Abstract
High NaCl rapidly activates p38 MAPK by phosphorylating it, the phosphorylation presumably being regulated by a balance of kinases and phosphatases. Kinases are known, but the phosphatases are uncertain. Our initial purpose was to identify the phosphatases. We find that in HEK293 cells transient overexpression of MAPK phosphatase-1 (MKP-1), a dual-specificity phosphatase, inhibits high NaCl-induced phosphorylation of p38, and that overexpression of a dominant negative mutant of MKP-1 does the opposite. High NaCl lowers MKP-1 activity by increasing reactive oxygen species, which directly inhibit MKP-1, and by reducing binding of MKP-1 to p38. Because inhibition of p38 is reported to reduce hypertonicity-induced activation of the osmoprotective transcription factor, TonEBP/OREBP, we anticipated that MKP-1 expression might also. However, overexpression of MKP-1 has no significant effect on Ton EBP/OREBP activity. This paradox is explained by opposing effects of p38α and p38δ, both of which are activated by high NaCl and inhibited by MKP-1. Thus, we find that overexpression of p38α increases high NaCl-induced TonEBP/OREBP activity, but overexpression of p38δ reduces it. Also, siRNA-mediated knockdown of p38δ enhances the activation of TonEBP/OREBP. We conclude that high NaCl inhibits MKP-1, which contributes to the activation of p38. However, opposing actions of p38α and p38δ negate any effect on TonEBP/OREBP activity. Thus, activation of p38 isoforms by hypertonicity does not contribute to activation of TonEBP/OREBP because of opposing effects of p38α and p38δ, and effects of inhibitors of p38 depend on which isoform is affected, which can be misleading.
Keywords: hypertonicity, reactive oxygen species, transcription, HEK293 cells, mIMCD3 cells
Cells in the renal inner medulla are normally exposed to extraordinarily high and variable levels of NaCl. High NaCl induces cell cycle delay (1, 2), oxidative stress (3), DNA damage (4, 5), and apoptosis (1, 2). Hypertonicity, which is caused by high NaCl, activates the transcription factor, TonEBP/OREBP, leading to increased expression of osmoprotective genes like those that code for aldose reductase and the glycine betaine transporter (BGT1) (6). Surviving TonEBP/OREBP null mice have impaired expression of the osmoprotective genes associated with profound atrophy of the renal inner medulla (7), illustrating the critical role of TonEBP/OREBP in protection of cells from hypertonic injury.
Several different pathways contribute to signaling hypertonicity-induced activation of TonEBP/OREBP (6), and the p38 MAPK pathway has received prominent attention in this regard (8–10). Studies of yeast first suggested the possible importance of this pathway in mammals. Osmotic signaling in yeast passes through HOG-1, a yeast homolog of p38, and mutants in HOG-1 or of other members in its pathway, inhibit osmoprotection (11). A similar role for p38 in mammalian cells was suggested by observations that p38 complements HOG-1 mutations in yeast (12), hypertonicity results in phosphorylation and activation of p38 (12), and chemical (9) or dominant negative (8) inhibition of p38 reduces the activation of TonEBP/OREBP by hypertonicity. Accordingly, it was concluded that hypertonicity activates p38, which, in turn, contributes to the activation of TonEBP/OREBP. Hypertonicity-induced phosphorylation and activation of p38 is signaled by a MAPK pathway, Rac-MEKK3-MKK3-p38, in association with actin and the scaffolding protein, OSM (CCM2) (13). Given the probability that the effect of the MAPK to phosphorylate p38 is balanced by opposing effects of phosphatases, our initial purpose was to identify the phosphatases.
A role of MAPK phosphatase-1 (MKP-1) in hyperosmotic activation of p38 was suggested by a study of MKP−/− mouse embryonic fibroblasts in which p38 is more active than in MKP+/+ mouse embryonic fibroblasts after osmotic stress (14). In agreement, we found in our present studies that expression of MKP-1 reduces hypertonicity-induced phosphorylation of p38 (see Results), so we anticipated that it would also reduce the hypertonicity-induced activation of TonEBP/OREBP. However, it does not (see Results). At that point we were faced with the question of why some ways of inhibiting p38 reduce the activation of TonEBP/OREBP (8, 9), but others do not (present study and ref. 15). Evidently, the role of p38 in signaling hypertonicity in mammalian cells is more complicated than the role of HOG-1 in yeast. One possible difference is that, whereas HOG-1 is the sole isoform in yeast, there are four isoforms of p38 in mammalian cells, p38α (often called p38), p38β, p38γ, and p38δ (16). The existence of these isoforms provides a potential complication, especially if more than one is activated by hypertonicity and if their effects differ. This scenario seemed possible because p38α enhances IL-1β-induced inducible NO synthase (iNOS) mRNA expression, whereas p38β inhibits it (17). With this in mind, we examined in detail the roles of two isoforms, p38α and p38δ.
p38α is ubiquitously expressed in mammalian tissues, whereas p38δ expression is limited to several organs, including the kidney (16). p38α and p38δ are coded by different genes and share ≈60% amino acid homology (16). Both become activated upon exposure to hypertonicity (12, 16). Previous studies of the role of p38 in hypertonicity-induced activation of TonEBP/OREBP used p38 inhibitors that affect only certain isoforms of p38. For example, SB203580, which inhibits hypertonicity-induced activation of TonEBP/OREBP (9), inhibits p38α and 38β, but not p38δ (16, 18). Also, a dominant negative p38 that inhibits hypertonicity-induced activation of TonEBP/OREBP is specific for p38α (8). Given the possibility of opposite effects of p38α and p38δ, we decided to study their specific roles in detail.
In the present studies we confirm that hypertonicity activates both p38α and p38δ. Also, in contrast to the limitation of some other inhibitors of p38 to specific isoforms of p38, MKP-1 inhibits both p38α and p38δ. Further, we find that p38α and p38δ have opposite effects on hypertonicity-induced activation of TonEBP/OREBP. p38α enhances it, but p38δ inhibits it. We conclude that inhibition of p38 by MKP-1 does not affect TonEBP/OREBP transcriptional activity because it inhibits the opposing effects of p38α and p38δ, resulting in no net change.
Results
Effect of MKP-1 on High NaCl-Induced Phosphorylation of p38.
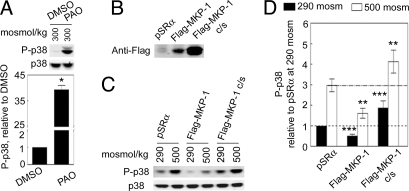
To confirm that protein tyrosine phosphatases (PTPs) are involved in regulation of phosphorylation of p38 in renal cells, we treated mIMCD3 cells with phenylarsine oxide (PAO; 250 nM), a general inhibitor of PTPs. PAO greatly enhances phosphorylation of p38 (Fig. 1A). It is important to note that the antibody used here does not distinguish phosphorylated p38α from phosphorylated p38δ. Then, we tested two PTPs to see whether either might be involved by transiently transfecting cDNAs coding for them into HEK293 cells. Overexpression of Flag-MKP-1 (Fig. 1B) significantly inhibits p38 phosphorylation at both 290 and 500 mosmol/kg (NaCl added) (Fig. 1 C and D), whereas overexpression of Flag-MKP-1 dominant negative mutant (active cysteine mutated to serine; Fig. 1B) does the opposite (Fig. 1 C and D). Neither the WT nor the mutant has a significant effect on abundance of p38 (Fig. 1C). In contrast, overexpression of the PTP cdc25c has no significant effect on phosphorylation of p38 at either osmolality [supporting information (SI) Fig. S1]. We conclude that MKP-1 reduces phosphorylation of p38, but that cdc25c does not.
Fig. 1.
MKP-1 inhibits phosphorylation of p38. (A) PAO increases phosphorylation of p38 at 300 mosmol/kg in mIMCD3 cells. Cells were incubated with PAO (250 nM) for 75 min (*, P < 0.005, paired t test, n = 3). (B–D) Overexpression of Flag-MKP-1 decreases phosphorylation of p38, whereas overexpression of the dominant negative mutant of Flag-MKP-1 (Flag-MKP-1 c/s) increases phosphorylation of p38. HEK293 cells were transfected with Flag-MKP-1, Flag-MKP-1 c/s, or the empty vector pSRα (Control) for 48 h. Then, osmolality was increased to 500 mosmol/kg (NaCl added) for 2 h (***, P < 0.05 compared with the group pSRα at 290 mosmol/kg; **, P < 0.05 compared with the pSRα group at 500 mosmol/kg, n = 3).
Effect of MKP-1 on High NaCl-Induced Phosphorylation of p38α and p38δ.
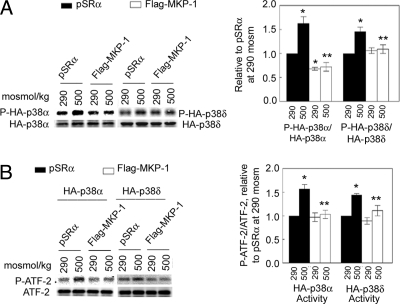
Knowing that hypertonicity increases phosphorylation of both p38α and p38δ (12, 16), we next tested whether MKP-1 inhibits their increased phosphorylation. We transiently overexpressed Flag-MKP-1 with either HA-p38α or HA-p38δ in HEK293FT cells (used because they generally have higher transfection efficiency than HEK293) and immunoprecipitated the HA-p38α and HA-p38δ, using anti-HA antibody. Overexpression of MKP-1 reduces phosphorylation of HA-p38α at 290 mosmol/kg and reduces phosphorylation of both HA-p38α and HA-p38δ at 500 mosmol/kg (Fig. 2A). We conclude that MKP-1 inhibits high NaCl-induced phosphorylation of both p38α and p38δ.
Fig. 2.
Overexpression of Flag-MKP-1 inhibits high NaCl-induced phosphorylation and activity of p38α and p38δ. HEK293FT cells were cotransfected with HA-p38α or HA-p38δ and with Flag-MKP-1 or the empty vector pSRα (Control) for 48 h. Then, osmolality was increased from 290 to 500 mosmol/kg (NaCl added) for 30 min. HA-p38α and HA-p38δ were immunoprecipitated. (A) Phosphorylation of HA-p38α and HA-p38δ. (B) Kinase activities of HA-p38α and HA-p38δ, measured with ATF-2 as a substrate. *, P < 0.05 compared with the group pSRα at 290 mosmol/kg; **, P < 0.05 compared with the pSRα group at 500 mosmol/kg, n = 3.
Effect of MKP-1 on High NaCl-Induced Activation of p38α and p38δ.
We next tested whether MKP-1 affects the high NaCl-induced increase of kinase activity of p38α and p38δ, as might be expected from its effect on their phosphorylation. As above, we transiently overexpressed Flag-MKP-1 with either HA-p38α or HA-p38δ in HEK293FT cells. Then, we measured the kinase activity of the immunoprecipitated HA-p38α and HA-p38δ, using GST-ATF-2 as substrate. We found that overexpression of MKP-1 inhibits high NaCl-induced kinase activity of both p38α and p38δ (Fig. 2B).
Effect of Antioxidant Treatment on High NaCl-Induced Inhibition of MKP-1 Activity.
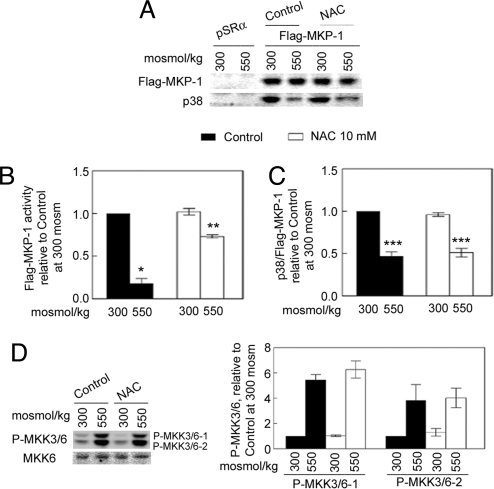
High NaCl increases reactive oxygen species (ROS) (3, 19), and ROS inhibit MKP-1 activity (20). To determine whether high NaCl inhibits MKP-1 enzymatic activity by increasing ROS, we established stable clones overexpressing Flag-MKP-1 in mIMCD3 cells and measured the phosphatase activity of MKP-1 immunoprecipitated from the cells, using 3-O-methylfluorescein phosphate as substrate. We found that high NaCl inhibits MKP-1 activity, and that this effect is attenuated by treating the cells with the antioxidant, N-acetylcysteine (NAC) (Fig. 3 A and B). We conclude that the ROS produced in response to high NaCl inhibit MKP-1 activity. We also found that high NaCl reduces binding of Flag-MKP-1 to p38; however, NAC does not affect this binding (Fig. 3 A and C).
Fig. 3.
High NaCl-induced inhibition of MKP-1 activity is reversed by NAC, but binding of Flag-MKP-1 to p38 is not altered by NAC. mIMCD3 cells stably expressing Flag-MKP-1 were preincubated either with NAC or an equal volume of PBS (Control) overnight, then osmolality was increased to 550 mosmol/kg (NaCl added) for 30 min. (A) Western analysis of Flag-MKP-1 and p38 abundances in anti-Flag immunoprecipitates. (B) Activity of immunoprecipitated Flag-MKP-1, measured with 3-O-methylfluorescein phosphate as substrate and normalized to the abundance of Flag-MKP-1 protein, measured by Western analysis (*, P < 0.005, as compared with the control at 300 mosmol/kg; **, P < 0.01, as compared with the control at 550 mosmol/kg, n = 3). (C) Amounts of Flag-MKP-1 and coimmunoprecipitated p38, measured by Western analysis. (***, P < 0.01 vs. control at 300 mosmol/kg, n = 3). (D) In nontransfected mIMCD3 cells elevating osmolality for 30 min by adding NaCl increases phosphorylation of MKK3/6, which is not affected by NAC.
Mechanism by Which High NaCl-Induced ROS Activate p38.
Previous studies (21, 22) showed that NAC inhibits high NaCl-induced phosphorylation of p38. We confirmed this finding in our present studies of mIMCD3 cells (data not shown). It was also previously shown that hypertonicity activates MKK3 (23), which is redox-sensitive (24). This finding raised the possibility that greater activation of MKK3 might contribute to the ROS-mediated, high NaCl-induced increased phosphorylation of p38. However, NAC has no significant effect on high NaCl-induced phosphorylation of MKK3/6 (Fig. 3D), which is not consistent with this hypothesis. We also find that NAC has no significant effect on high NaCl-induced phosphorylation of apoptosis signaling kinase-1 (ASK-1), the kinase upstream of MKK3 (data not shown). We conclude that ROS contribute to hypertonicity-induced phosphorylation of p38 by inactivating MKP-1, not by activating the MAPK cascade.
Effect of High NaCl on Binding of MKP-1 to p38.
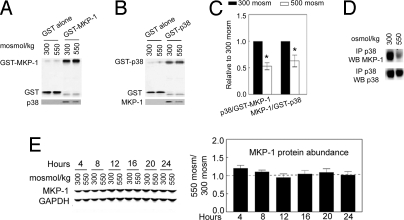
MKP-1 binds to its substrates (25, 26). To ascertain whether high NaCl affects binding of MKP-1 to p38, we established clonal lines of mIMCD3 cells that stably express GST, GST-MKP-1, or GST-p38α. GST-MKP-1 that is pulled down from these cells is accompanied by native p38 (Fig. 4A) and vice versa (Fig. 4B). In both cases, elevating osmolality to 550 mosmol/kg by adding NaCl reduces the binding (Fig. 4 A–C). We were unable to test for an effect of NAC on the binding because it elevates reduced glutathione, which interferes with the GST pull-down. In addition, native p38 that is immunoprecipitated from nontransfected mIMCD3 cells is also accompanied by MKP-1, and high NaCl reduces this association (Fig. 4D). We conclude that the inhibition of MKP-1 activity that contributes to high NaCl-induced phosphorylation of p38 includes reduced binding of MKP-1 to p38 and direct ROS-mediated inhibition of MKP-1 activity.
Fig. 4.
High NaCl reduces binding of MKP-1 to p38 and has no significant effect on MKP-1 protein expression. (A–C) High NaCl reduces binding of MKP-1 to p38. Osmolality bathing mIMCD3 cells stably expressing GST, GST-MKP-1, or GST-p38 was increased to 550 mosmol/kg (NaCl added) for 30 min, then GST-MKP-1 or GST-p38 was pulled down with glutathione Sepharose beads, and the amount of accompanying native MKP-1 or p38 was measured by Western analysis (*, P < 0.01, n = 4–5). (D). High NaCl reduces binding of native p38 to native MKP-1 in mIMCD3 cells. Osmolality was increased to 550 mosmol/kg (NaCl added) for 30 min, then p38 was immunoprecipitated, and the amounts of p38 and MKP-1 in the immunoprecipitate were measured by Western analysis. Results are representative of two independent experiments. (E) High NaCl does not affect expression of MKP-1 protein in mIMCD3 cells. MKP-1 protein expression was measured by Western analysis (n = 3).
Effect of High NaCl on MKP-1 Expression.
Acute elevation of NaCl in cell culture medium has no significant effect on MKP-1 protein abundance (Fig. 4E). TNF-α induces aggregation of MKP-1 by increasing ROS. The aggregates appear at ≈220 kDa in gels (20). However, high NaCl, which increases ROS, does not cause aggregation of MKP-1 protein (data not shown). Thus, we find no evidence that high NaCl affects MKP-1 protein expression.
Effect of Overexpression of MKP-1 on TonEBP/OREBP Transcriptional Activity.
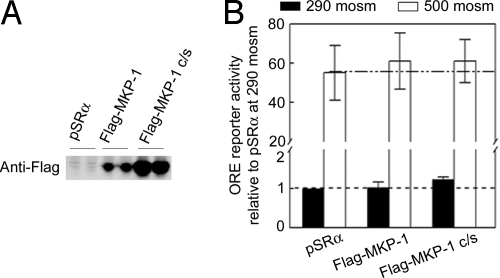
Because activation of p38 by hypertonicity is believed to contribute to hypertonicity-induced increase of TonEBP/OREBP transcriptional activity (8–10), we anticipated that overexpression of MKP-1, which reduces p38 activity (Figs. 1 B–D and 2), would reduce TonEBP/OREBP transcriptional activity. However, we do not find that overexpression of either Flag-MKP-1 or the dominant negative Flag-MKP-1 c/s affects the high NaCl-induced increase of TonEBP/OREBP transcriptional activity in HEK293 cells, as measured with an ORE reporter (Fig. 5). We conclude that MKP-1 does not regulate TonEBP/OREBP transcriptional activity.
Fig. 5.
Overexpression of Flag-MKP-1 has no significant effect on high NaCl-induced TonEBP/OREBP transcriptional activity. HEK293 cells were cotransfected with Flag-MKP-1, Flag-MKP-1 c/s (dominant negative mutant), or the empty vector pSRα (Control) and with an ORE reporter. Thirty-two hours later, osmolality was increased to 500 mosmol/kg (NaCl added) or left at 290 mosmol/kg for 16 h. (A) Western analysis of expression of Flag-MKP-1 and its mutant in two independent transfections. (B) ORE reporter assay (n = 3).
p38α and p38δ Have Opposite Effects on High NaCl-Induced TonEBP/OREBP Transcriptional Activity.
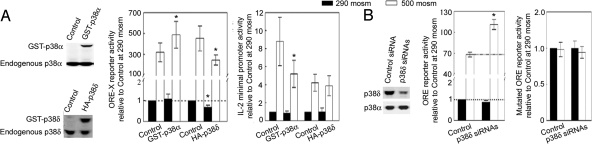
We questioned whether the lack of effect of MKP-1 on high NaCl-induced TonEBP/OREBP activity could be caused by opposing effects of the p38 isoforms that it inactivates. In agreement with this hypothesis, transient overexpression of GST-p38α increases high NaCl-induced TonEBP/OREBP transcriptional activity (ORE-X; Fig. 6A), but overexpression of HA-p38δ reduces it (ORE-X; Fig. 6A). Nonspecific general effects on transcription are ruled out because they do not occur when using reporters that lack TonEBP/OREBP binding sites (IL-2 minimal promoter; Fig. 6A). As an additional test, we knocked down p38δ with siRNAs (Fig. 6B). Knocking down protein expression of p38δ by 60% (Fig. 6B, immunoblot) increases high NaCl-induced ORE reporter activity (Fig. 6B). As a control, the siRNAs have no effect when the DNA sites in the reporter are mutated to prevent binding of TonEBP/OREBP (Mutated ORE reporter; Fig. 6B). We conclude that p38α enhances TonEBP/OREBP transcriptional activity and that p38δ inhibits it, which explains why there is no net effect when MKP-1 inactivates both of them.
Fig. 6.
p38α and p38δ have opposite effects on high NaCl-induced TonEBP/OREBP transcriptional activity. (A) HEK293 cells were cotransfected with GST-p38α, HA-p38δ, empty vector pEBG, or pcDNA 3.0 (Control) and with ORE-X or IL-2 minimal promoter reporter. Thirty-two hours later, osmolality was increased to 500 mosmol/kg (NaCl added) for 16 h. Overexpression of GST-p38α increases high NaCl-induced ORE-X activity, whereas overexpression of HA-p38δ decreases ORE-X activity. (B) siRNA-mediated knockdown of p38δ increases high NaCl-induced TonEBP/OREBP transcriptional activity. As in A, except that HEK293 cells stably expressing ORE or mutated ORE reporters were transfected with control siRNA or specific siRNAs against p38δ. The specific siRNAs, which decrease p38δ protein expression by 60% (immunoblot), increase high NaCl-induced transcriptional activity. *, P < 0.05, n = 3–4.
Discussion
PTP2 and PTP3 cocoordinately regulate osmotic activation of the p38 homolog, HOG-1, in Saccharomyce cerevisiae (27). StyI/Spc1, the functional equivalent of HOG-1 in Schizosaccharomyces pombe, is also regulated by two PTPs, Pyp1 and Pyp2 (27). Given the high level of conservation of MAPK pathways from yeast to human, it would be surprising if mammalian equivalents of these PTPs do not exist, but their identity has been uncertain. Our initial goal was to identify PTPs that might contribute to the regulation of hypertonicity-induced phosphorylation of p38.
MKP-1 Regulates High NaCl-Induced Activation of p38.
Our attention was directed to MKP-1 because MKP-1 is critical for the inactivation of p38 in mouse embryonic fibroblasts after osmotic stress (14), and because MKP-1 promotes survival of mouse embryonic fibroblasts by attenuating anisomycin-responsive MAPK-mediated apoptosis (14). In agreement with these results, we found in our present studies that overexpression of wild-type MKP-1 reduces high NaCl-induced phosphorylation of p38 and dominant negative MKP-1 increases it (Fig. 1 B–D). Further, high NaCl inhibits MKP-1 activity (Fig. 3 A and B). Thus, MKP-1 is involved in regulation of hypertonicity-induced phosphorylation of p38.
MKP-1 is the archetypal member of a family that includes 11 MKPs (14). Regulation of MKP-1 was previously studied extensively with regard to the innate immune response and inflammation (28). The MKPs contain a conserved PTP domain, characterized by a signature catalytic motif [C(X)5R] at the carboxyl terminus. Because of the unique structure of the catalytic domain, the pKa values for the active cysteine residues of MKPs are ≈5–6, as opposed to 8 for ordinary cysteines. Under physiological conditions, the thiol group of the active cysteine is a thiolate anion. The thiolate anion is necessary for nucleophilic attack on phosphate groups in the MAPKs and is vulnerable to oxidation. ROS inactivate MKPs by direct oxidation of the thiolate anion to sulfenic acid (20). High NaCl increases ROS (3, 19), and the high NaCl-induced ROS directly inhibit MKP-1 (Fig. 3 A and B).
Activation of MKPs also involves binding to their MAPK targets (25, 26). The amino termini of the MKPs are variable, but typically contain two conserved regions with significant homology with the CH2 domain of cdc25 phosphatase (14, 25, 26). Positively charged arginine residues in the noncatalytic amino terminus of MKP-1 interact with the negatively charged carboxyl terminus of their MAPK substrates via a modular docking surface located between the CH2A and CH2B domains of the MKPs (25, 26). High NaCl reduces binding of MKP-1 to p38 (Figs. 3 A and C and 4 A–D). Because binding of MKP-1 to its substrates activates it (25, 26), dissociation of p38 from MKP-1 should increase phosphorylation of p38. This association of MKP-1 with its p38 substrates is redox-insensitive (Fig. 3 A and C).
p38α and p38δ Are both Activated by Hypertonicity but Have Opposite Effects on TonEBP/OREBP.
High NaCl increases phosphorylation of p38α and p38δ and activates them (Fig. 2). However, expression of p38α increases TonEBP/OREBP activity, but p38δ inhibits it (Fig. 6A). Previously, opposite effects of some p38 isoforms had been noted. p38α contributes to IL-1β-induced iNOS mRNA expression, but p38β inhibits it (17). Our present finding of opposing effects of p38α and p38δ on TonEBP/OREBP casts a different light on some previous observations. The p38 inhibitor SB203580 inhibits p38α and p38β, but not p38δ (16, 18). SB203580 reduces high NaCl-induced TonEBP/OREBP transcriptional activity, which was interpreted as caused by inhibition of a positive effect of p38 (8, 9). In view of our present findings the situation evidently is more complicated. We now propose that inhibition of the positive effect of p38α by SB203580 unmasks an inhibitory effect of p38δ. Similarly, we propose that reduction of high NaCl-induced TonEBP/OREBP transcriptional activity by a dominant negative mutant of p38α (8) also involves unmasking of the inhibitory effect of p38δ. Further, the opposing effects of p38α and p38δ explain why dominant negative MKK3 prevents high NaCl-induced phosphorylation of p38, but does not affect TonEBP/OREBP transcriptional activity (15). Because MKK3 activates both p38α and p38δ (16, 29), the dominant negative should reduce both of their activities. In view of our present studies, we would expect that elimination of their opposing effects should not cause any net change in TonEBP/OREBP activity.
Perspective.
We cannot yet ascribe any physiological significance to the opposite effects of p38α and p38δ on TonEBP/OREBP. Most likely, some, as yet unidentified, additional stimuli may affect the hypertonicity-induced activation of one or the other, fine-tuning the activity of TonEBP/OREBP to an optimal level. In any event, because p38 isoforms can have different effects, it is necessary when inhibiting p38 to distinguish which isoforms are affected and what are the actions of the isoforms. Otherwise, incomplete or misleading interpretations can be reached.
Materials and Methods
Cells, Cell Culture, and Chemicals.
Murine inner medullary collecting duct cells (mIMCD3) (30), generously provided by S. Gullans (Harvard University, Boston), were cultured in DMEM/Ham's F-12 low glucose (1:1) containing 10% FBS and 2 mM l-glutamine in 5% CO2/95% air at 37°C and used between passages 14 and 20. HEK293 cells and HEK293FT cells, purchased from ATCC, were incubated in Eagle's MEM plus 10% FBS in 5% CO2/95% air at 37°C and used between passages 38 and 48. The initial osmolality of media was 300 mosmol/kg for mIMCD3 cells and 290 mosmol/kg for HEK293 cells. All experiments were performed on subconfluent cells. PAO was purchased from Calbiochem, and all other chemicals were purchased from Sigma. PAO (1 M) was dissolved in DMSO. NAC (300 mM) was dissolved in water.
Plasmids, siRNAs, and Transfections.
Flag-tagged full-length rat MKP-1 expression vector pSRα-Flag-MKP-1 and its dominant negative mutant with the active cysteine mutated to serine pSRα-Flag-MKP-1 C/S, HA-tagged p38α expression vector pSRα-HA-p38α, GST-tagged full-length rat MKP-1, p38α expression vectors (pEBG-Srf I-MKP-1, pEBG-Srf I-p38α), and empty vector (pEBG-Srf I) were constructed as described (25, 31). Myc-tagged human Cdc25c cDNA and its mutant cloned into pcDNA3.1 (Invitrogen) (32), were kindly provided by H. Piwnica-Worms (Washington University, St. Louis) through T. Finkel (National Heart, Lung, and Blood Institute). HA-tagged human p38δ, kindly provided by O. Livnah (Hebrew University, Jerusalem), was cloned into pcDNA3.0 (18). ORE-X, IL-1 minimal promoter, ORE, and mutated ORE luciferase reporters have been described (19). siRNAs against human p38δ (FlexiTube with the material number 1027415) were purchased from Qiagen. The control siRNA has been described (33). Transient transfection of plasmids into HEK293 cells and HEK293FT cells used Effectene, according to the manufacturer's protocol (Qiagen). In some cases, the plasmids were also reverse-transfected into HEK293 cells (cells added to plasmids and Effectene, rather than vice versa). Reverse transfection of siRNAs into HEK293 cells used Lipofectamine 2000 with the recommended ratio of siRNA to Lipofectamine 2000 (Invitrogen).
To get stable expression, Flag-MKP-1, GST-MKP-1, and GST-p38 vectors were transfected into mIMCD3 cells together with pcDNA 3.0 (10:1), using Lipofectamine 2000 (Invitrogen). Selection was initially made with 600 μg/ml G418, then the cells were maintained in 200 μg/ml G418. Positive clones were identified by immunocytochemistry (34).
Immunoprecipitation and GST Pull-Down.
Flag-MKP-1 was immunoprecipitated from mIMCD3 cells stably expressing it. Osmolality was raised from 300 to 550 mosmol/kg by adding NaCl for 30 min, then the cells were washed once with ice-cold PBS, adjusted to the same osmolality as the medium by adding NaCl, and lysed on ice with a buffer containing 30 mM Tris·HCl (pH 7.5), 5 mM EDTA, 50 mM β-glycerolphosphate, 10% glycerol, 1 mM β-mercaptoethanol, 1% Triton X-100, and plus protease inhibitor, 1 tablet to 10 ml) (31). Protein concentration was determined with BCA. Cell extracts were precleared with rabbit IgG plus Protein A/G-PLUS Agarose (Santa Cruz Biotechnologies) at 4°C for 1 h and then incubated with anti-Flag antibody-Protein A/G-PLUS Agarose at 4°C overnight. The agarose beads were washed once with lysis buffer and twice with MKP-1 activity assay buffer. The beads were divided to measure separately MKP-1 activity and MKP-1 protein by Western analysis. Native p38 was immunoprecipitated from mIMCD3 cells with a mAb (Cell Signaling Technology). HA-p38α and HA-p38δ were immunoprecipitated from transiently transfected HEK293FT cells. The cells were cotransfected with Flag-MKP-1 plus either HA-p38α or HA-p38δ for 48 h before increasing osmolality to 500 mosmol/kg by adding NaCl for 30 min. The cells were collected in the lysis buffer provided with the p38 Kinase Assay Kit (Cell Signaling Technology) plus protease inhibitor mixture tablet (Roche), 2.5 μM NaF, and 2.5 μM Na3VO4. HA-p38α and HA-p38δ were immunoprecipitated with biotinylated goat anti-HA antibody (GenScript) on streptavidin-conjugated magnetic Dynabeads M-280 (Invitrogen).
GST pull-down of recombinant GST, GST-MKP-1, and GST-p38 was as described (31). Cells were washed once with ice-cold PBS, then lysed as in the immunoprecipitation of Flag-MKP-1. Cell extracts were incubated with Glutathione Sepharose High Performance beads (Amersham Biosciences) at 4°C overnight with gentle shaking. Then, the beads were washed with lysis buffer four times. Proteins were eluted with 50 mM Tris·HCl (pH 8.0), 10 mM glutathione, and 10 mM β-mercaptoethanol.
Western Analysis, p38 Activity, and Antibodies.
After treatment, cells were washed once with ice-cold PBS adjusted to the osmolality of the medium by adding NaCl. The samples were collected and analyzed by Western analysis (35). The activities of HA-p38α and HA-p38δ were measured with a p38 MAPK assay kit (Cell Signaling Technology), according to the manufacturer's protocol. All antibodies for Western analysis were purchased from Cell Signaling Technology, except for the antibody against MKP-1, which was from Upstate.
Luciferase and MKP-1 Activities.
Luciferase activity was measured as before (19). Flag-MKP-1 was immunoprecipitated and washed as described above. The immunoprecipitates were incubated in an assay buffer containing 30 mM Tris·HCl (pH 7.5), 75 mM NaCl, 0.5 mM EDTA, 5 mM 2-mercaptoethanol, 0.04% BSA, and 20 μM 3-O-methylfluorescein phosphate at 37°C for 5 h in a black 96-well plate (36). Fluorescence emission from the reaction was measured in a Victor3 1420 multilabel counter with excitation at 485 nm and emission at 530 nm.
Statistics.
Data are expressed as mean ± SEM. Analyses were performed by paired t test. P < 0.05 is considered significant.
Acknowledgments.
We thank Dr. Oded Livnah for the HA-p38δ plasmid and Dr. H. Piwnica-Worms for the cdc25c constructs. This study was supported by grants from the National Kidney Foundation/National Capital Area and Uniformed Services University (to X.Z.) and the Intramural Research Program of the National Heart, Lung, and Blood Institute, National Institutes of Health.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/cgi/content/full/0801453105/DCSupplemental.
References
- 1.Santos BC, Chevaile A, Hebert MJ, Zagajeski J, Gullans SR. A combination of NaCl and urea enhances survival of IMCD cells to hyperosmolality. Am J Physiol. 1998;274:F1167–F1173. doi: 10.1152/ajprenal.1998.274.6.F1167. [DOI] [PubMed] [Google Scholar]
- 2.Michea L, et al. Cell cycle delay and apoptosis are induced by high salt and urea in renal medullary cells. Am J Physiol. 2000;278:F209–F218. doi: 10.1152/ajprenal.2000.278.2.F209. [DOI] [PubMed] [Google Scholar]
- 3.Zhang Z, Dmitrieva NI, Park JH, Levine RL, Burg MB. High urea and NaCl carbonylate proteins in renal cells in culture and in vivo, and high urea causes 8-oxoguanine lesions in their DNA. Proc Natl Acad Sci USA. 2004;101:9491–9496. doi: 10.1073/pnas.0402961101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kultz D, Chakravarty D. Hyperosmolality in the form of elevated NaCl but not urea causes DNA damage in murine kidney cells. Proc Natl Acad Sci USA. 2001;98:1999–2004. doi: 10.1073/pnas.98.4.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dmitrieva NI, Bulavin DV, Burg MB. High NaCl causes Mre11 to leave the nucleus, disrupting DNA damage signaling and repair. Am J Physiol. 2003;285:F266–F274. doi: 10.1152/ajprenal.00060.2003. [DOI] [PubMed] [Google Scholar]
- 6.Burg MB, Ferraris JD, Dmitrieva NI. Cellular response to hyperosmotic stresses. Physiol Rev. 2007;87:1441–1474. doi: 10.1152/physrev.00056.2006. [DOI] [PubMed] [Google Scholar]
- 7.Lopez-Rodriguez C, et al. Loss of NFAT5 results in renal atrophy and lack of tonicity-responsive gene expression. Proc Natl Acad Sci USA. 2004;101:2392–2397. doi: 10.1073/pnas.0308703100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ko BC, et al. Fyn and p38 signaling are both required for maximal hypertonic activation of the OREBP/TonEBP. J Biol Chem. 2002;277:46085–46092. doi: 10.1074/jbc.M208138200. [DOI] [PubMed] [Google Scholar]
- 9.Nadkarni V, Gabbay KH, Bohren KM, Sheikh-Hamad D. Osmotic response element enhancer activity: Regulation through p38 kinase and mitogen-activated extracellular signal-regulated kinase kinase. J Biol Chem. 1999;274:20185–20190. doi: 10.1074/jbc.274.29.20185. [DOI] [PubMed] [Google Scholar]
- 10.Padda R, et al. MEKK3-mediated signaling to p38 kinase and TonE in hypertonically stressed kidney cells. Am J Physiol. 2006;291:F874–F881. doi: 10.1152/ajprenal.00377.2005. [DOI] [PubMed] [Google Scholar]
- 11.O'Rourke SM, Herskowitz I, O'Shea EK. Yeast go the whole HOG for the hyperosmotic response. Trends Genet. 2002;18:405–412. doi: 10.1016/s0168-9525(02)02723-3. [DOI] [PubMed] [Google Scholar]
- 12.Han J, Lee JD, Bibbs L, Ulevitch RJ. A MAP kinase targeted by endotoxin and hyperosmolarity in mammalian cells. Science. 1994;265:808–811. doi: 10.1126/science.7914033. [DOI] [PubMed] [Google Scholar]
- 13.Uhlik MT, et al. Rac-MEKK3-MKK3 scaffolding for p38 MAPK activation during hyperosmotic shock. Nat Cell Biol. 2003;5:1104–1110. doi: 10.1038/ncb1071. [DOI] [PubMed] [Google Scholar]
- 14.Wu JJ, Bennett AM. Essential role for mitogen-activated protein (MAP) kinase phosphatase-1 in stress-responsive MAP kinase and cell survival signaling. J Biol Chem. 2005;280:16461–16466. doi: 10.1074/jbc.M501762200. [DOI] [PubMed] [Google Scholar]
- 15.Kultz D, Garcia-Perez A, Ferraris JD, Burg MB. Distinct regulation of osmoprotective genes in yeast and mammals: Aldose reductase osmotic response element is induced independent of p38 and stress-activated protein kinase/Jun N-terminal kinase in rabbit kidney cells. J Biol Chem. 1997;272:13165–13170. doi: 10.1074/jbc.272.20.13165. [DOI] [PubMed] [Google Scholar]
- 16.Jiang Y, et al. Characterization of the structure and function of the fourth member of p38 group mitogen-activated protein kinases, p38d. J Biol Chem. 1997;272:30122–30128. doi: 10.1074/jbc.272.48.30122. [DOI] [PubMed] [Google Scholar]
- 17.Lui P, et al. Effects of p38MAPK isoforms on renal mesangial cell inducible nitric oxide synthase expression. Am J Physiol. 2004;286:C145–C152. doi: 10.1152/ajpcell.00233.2003. [DOI] [PubMed] [Google Scholar]
- 18.Avitzour M, et al. Intrinsically active variants of all human p38 isoforms. FEBS J. 2007;274:963–975. doi: 10.1111/j.1742-4658.2007.05644.x. [DOI] [PubMed] [Google Scholar]
- 19.Zhou X, Ferraris JD, Cai Q, Agarwal A, Burg MB. Increased reactive oxygen species contribute to high NaCl-induced activation of the osmoregulatory transcription factor TonEBP/OREBP. Am J Physiol. 2005;289:F377–F385. doi: 10.1152/ajprenal.00463.2004. [DOI] [PubMed] [Google Scholar]
- 20.Kamata H, et al. Reactive oxygen species promote TNFα-induced death and sustained JNK activation by inhibiting MAP kinase phosphatases. Cell. 2005;120:649–661. doi: 10.1016/j.cell.2004.12.041. [DOI] [PubMed] [Google Scholar]
- 21.Yang T, et al. Hypertonic induction of COX-2 in collecting duct cells by reactive oxygen species of mitochondrial origin. J Biol Chem. 2005;280:34966–34973. doi: 10.1074/jbc.M502430200. [DOI] [PubMed] [Google Scholar]
- 22.Robinson KA, et al. Redox-sensitive protein phosphatase activity regulates the phosphorylation state of p38 protein kinase in primary astrocyte culture. J Neurosci Res. 1999;55:724–732. doi: 10.1002/(SICI)1097-4547(19990315)55:6<724::AID-JNR7>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 23.Umenishi F, Schrier RW. Hypertonicity-induced aquaporin-1 (AQP1) expression is mediated by the activation of MAPK pathways and hypertonicity-responsive element in the AQP1 gene. J Biol Chem. 2003;278:15765–15770. doi: 10.1074/jbc.M209980200. [DOI] [PubMed] [Google Scholar]
- 24.Hashimoto S, Gon Y, Matsumoto K, Takeshita I, Horie T. N-acetylcysteine attenuates TNF-α-induced p38 MAP kinase activation and p38 MAP kinase-mediated IL-8 production by human pulmonary vascular endothelial cells. Br J Pharmacol. 2001;132:270–276. doi: 10.1038/sj.bjp.0703787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hutter D, Chen P, Barnes J, Liu Y. Catalytic activation of mitogen-activated protein (MAP) kinase phosphatase-1 by binding to p38 MAP kinase: Critical role of the p38 C-terminal domain in its negative regulation. Biochem J. 2000;352:155–163. [PMC free article] [PubMed] [Google Scholar]
- 26.Slack DN, Seternes OM, Gabrielsen M, Keyse SM. Distinct binding determinants for ERK2/p38α and JNK MAP kinases mediate catalytic activation and substrate selectivity of MAP kinase phosphatase-1. J Biol Chem. 2001;276:16491–16500. doi: 10.1074/jbc.M010966200. [DOI] [PubMed] [Google Scholar]
- 27.Keyse SM. Protein phosphatases and the regulation of mitogen-activated protein kinase signaling. Curr Opin Cell Biol. 2000;12:186–192. doi: 10.1016/s0955-0674(99)00075-7. [DOI] [PubMed] [Google Scholar]
- 28.Wang X, Liu Y. Regulation of innate immune response by MAP kinase phosphatase-1. Cell Signal. 2007;19:1372–1382. doi: 10.1016/j.cellsig.2007.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Enslen H, Raingeaud J, Davis RJ. Selective activation of p38 mitogen-activated protein (MAP) kinase isoforms by the MAP kinase kinases MKK3 and MKK6. J Biol Chem. 1998;273:1741–1748. doi: 10.1074/jbc.273.3.1741. [DOI] [PubMed] [Google Scholar]
- 30.Rauchman MI, Nigam SK, Delpire E, Gullans SR. An osmotically tolerant inner medullary collecting duct cell line from an SV40 transgenic mouse. Am J Physiol. 1993;265:F416–F424. doi: 10.1152/ajprenal.1993.265.3.F416. [DOI] [PubMed] [Google Scholar]
- 31.Chen P, Hutter D, Liu P, Liu Y. A mammalian expression system for rapid production and purification of active MAP kinase phosphatases. Protein Expression Purif. 2002;24:481–488. doi: 10.1006/prep.2001.1599. [DOI] [PubMed] [Google Scholar]
- 32.Savitsky PA, Finkel T. Redox regulation of Cdc25C. J Biol Chem. 2002;277:20535–20540. doi: 10.1074/jbc.M201589200. [DOI] [PubMed] [Google Scholar]
- 33.Irarrazabal CE, Burg MB, Ward SG, Ferraris JD. Phosphatidylinositol 3-kinase mediates activation of ATM by high NaCl and by ionizing radiation: Role in osmoprotective transcriptional regulation. Proc Natl Acad Sci USA. 2006;103:8882–8887. doi: 10.1073/pnas.0602911103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dmitrieva NI, Cai Q, Burg MB. Cells adapted to high NaCl have many DNA breaks and impaired DNA repair both in cell culture and in vivo. Proc Natl Acad Sci USA. 2004;101:2317–2322. doi: 10.1073/pnas.0308463100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhou X, Ferraris JD, Burg MB. Mitochondrial reactive oxygen species contribute to high NaCl-induced activation of the transcription factor TonEBP/OREBP. Am J Physiol. 2006;290:F1169–F1176. doi: 10.1152/ajprenal.00378.2005. [DOI] [PubMed] [Google Scholar]
- 36.Rice RL, et al. A targeted library of small-molecule, tyrosine, and dual-specificity phosphatase inhibitors derived from a rational core design and random side-chain variation. Biochemistry. 1997;36:15965–15974. doi: 10.1021/bi971338h. [DOI] [PubMed] [Google Scholar]