Abstract
A recent study described a recessive ATPase activating germ-line mutation in smooth-muscle myosin (smmhc/myh11) underlying the zebrafish meltdown (mlt) phenotype. The mlt zebrafish develops intestinal abnormalities reminiscent of human Peutz–Jeghers syndrome (PJS) and juvenile polyposis (JP). To examine the role of MYH11 in human intestinal neoplasia, we searched for MYH11 mutations in patients with colorectal cancer (CRC), PJS and JP. We found somatic protein-elongating frameshift mutations in 55% of CRCs displaying microsatellite instability and in the germ-line of one individual with PJS. Additionally, two somatic missense mutations were found in one microsatellite stable CRC. These two missense mutations, R501L and K1044N, and the frameshift mutations were functionally evaluated. All mutations resulted in unregulated molecules displaying constitutive motor activity, similar to the mutant myosin underlying mlt. Thus, MYH11 mutations appear to contribute also to human intestinal neoplasia. Unregulated MYH11 may affect the cellular energy balance or disturb cell lineage decisions in tumor progenitor cells. These data challenge our view on MYH11 as a passive differentiation marker functioning in muscle contraction and add to our understanding of intestinal neoplasia.
Keywords: colorectal cancer, microsatellite instability, Peutz–Jeghers syndrome, smmhc/myh11
Germline mutations in human myosin heavy chain genes contribute to multiple inherited human diseases (1). In addition, smooth-muscle myosin (MYH11) participates in formation of an oncogenic fusion gene CBFB/MYH11 in acute myeloid leukaemia (2).
Microsatellite instability (MSI) occurs in ≈15% of colorectal cancers (CRC), and is a consequence of defects in the DNA mismatch repair (MMR) system (3). Mutations occurring during DNA replication are not repaired, resulting in genetic instability that particularly affects short repetitive DNA sequences. Mutations that result in malignant progression are selected and thus detected in surgically removed MSI cancers. Passenger mutations also occur frequently in this mutator tumor type (4–6).
By examining gene expression data (7), we had identified MYH11 as a gene with reduced expression in MSI CRCs compared with normal colon samples. The reduced expression of MYH11 in CRCs could simply reflect a larger smooth-muscle component in our normal colon specimens. Nevertheless, the differential expression prompted us to examine whether MYH11 has a mononucleotide tract in the coding sequence, because mononucleotide tracts are prone to mutations under MSI. Two splice variants, SM1 and SM2, which are distinct in the C-terminal tailpiece, are produced from the MYH11 gene. Indeed, the SM2 isoform of MYH11 was observed to harbor a mononucleotide repeat of 8 cytosines (C8). Thus, MYH11 was identified as a candidate MSI colon cancer gene. Dominant-negative germ-line mutations in MYH11 occur in a syndrome predisposing to thoracic aortic aneurysm/aortic dissection (TAAD) and patent ductus arteriosus (8). The TAAD phenotype closely resembles the one caused by inactivating TGF-beta type 2 receptor (TGFBR2) germ-line mutations (9, 10). Therefore, it appeared that MYH11 and TGFBR2, might have overlapping functions. Somatic inactivating TGFBR2 mutations are a fundamental step in MSI CRC formation (11), and thus examining the possible role of somatic MYH11 mutations in MSI CRCs was of particular interest.
H.L.S. participated in a recent study describing a recessive ATPase activating MYH11 mutation underlying the zebrafish meltdown (mlt) phenotype (12), characterized by intestinal abnormalities, such as invasion of the intestinal epithelium into underlying stroma, desmoplastic reaction, and formation of cysts. This phenotype was said to resemble Peutz–Jeghers syndrome (PJS) and juvenile polyposis (JP) (12), rendering MYH11 a plausible candidate gene for these human intestinal polyp and cancer syndromes.
The aim of this study was to examine the possible role of inherited and somatic MYH11 mutations in human intestinal neoplasia. Somatic mutations were investigated in colorectal cancers, especially in tumors displaying microsatellite instability. In inherited intestinal cancer syndromes, we focused on PJS and JP.
Results
MYH11 Mutations in Intestinal Neoplasia.
Altogether, 101 MSI CRC samples were screened for the MYH11 C8 microsatellite residing in the last exon of the MYH11 SM2 isoform. In total, 56 (56/101, 55%) cancers harbored mutations in the C8 repeat (C7; c.5798delC, C6; c.5797_5798delCC, or C9; c.5798_5799insC). A subset of CRCs had a biallelic mutation in C8. Somatic origin was always confirmed (Fig. 1 and Table 1). The mutation frequency in the MYH11 C8 tract was significantly higher than in 25 neutral intronic C8 control repeats [P = 3.8 × 10−10, Fisher's Exact test, supporting information (SI) Table S1]. The MYH11 C8 tract mutations lead to a frameshift and are predicted to elongate the SM2 protein rather than cause a premature stop codon (Fig. 1).
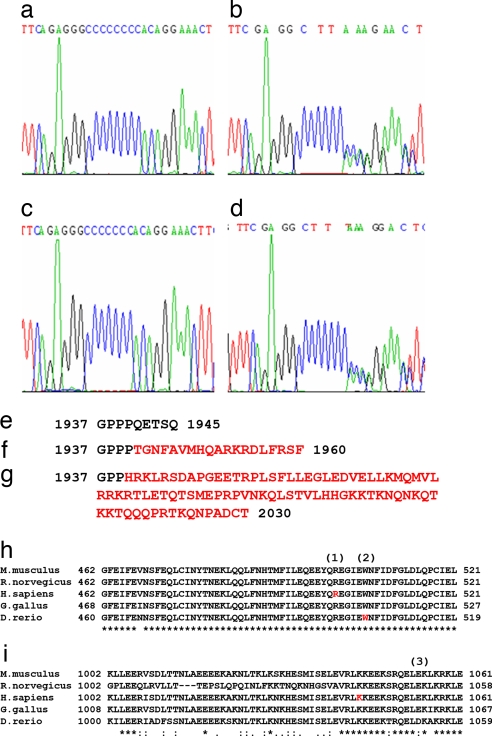
Fig. 1.
Examples of mutations observed in the study. Mutations found in the C8 mononucleotide repeat of MYH11. (a) WT sequence. (b) Deletion of one cytosine in a MSI CRC sample. (c) A homozygous deletion of one cytosine in HCA7 MSI CRC cell line. (d) Germ-line insertion of one cytosine in a patient with PJS. (e) WT amino acid sequence of the last exon of MYH11 SM2. (f and g) Consequences of insertion (f) and deletion (g) of one cytosine to the amino acid sequence. Both mutations disrupt the C-terminal amino acids and elongate the predicted peptide. (h and i) Homology comparison between species shows that MYH11 amino acids R501 (1) and K1044 (3) are highly conserved. (2) indicates the amino acid mutated in zebrafish meltdown phenotype. Peptide sequences: Mm, ENSMUSP00000087756; Rn, ENSRNOP00000047484; Hs, ENSP00000300036; Gg, ENSGALP00000032964; Dr, ENSDARP00000041141.
Table 1.
Somatic mutations detected in the C8 repeat of MYH11 in 101 primary MSI CRCs
| Samples, no. (%) | Mutation type |
|---|---|
| 45 (44.6) | C8/C8 (WT) |
| 42 (41.6) | C7/C8 |
| 4 (3.9) | C7/C7 |
| 6 (5.9) | C8/C9 |
| 3 (3.0) | C7/C9 |
| 1 (1.0) | C6/C8 |
Subsequently, 32 MSI CRCs with mutation in C8 were sequenced for the entire coding region of MYH11 to detect possible additional hits. Somatic changes identified are listed in Table 2. These changes were not further assessed and remain of unknown significance. However, R501C was of particular interest, because it lies in immediate vicinity of the ATPase activating missense mutation underlying the zebrafish mlt phenotype (Fig. 1) (12). The mlt mutation in Danio rerio sequence (W504R ENSDARP00000041141, or W512R in ref. 12) resides only five codons apart from the human R501C mutation.
Table 2.
Somatic changes found in CRCs analyzed for the entire coding region of MYH11
| Type of sample | Sample ID | MSI status | Sequence variant | Exon | Germline* |
|---|---|---|---|---|---|
| Patient sample | S1160T | MSS | R501L, K1044N | 12, 24 | WT |
| C451T | MSI | c.3766_3768delAAG, c.5798delC | 27, 40 (SM2) | WT | |
| C621T | MSI | c.5798delC, IVS13 + 3A > G | 40 (SM2) | WT | |
| C840T | MSI | c.5798delC, R1339C | 40 (SM2), 29 | WT | |
| C426T | MSI | c.5798delC, c.3766_3768delAAG, R501C | 40 (SM2), 27, 12 | WT | |
| C698T | MSI | c.5798delC, R1862H | 40 (SM2), 38 | WT | |
| C204T | MSI | c.5797_5798delCC | 40 (SM2) | WT | |
| C340T | MSI | c.5798_5799insC | 40 (SM2) | WT | |
| C1023 | MSI | c.5798delC, c.5798delC | 40 (SM2) | WT | |
| C484T | MSI | c.5798delC, T672T | 40 (SM2), 15 | WT | |
| C440T | MSI | c.5798delC, D1100D | 40 (SM2), 25 | WT | |
| † | MSI | c.5798delC | 40 (SM2) | WT | |
| Cell line | GP5D | MSI | c.5798delC, c.1094_1097delGAAA, p.R1732H | 40 (SM2), 9, 36 | ND |
| LS174T | MSI | c.5798delC, G258E | 40 (SM2), 6 | ND | |
| HCT15 | MSI | A1766V | 37 | ND | |
| HCT116 | MSI | A1817V | 37 | ND | |
| SNUC2B | MSI | R1895C | 39 | ND | |
| LoVo, CCL231, RKO, VACO5 | MSI | c.5798delC | 40 (SM2) | ND |
ND, not determined because no normal tissue was available.
*When a nucleotide change in tumor tissue was observed, the respective normal tissue DNA was sequenced.
†Twenty-two samples total.
Fifteen MSI CRC cell lines were sequenced for the coding region of MYH11. The repeat mutation frequency was 50%, consisting of monoallelic deletions (C7/C8) in 6/14 cell lines, and a biallelic deletion (C7/C7) in one. Seven cell lines were WT for C8. Five cell lines displayed missense changes of unknown significance (Table 2).
Tumor samples from 30 patients with microsatellite stable (MSS) CRC were sequenced for the MYH11 coding sequence. Two somatic mutations were identified, both in the same cancer: R501L in exon 12 and K1044N in exon 24 (Fig. 1 and Table 2). Somatic mutation prevalence in this tumor type is low, ≈1.2 mutations in one million base pairs (4). Therefore, finding these two missense changes in one cancer would have been unlikely by chance, and they, in addition to the C7/C9 mutations, appeared to be particularly attractive candidates for being causative.
Screening of 25 LKB1 mutation negative PJS patients for the coding region of MYH11 identified a U.K. Caucasian patient with a heterozygous c.5798_5799insC (C9) germ-line mutation. C9 was not found in 1015 population-matched controls. The patient had had a cystic astrocytoma at 13 years. Intussusception and hemicolectomy at the age of 23 years revealed PJS, with multiple intestinal polyps of typical Peutz–Jeghers morphology. The patient had slight perioral freckling, which had faded somewhat over the years. The C9 change was detected also in the patient's father. By the age of 59 years, he had never undergone endoscopic examination, had no PJS pigmentation, and had no history of malignancy. Neither individual had a history of TAAD, and cardiologic examinations, including echocardiogram, showed no pathological changes. Apart from the index case, no other family members had been diagnosed with PJS. Based on the zebrafish model, recessive inheritance of the trait and a second mutation undetectable by sequencing may explain the difference between the index case and the father. Also, penetrance might be incomplete or modifying genetic factors required for the phenotype.
Samples from seven JP patients were screened for MYH11 mutations, with negative results. To complete the mutation search, we focused on the exons that had displayed MYH11 mutations in the above efforts. An additional 66 samples from patients with unexplained hamartomatous or hyperplastic polyposis were examined for germ line mutations, and 185 MSS CRC samples were examined for somatic mutations, both with negative results. Moreover, MYH11 exons 12 and 24 were analyzed in 800 unselected CRCs. Novel somatic alterations E502K and A1079K were identified in two different MSI cancers.
MYH11 Mutations Reside in the Epithelial Compartment of CRCs.
Clinical and histopathological parameters could not distinguish MYH11 mutation-positive and -negative MSI cancers. In six cancers, the mutant (c.3766_3768delAAG, c.5798delC, and R501C) cell population was studied by using microdissection. DNAs extracted from the normal tissue compartment (n = 4) were WT for mutations and showed normal genotypes at the BAT25 and BAT26 microsatellite markers. Of the 28 tumor stroma samples studied, 21 were WT, and 7 showed some evidence for presence of a mutant allele best fitting minor contamination by tumor epithelium. Indeed, all stromal samples proved to be WT in MSI analysis. All 10 samples from malignant epithelium harbored the expected MYH11 mutations and showed MSI.
MYH11 mRNA and Protein Expression.
MYH11 mRNA was expressed in all 11 MSI CRC cell lines examined, regardless of their C8 status. Expression of the SM2 isoform was examined by quantitative real-time PCR, and no expression could be detected in the eight cell lines studied. MYH11 protein from five MSI CRC cell lines was examined by immunoblotting, using antibodies against the WT protein. Cells with or without frameshift MYH11 alleles (HCT8, HCA7, CCL231, LoVo, and HCT116) did not express MYH11 at detectable levels. In immunohistochemistry with two different MYH11 antibodies, normal and malignant colonic epithelium typically displayed no staining regardless of mutation status, although examples of samples with positive epithelial staining, particularly in nonneoplastic epithelium adjacent to tumor tissue, were seen.
ATPase and Motor Activity in MYH11 Mutant Proteins.
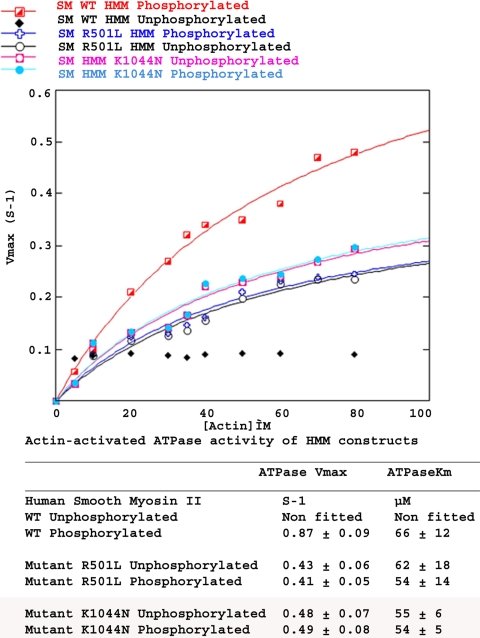
We next studied the possible effects of the R501L and K1044N mutations on MYH11 ATPase activity and motor function. A soluble, regulated fragment, known as a heavy meromyosin (HMM) fragment was created for WT and mutant proteins. This fragment is more easily expressed and analyzed than is the full-length protein. Smooth muscle myosin activity is regulated by phosphorylation of the regulatory light chains that are bound to the myosin heads (13). The steady-state actin-activated ATPase activity of WT HMM was 0.87 s−1 for the phosphorylated form, with a speed of 0.36 μm/sec in an in vitro motility assay (14). When not phosphorylated, the WT protein displayed low residual ATPase activity and no movement (Fig. 2 and Table 3), demonstrating that the WT HMM is regulated by phosphorylation. Although both the R501L and K1044N mutations lowered the maximal activity and the speed of actin filament translocation, the more profound impact was the total loss of regulation in both cases (Fig. 2 and Table 3). The affinity for actin (KATPase) did not differ significantly between WT and mutants.
Fig. 2.
ATPase activity measurements. ATPase activity of WT, R501L, and K1044N mutant smooth-muscle heavy meromyosins.
Table 3.
Maximal ATPase activity and in vitro motility of WT and mutant MYH11 constructs
| Construct | PHOS | Maximal actin-activated ATPase,* sec−1 per head ± SD† | In vitro motility,‡ μm/sec ± SD† |
|---|---|---|---|
| MYH11-HMM WT | −PHOS | ≈0 | 0 (no observed motility) |
| + PHOS | 0.87 ± 0.06† | 0.36 ± 0.09† | |
| MYH11-HMM R501L | −PHOS | 0.43 ± 0.02 | 0.12 ± 0.03 |
| + PHOS | 0.41 ± 0.03 | 0.13 ± 0.04 | |
| MYH11-HMM K1044N | −PHOS | 0.48 ± 0.03 | 0.14 ± 0.04 |
| + PHOS | 0.49 ± 0.04 | 0.16 ± 0.04 | |
| MYH11− full-length WT | − PHOS | ND | 0 (no observed motility) |
| + PHOS | ND | 0.35 ± 0.11† | |
| MYH11− full-length with C9 mutation | −PHOS | ND | 0.17 ± 0.06 |
| +PHOS | ND | 0.13 ± 0.04 | |
| MYH11− full-length with C7 mutation | −PHOS | ND | 0.26 ± 0.05 |
| +PHOS | ND | 0.35 ± 0.09 |
ND, not determined. PHOS, phosphorylation status.
*Data pooled from triplicate assays on three independent protein preparations.
†Significant difference (P < 0.01) between unphosphorylated and phosphorylated myosin, demonstrating the presence of regulation. P values were calculated using Student's t test, comparing the unphosphorylated and the phosphorylated data groups for each mutant.
‡Data pooled from three independentprotein preparations.
The full-length human MYH11 (SM2 isoform) was created for WT, C7 and C9 mutants, to examine the impact of extending the C-terminal tail of the molecule. Again, the WT construct showed phosphorylation-dependent regulation, but the C7 and C9 constructs displayed unregulated motor activity that was not modulated by phosphorylation (Table 3). This was as measured by an actin-gliding in vitro motility assay (14). In this assay, no directed actin filament movement is seen for WT smooth-muscle myosin (either HMM or full-length) in the absence of phosphoryation (Table 3). However, for the mutant myosins, there was no change in the number of moving actin filaments, the average velocity of movement, or in the quality of movement after phosphorylation, suggesting that regulation was abolished. Direct actin-activated ATPase assays could not be performed on the full-length myosin, due to lack of sufficient quantities of expressed full-length myosin to perform the assay.
Discussion
We found protein-elongating MYH11 mutations in most MSI CRCs and in the germ line of one individual with PJS. Additionally, two somatic missense mutations were found in one MSS CRC. These two missense mutations, R501L and K1044N, and the two frameshift mutation types were functionally evaluated. The zebrafish mlt germ-line missense mutation resulting in intestinal abnormalities (12) resides no more than five codons from one of the two somatic missense mutations functionally studied, R501L. Furthermore, two other cancers harbored somatic missense mutations in this region (R501C, E502K) (Table 2), and thus these mutations were of particular interest. Interestingly, the germ-line mutation observed in a PJS patient was identical to the somatic mutations observed in multiple MSI CRCs.
We performed ATPase activity and motility assays, using a protein construct harboring R501L and demonstrated that at least one functional consequence of this MYH11 mutation was the formation of a protein with unregulated actin-activated motor activity. The loss of regulation causes a major impact on the cell. Changes in force and speed can be overcome in a cell by modulating the regulatory light chain phosphorylation. The loss of ability to turn off the motor, however, cannot be compensated by any cellular mechanisms. In the mlt zebrafish (12), only ATPase activity, but not motor function was measured. Based on our present results, it is likely that also in the mlt zebrafish, motor activity is constitutively activated and unregulated. The consequences of the human mutations examined were even more dramatic in that absolutely no regulation was detected.
The mutation within the S2 region of the myosin rod (K1044N), is in a region known to be critical for regulation of MYH11 (15). Functional analysis demonstrated that K1044N resulted in total loss of regulation, similar to R501L. Studies have indicated that the coiled–coiled carboxyterminal myosin tail determines, and the short nonhelical sequence further enhances, filament formation (16–18). Based on what is known about the function of the C-terminal tail of MYH11, we hypothesized that the C7 and C9 mutations might result in an altered nonhelical tail region that disturbs communication between the myosin heads and filament backbones necessary for regulation within a filament (19) and might interfere with the folded monomer structure necessary for regulation (13). The results of the C7 and C9 motor assays validate this speculation and demonstrate that the consequence of perturbing the C-terminal tail is similar to the missense mutations.
MSI cancers harbor many passenger mutations (4–6). Real MSI tumor suppressor genes have been suggested ideally to display the following characteristics: a high mutation frequency, biallelic inactivation, a role in a growth suppressor pathway, mutations in MSS tumors, and altered characteristics in functional studies (3). The general requirements presented in these very stringent criteria are fulfilled by our present work. MYH11 also survived statistical approaches, premised on comparison of mutation rates in neutral and coding repeats. Higher mutation frequencies than that observed in MYH11 (55%) have been reported for a few bona fide MSI target genes. The most frequently mutated MSI CRC target gene is TGFBR2 with a mutation frequency up to 90% (20–22). Genes with similar mutation frequency to that observed in MYH11 are ACVRII (58–83%), BAX (37–64%), MSH3 (26–55%), and CASP5 (48–66%) (20, 21, 23–26); to our knowledge, the latter four have not been shown to be mutated in MSS CRC. Thus, the genetic, functional, and animal model-derived evidence linking MYH11 to intestinal neoplasia is comparable with or superior to established MSI target genes.
It is impossible to predict the significance of the sequence changes (Table 2) that were not functionally tested. The identification of three somatic mutations in codons 501–502, one of which was functionally tested, suggests that all three mutations, in addition to the K1044N and the C7 and C9 mutations, are causally connected to colorectal tumorigenesis. This assertion is further supported by the fact that most mutations in the MYH11 head or in the rod would not be expected to abolish regulation based on the current structural models of regulation (27).
Microdissection demonstrated that the MYH11 mutations were epithelial in accordance with studies reporting that MMR deficiency targets CRC epithelium. MYH11 is typically expressed in stromal elements and not expected to be expressed in the epithelium. Here, we could demonstrate MYH11 mRNA expression in all CRC samples analyzed, albeit at very low levels. The possible translated products, if any, would be difficult to detect. It is possible that low amounts of unregulated MYH11 play a role in disturbing the epithelial homeostasis, such as energy balance. A recent study demonstrated that myosin regulatory light chain (MRLC) is phosphorylated by AMP-activated protein kinase (AMPK), a metabolic regulator activated in response to energy deprivation (28). Furthermore, MRLC was shown to control cell polarity and mitosis as an important functional mediator of AMPK and LKB1, the protein underlying PJS (29). These new findings firmly link the established PJS gene LKB1 to myosin functions. Although our intriguing results on PJS still require further confirmation, this work suggests an altered MYH11 function in PJS pathogenesis. Subsequent studies on smooth-muscle signaling in PJS may provide clues to understanding the presently unknown mechanisms of tumorigenesis underlying PJS.
In addition to possible effects on epithelial cell structural and energy balance, unregulated MYH11 could play a role in tumor progenitor stem cells, which for gastrointestinal adenocarcinoma can be epithelial stem cells or, as suggested in model systems, bone marrow derived mesenchymal stem cells (MSCs) (30). The loss of TGFβ signaling owing to mutations in TGFBR2 together with constitutively active MYH11 in a stem cell could lead to a phenotype resembling poorly differentiated adenocarcinoma, which is typical of MSI CRCs (31). The overlap in phenotypes associated with both somatic (MSI CRC) and germ-line (TAAD) MYH11 and TGFBR2 mutations is noteworthy and might implicate that these proteins have interacting cellular functions, possibly relevant for stem cell differentiation (32). The tumorigenic effect associated with functionally aberrant MYH11 might be exerted through transient expression of mutant MYH11 in tumor progenitor cells, leading to aberrant cell lineage decisions. Low MYH11 expression in colonic neoplasia is compatible with epithelial differentiation of the malignant cells and speaks in favor of a cell lineage decision problem, rather than altered epithelial structural or energy balance, as the abnormal process underlying MYH11 activation-related malignancy.
Although it requires extensive further work to prove or refute it, this intriguing possibility is compatible with our recent finding showing the importance of nonmuscle myosin II in cell fate decisions in MSC differentiation (33). Thereafter, we have probed MSCs grown on different substrates for the expression of MYH11. We detected MYH11 expression (data not shown) in cells grown on substrates with stiffness equivalent to or greater than that of muscle but not in cells grown on very soft substrates that mimic neuronal tissues (33). Because we have demonstrated that myosin II contractile activity is necessary to correctly interpret matrix properties and drive differentiation decisions, expression of an unregulated smooth-muscle myosin in the context of naïve stem cells would potentially alter their cell fate.
In conclusion, we suggest that mutational activation of MYH11 is involved in human intestinal tumorigenesis. Subsequent research into the detailed mechanisms of myosin functions in CRC and stem cells should significantly enhance our understanding of the role of myosin in cellular differentiation processes and the development of colorectal tumors.
Materials and Methods
Patient Samples.
A set of 25 Caucasian patients with LKB1 mutation negative PJS (34), samples from 2 Finnish and 5 U.K. patients with mutation-negative JP, and a collection of 66 Caucasian patients with unspecific hamartomatous polyposis [ref. 35 and unpublished data], were included in the study. Two hundred eighty-eight DNAs from population-matched healthy controls were obtained from the Human Random Control DNA panel representing U.K. Caucasian blood donors (Sigma) and an additional 727 cancer-free controls from the U.K. with no family history of CRC were available, totalling 1,015 control samples. Colorectal adenocarcinoma and corresponding normal tissue samples were from a Finnish sample series (36, 37). Before DNA and RNA extraction the samples were evaluated by a pathologist to confirm the presence of malignant tissue. MSI had been determined earlier (36, 37). Patient information and samples were obtained after informed consent and Ethical Review Board approval.
Mutation Detection.
This was performed by genomic sequencing (34). Primer sequences are available upon request. MYH11 primers were designed by using reference sequences NM_002474 and NM_022844. Fragments with the C8 repeat were amplified by using Phusion proof-reading enzyme (Finnzymes).
Microdissection.
Six CRC specimens with MYH11 mutations were scrutinized. Multiple small tissue regions of ≈200–400 cells were isolated by selective UV radiation fractionation from paraffin-embedded tissue sections (altogether, 42 regions from the six specimens) as described in ref. 38. Fluorescence-labeled BAT25 and BAT26 microsatellite markers (primer sequences: BAT25: forward, TCGCCTCCAAGAATGTAAGT and reverse, TCTGGATTTTAACTATGGCTC and BAT26: forward, TGACTACTTTTGACTTCAGCC and reverse, AACCATTCAACATTTTTAACC) were used in MSI analysis and the size of the amplified PCR products was assessed by using an Applied Biosystems ABI3730 Automatic DNA sequencer.
cDNA.
Primers were designed to evaluate the expression of the MYH11 isoforms SM1 and SM2. PCR fragments were visualized by using 2% agarose gel electrophoresis. Results were further confirmed by direct sequencing of the fragments. Samples from the multiple tissue cDNA panel (Clontech) were used as positive controls.
Cell Culture.
Cell culture was carried out by using standard protocols. Cell lines were purchased from American Type Culture Collection and the European Collection of Cell Cultures or provided by I. Tomlinson (London Research Institute, London). The following MSI CRC cell lines were used in the study: LoVo, SW948, HCT15, CCL231, HCT116, LS174T, RKO, HCT8, GP5D, HUTU80, LS180, SNUC2B, VACO5, HCA7, and LIM1215.
Real-Time Quantitative PCR.
RNA levels were measured by using Taqman assay Hs00224610_m1 in a GeneAmp 7500 sequence detection system (Applied Biosystems) as described in ref. 39. Expression of beta-actin served as a control. The cell lines studied were HUTU80, GD5D, HCT8, RKO, CCl231, HCT15, HCA7, HCT116, VACO5, SNUC2B, and LS180.
Western Blot Analysis and Immunohistochemistry.
Proteins extracted from MSI CRC cell lines HCT8, HCA7, CCL231, LoVo, and HCT116 were analyzed. A fresh-frozen tissue specimen from uterine myometrium served as positive control. Cell pellets were lysed into RIPA buffer (Sigma), and the fresh-frozen tissue sample were lysed into T-Per buffer (Pierce), both supplemented with a protease inhibitor mixture (Roche Diagnostics). Protein aliquots were loaded onto a 5% polyacrylamide gel (Bio-Rad), and fractionated proteins were transferred onto a polyvinylidene fluoride membrane (Millipore). Immunostaining with anti-MYH11 antibody (Chemicon International and Sigma) was carried out, after which protein expression was detected by using Western Breeze Chemiluminescent Immunodetection System (Invitrogen Life Technologies). Ponceau-S staining was used as a loading control. Immunohistochemistry was performed as described in ref. 39, using two different anti-MYH11 antibodies (Chemicon International Sigma). Paraffin-embedded sections from 28 MSI and 39 MSS CRCs were immunostained. Most sections contained both tumor tissue and normal intestinal epithelium.
Construction, Expression, and Purification of Human Smooth Myosin II (SMII).
The cDNA for human SMII (SMA isoform) was purchased from Open Biosystems (cDNA clone MGC:126726, accession no. BC101677). To create the HMM construct, the sequence was truncated at the codon for aspartate 1063, after which a glycine plus FLAG peptide (DYKDDDDK) was appended. For the full-length human MYH11 SM2 constructs, a FLAG peptide was appended to the N terminus, and the SM2 sequence continued through either the WT or mutant (C9) stop codon. The constructs were subcloned into the baculovirus transfer vector, p2Bac (Invitrogen). Site-directed mutagenesis (for R501L and K1044N) was performed by using QuikChange XLII kit (Stratagene). Protein expression and purification were as described in ref. 40.
ATPase Assay.
The actin-activated ATPase activity assay was performed at 23°C in buffer 20/20 [20 mM KCl, 5 mM Mg2+, 1 mM EGTA, and 20 mM Mops (pH 7.0)], 1 mM (final concentration) ATP, and actin concentrations ranging from 0 to 80 μM. Actin was purified from rabbit skeletal muscle and stabilized by phalloidin. Phosphorylation of HMM WT and mutant constructs was performed as described in ref. 41. Both unphosphorylated and phosphorylated forms of WT and mutant human smooth myosin 2 HMM constructs were assayed at 0.2 nM final concentration. Curves were fitted with Kaleidagraph software. Triplicate assays were performed with three different preparations of each protein.
In Vitro Motility Assay.
The sliding actin filament assay was used to test the functionality of the recombinant proteins. The assay was performed at 30°C, following procedures published in refs. 42 and 43. The data from ≥20 filaments (per preparation and phosphorylation state) were analyzed with Volocity software (Improvision). See also SI Text.
Statistical Analyses.
Independent variables were compared with the Pearson's χ2 test or Fisher's exact test. Analyses of survival were performed by using the method of Kaplan and Meier. P < 0.05 was considered significant.
Acknowledgments.
We thank Mikko Aho, Päivi Hannuksela, Mairi Kuris, Sini Marttinen, Inga-Lill Svedberg, and Iina Vuoristo for technical assistance. The work was supported by the Academy of Finland; the Finnish Cancer Society; the Sigrid Jusélius Foundation; the Association for International Cancer Research; the European Commission; grants from the Research and Science Foundation of Farmos, AstraZeneca, the Finnish Medical Society Duodecim, the Lilly Foundation, The Research Foundations of Finnish Gastroenterology and Oncology Society, the Paulo Foundation, the Maud Kuistila Foundation, and the Ida Montin foundation (to P.A.); a National Institutes of Health grant (to H.L.S.); Spanish Fondo de Investigaciones Sanitarias Grants FIS 05/1394 and CP05/00256 (to D.A.); and Fundacion de Investigacion Medica Mutua Madrilena. C.E. is a recipient of the Doris Duke Distinguished Clinical Scientist Award.
Footnotes
The authors declare no conflict of interest.
Data deposition: The sequence reported in this paper has been deposited in the GenBank database (accession no. EU489063).
This article contains supporting information online at www.pnas.org/cgi/content/full/0801213105/DCSupplemental.
References
- 1.Laing NG, Nowak KJ. When contractile proteins go bad: The sarcomere and skeletal muscle disease. BioEssays. 2005;27:809–822. doi: 10.1002/bies.20269. [DOI] [PubMed] [Google Scholar]
- 2.Liu P, et al. Fusion between transcription factor CBF beta/PEBP2 beta and a myosin heavy chain in acute myeloid leukemia. Science. 1993;261:1041–1044. doi: 10.1126/science.8351518. [DOI] [PubMed] [Google Scholar]
- 3.Boland CR, et al. National Cancer Institute workshop on microsatellite instability for cancer detection and familial predisposition: development of international criteria for the determination of microsatellite instability in colorectal cancer. Cancer Res. 1998;58:5248–5257. [PubMed] [Google Scholar]
- 4.Greenman C, et al. Patterns of somatic mutation in human cancer genomes. Nature. 2007;446:153–158. doi: 10.1038/nature05610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hienonen T, et al. Mutations in two short noncoding mononucleotide repeats in most microsatellite-unstable colorectal cancers. Cancer Res. 2005;65:4607–4613. doi: 10.1158/0008-5472.CAN-05-0165. [DOI] [PubMed] [Google Scholar]
- 6.Sammalkorpi H, et al. Background mutation frequency in microsatellite-unstable colorectal cancer. Cancer Res. 2007;67:5691–5698. doi: 10.1158/0008-5472.CAN-06-4314. [DOI] [PubMed] [Google Scholar]
- 7.Kruhoffer M, et al. Gene expression signatures for colorectal cancer microsatellite status and HNPCC. Br J Cancer. 2005;92:2240–2248. doi: 10.1038/sj.bjc.6602621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhu L, et al. Mutations in myosin heavy chain 11 cause a syndrome associating thoracic aortic aneurysm/aortic dissection and patent ductus arteriosus. Nat Genet. 2006;38:343–349. doi: 10.1038/ng1721. [DOI] [PubMed] [Google Scholar]
- 9.Mizuguchi T, et al. Heterozygous TGFβR2 mutations in Marfan syndrome. Nat Genet. 2004;36:855–860. doi: 10.1038/ng1392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pannu H, et al. Mutations in transforming growth factor-β receptor type II cause familial thoracic aortic aneurysms and dissections. Circulation. 2005;112:513–520. doi: 10.1161/CIRCULATIONAHA.105.537340. [DOI] [PubMed] [Google Scholar]
- 11.Markowitz S, et al. Inactivation of the type II TGF-beta receptor in colon cancer cells with microsatellite instability. Science. 1995;268:1336–1338. doi: 10.1126/science.7761852. [DOI] [PubMed] [Google Scholar]
- 12.Wallace KN, et al. Mutation of smooth muscle myosin causes epithelial invasion and cystic expansion of the zebrafish intestine. Dev Cell. 2005;8:717–726. doi: 10.1016/j.devcel.2005.02.015. [DOI] [PubMed] [Google Scholar]
- 13.Trybus KM. Regulation of smooth muscle myosin. Cell Motil Cytoskeleton. 1991;18:81–85. doi: 10.1002/cm.970180202. [DOI] [PubMed] [Google Scholar]
- 14.Kron SJ, Spudich JA. Fluorescent actin filaments move on myosin fixed to a glass surface. Proc Natl Acad Sci USA. 1986;83:6272–6276. doi: 10.1073/pnas.83.17.6272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Trybus KM, Freyzon Y, Faust LZ, Sweeney HL. Spare the rod, spoil the regulation: Necessity for a myosin rod. Proc Natl Acad Sci USA. 1997;94:48–52. doi: 10.1073/pnas.94.1.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hodge TP, Cross R, Kendrick-Jones J. Role of the COOH-terminal nonhelical tailpiece in the assembly of a vertebrate nonmuscle myosin rod. J Cell Biol. 1992;118:1085–1095. doi: 10.1083/jcb.118.5.1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ikebe M, et al. The tip of the coiled-coil rod determines the filament formation of smooth muscle and nonmuscle myosin. J Biol Chem. 2001;276:30293–30300. doi: 10.1074/jbc.M101969200. [DOI] [PubMed] [Google Scholar]
- 18.Rovner AS, Fagnant PM, Lowey S, Trybus KM. The carboxyl-terminal isoforms of smooth muscle myosin heavy chain determine thick filament assembly properties. J Cell Biol. 2002;156:113–123. doi: 10.1083/jcb.200107131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Woodhead JL, et al. Atomic model of a myosin filament in the relaxed state. Nature. 2005;436:1195–1199. doi: 10.1038/nature03920. [DOI] [PubMed] [Google Scholar]
- 20.Jung B, et al. Loss of activin receptor type 2 protein expression in microsatellite unstable colon cancers. Gastroenterology. 2004;126:654–659. doi: 10.1053/j.gastro.2004.01.008. [DOI] [PubMed] [Google Scholar]
- 21.Mori Y, et al. Instabilotyping: Comprehensive identification of frameshift mutations caused by coding region microsatellite instability. Cancer Res. 2001;61:6046–6049. [PubMed] [Google Scholar]
- 22.Parsons R, et al. Microsatellite instability and mutations of the transforming growth factor beta type II receptor gene in colorectal cancer. Cancer Res. 1995;55:5548–5550. [PubMed] [Google Scholar]
- 23.Calin GA, et al. Genetic progression in microsatellite instability high (MSI-H) colon cancers correlates with clinico-pathological parameters: A study of the TGRbetaRII, BAX, hMSH3, hMSH6, IGFIIR and BLM genes. Int J Cancer. 2000;89:230–235. [PubMed] [Google Scholar]
- 24.Miyaki M, et al. Alterations of repeated sequences in 5′ upstream and coding regions in colorectal tumors from patients with hereditary nonpolyposis colorectal cancer and Turcot syndrome. Oncogene. 2001;20:5215–5218. doi: 10.1038/sj.onc.1204578. [DOI] [PubMed] [Google Scholar]
- 25.Rampino N, et al. Somatic frameshift mutations in the BAX gene in colon cancers of the microsatellite mutator phenotype. Science. 1997;275:967–969. doi: 10.1126/science.275.5302.967. [DOI] [PubMed] [Google Scholar]
- 26.Trojan J, et al. BAX and caspase-5 frameshift mutations and spontaneous apoptosis in colorectal cancer with microsatellite instability. Int J Colorectal Dis. 2004;19:538–544. doi: 10.1007/s00384-004-0597-1. [DOI] [PubMed] [Google Scholar]
- 27.Wendt T, Taylor D, Trybus KM, Taylor K. Three-dimensional image reconstruction of dephosphorylated smooth muscle heavy meromyosin reveals asymmetry in the interaction between myosin heads and placement of subfragment 2. Proc Natl Acad Sci USA. 2001;98:4361–4366. doi: 10.1073/pnas.071051098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lee JH, et al. Energy-dependent regulation of cell structure by AMP-activated protein kinase. Nature. 2007;447:1017–1020. doi: 10.1038/nature05828. [DOI] [PubMed] [Google Scholar]
- 29.Hemminki A, et al. A serine/threonine kinase gene defective in Peutz–Jeghers syndrome. Nature. 1998;391:184–187. doi: 10.1038/34432. [DOI] [PubMed] [Google Scholar]
- 30.Houghton J, et al. Gastric cancer originating from bone marrow-derived cells. Science. 2004;306:1568–1571. doi: 10.1126/science.1099513. [DOI] [PubMed] [Google Scholar]
- 31.Yearsley M, et al. Histologic features distinguish microsatellite-high from microsatellite-low and microsatellite-stable colorectal carcinomas, but do not differentiate germline mutations from methylation of the MLH1 promoter. Hum Pathol. 2006;37:831–838. doi: 10.1016/j.humpath.2006.02.009. [DOI] [PubMed] [Google Scholar]
- 32.Mishra L, Derynck R, Mishra B. Transforming growth factor-beta signaling in stem cells and cancer. Science. 2005;310:68–71. doi: 10.1126/science.1118389. [DOI] [PubMed] [Google Scholar]
- 33.Engler AJ, Sen S, Sweeney HL, Discher DE. Matrix elasticity directs stem cell lineage specification. Cell. 2006;126:677–689. doi: 10.1016/j.cell.2006.06.044. [DOI] [PubMed] [Google Scholar]
- 34.Alhopuro P, et al. Mutation analysis of three genes encoding novel LKB1-interacting proteins, BRG1, STRADalpha, and MO25alpha, in Peutz–Jeghers syndrome. Br J Cancer. 2005;92:1126–1129. doi: 10.1038/sj.bjc.6602454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sweet K, et al. Molecular classification of patients with unexplained hamartomatous and hyperplastic polyposis. J Am Med Assoc. 2005;294:2465–2473. doi: 10.1001/jama.294.19.2465. [DOI] [PubMed] [Google Scholar]
- 36.Aaltonen LA, et al. Incidence of hereditary nonpolyposis colorectal cancer and the feasibility of molecular screening for the disease. N Engl J Med. 1998;338:1481–1487. doi: 10.1056/NEJM199805213382101. [DOI] [PubMed] [Google Scholar]
- 37.Salovaara R, et al. Population-based molecular detection of hereditary nonpolyposis colorectal cancer. J Clin Oncol. 2000;18:2193–2200. doi: 10.1200/JCO.2000.18.11.2193. [DOI] [PubMed] [Google Scholar]
- 38.Shibata D, et al. Specific genetic analysis of microscopic tissue after selective ultraviolet radiation fractionation and the polymerase chain reaction. Am J Pathol. 1992;141:539–543. [PMC free article] [PubMed] [Google Scholar]
- 39.Alhopuro P, et al. SMAD4 levels and response to 5-fluorouracil in colorectal cancer. Clin Cancer Res. 2005;11:6311–6316. doi: 10.1158/1078-0432.CCR-05-0244. [DOI] [PubMed] [Google Scholar]
- 40.Sweeney HL, et al. Kinetic tuning of myosin via a flexible loop adjacent to the nucleotide binding pocket. J Biol Chem. 1998;273:6262–6270. doi: 10.1074/jbc.273.11.6262. [DOI] [PubMed] [Google Scholar]
- 41.Yang Z, Sweeney HL. Restoration of phosphorylation-dependent regulation to the skeletal muscle myosin regulatory light chain. J Biol Chem. 1995;270:24646–24649. doi: 10.1074/jbc.270.42.24646. [DOI] [PubMed] [Google Scholar]
- 42.Umemoto S, Sellers JR. Characterization of in vitro motility assays using smooth muscle and cytoplasmic myosins. J Biol Chem. 1990;265:14864–14869. [PubMed] [Google Scholar]
- 43.Sellers JR, Cuda G, Wang F, Homsher E. Myosin-specific adaptations of the motility assay. Methods Cell Biol. 1993;39:24–49. doi: 10.1016/s0091-679x(08)60159-4. [DOI] [PubMed] [Google Scholar]