Abstract
Objective
To investigate changes in depressive symptoms in hypertensive individuals participating in an exercise and weight loss intervention.
Methods
133 sedentary men and women with high blood pressure (130–180 mmHg SBP and/or 85–110 mmHg DBP) who participated in a 6 month intervention consisting of three groups: aerobic exercise, aerobic exercise and weight loss, and a waiting list control.
Results
Participants in both treatment groups demonstrated significant improvements in aerobic capacity and lower BP compared with participants in the control group. Participants in the active treatment groups who had mild to moderate depressive symptoms at baseline also exhibited greater reductions in depressive symptoms compared to participants in the control group. Conclusion: Results from the present study suggest that an exercise, alone or combined with weight management, may reduce self-reported depressive symptoms among patients with hypertension.
Keywords: Hypertension, depression, exercise, weight loss, aerobic fitness
INTRODUCTION
Depression is a major health burden worldwide and is relatively common among individuals with chronic health conditions [1–3]. Although there have been important advances in pharmacological treatment of depression [4], a significant number of patients do not respond to antindepressant medication [5]. Consequently, there has been considerable interest in the development of new and effective treatments for depression. A growing body of research indicates that exercise may be a safe and effective treatment for depression [6–8] comparable to psychotherapy [9,10] and pharmacologic treatment [11,12]. The majority of previous studies have found exercise to be effective in reducing depressive symptoms both among healthy populations [7,13–17] as well as patients with heart disease [18–26]. However, it also has been noted that many studies have been plagued by methodological weaknesses, such as small samples, improper statistical analyses, and a lack of a control group, leaving uncertainty about the value of exercise as a treatment of depression [27].
The present study examines the effects of exercise on depressive symptoms among individuals with elevated blood pressure (BP). Several previous studies have shown that depression and hypertension may often exist comorbidly [28,29] and that depression may be a risk factor for the development of coronary heart disease (CHD) [30,31]. Given the increased risk of CHD among individuals with depression, interventions that reduce depressive symptoms among patients with hypertension may be especially important given the increased risk of CHD in this population. Furthermore, depressive symptoms have been associated with a greater risk for hypertension-related morbidity, cerebrovascular events, and mortality in hypertensive individuals, even at subclinical levels [32,33].
Methods
Participants
This study represents a secondary analysis of a previously reported clinical trial examining the effects of exercise and weight loss in a sample of patients with high blood pressure [34]. Briefly, participants had unmedicated high normal BP or stage 1 to 2 hypertension and were sedentary, overweight or obese, and were at least 29 years old. Exclusion criteria included current anti-depressant use, current substance abuse, medical contraindications to exercise, a major psychiatric disorder requiring treatment, and ongoing participation in regular aerobic exercise. Participants were recruited from advertisements placed in local newspapers, television, and radio stations. Additionally, referrals from local clinics and BP screenings at community health fairs and local shopping centers contributed to participant recruitment.
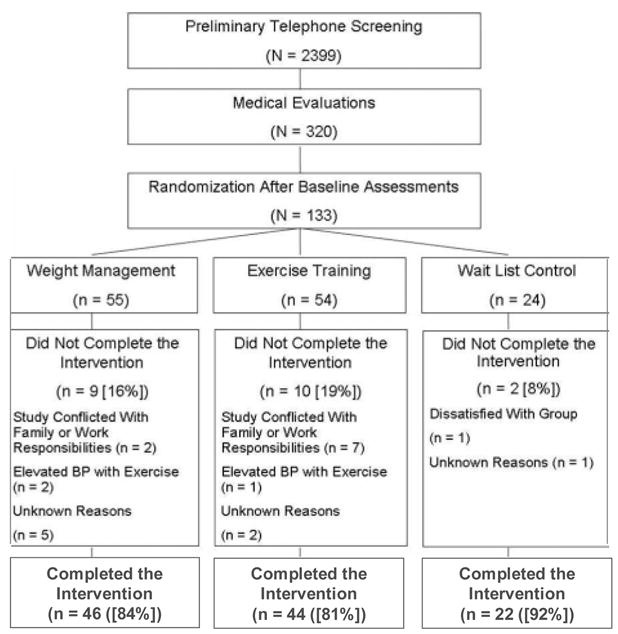
As reported previously, 2,399 individuals were initially screened through a preliminary telephone conversation and 320 individuals underwent a subsequent medical evaluation (Figure 1). One hundred thirty three individuals met the study criteria and were randomized following a 2:2:1 schema to one of three groups for the 6-month treatment program: aerobic exercise (EX; N=54), weight management including aerobic exercise (WM; N=55), and wait list control (WLC; N=24). All participants who consented to participate in the trial were eligible for assignment to any one of the three treatment groups.
Interventions
Aerobic Exercise Only participants exercised 3 to 4 times per week at a level of 70% to 85% of their initial heart rate (HR) reserve [35] determined at the time of their baseline treadmill test. The exercise program consisted of 10 minutes of warm-up exercises, 35 minutes of cycle ergometry and walking (and eventually jogging), and 10 minutes of cool-down exercises. Participants were asked to maintain their pre-treatment diets.
Weight Management participants exercised 3 to 4 times per week using the identical protocol as previously described. In addition, participants took part in a weight management program in small groups of 3 to 4 members based on the LEARN manual [36]. The primary goal of the intervention was a weight loss of 0.5 to 1.0 kg/wk, achieved gradually by decreasing caloric consumption and fat intake through lifestyle changes.
The group format consisted of 26 weekly group sessions. Weight was recorded at each session and participants’ food diaries and behavioral modification targets were reviewed. Group participation was encouraged during this process in supporting fellow group members and in problem solving around obstacles and lapses that they may have encountered. During the last 6 weeks of the program, sessions focused increasingly on weight maintenance, and group members worked on individualized plans for maintaining the changes they had made during the past 6 months.
Participants in both the EX and WM groups were instructed in how to monitor their radial pulses and maintain their heart rates (HRs) at, or above, their target level for at least 30 minutes. A trained exercise physiologist supervised all exercise sessions, and performed 2 to 3 random checks of HRs per session to ensure that participants were exercising at a sufficient intensity.
Waiting List Control participants were asked to maintain their usual dietary and exercise habits for 6 months until they were reexamined. On completion of their posttreatment assessment, participants were then allowed to select either of the 2 active treatments and could participate under supervision for 6 months.
Depression Measures
In order to investigate the effects of the intervention in alleviating depressive symptoms, participants completed the Beck Depression Inventory (BDI) [37] at baseline and after 6 months. The BDI is a standardized 21-item self-report questionnaire consisting of symptoms and attitudes related to depression including items such as self-dislike, suicidal ideation, insomnia, and sadness. The items are summed with total scores ranging from 0 to 63 with higher scores indicating higher levels depression. The BDI has been shown to be a valid and reliable measure of depression severity and a previous meta-analysis have shown the internal consistency of the BDI to yield a mean coefficient α of .86 for psychiatric patients [38].
Peak Oxygen Consumption
Maximal exercise testing was performed using the Duke-Wake Forest protocol in which graded exercise began at 3.2 kilometers per hour and 0% grade and workload was increased at a rate of 1 metabolic equivalent per minute (oxygen, 3.5 mL/kg per minute) [22]. Expired gases were collected for the determination of peak oxygen consumption using a metabolic cart (model 2900; Sensormedics, Yorba Linda, Calif). Assessments were made at baseline and again following 6 months of treatment.
Weight and Body Composition Assessment
Weight was measured by a standard balance scale. Body fat measurements were performed using a bioelectrical impedance analyzer (BIA-101Q; Quantum, Highland Heights, Ohio) in conjunction with bioelectrical impedance analyzer interpretation software (RJL Systems, Inc, Clinton Township, Mich). Measurements were done using standard right-sided, tetrapolar electrode placement with each subject in a supine position. Studies were conducted between 3 and 5pm at ambient temperature following a standard protocol in which participants had refrained from eating or drinking for at least 3 hours before testing (NIH Technol Assess Statement, 1994).
Clinic Blood Pressure
A trained technician obtained BP measurements using a random zero sphygomomanometer, standardized for cuff size and position. Measurements were made on 4 separate visits during a 3-week period. At each visit, BP was measured in the nondominant arm in the sitting position after an initial rest period of 5 minutes. BP was measured 4 successive times at 2-minute intervals. The first BP measurement of each visit was discarded and the average of the remaining 3 measurements represented the clinic visit BP. The overall clinic BP was then determined by averaging the mean BPs over 4 visits. Measurements were obtained in this standardized manner at baseline and after 6 months.
Data Analysis
Baseline differences among treatment groups were examined using 1-way analysis of variance (ANOVA) for continuous variables and χ2 tests for categorical variables. Treatment effects were evaluated using analysis of covariance (ANCOVA), with posttreatment values serving as the dependent variables and pretreatment values, age, gender, and treatment as the predictors. Within each model, planned contrasts were used to compare 1) the 2 treatment groups with controls and 2) WM participants with EX subjects. Separate ANCOVA models were used to determine the mediating effects of VO2 peak and weight loss in predicting improvement in depressive symptoms. Following the intent-to-treat principle, participants were analyzed regardless of adherence to the study protocol.
Results
One hundred thirty three participants were initially randomized for participation (54 in EX, 55 in WM, and 24 in WLC) (Figure 1). Twenty one participants dropped out of the study prior to completion (10 in EX, 9 in WM, and 2 in WLC), nine of whom returned for follow-up assessments. Dropouts did not differ from completers in age, gender, or severity of depressive symptoms, although there was a higher percentage of Caucasian participants among completers (P < .01). Participants in the EX group had lower SBP compared with their WM counterparts (SBP = 138.0 vs. 143.6, p = .024). No other group differences were observed for any clinical or demographic variables.
Adherence
Participants in the active treatment groups attended an average of 77 exercise sessions (mean sessions = 77.5 (SD = 27.2)) and were exercising at or above their target heart range during approximately 83% of their heart rate checks. Among participants completing the study, participants in the EX group attended approximately 74.8% of exercise sessions and exercised at or above their target heart rate during approximately 84.6% of their sessions. Participants in the WM group attended an average of 78.7% of exercise sessions and exercised at or above their target heart rate during approximately 81.2% of their sessions. There were no significant differences between the EX and WM groups in attendance or adherence to the exercise protocol, although younger participants tended to have lower attendance (p = .06). Adherence to the exercise protocol was not associated with pretreatment levels of depressive symptoms (p = .411).
Changes in Blood Pressure, Body Weight, and Aerobic Capacity
EX and WM groups showed significant reductions in systolic and diastolic blood pressures at the completion of the study compared with control participants (F1,127 = 10.88, P < .001; F1,127 = 29.46, p < .001), although there were no significant differences between the EX and WM groups (F1,127 = 1.95, P = .165; F1,127 = 1.72, P < .192). There were significant differences between the 3 groups in weight loss such that participants in the active treatment groups demonstrated significantly greater weight loss compared with control participants (F1,114 = 30.44, P < .001) and WM participants showed greater weight loss compared with their EX counterparts (F1,114 = 48.68, P < .001). Participants in the EX and WM participants groups lost an average of 1.8 and 7.9 kilograms (kgs) while control participants gained an average of .71 kgs.
Participants in the active treatment groups also showed greater improvement in exercise treadmill time (ETT) (F1,115 = 50.83, P < .001) and peak oxygen consumption (VO2) (F1,106 = 35.35, P < .001) compared with controls. In addition, WM participants experienced greater improvements in ETT compared with EX participants (F1,115 = 14.68, P < .01), although the groups did not differ significantly in VO2 improvement (F1,106 = 1.08, P = .30). Analysis of pre-to-post treatment mean differences for treadmill time showed that EX and WM participants increased their ETT by 77 and 136 seconds, respectively, while WLC participants showed a 8 second decline. Similarly, pre-to-post treatment mean gains for VO2 peak were .25 and .29 mL/kgs/min for EX and WM participants, whereas WLC participants exhibited a −.05 mL/kgs/min decline.
Changes in depression
Participants exhibited low levels of depression at baseline (mean BDI = 4.8 (SD = 4.3)) and only 21 participants (16%) reported at least “mild” levels of depression[39]. Planned contrasts examining treatment effects on posttreatment depression showed no differences between the EX and WM groups compared with the WLC group (F 1,112 = 0.92, p = .339). Similarly, the EX and WM groups did not differ significantly (F 1,112 = 0.08, P = .773) in post-treatment levels of depression.
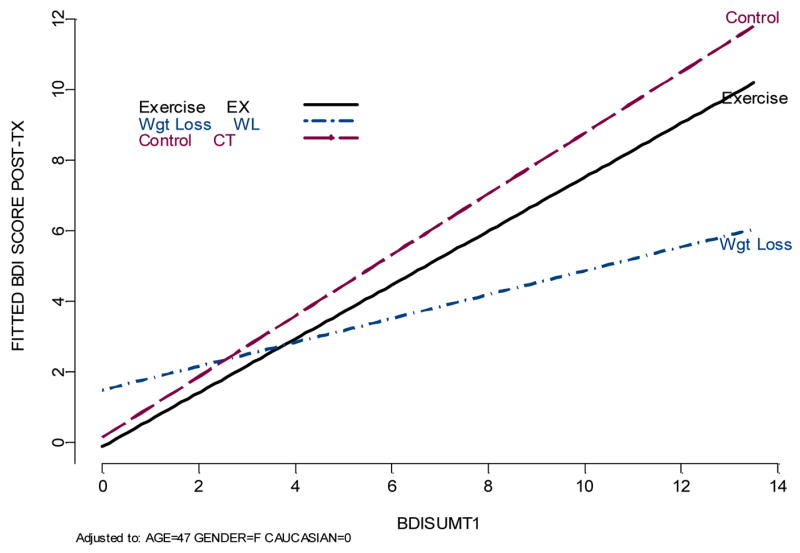
Further analysis of treatment differences in depression revealed a significant treatment group by baseline depression severity interaction (F2,106 = 5.75, P < .01) in predicting posttreatment levels of depression, such that participants in the WM and EX groups with higher BDI scores at baseline demonstrated significantly greater reductions in BDI scores compared with controls. The reduction in depressive symptoms among participants in the active treatment groups was more pronounced in the WM group, although EX participants also improved significantly (Figure 2). In order to examine the magnitude of this effect, we examined the number of participants who exhibited a > 25% reduction in BDI scores, consistent with previous clinical investigations. Excluding those participants who reported no depressive symptoms at baseline, and could therefore not improve (n = 19), approximately 35.4% of EX participants and 38.1% of WM participants showed significant reductions in depressive symptoms, compared to only 21.7% of WLC participants.
Mediators of Change in Depressive Symptoms
In order to investigate potential mediators to the improvement in depressive symptoms, the relationships between improvement in VO2 peak, weight loss, and depression were examined. Analysis of changes in VO2 revealed a significant three-way interaction between baseline depression severity, group assignment, and change in VO2 (F2,92 = 4.64, P = .01) such that posttreatment levels of depression were related to increased aerobic fitness among participants in the active treatment groups with elevated BDI scores at baseline. An analysis of raw change scores in depression and aerobic capacity among this group of participants showed a significant association between improvement in depression and increased VO2 peak (r = .36, P =.02), indicating that reduced levels of depression were mediated by increases in aerobic capacity. Neither weight loss, nor blood, pressure was associated with reduced levels of depression.
Discussion
Results from the present analysis demonstrate that for men and women with high blood pressure and mild to moderate levels of depression, depressive symptoms may be reduced to a greater extent by an exercise program, either alone or combined with a weight management intervention, compared with participants receiving no treatment. Furthermore, among participants in the active treatment groups, reduced levels of depression were associated with increased aerobic capacity such that participants with greater improvements in VO2 peak showed greater reductions in depression.
Our results are consistent with previous studies, which indicated that exercise can reduce depressive symptoms in healthy individuals [15,16,40–42] as well as patients with conditions such as heart disease [18–25,43] and arthritis [44]. Because both hypertension [28,29] and elevated depressive symptoms [45,46] are independent risk factors for CHD, an exercise intervention that reduces blood pressure and depression may be especially beneficial among hypertensive patients, a group at elevated risk for the development of CHD.
We observed that the antidepressant effect of exercise was moderated by pretreatment levels of depression. Participants with initially elevated levels of depressive symptoms benefited from exercise, whereas exercise resulted in minimal changes in depression for participants who were initially non-depressed. It is possible that there was a “floor effect” such that participants with low depressive symptoms had no room for improvement, hence only those participants with elevated depressive scores could improve. Alternatively, it is possible that, among individuals with elevated levels of depressive symptoms at baseline, those whose depressive symptoms were more rapidly reduced were able to engage in the exercise portion of the intervention more effectively.
Exercise has repeatedly been shown to reduce depressive symptoms in a variety of depressed populations[7] and to be equally effective[11] compared to anti-depressant therapy. In one of the first clinical trials to evaluate the use of aerobic exercise as a treatment for major depressive disorder (MDD), Blumenthal and colleagues [11] found that a 16-week aerobic exercise intervention was equally effective to treatment with Sertraline in alleviating depressive symptoms. Moreover, in a follow-up analysis from this original trial, Babyak and colleagues [12] found individuals who participated in the exercise portion of the study were less likely to have a recurrent depressive episode 10 months following study completion and that reduced risk was associated with higher levels of physical activity in this subset of patients. Similarly, in a recent meta-analysis of 11 treatment outcome studies utilizing physical activity in the treatment of depression, Stathopoulou and colleagues [7] found a clear advantage of exercise compared to control conditions.
Mechanisms by which exercise may reduce depressive symptoms by affecting changes in central monoamines, increasing hypothalamic-pituitary-adrenal (HPA) axis regulation, altering endorphin levels, increasing positive self-evaluations, and by enhancing cardiopulmonary functioning. Improved aerobic capacity assessed by VO2 peak has been associated with improvement in depression in several previous studies among both cardiac patients [18,19,47] and healthy patients [48,49]. Recent studies have shown that decreased aerobic capacity predicts increased levels of depressive symptoms as early as two weeks following exercise withdrawal among healthy individuals [50]. Among individuals with depression, Dunn and colleagues (CIT) recently demonstrated that a dose response relationship may exist between increased levels of aerobic fitness and decreased levels of depression. Our results provide further evidence to support this relationship by showing that training-induced improvement in aerobic capacity was associated with a reduction in symptoms of depression. These findings suggest that improved fitness may have contributed to the antidepressant effects of the exercise interventions. In contrast to previous findings [51], weight reduction was not significantly associated with improvement in depression.
The findings of this study had several notable limitations. First, because participants exhibited relatively low levels of depression at baseline our findings may not be generalizable to individuals with more severe depressive symptoms. Second, because depressive symptoms were assessed currently at two time points, the direction of the relationship was between changes in depressive symptoms and changes in aerobic fitness is unclear: improved aerobic capacity could result in reduced depression, or reduced depression may contribute to improved aerobic capacity. Finally, the WLC group in our study did not receive as much attention from the study staff as the active treatment groups, which may have affected their mood over the course of the 6-month treatment program. Future studies investigating this relationship would be improved with the addition of an attentional control group.
Results of this study may have important clinical implications. Previous studies have shown that depression is associated with adverse medical outcomes [52]. Because depressive symptoms have been associated with a greater incidence of adverse events in the presence of hypertension [33,53], we feel that our findings may have important implications for the management of depression among hypertensive individuals. Furthermore, given that depression has been associated with a greater risk for both the development of CHD [54] and increased mortality following cardiac events [55], interventions that alleviate depressive symptoms in this at-risk population may have important public health implications. Future research should examine the benefits of exercise on clinical outcomes among depressed hypertensive patients, including assessment of other important biomarkers of risk.
Table 1.
Background characteristics*
| Variable | EX (n = 54) | WM (n = 55) | WLC (n = 24) | Total (n = 133) |
|---|---|---|---|---|
| Age, mean (SD), y | 46.6 (8.2) | 48.5 (9.9) | 47.2 (7.4) | 47.5 (8.8) |
| Males | 25 (46.3) | 21 (38.2) | 13 (54.2) | 59 (44.4) |
| Caucasians | 41 (75.9) | 44 (80.0) | 15 (62.5) | 100 (75.2) |
| College Degree | 38 (70.4) | 34 (61.8) | 15 (62.5) | 87 (65.4) |
| SBP, mean (SD), mm Hg | 141.1 (14.8) | 145.5 (16.3) | 145.7 (14.0) | 143.7 (15.3) |
| DBP, mean (SD), mm Hg | 91.0 (8.1) | 90.6 (7.6) | 91.5 (7.1) | 90.9 (7.7) |
| Body weight, mean (SD), KGS | 95.5 (14.5) | 93.4 (16.9) | 93.1 (16.9) | 94.2 (15.9) |
| BDI, mean (SD) | 5.2 (4.1) | 4.0 (4.1) | 5.7 (5.0) | 4.8 (4.5) |
Data are given as number (percentage) unless otherwise indicated. SBP indicates systolic blood pressure; DBP indicates diastolic blood pressure; mm Hg indicates millimeters of mercury; KGS indicates kilograms; BDI indicates Beck Depression Inventory.
Acknowledgments
This study was supported by grants HL 49572, HL 59672, HL 65503, HC 55142, MH 49679, and HL 59672 from the National Institutes of Health and grant M01-RR-30 from the General Clinical Research Center Program, National Center for Research Resources, National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Patrick J. Smith, Duke University Department of Psychiatry and Behavioral Sciences
James A. Blumenthal, Duke University Department of Psychiatry and Behavioral Sciences
Michael A. Babyak, Duke University Department of Psychiatry and Behavioral Sciences
Anastasia Georgiades, Duke University Department of Psychiatry and Behavioral Sciences
Alan Hinderliter, University of North Carolina at Chapel Hill Department of Medicine
Andrew Sherwood, Duke University Department of Psychiatry and Behavioral Sciences
Reference List
- 1.Katon WJ. Clinical and health services relationships between major depression, depressive symptoms, and general medical illness. Biological Psychiatry. 2003;54:216–226. doi: 10.1016/s0006-3223(03)00273-7. [DOI] [PubMed] [Google Scholar]
- 2.Simon GE. Social and economic burden of mood disorders. Biological Psychiatry. 2003;54:208–215. doi: 10.1016/s0006-3223(03)00420-7. [DOI] [PubMed] [Google Scholar]
- 3.Krishnan KR. Treatment of depression in the medically ill. Journal of Clinical Psychopharmacology. 2005;25:S14–S18. doi: 10.1097/01.jcp.0000162808.92194.2a. [DOI] [PubMed] [Google Scholar]
- 4.Hollon SD, Thase ME, Markowitz JC. Treatment and prevention of depression. Psychological Science in the Public Interest. 2002;3:39–77. doi: 10.1111/1529-1006.00008. [DOI] [PubMed] [Google Scholar]
- 5.Baldessarini RJ. Current status of antidepressants: clinical pharmacology and therapy. Journal of Clinical Psychiatry. 1989;50:117–126. [PubMed] [Google Scholar]
- 6.Dunn AL, Trivedi MH, Kampert JB, Clark CG, Chambliss HO. The DOSE study: a clinical trial to examine efficacy and dose response of exercise as treatment for depression. Control Clin Trials. 2002;23:584–603. doi: 10.1016/s0197-2456(02)00226-x. [DOI] [PubMed] [Google Scholar]
- 7.Stathopoulou G, Powers MB, Berry AC, Smits JAJ, Otto MW. Exercise interventions for mental health: a quantitative and qualitative review. Clinical Psychology: Science and Practive. 2006;13:179–193. [Google Scholar]
- 8.Lawlor DA, Hopker SW. The effectiveness of exercise as an intervention in the management of depression: systematic review and meta-regression analysis of randomised controlled trials. BMJ. 2001;322:763–767. doi: 10.1136/bmj.322.7289.763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fremont J, Craighead LW. Aerobic exercise and cognitive therapy in the treatment of dysphoric moods. Cognitive Therapy and Research. 1987;11:241–251. [Google Scholar]
- 10.Klein MH, Greist JH, Gurman AS, Neimeyer RA, Lesser DP, Bushnell NJ. A comparative outcome study of group psychotherapy vs. exercise treatments for depression. International Journal of Mental Health. 1985;13:148–177. [Google Scholar]
- 11.Blumenthal JA, Babyak M, Moore KA, Craighead WE, Herman S, Khatri P, Waugh R, Napolitano MA, Forman LM, Appelbaum M, Doraiswamy PM, Krishnan KR. Effects of exercise training on older patients with major depression. Archives of Internal Medicine. 1999;159:2349–2356. doi: 10.1001/archinte.159.19.2349. [DOI] [PubMed] [Google Scholar]
- 12.Babyak M, Blumenthal JA, Herman S, Khatri P, Doraiswamy M, Moore K, Craighead WE, Baldewicz TT, Krishnan KR. Exercise treatment for major depression: maintenance of therapeutic benefit at 10 months. Psychosom Med. 2000;62:633–638. doi: 10.1097/00006842-200009000-00006. [DOI] [PubMed] [Google Scholar]
- 13.Blumenthal JA, Emery CF, Madden DJ, George LK, Coleman RE, Riddle MW, McKee DC, Reasoner J, Williams RS. Cardiovascular and behavioral effects of aerobic exercise training in healthy older men and women. Journal of Gerontology. 1989;44:M147–M157. doi: 10.1093/geronj/44.5.m147. [DOI] [PubMed] [Google Scholar]
- 14.DiLorenzo TM, Bargman EP, Stucky-Ropp R, Brassington GS, Frensch PA, LaFontaine T. Long-term effects of aerobic exercise on psychological outcomes. Preventive Medicine. 1999;28:75–85. doi: 10.1006/pmed.1998.0385. [DOI] [PubMed] [Google Scholar]
- 15.King AC, Taylor CB, Haskell WL, DeBusk RF. Influence of regular aerobic exercise on psychological health: a randomized, controlled trial of healthy middle-aged adults. Health Psychology. 1989;8:305–324. doi: 10.1037//0278-6133.8.3.305. [DOI] [PubMed] [Google Scholar]
- 16.Norvell N, Martin D, Salamon A. Psychological and physiological benefits of passive and aerobic exercise in sedentary middle-aged women. Journal of Gerontology. 1991;179:573–574. doi: 10.1097/00005053-199109000-00011. [DOI] [PubMed] [Google Scholar]
- 17.Roth DL, Holmes DS. Influence of aerobic exercise training and relaxation training on physical and psychologic health following stressful life events. Psychosomatic Medicine. 1987;49:355–365. doi: 10.1097/00006842-198707000-00004. [DOI] [PubMed] [Google Scholar]
- 18.Lavie CJ, Milani RV. Effects of cardiac rehabilitation and exercise training programs in patients > or = 75 years of age. American Journal of Cardiology. 1996;78:675–677. doi: 10.1016/s0002-9149(96)00393-1. [DOI] [PubMed] [Google Scholar]
- 19.Lavie CJ, Milani RV. Benefits of cardiac rehabilitation and exercise training in elderly women. American Journal of Cardiology. 1997;79:664–666. doi: 10.1016/s0002-9149(96)00835-1. [DOI] [PubMed] [Google Scholar]
- 20.Lavie CJ, Milani RV. Effects of cardiac rehabilitation and exercise training on exercise capacity, coronary risk factors, behavioral characteristics, and quality of life in women. American Journal of Cardiology. 1995;75:340–343. doi: 10.1016/s0002-9149(99)80550-5. [DOI] [PubMed] [Google Scholar]
- 21.Benjamini Y, Rubenstein JJ, Zaichkowsky LD. Effects of high-intensity strength training on quality-of-life parameters in cardiac rehabilitation patients. American Journal of Cardiology. 1997;75:340–343. doi: 10.1016/s0002-9149(97)00533-x. [DOI] [PubMed] [Google Scholar]
- 22.Blumenthal JA, Rejeski WJ, Walsh-Riddle M, Emery CF, Miller H, Roark S, Ribisl PM, Morris PB, Brubaker P, Williams RS. Comparison of high- and low- intensity exercise training early after acute myocardial infarction. American Journal of Cardiology. 1988;61:26–30. doi: 10.1016/0002-9149(88)91298-2. [DOI] [PubMed] [Google Scholar]
- 23.Lavie CJ, Milani RV, Cassidy MM. Effects of cardiac rehabilitation and exercise training programs in women with depression. American Journal of Cardiology. 1999;83:1480–1483. doi: 10.1016/s0002-9149(99)00127-7. [DOI] [PubMed] [Google Scholar]
- 24.Maines TY, Lavie CJ, Milani RV. Effects of cardiac rehabilitation and exercise programs on exercise capacity, coronary risk factors, behavior, and quality of life in patients with coronary artery disease. Southern Medical Journal. 1997;90:43–49. doi: 10.1097/00007611-199701000-00010. [DOI] [PubMed] [Google Scholar]
- 25.Taylor CB, Houston-Miller N, Ahn DK. The effects of exercise training programs on psychosocial improvement in uncomplicated postmyocardial infarction patients. Journal of Psychosomatic Research. 1986;30:581–587. doi: 10.1016/0022-3999(86)90031-0. [DOI] [PubMed] [Google Scholar]
- 26.Milani RV, Lavie CJ. Prevalence and effects of cardiac rehabilitation on depression in the elderly with coronary heart disease. American Journal of Cardiology. 1998;81:1233–1236. doi: 10.1016/s0002-9149(98)00121-0. [DOI] [PubMed] [Google Scholar]
- 27.Lawlor DA, Hopker SW. The effectiveness of exercise as an intervention in the management of depression: systematic review and meta-regression analysis of randomised controlled trials. British Medical Journal. 2001;322:763–767. doi: 10.1136/bmj.322.7289.763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Levenstein S, Smith MW, Kaplan GA. Psychosocial predictors of hypertension in men and women. Archives of Internal Medicine. 2001;161:1341–1346. doi: 10.1001/archinte.161.10.1341. [DOI] [PubMed] [Google Scholar]
- 29.Rabkin J, Charles E, Kass F. Hypertension and DSM-III depression and psychiatric outpatients. American Journal of Psychiatry. 1983;140:1072–1074. doi: 10.1176/ajp.140.8.1072. [DOI] [PubMed] [Google Scholar]
- 30.Rugulies R. Depression as a predictor for coronary heart disease a review and meta-analysis. American Journal of Preventive Medicine. 2002;23:51–61. doi: 10.1016/s0749-3797(02)00439-7. [DOI] [PubMed] [Google Scholar]
- 31.Frasure-Smith N, Lesperance F. Reflections on depression as a cardiac risk factor. Psychosomatic Medicine. 2005;67(Suppl 1):S19–S25. doi: 10.1097/01.psy.0000162253.07959.db. [DOI] [PubMed] [Google Scholar]
- 32.Simonsick EM, Wallace RB, Blazer DG, Berkman LF. Depressive symptomatology and hypertension-associated morbidity and mortality in older adults. Psychosom Med. 1995;57:427–435. doi: 10.1097/00006842-199509000-00003. [DOI] [PubMed] [Google Scholar]
- 33.Gump BB, Matthews KA, Eberly LE, Chang YF. Depressive symptoms and mortality in men: results from the Multiple Risk Factor Intervention Trial. Stroke. 2005;36:98–102. doi: 10.1161/01.STR.0000149626.50127.d0. [DOI] [PubMed] [Google Scholar]
- 34.Blumenthal JA, Sherwood A, Gullette ECD, Babyak M, Waugh R, Georgiades A, Craighead LW, Tweedy D, Fienglos M, Appelbaum M, Hayano J, Hinderliter A. Exercise and weight loss reduce blood pressure in men and women with mild hypertension: effects on cardiovascular, metabolic, and hemodynamic functioning. Archives of Internal Medicine. 2000;160:1947–1958. doi: 10.1001/archinte.160.13.1947. [DOI] [PubMed] [Google Scholar]
- 35.Karovenen M, Kentala K, Mustala O. The effects of training heart rate: a longitudinal study. Annals of Medicine and Experimental Biology Fenn. 1957;35:307–315. [PubMed] [Google Scholar]
- 36.Brownwell KD. The LEARN Program for Weight Control. 1994 [Google Scholar]
- 37.Beck AT, Ward CH, Mendelsohn M, Mock J, Erbaugh J. An inventory for measuring depression. Archives of General Psychiatry. 1961;4:561–571. doi: 10.1001/archpsyc.1961.01710120031004. [DOI] [PubMed] [Google Scholar]
- 38.Beck AT, Steer RA, Garbin MG. Psychometric properties of the Beck Depression Inventory: twenty-five years of evaluation. Clinical Psychology Review. 1988;8:77–100. [Google Scholar]
- 39.Beck AT, Steer RA, Brown GK. Beck Depression Inventory Manual. Annals of Behavioral Medicine; San Antonio (TX): 1996. p. 2. [Google Scholar]
- 40.Blumenthal JA, Emery CF, Madden DJ, George LK, Coleman RE, Riddle MW, McKee DC, Reasoner J, Williams RS. Cardiovascular and behavioral effects of aerobic exercise training in healthy older men and women. Journal of Gerontology. 1989;44:M147–M157. doi: 10.1093/geronj/44.5.m147. [DOI] [PubMed] [Google Scholar]
- 41.DiLorenzo TM, Bargman EP, Stucky-Ropp R, Brassington GS, Frensch PA, LaFontaine T. Long-term effects of aerobic exercise on psychological outcomes. Preventive Medicine. 1999;28:75–85. doi: 10.1006/pmed.1998.0385. [DOI] [PubMed] [Google Scholar]
- 42.Roth DL, Holmes DS. Influence of aerobic exercise training and relaxation training on physical and psychologic health following stressful life events. Psychosomatic Medicine. 1987;49:355–365. doi: 10.1097/00006842-198707000-00004. [DOI] [PubMed] [Google Scholar]
- 43.Milani RV, Lavie CJ, Cassidy MM. Effects of cardiac rehabilitation and exercise training programs on depression in patients after major coronary events. American Heart Journal. 1996;132:726–732. doi: 10.1016/s0002-8703(96)90304-x. [DOI] [PubMed] [Google Scholar]
- 44.Penninx BW, Rejeski WJ, Pandya J, Miller ME, Di Bari M, Applegate WB, Pahor M. Exercise and depressive symptoms: a comparison of aerobic and resistance exercise effects on emotional and physical function in older persons with high and low depressive symptomatology. Journals of Gerontology Series B: Psychological Sciences and Social Sciences. 2002;57:124–132. doi: 10.1093/geronb/57.2.p124. [DOI] [PubMed] [Google Scholar]
- 45.Rugulies R. Depression as a predictor for coronary heart disease. a review and meta-analysis. American Journal of Preventive Medicine. 2002;23:51–61. doi: 10.1016/s0749-3797(02)00439-7. [DOI] [PubMed] [Google Scholar]
- 46.Frasure-Smith N, Lesperance F. Reflections on depression as a cardiac risk factor. Psychosomatic Medicine. 2005;67(Suppl 1):S19–S25. doi: 10.1097/01.psy.0000162253.07959.db. [DOI] [PubMed] [Google Scholar]
- 47.Yoshida T, Masahiro K, Yoshida K, Hiwatari M, Kamimoto M, Yamamoto C, Meguro S, Endo N, Kato A, Kanazawa M, Sato T. Physical and psychological improvements after phase II cardiac rehabilitation in patients with myocardial infarction. Nursing and Health Sciences. 1999;1:163–170. doi: 10.1046/j.1442-2018.1999.00021.x. [DOI] [PubMed] [Google Scholar]
- 48.Galper DI, Trivedi MH, Barlow CE, Dunn AL, Kampert JB. Inverse association between physical inactivity and mental health in men and women. Medicine & Science in Sports & Exercise. 2006;38:173–178. doi: 10.1249/01.mss.0000180883.32116.28. [DOI] [PubMed] [Google Scholar]
- 49.McCann IL, Holmes DS. Influence of aerobic exercise on depression. Journal of Personality and Social Psychology. 1984;46:1142–1147. doi: 10.1037//0022-3514.46.5.1142. [DOI] [PubMed] [Google Scholar]
- 50.Berlin AA, Kop WJ, Deuster PA. Depressive mood symptoms and fatigue after exercise withdrawal: the potential role of decreased fitness. Psychosomatic Medicine. 2006;68:224–230. doi: 10.1097/01.psy.0000204628.73273.23. [DOI] [PubMed] [Google Scholar]
- 51.King AC, Taylor CB, Haskell WL. Effects of differing intensities and formats of 12 months of exercise training on psychological outcomes in older adults. Health Psychology. 1993;12:292–300. doi: 10.1037//0278-6133.12.4.292. [DOI] [PubMed] [Google Scholar]
- 52.Lett HS, Blumenthal JA, Babyak MA, Sherwood A, Strauman T, Robins C, Newman MF. Depression as a risk factor for coronary artery disease: evidence, mechanisms, and treatment. Psychosomatic Medicine. 2004;66:305–315. doi: 10.1097/01.psy.0000126207.43307.c0. [DOI] [PubMed] [Google Scholar]
- 53.Simonsick EM, Wallace RB, Blazer DG, Berkman LF. Depressive symptomatology and hypertension-associated morbidity and mortality in older adults. Psychosom Med. 1995;57:427–435. doi: 10.1097/00006842-199509000-00003. [DOI] [PubMed] [Google Scholar]
- 54.Rugulies R. Depression as a predictor for coronary heart disease: a review and meta-analysis. American Journal of Preventive Medicine. 2002;23:51–61. doi: 10.1016/s0749-3797(02)00439-7. [DOI] [PubMed] [Google Scholar]
- 55.Frasure-Smith N, Lesperance F, Talajic M. Depression following myocardial infarction. Impact on 6-month survival. JAMA. 1993;270:1819–1825. [PubMed] [Google Scholar]


