutes.
2.4. Measurements of tissue content of 5-HT
High performance liquid chromatography (HPLC) with a fluorescent detector was employed to determine the content of 5-HT in the spinal cord, sciatic nerve and skin of 5,7-DHT and vehicle treated rats [14].
2.5. Statistics
For measurements of protein levels by Western blotting and formalin test, four to seven animals were included per group. Differences between groups were compared with one-way ANOVA and Bonferroni post hoc test.
3. Results
3.1. Injection of formalin into the paw evokes phosphorylation of spinal ERK1/2
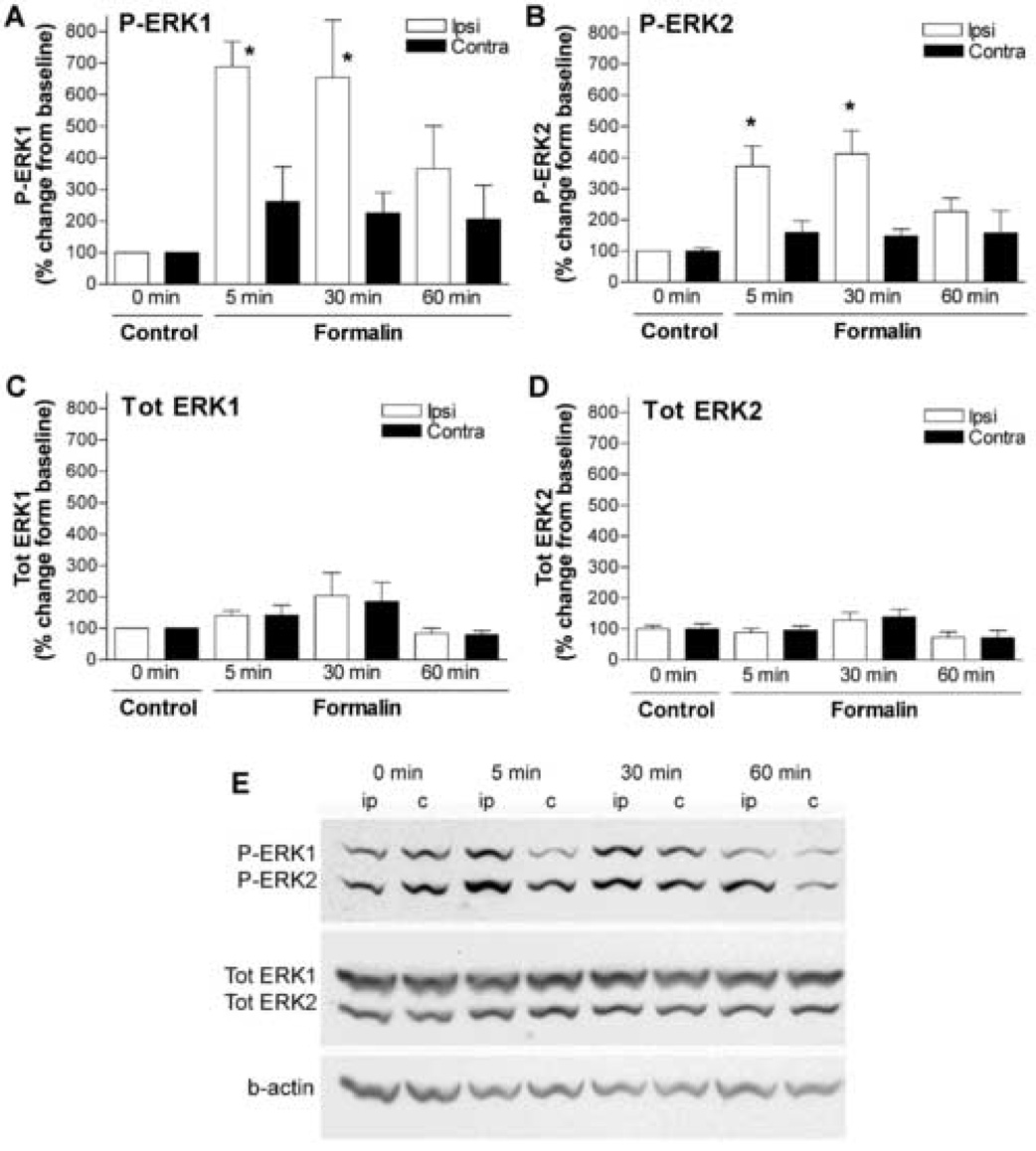
The formalin model is frequently used to study tissue injury induced facilitated pain states in rodents [13]. Formalin-induced hyperalgesia (i.e., phase II flinches, see below) is thought to represent spinal facilitation initiated by afferent input [15]. In agreement with the immunocytochemical studies [1,16], we showed by western blots that formalin paw injection produced an increase in phosphorylation of spinal ERK1/2. Both P-ERK1 and P-ERK2 increased profoundly in the ipsilateral spinal cord 5 min after formalin injection with the peak effects at 30 min (4–7 fold, p < 0.05), and returned towards baseline levels at 60 min (Fig. 1A, B, E). There were no significant changes in P-ERK1/2 in the contralateral side (Fig. 1A, B, E) or total protein expression of ERK1/2 at the time points examined (Fig. 1C, D).
Figure 1.
Injection of formalin to the paw activates ERK1/2 in the spinal cord. Percentage change of phosphorylated (P) ERK1 (A), P-ERK2 (B), Total (Tot) ERK1 (C) and Tot-ERK2 (D) in the lumbar dorsal cord ipsilateral (ipsi) or contralateral (contra) to formalin paw injection at 5, 30 and 60 min compared to naïve control. The data were normalized against beta-actin (b-actin) expressed in each sample. N=4–7/group, *p < 0.05, 5 or 30 min vs. 0 min, one-way ANOVA. (E) Representative western blots showing the levels of P-ERK1/2, tot ERK1/2 and b-actin in ipsilateral (ip) and contraleteral (c) spinal cords after formalin injections at the time points of 5, 30 and 60 min (0 min = naïve rat).
3.2. Inhibition of spinal ERK1/2 attenuates formalin induced flinching
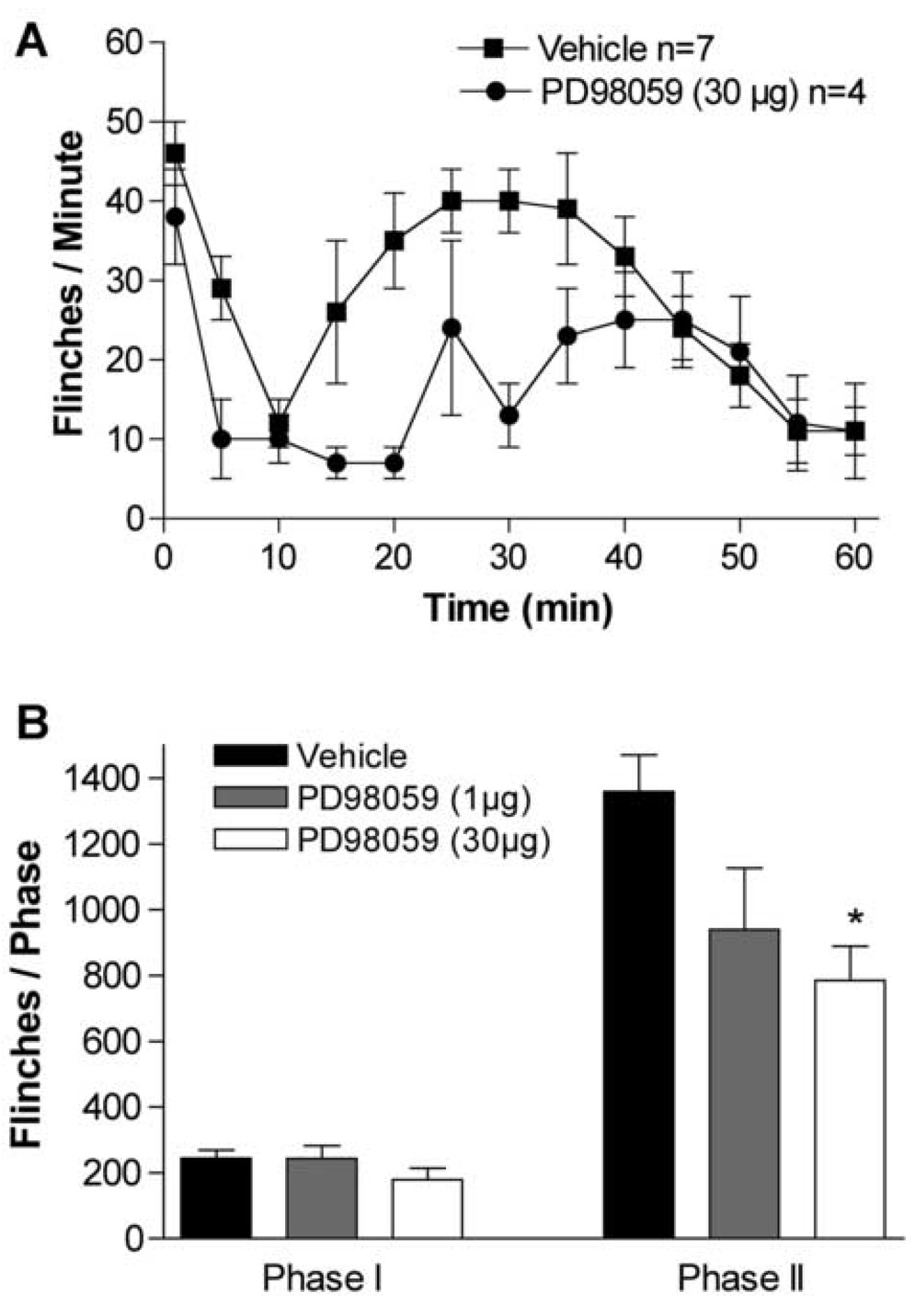
After formalin injection, the total number of flinches in the vehicle group was 224 ± 25 in phase 1 (1–9 min), and 1360 ± 111 in phase 2 (10–60 min) (Fig. 2A). Pretreatment with the MEK1 inhibitor PD98059 (IT, 1–30µg, 15 min prior to formalin injection) produced a dose-dependent inhibition of the phase 2 flinching (Fig. 2 A, B). The highest dose of IT PD98059 (30 µg) reduced the second phase response in comparison with the vehicle group (786±103 flinches, p < 0.05 vs. vehicle group) without causing any sign of sedation or motor weakness.
Figure 2.
Inhibition of ERK1/2 activity by blocking MEK1 activity attenuates formalin evoked formalin induced flinching. (A) Number of flinches/minute plotted versus time following injection of formalin to the paw and IT injection of PD98059 (30 µg), or vehicle (10 µl). (B) Histogram showing the dose-response effect of IT PD98059 (1–30 µg) on formalin test phase I and phase II flinching behavior. The bars represent the total number of flinches in each phase: Phase I, 1–9 min; Phase II, 10–60 min. The data are presented as mean ± SEM of 4–7 rats/group. * p < 0.05, PD98059 (30 µg) vs. vehicle, one-way ANOVA.
3.3. Depletion of spinal 5-HT prevents formalin-evoked spinal ERK1/2 phosphorylation and hyperalgesia
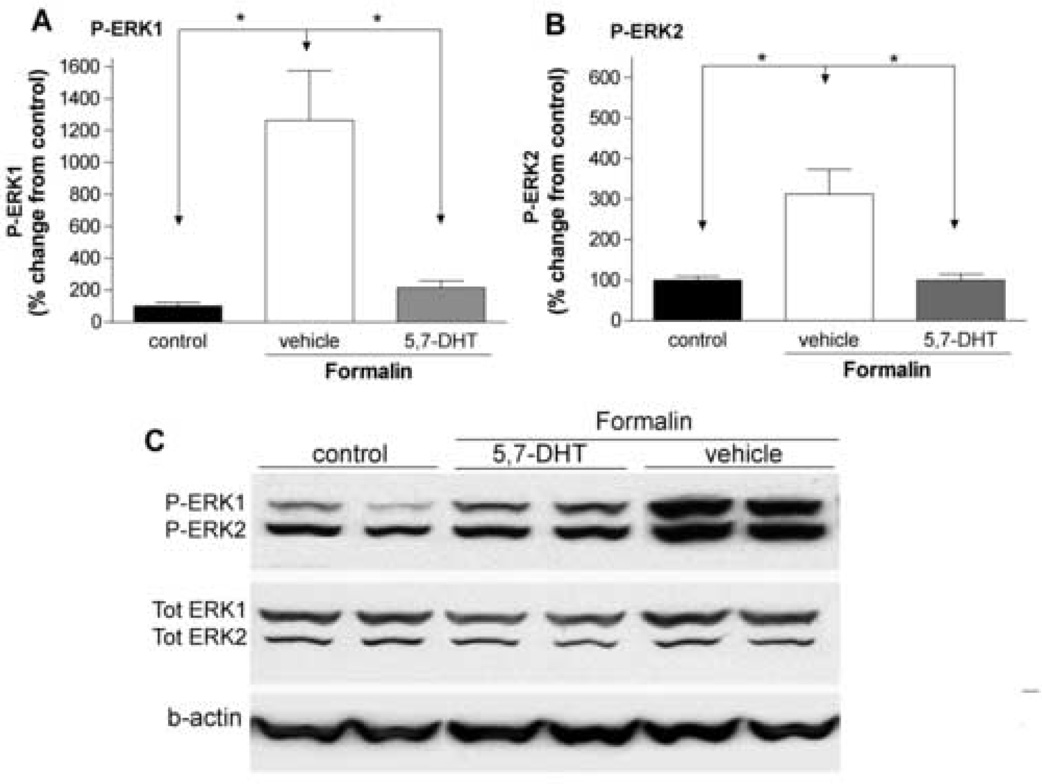
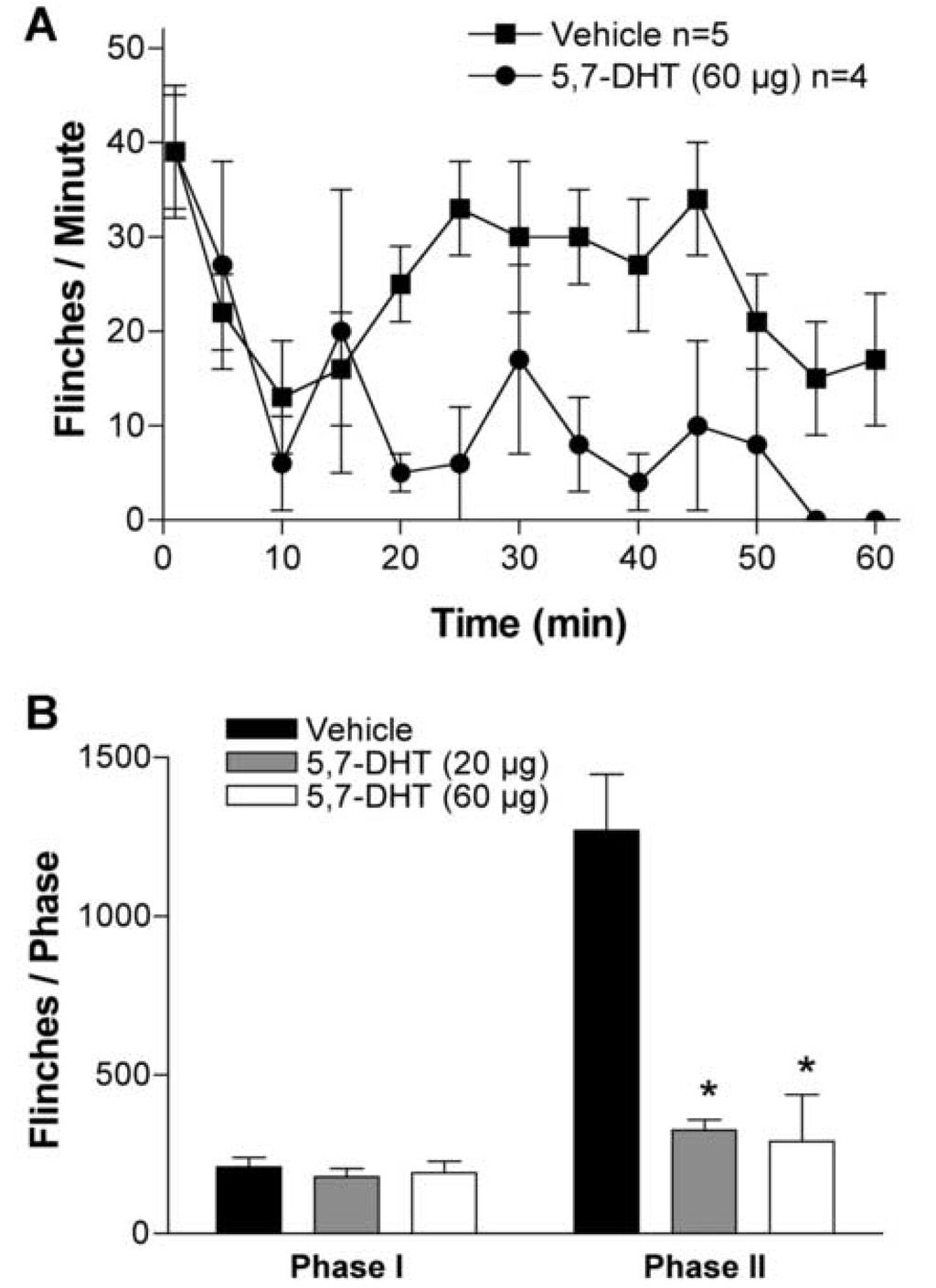
Two days after IT treatment with 5,7-DHT (20–60 µg), the 5-HT level in rat spinal cord was significantly reduced, i.e., 5-HT (nmol/mg tissue): 2.8 ± 0.5 after vehicle treatment, 0.6 ± 0.2 and 0.5 ± 0.1 after 5,7-DHT 20 µg and 60 µg respectively (78–84% reduction, p < 0.05). 5,7-DHT (20 µg, IT) which effectively depleted the 5-HT in the spinal cord, did not affect the 5-HT content in two peripheral tissues examined, sciatic nerve and skin (data not shown). No abnormal behavior or motor functions were observed in 5,7-DHT or vehicle treated animals. In rats pretreated with IT vehicle there was a robust increase in spinal P-ERK1/2 observed 30 min after injection of formalin into the paw. This elevation of spinal P-ERK1/2 was diminished in rats treated with 5,7-DHT (60 µg, p < 0.05, Fig. 3). In parallel with the potent inhibitory effect on activation of spinal ERK1/2, formalin-induced second phase flinching, but not first phase, was profoundly attenuated in 5,7-DHT treated animals (p < 0.05, Fig. 4).
Figure 3.
Intrathecal pretreatment with 5,7-DHT blocks formalin-evoked phosphorylation of spinal ERK1/2. Percentage change of P-ERK1 (A), P-ERK2 (B) in the ipsilateral lumbar spinal cord 30 min after formalin paw injection in the control (no formalin injection), vehicle (10 µl) and 5,7-DHT (60 µg) group. The data were normalized against beta-actin (b-actin) expressed in each sample and presented as mean ± SEM of 4–7 rats/group. *p < 0.05, one-way ANOVA. (C) Representative western blots showing the levels of P-ERK1/2, tot ERK1/2 and b-actin in ipsilateral spinal cords after formalin injections in rats.
Figure 4.
Depletion of spinal 5-HT prevents formalin-evoked hyperalgesia. (A) Flinches/minute plotted veresus time after injection of formalin in rats treated with 5,7-DHT (60 µg, IT) or vehicle (10 µl, IT). (B) Histogram showing the dose-response effect of 5,7-DHT (20–60 µg, IT) on formalin test phase I and phase II flinching behavior. The bars represent the total number of flinches in each phase. The data are presented as mean ± SEM of 4–5 rats /group. * p < 0.05, 5,7-DHT vs. vehicle, one-way ANOVA.
3.4. Inhibition of spinal 5-HT3 receptors attenuates formalin-induced ERK activation and pain behavior
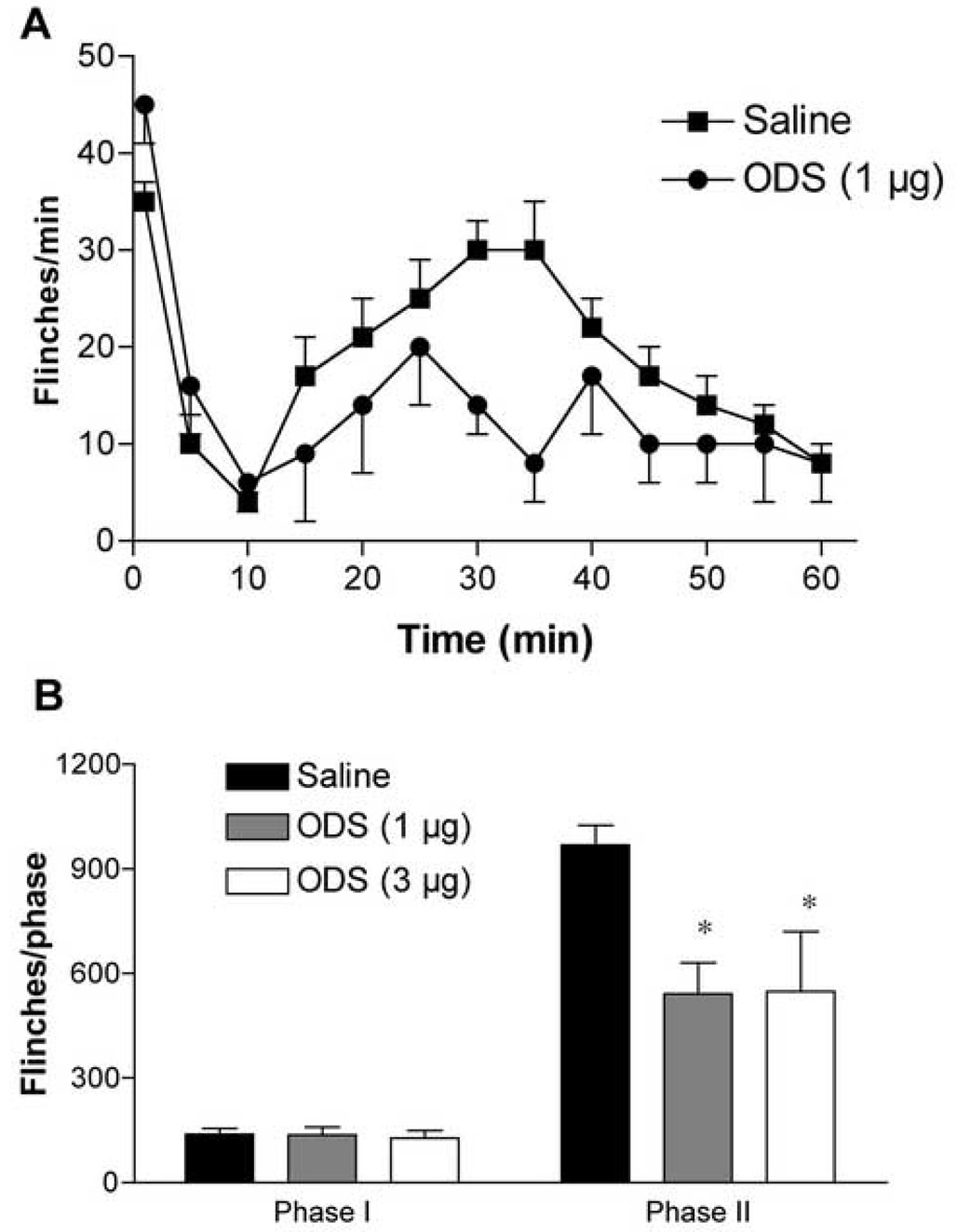
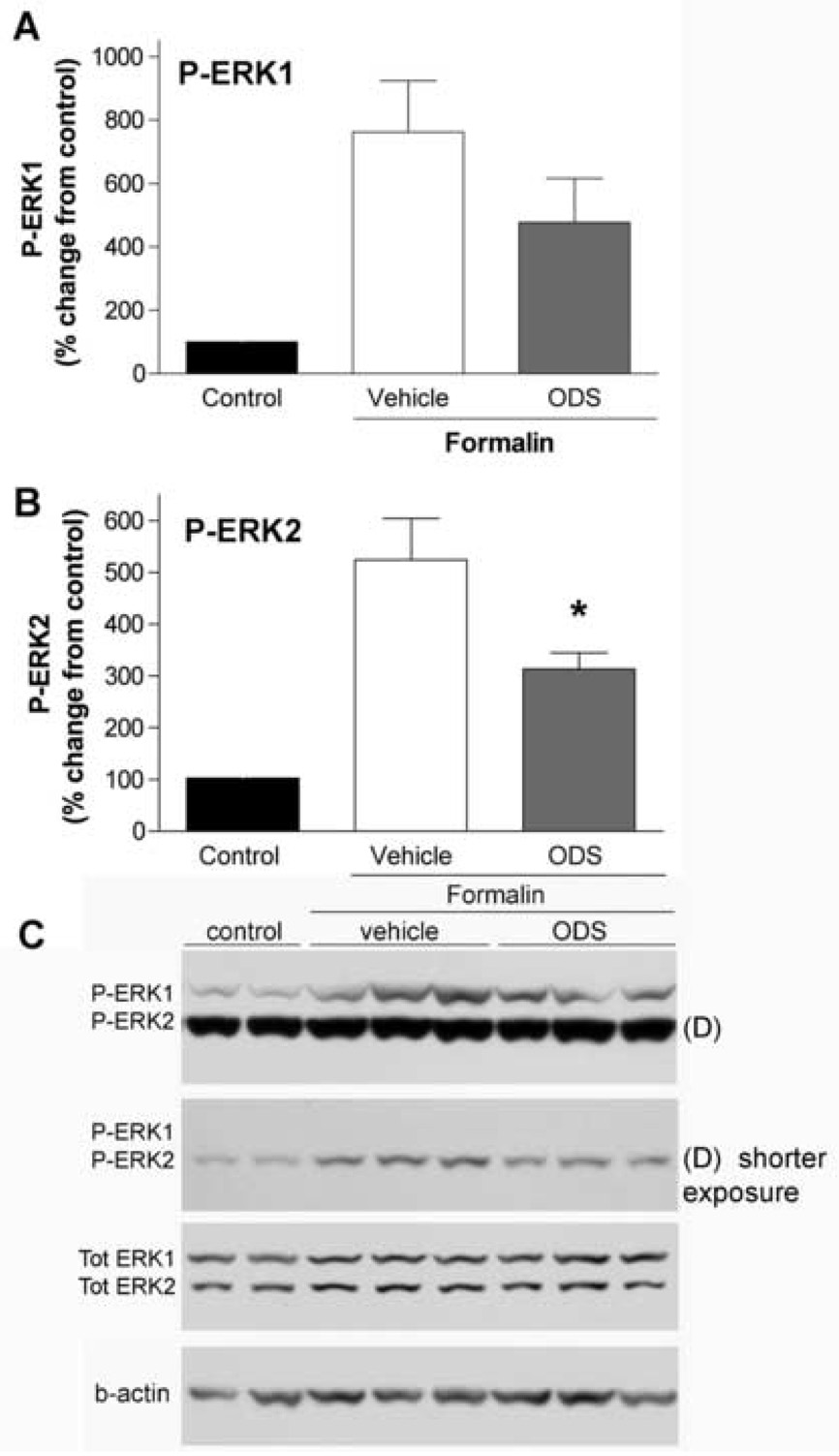
Intrathecal ondansetron inhibited both pain behavior and ERK activation, in agreement with the 5,7-DHT data. Ondansetron, at a dose of 1–3 µg, inhibited the second phase of formalin (but not the first phase) flinching by 30–40% compared to saline group (P< 0.05, Fig. 6). Expression of P-ERK1/2 was attenuated by ondansetron (1 µg), however, only inhibition of P-ERK2 reached statistical significance (Fig. 5).
Figure 6.
Blockade of spinal 5-HT3 receptors attenuates formalin-evoked hyperalgeisa. (A) Flinches/minute plotted versus time after injection of formalin in rats treated with IT injection of ondansetron (ODS, 1 µg), or vehicle (saline, 10 µl). Each point represents the number of flinches per min. (B) Histogram shows inhibitory effects of IT ondasertron at doses of 1 and 3 µg on phase I and phase II flinching behavior. The bars represent the total number of flinches in each phase. The data are presented as mean ± SEM of 4–11 rats/group. * p < 0.05, ODS vs. saline, one-way ANOVA.
Figure 5.
Intrathecal injection of ondansetron reduces formalin-evoked phosphorylation of spinal ERK1/2. Percentage change of P-ERK1 (A) and P-ERK2 (B) in ipsilateral lumbar spinal cord 30 min after formalin injection in the control (no formalin injection), vehicle (saline, 10 µl) and ondansetron (ODS, 1 µg) group. The vehicle and ondansetron were delivered IT 15 min before the formalin injection. Reduction of formalin-evoked P-ERK1/2 by ondansetron was observed, however, only P-ERK2 reduction reached statistical significance (* p < 0.05, one-way ANOVA, N=4–7/group). (C) Representative western blots showing the levels of P-ERK1/2, tot ERK1/2 and b-actin in ipsilateral spinal cords after formalin injections in rats with and without IT ondansetron. (D) P-ERK2 showed a higher level of phosphorylation in this experiment and in order to avoid saturation during quantification two different exposure times was used for this membrane.
4. Discussion
The present studies indicate that injury-evoked spinal P-ERK expression is dependent on the presence of an excitatory descending 5-HT pathway. It is known that serotonergic innervation of the spinal cord is derived largely from supraspinal sources [17]. Thus, 5,7-DHT, a neurotoxin selective for serotonergic terminals [18], at IT doses that produced a 70–80% reduction in dorsal horn 5-HT, prevented both the formalin-evoked increase in P-ERK in the dorsal horn and the phase II flinching behavior. The involvement of spinal 5-HT3 receptors is supported by the attenuation of P-ERK after IT delivery of ondansetron, a selective 5-HT3 antagonist, at a dose that suppressed formalin-evoked flinching.
Immunocytochemical studies [1,16] have demonstrated that shortly after peripheral formalin injection there is a marked increase in the number of neurons in the dorsal horn ipsilateral to the stimulus containing the activated (phosphorylated) form of ERK1/2. An important issue relates to the organization of the input that initiates phosphorylation of dorsal horn ERK1/2. It has been shown in a variety of neuronal systems that membrane depolarization triggered by excitatory transmitters activates ERK [8]. Therefore, in the present context, it may be argued that activation of ERK in the dorsal horn is evoked by substance P and glutamate released from the central terminals of primary afferents. This suggestion is supported by the observations that afferent-evoked activation of spinal ERK1/2 is blocked by antagonism of neurokinin 1 (NK1) and N-methyl-D-aspartate receptors [1,16]. While it is evident that ERK activation could be initiated by primary afferent input, spinal neuronal excitability is also regulated by input from supraspinal sources [8]. Apart from the presence of descending inhibitory pathways altering pain thresholds [17,19,20], there is a growing body of data indicating the importance of descending facilitatory pathways [6,17,21]. The descending serotonergic system arises in the rostroventral mediulla, projects to the dorsal horn and exerts a potent facilitatory effect through dorsal horn 5-HT3 receptors. Thus, formalin-induced spinal neuronal activation (assessed as cFOS expression) and nocifensive behaviors are significantly reduced after either functional elimination of 5-HT3 receptors [10] or by spinal delivery of the 5-HT3 receptor antagonists [6,7]. It is currently thought that this facilitatory drive is initiated by small afferent input leading to activation of superficial dorsal horn NK1 receptor-bearing neurons projecting to the periaqueductal grey and parabrachial area [22]. These areas connect to spinopetal serotonergic projections via the rostral ventromedial medulla [6,9] and serotonin is released in the spinal cord through this spino-bulbo-spinal loop upon high intensity afferent input [23]. These observations along with our present finding that 5,7-DHT abolish formalin-induced ERK activation highlights the importance of descending facilitatory systems and suggests that enhancement of afferent-initiated spinal ERK activation as one mechanism underlying this descending facilitation.
Although there are conflicting reports, spinal 5-HT3 receptors are considered to have predominantly excitatory functions [6,17,24]. Immunocytochemical and in situ hybridization studies have revealed that 5-HT3 receptors are present on superficial dorsal horn neurons and terminals [10,25–28], a finding consistent with the processing of nociceptive information in the spinal cord. A proportion of the 5-HT3 receptors are located on terminals of primary afferents while others are on dorsal horn interneurons, in particular on a subgroup of excitatory interneurons in lamina II that uses glutamate as neurotransmitter. It is known that 5-HT3 receptor channels enhance calcium permeability and phospholipase C activity and both events can reinforce ERK activation that is initially triggered by neurotransmitters released from sensory afferents [1,16,17]. Our data indicate that the 5-HT3 receptors are possibly involved in descending 5-HT–mediated facilitation of spinal ERK signaling.
In conclusion, the present findings emphasize the role of a spino-bulbo-spinal serotonergic pathway activated by nociceptive input in initiating a facilitated state of processing at the level of spinal dorsal horn. This spinopetal input acts by the release of spinal 5-HT which works through a local 5-HT3 receptor to activate ERK-containing cell systems. These spinal ERK systems then serve to enhance nociceptive transmission. These studies thus show the importance of an extra-spinal linkage in mediating afferent evoked changes in local dorsal horn excitability. It is evident that this loop pathway must now be routinely considered in interpreting the effects of afferent stimulation on dorsal horn function and particularly those indices such as ERK, which mediate facilitated processing.
ACKNOWLEDGEMENT
This work was supported by NIH NS 16541. The authors wish to thank Alan Moore and Steve Rossi for performing 5-HT assay.
Abbreviations
- ERK
extracellular signal-regulated protein kinase
- IT
intrathecal
- NK1
neurokinin 1
- P-ERK
phosphorylated extracellular signal-regulated protein kinase
- SP-SAP
substance P and saporin
- 5-HT
5-hydroxytrytamine
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Ji RR, Baba H, Brenner GJ, Woolf CJ. Nociceptive-specific activation of ERK in spinal neurons contributes to pain hypersensitivity. Nat Neurosci. 1999;2:1114–1119. doi: 10.1038/16040. [DOI] [PubMed] [Google Scholar]
- 2.Ji RR, Befort K, Brenner GJ, Woolf CJ. ERK MAP kinase activation in superficial spinal cord neurons induces prodynorphin and NK-1 upregulation and contributes to persistent inflammatory pain hypersensitivity. J Neurosci. 2002;22:478–485. doi: 10.1523/JNEUROSCI.22-02-00478.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Karim F, Wang CC, Gereau RWt. Metabotropic glutamate receptor subtypes 1 and 5 are activators of extracellular signal-regulated kinase signaling required for inflammatory pain in mice. J Neurosci. 2001;21:3771–3779. doi: 10.1523/JNEUROSCI.21-11-03771.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cruz CD, Avelino A, McMahon SB, Cruz F. Increased spinal cord phosphorylation of extracellular signal-regulated kinases mediates micturition overactivity in rats with chronic bladder inflammation. Eur J Neurosci. 2005;21:773–781. doi: 10.1111/j.1460-9568.2005.03893.x. [DOI] [PubMed] [Google Scholar]
- 5.Zhuang ZY, Gerner P, Woolf CJ, Ji RR. ERK is sequentially activated in neurons, microglia, and astrocytes by spinal nerve ligation and contributes to mechanical allodynia in this neuropathic pain model. Pain. 2005;114:149–159. doi: 10.1016/j.pain.2004.12.022. [DOI] [PubMed] [Google Scholar]
- 6.Suzuki R, Morcuende S, Webber M, Hunt SP, Dickenson AH. Superficial NK1-expressing neurons control spinal excitability through activation of descending pathways. Nat Neurosci. 2002;5:1319–1326. doi: 10.1038/nn966. [DOI] [PubMed] [Google Scholar]
- 7.Green GM, Scarth J, Dickenson A. An excitatory role for 5-HT in spinal inflammatory nociceptive transmission; state-dependent actions via dorsal horn 5-HT(3) receptors in the anaesthetized rat. Pain. 2000;89:81–88. doi: 10.1016/S0304-3959(00)00346-8. [DOI] [PubMed] [Google Scholar]
- 8.Hunt SP, Mantyh PW. The molecular dynamics of pain control. Nat Rev Neurosci. 2001;2:83–91. doi: 10.1038/35053509. [DOI] [PubMed] [Google Scholar]
- 9.Khasabov SG, Ghilardi JR, Mantyh PW, Simone DA. Spinal neurons that express NK-1 receptors modulate descending controls that project through the dorsolateral funiculus. J Neurophysiol. 2005;93:998–1006. doi: 10.1152/jn.01160.2003. [DOI] [PubMed] [Google Scholar]
- 10.Zeitz KP, et al. The 5-HT3 subtype of serotonin receptor contributes to nociceptive processing via a novel subset of myelinated and unmyelinated nociceptors. J Neurosci. 2002;22:1010–1019. doi: 10.1523/JNEUROSCI.22-03-01010.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rahman W, Suzuki R, Webber M, Hunt SP, Dickenson AH. Depletion of endogenous spinal 5-HT attenuates the behavioural hypersensitivity to mechanical and cooling stimuli induced by spinal nerve ligation. Pain. 2006;123:264–274. doi: 10.1016/j.pain.2006.02.033. [DOI] [PubMed] [Google Scholar]
- 12.Yaksh TL, Rudy TA. Chronic catheterization of the spinal sub-arachnoid space. Physiology and Behavior. 1976;17:1031–1036. doi: 10.1016/0031-9384(76)90029-9. [DOI] [PubMed] [Google Scholar]
- 13.Yaksh TL, Ozaki G, McCumber D, Rathbun M, Svensson C, Malkmus S, Yaksh MC. An automated flinch detecting system for use in the formalin nociceptive bioassay. J Appl Physiol. 2001;90:2386–2402. doi: 10.1152/jappl.2001.90.6.2386. [DOI] [PubMed] [Google Scholar]
- 14.Anderson GM, Hall LM, Yang JX, Cohen DJ. Platelet dense granule release reaction monitored by high-performance liquid chromatography-fluorometric determination of endogenous serotonin. Anal Biochem. 1992;206:64–67. doi: 10.1016/s0003-2697(05)80011-9. [DOI] [PubMed] [Google Scholar]
- 15.Dickenson AH, Sullivan AF. Subcutaneous formalin-induced activity of dorsal horn neurones in the rat: differential response to an intrathecal opiate administered pre or post formalin. Pain. 1987;30:349–360. doi: 10.1016/0304-3959(87)90023-6. [DOI] [PubMed] [Google Scholar]
- 16.Wei F, et al. Calcium calmodulin-stimulated adenylyl cyclases contribute to activation of extracellular signal-regulated kinase in spinal dorsal horn neurons in adult rats and mice. J Neurosci. 2006;26:851–861. doi: 10.1523/JNEUROSCI.3292-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Millan MJ. Descending control of pain. Prog Neurobiol. 2002;66:355–474. doi: 10.1016/s0301-0082(02)00009-6. [DOI] [PubMed] [Google Scholar]
- 18.Howe JR, Yaksh TL. Changes in sensitivity to intrathecal norepinephrine and serotonin after 6-hydroxydopamine (6-OHDA), 5,6-dihydroxytryptamine (5,6-DHT) or repeated monoamine administration. J Pharmacol Exp Ther. 1982;220:311–321. [PubMed] [Google Scholar]
- 19.Yaksh TL, Rudy TA. Narcotic analgestics: CNS sites and mechanisms of action as revealed by intracerebral injection techniques. Pain. 1978;4:299–359. doi: 10.1016/0304-3959(77)90145-2. [DOI] [PubMed] [Google Scholar]
- 20.Basbaum AI, Fields HL. Endogenous pain control systems: brainstem spinal pathways and endorphin circuitry. Annu Rev Neurosci. 1984;7:309–338. doi: 10.1146/annurev.ne.07.030184.001521. [DOI] [PubMed] [Google Scholar]
- 21.Porreca F, Ossipov MH, Gebhart GF. Chronic pain and medullary descending facilitation. Trends Neurosci. 2002;25:319–325. doi: 10.1016/s0166-2236(02)02157-4. [DOI] [PubMed] [Google Scholar]
- 22.Todd AJ. Anatomy of primary afferents and projection neurones in the rat spinal dorsal horn with particular emphasis on substance P and the neurokinin 1 receptor. Exp Physiol. 2002;87:245–249. doi: 10.1113/eph8702351. [DOI] [PubMed] [Google Scholar]
- 23.Tyce GM, Hammond DL, Rorie DK, Yaksh TL. In: Phenolsulfo-transferase in Mental Health. Sandler M, Usdin E, editors. London and Basingstoke: Macmillian Pub. Inc.; 1981. pp. 152–163. [Google Scholar]
- 24.Suzuki R, Rahman W, Rygh LJ, Webber M, Hunt SP, Dickenson AH. Spinal-supraspinal serotonergic circuits regulating neuropathic pain and its treatment with gabapentin. Pain. 2005;117:292–303. doi: 10.1016/j.pain.2005.06.015. [DOI] [PubMed] [Google Scholar]
- 25.Kia HK, Miquel MC, McKernan RM, Laporte AM, Lombard MC, Bourgoin S, Hamon M, Verge D. Localization of 5-HT3 receptors in the rat spinal cord: immunohistochemistry and in situ hybridization. Neuroreport. 1995;6:257–261. doi: 10.1097/00001756-199501000-00008. [DOI] [PubMed] [Google Scholar]
- 26.Morales M, Battenberg E, Bloom FE. Distribution of neurons expressing immunoreactivity for the 5HT3 receptor subtype in the rat brain and spinal cord. J Comp Neurol. 1998;402:385–401. [PubMed] [Google Scholar]
- 27.Maxwell DJ, Kerr R, Rashid S, Anderson E. Characterisation of axon terminals in the rat dorsal horn that are immunoreactive for serotonin 5-HT3A receptor subunits. Exp Brain Res. 2003;149:114–124. doi: 10.1007/s00221-002-1339-7. [DOI] [PubMed] [Google Scholar]
- 28.Conte D, Legg ED, McCourt AC, Silajdzic E, Nagy GG, Maxwell DJ. Transmitter content, origins and connections of axons in the spinal cord that possess the serotonin (5-hydroxytryptamine) 3 receptor. Neuroscience. 2005;134:165–173. doi: 10.1016/j.neuroscience.2005.02.013. [DOI] [PubMed] [Google Scholar]