Abstract
β-Site APP-cleaving enzyme 1 (BACE1) is required for the penultimate cleavage of the amyloid-β precursor protein (APP) leading to the generation of amyloid-β peptides that is central to the pathogenesis of Alzheimer's disease. In addition to its role in endoproteolysis of APP, BACE1 participates in the proteolytic processing of neuregulin 1 (NRG1) and influences the myelination of central and peripheral axons. Although NRG1 has been genetically linked to schizophrenia and NRG1+/− mice exhibit a number of schizophrenia-like behavioral traits, it is not known whether altered BACE1-dependent NRG1 signaling can cause similar behavioral abnormalities. To test this hypothesis, we analyze the behaviors considered to be rodent analogs of clinical features of schizophrenia in BACE1−/− mice with impaired processing of NRG1. We demonstrate that BACE1−/− mice exhibit deficits in prepulse inhibition, novelty-induced hyperactivity, hypersensitivity to a glutamatergic psychostimulant (MK-801), cognitive impairments, and deficits in social recognition. Importantly, some of these manifestations were responsive to treatment with clozapine, an atypical antipsychotic drug. Moreover, although the total amount of ErbB4, a receptor for NRG1 was not changed, binding of ErbB4 with postsynaptic density protein 95 (PSD95) was significantly reduced in the brains of BACE1−/− mice. Consistent with the role of ErbB4 in spine morphology and synaptic function, BACE1−/− mice displayed reduced spine density in hippocampal pyramidal neurons. Collectively, our findings suggest that alterations in BACE1-dependent NRG1/ErbB4 signaling may participate in the pathogenesis of schizophrenia and related psychiatric disorders.
Keywords: clozapine, dizocilpine, neuregulin, prepulse inhibition, spine density
BACE1 (β-site APP-cleaving enzyme 1), is the rate-limiting enzyme that makes the initial cleavage of the amyloid-β (Aβ) precursor protein (APP) and, in concert with γ-secretase, gives rise to the plaque-forming β-amyloid peptides in Alzheimer's disease (AD) (1). Deletion of BACE1 prevents the formation of Aβ in vitro and in vivo and the cognitive abnormalities in mouse models of Aβ amyloidosis. These findings strongly support BACE1 as an attractive therapeutic target for AD (1–3). In addition to APP, a number of other putative substrates for BACE1 have been identified, suggesting that BACE1 has multiple physiological functions (2). For example, recent studies indicate that BACE1 participates in the proteolytic processing of neuregulin 1 (NRG1) (4, 5), a ligand for members of the ErbB family of receptor-tyrosine kinases. This signaling pathway have numerous roles in CNS development and functions, including synapse formation, plasticity, neuronal migration, myelination of central and peripheral axons, and the regulation of neurotransmitter expression and function (6, 7). In addition to these physiological functions, NRG1 is one of the first genes that has been linked to an increased risk of schizophrenia (8). The disease-associated single-nucleotide polymorphisms (SNPs) are all noncoding regions of NRG1 (9, 10), which has led to the suggestion that SNPs associated with schizophrenia are regulatory and may affect putative binding sites for transcriptional factors (such as serum response factor or myelin-transcription factor 1) and, thereby alter levels of NRG1 isoforms (9). Supporting the idea that genetic variations in noncoding regions of NRG1 can affect brain function is the finding of a strong association of SNPs in the NRG1 promoter in subjects at high risk of schizophrenia to abnormalities in cortical function and psychotic symptoms and cognitive impairments (11).
A functional role of NRG1 has been further clarified in a number of mouse models with various NRG1 deletions that showed multiple behaviors of putative relevance to schizophrenia: impaired prepulse inhibition, spontaneous hyperactivity, and reversal by clozapine of such hyperactivity (8, 12). Recent in vivo and in vitro studies indicate that NRG1/ErbB4 signaling plays a key role in structural and functional plasticity of glutamatergic synapses (13, 14) and regulates GABAergic transmission (15). Both glutamate and GABA systems are thought to be of pathophysiological relevance in psychiatric diseases, particularly in schizophrenia (16).
Given the strong genetic and functional links of NRG1 to schizophrenia (8, 10, 11) and roles for BACE1 in the biology of NRG1, the observation that NRG1 processing is altered in BACE1 knockout mice (4, 5) raises the possibility that perturbations in NRG1 signaling in these mice may result in the behavioral phenotypes reminiscent of some of the features of schizophrenia. Here, we demonstrate that BACE1 knockout mice show a sensorimotor-gating deficiency, behavioral signs of glutamatergic hypofunction, and other typical endophenotypes of schizophrenia. Taken together with our observations that postsynaptic density protein 95 (PSD95)-associated ErbB4 and spine densities are reduced in BACE1−/− mice, our findings suggest that altered BACE1-dependent NRG1/ErbB4 signaling leads to schizophrenic-like phenotypes. These observations identify a unique function of BACE1 and suggest a new drug target for schizophrenia and other mental disorders.
Results
To test whether alteration in BACE1-dependent NRG1 signaling impacts on behaviors resembling features of schizophrenia, we analyzed BACE1−/− mice with altered processing of NRG1. Schizophrenia is characterized by psychotic and nonpsychotic episodes with positive and negative features, respectively, and by cognitive deficits. Because it is not possible to recapitulate the entire clinical syndrome in an animal model (17), we assessed specific behaviors in BACE1−/− mice that are considered to be analogous to some of those occurring in patients with schizophrenia [supporting information (SI) Table S1].
Impaired Prepulse Inhibition.
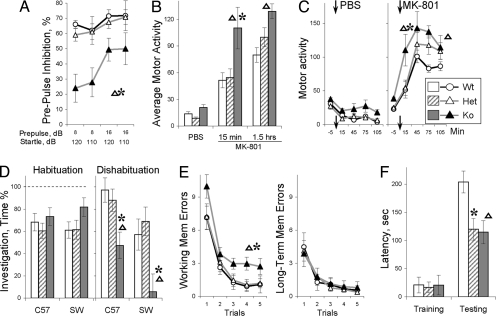
We first used prepulse inhibition (PPI), the paradigm most widely used in animal models relevant to schizophrenia because PPI can be measured easily in rodents in a fashion almost identical to procedures used in humans. PPI, a preattentive process that results in inhibitory “gating” to physiological responses, is impaired in schizophrenic patients (18). In this paradigm, a brief, low-intensity acoustic stimulus (the prepulse) inhibits the startle reflex caused by a loud stimulus (18). BACE1−/− mice show significant deficits in PPI compared with wild-type (WT) and BACE1+/− littermates (ANOVA, F2,39 = 6.05, P < 0.011; Newman–Keuls post hoc tests, P < 0.01; Fig. 1A). Startle amplitudes were not significantly different between BACE1−/− mice and their WT littermates (Fig. S1). That BACE1−/− mice exhibit hypomyelination of central and peripheral axons (4, 5) raises the possibility that the speed of signal transduction is altered in long axons (19). Indeed, the latencies of startle reaction were longer in BACE1−/− mice compared with those of WT and BACE1+/− (Fig. S1; F2,39 = 3.87, P < 0.035; Newman–Keuls post hoc tests, P < 0.03). However, there was no correlation between latency of startle reaction and deficit in prepulse inhibition (Fig. S1), indicating that these two phenotypes observed in BACE1−/− mice are independent.
Fig. 1.
Schizophrenia-like phenotypes of BACE1−/− mice. (A) Deficit in prepulse inhibition of acoustic startle reaction. (B) Hypersensitivity to the effects of (+)-MK-801 (0.3 mg/kg, i.p.) on locomotor activity. (C) Dynamics of locomotor activity after PBS or MK-801 injection. (D) Deficits in social recognition. Changes in time of social investigation are shown for habituation and dishabituation phases of the task (see SI Materials and Methods). C57B6 and SW denote a strain background of juvenile stimulus mice. Test mice were C57B6 background. (E) Working-memory deficits tested in the radial water maze (2). Note that the BACE1−/− mice were impaired in working-memory errors (reentries into previously visited arms) but were not different from other genotypes in long-term memory errors. (F) Deficit in the inhibitory avoidance. The latencies to stepdown from an elevated platform are shown for training and testing trial separated by 48-h delay. BACE1−/− mice had preserved sensitivity to a foot shock (Fig. S2). Data are expressed as means ± SEM. Triangles and asterisks indicate significant differences of BACE1−/− mice from WT or BACE1+/− littermates, respectively, as a result of Newman–Keuls post hoc test applied to significant effect of genotype or interactions (ANOVA, n = 8–12 mice per genotype).
Novelty-Induced Hyperactivity.
Novelty-induced hyperactivity has been viewed as a preclinical model of the positive symptoms of schizophrenia and psychomotor agitation in particular (17). We used several tasks (the open-field, plus maze, corner test, and Y maze) to assess locomotor response to novelty in BACE1−/− mice. Compared with WT and BACE1+/− mice, BACE1−/− mice exhibit significantly higher novelty-induced activity in all of these tasks (Fig. S2), indicating that hyperactivity in BACE1−/− mice is a highly reproducible trait generalized over situations that differ in terms of cognitive demands, spatial dimensions, and ability to evoke anxiety.
Supersensitivity to a Psychostimulant.
Sensitivity to a psychostimulant is another preclinical test used extensively in animal models as an analog of positive symptoms of schizophrenia (17). In particular, the psychotomimetic effects of N-methyl-d-aspartate (NMDA) receptor antagonists in healthy humans and their ability to exacerbate psychotic symptoms in schizophrenic patients suggested that glutamatergic neurotransmission may be critical in schizophrenia (16). Moreover, genetic deficits in NRG1/ErbB4 signaling has been thought to lead to glutamatergic hypofunction (13, 14). To assess the sensitivity of BACE1−/− mice to a psychostimulant, we tested the effects of dizocilpine (MK-801), a highly selective noncompetitive NMDA antagonist. A systemic injection of dizocilpine (0.3 mg/kg, i.p.) increased locomotor activity in all groups of mice (Fig. 1 B and C, and Fig. S2B; ANOVA, F1,20 = 37.76, P < 0.0001). However, BACE1−/− mice showed significantly higher levels of drug-stimulated motor activation compared with that of BACE1+/− or WT mice (Fig. 1 B and C; genotype F2,20 = 6.34, P < 0.01 and genotype × treatment interaction F2,20 = 3.54, P < 0.05). Note that the difference between BACE1−/− and WT mice was significant throughout the entire period (1.5 h) of observation (Fig. 1B). Thus, pharmacological inhibition of NMDA receptors in BACE1−/− mice revealed a marked hypersensitivity to changes in glutamatergic state in this animal model.
Alterations in Social Recognition.
To examine the impact of deletion of BACE1 on social interactions and memory, we used a social habituation–dishabituation paradigm (20) (Fig. S2), in which the test subject is exposed to a novel stimulus (a juvenile mouse) repeatedly. In a habituation stage of the experiment, BACE1−/− mice were indistinguishable from WT and BACE1+/− littermates showing similar decrease in social investigation of the same juvenile (Fig. 1D). To rule out the possibility that the reduced interest in the stimulus mouse is caused by changes in social motivation (habituation) we conducted the fourth trial in which a second novel stimulus mouse is presented. It is expected that in this “dishabituation” trial there will be an increase in time of investigation of the new stimulus mouse. However, BACE1−/− mice showed significant deficits in the dishabituation trial (F2,41 = 3.63, P < 0.035; Newman–Keuls post hoc tests P < 0.03 and 0.02, respectively; Fig. 1D). Importantly, an inability of BACE1−/− mice to dissociate between the new and familiar mouse was accentuated when the genetic background of the stimulus mice (Swiss–Webster strain, SW) differed from that of the BACE1−/− mice (C57BL6/J strain; Fig. 1D; and Fig. S2). In this situation, mice were required to dissociate relatively weak individual cues on the background of cues from the different genetic strain. Control tests showed that BACE1−/− mice successfully recognized differences between the two strains (Fig. S2), indicating that impairments in social recognition of BACE1−/− mice were not caused by deficits in perception of strain-specific cues. Thus, the habituation–dishabituation paradigm revealed an impaired ability of BACE1−/− mice to dissociate individual differences that can result in a lack of social recognition if individual cues are overshadowed by salient strain-specific cues.
Cognitive Deficits.
Many schizophrenic patients display various cognitive deficits, including impairments in working memory. These problems can be assessed in animal models (17). Consistent with previous findings that BACE1−/− mice were impaired in a number of working memory tasks (2, 3), we demonstrate that BACE1−/− mice were significantly impaired in the radial water maze task; deficits were specific to errors in working but not long-term memory (Fig. 1E). We also tested fear memory in inhibitory avoidance, a task widely used in animal models relevant to the associative deficits in schizophrenia patients (17). Indeed, compared with WT, both BACE1+/− and BACE1−/− mice were impaired in the inhibitory avoidance memory task (Fig. 1F).
Amelioration of PPI Deficits and Hyperactivity by Clozapine.
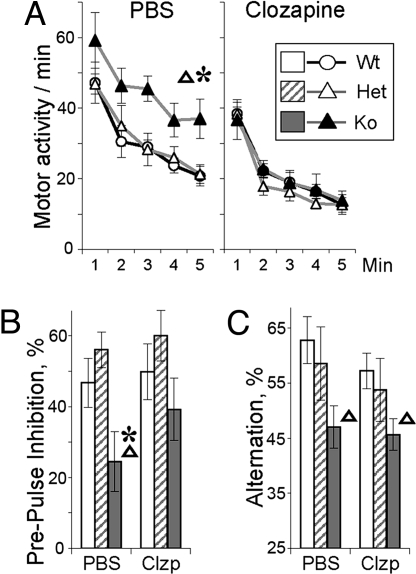
Clozapine, an atypical antipsychotic, has been shown to reduce psychotic symptoms, to normalize PPI deficits, and to counteract effects of NMDA antagonists in schizophrenic patients (18, 21). Moreover, acute administration of clozapine in NRG1+/− mice was effective in amelioration of some of the schizophrenia-like phenotypes, such as hyperactivity (8). We analyzed the effects of acute administration of clozapine in BACE1−/− mice. In WT controls, treatment with clozapine (2 mg/kg, i.p.) had no significant effect on amplitude of startle reaction (Fig. S3) or on prepulse inhibition (Fig. 2). Motor activity of WT mice was mildly reduced within 20 min of the treatment (effect of treatment F1,16 = 12.94, P < 0.005). However, there was no significant interaction of treatment and novelty-induced exploration (F4,64 = 0.63, P > 0.99), indicating that this dose was not sedative (Fig. 2). When administered to BACE1−/− mice, the same dose of clozapine ameliorated PPI deficits and negated novelty-induced hyperactivity (Fig. 2 A and B). These findings are in agreement with clinical effects of clozapine on sensorimotor gating deficits and positive symptoms (18). However, consistent with the observations that clozapine has limited efficacy in attenuating cognitive deficits in schizophrenic patients (22), no significant amelioration of deficits in working memory was demonstrated in BACE1−/− mice treated with clozapine (Fig. 2C). These results show that clozapine selectively attenuates novelty-induced hyperactivity and normalizes PPI deficits occurring in BACE1−/− mice.
Fig. 2.
Clozapine attenuates PPI deficits and hyperactivity in BACE−/−mice. Effect of clozapine (2 mg/kg, i.p.) on hyperactivity (A), PPI (B), and working-memory deficits (C) in BACE1−/− mice. PPI shows an overall percentage of inhibition (±SEM) observed in four types of trials with two different levels of startle and prepulse stimuli (Fig. S3). Abbreviations and signs are as in Fig. 1 (n = 8–12 per genotype per treatment).
Altered NRG1 Proteolysis and Reduced PSD95-Associated ErbB4 in BACE1−/− Mice.
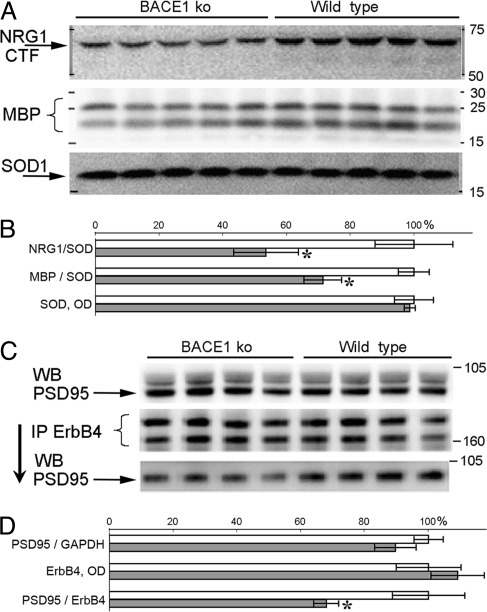
Although BACE1 has several substrates whose biology could play roles in the phenotypes observed in BACE1−/− mice, altered BACE1-dependent NRG1 signaling (4, 5) can be one plausible mechanism mediating schizophrenic-like behavioral traits. To test this possibility, we analyzed the effects of altered BACE1-dependent proteolytic processing of NRG1 on levels of ErbB4, a receptor-tyrosine kinase of NRG1 in the brains of BACE1−/− mice (7, 23). Similar to previous findings (4, 5), protein blot analyses of brain homogenates from BACE1−/− mice revealed a significant decrease in NRG1 proteolytic fragments probed by C-terminal (F1,8 = 37.94, P < 0.001; Fig. 3A) and N-terminal antibodies (Fig. S4), and a significant reduction in the amounts of myelin basic protein (MBP; F1,8 = 13.64, P < 0.01; Fig. 3A).
Fig. 3.
BACE1-related changes in NRG1 processing and ErbB4–PSD95 association. (A) Western blots of cortex homogenates from BACE1−/− and WT littermates stained with antibodies for C-terminal fragment of NRG1, MBP, and SOD1. (B) Bar graphs of relative NRG1-CTF, MBP, and SOD1 protein levels (mean ± SEM) based on results in A. (C) Western blot of cortex homogenates stained for PSD95 before (Top) and after (Bottom) immunoprecipitation with ErbB4 antibody. Middle shows Western blot for ErbB4 in samples after immunoprecipitation with ErbB4 antibody. Two bands represent full-length mature ErbB4 receptor (≈180 kDa) and its less-glycosylated intermediate (≈160 kDa) (39). (D) Bar graphs of PSD95 protein levels relative to GAPDH (Top) or ErbB4 (Bottom) and protein levels of ErbB4 (Middle). Open and closed bars represent measures for WT and BACE−/− mice, respectively. WB, Western blotting; IP, immunoprecipitation.
NRG1 function is mediated by a class of receptor-tyrosine kinases including ErbB2, ErbB3, and ErbB4 (7, 23). In adult brain, ErbB4 accumulates in regions enriched in dendritic processes (24) and is likely to be the major mediator of NRG1 functions related to myelination (19) and schizophrenia (10, 14). To examine the impact of impaired processing of NRG1 on ErbB4 signaling in BACE1−/− mice, we assessed ErbB4 interaction with PSD95, a downstream event critical for NRG1/ErbB4 signaling and for modulation of other PSD95-dependent activities (13, 14, 23). Lysates of cortices from BACE1−/− mice immunoprecipitated for ErbB4 and then immunoblotted with an antibody to PSD95 (Fig. 3C) showed significantly less recovery of PSD95 compared with that of controls (F1,6 = 7.13, P < 0.04; Fig. 3 C and D). Because there were no changes in overall levels of PSD95 (Fig. 3C) or ErbB4 proteins (data not shown) (4), this finding indicates that alterations in BACE1-dependent processing of NRG1 leads to a decreased interaction of ErbB4 with the synaptic protein PSD95.
Changes in Spine Density and Morphology in Hippocampal Pyramidal Neurons of BACE1−/− Mice.
It has been shown that ErbB4 is enriched on the surface of dendrites, is regulated by neuronal activity, and plays a critical role in maintaining spine morphology (13). It is possible that BACE1-dependent decrease in PSD95-associated ErbB4 can affect the number or morphology of dendritic spines. To test this notion, we analyzed dendritic spines in the CA3–CA1 pathway of the hippocampus where BACE1 and NRG1 are enriched presynaptically (2, 10), whereas ErbB4 is enriched postsynaptically on the surface of spines (13).
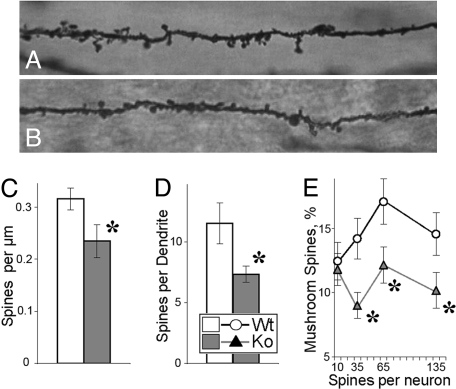
A significant decrease in the spine densities of CA1 pyramidal neurons was observed in BACE1−/− mice compared with that of control littermates (F1,10 = 5.47, P < 0.05; Fig. 4 A–C). Associated with a lower spine density, BACE1−/− mice exhibited a significant reduction in the number of spines per dendrite, a measure of total excitatory input to a dendrite as a signal integration unit (25) (F1,10 = 6.42, P < 0.03; Fig. 4D). To analyze spine morphology, the spines were classified into thin or mushroom-shaped types; the latter has been thought to represent stronger synaptic connections than thin spines (26). The proportions of mushroom-shaped types were calculated for each neuron (Fig. 4E). Because the number of spines per neuron varies, we used cluster analysis to generate objectively groups (clusters) of neurons with a different spine load. This analysis showed that in addition to deficits in the total number of spines, dendrites of BACE1−/− mice displayed a smaller proportion of mushroom-shaped spines (F1,399 = 4.34, P < 0.03), particularly in neurons with medium to high spine loads (Fig. 4E). In contrast to changes in spine density and morphology, dendritic arborization (the number of dendrites, dendritic nodes, and termination points) were similar in BACE1−/− mice compared with those of WT mice (data not shown). Thus, BACE1-related defects in fine structure of dendrites include specific loss of spines, particularly its most mature mushroom-shaped type, with dendritic arborization being relatively spared.
Fig. 4.
Fine structure of CA1 dendrites in the hippocampus of BACE1−/− mice. (A and B) Representative images of dendrites from CA1 pyramidal neurons of WT (A) and BACE1−/− mice (B). (C) Spine density (per millimole) for WT and BACE1−/− neurons (n = 241 and 182, respectively). (D) Total spine number per dendrite (n = 909 and 658 dendrites for WT and BACE1−/−, respectively). (E) Proportions of mushroom-shaped spines (from a total pool of 10,637 and 4,648 spines for WT and BACE1−/−, respectively) were calculated for each neuron and are shown as function of neurons with different spine load. Asterisks indicate significant differences between WT and BACE1−/− measures (P < 0.05) as a result of ANOVA (C and D) or post hoc tests applied to a significant effect of ANOVA (E).
Discussion
Recent advances in genetics of schizophrenia allowed for development of a number of animal models that test functional significance of susceptibility genes (for review, see ref. 27). Coupled with previous pharmacological models (for review, see ref. 28), these new efforts have begun to clarify our understanding of underlying pathophysiological mechanisms of this complex disease. Because schizophrenia is a heterogeneous disorder, no single animal model can recapitulate the full spectrum of symptoms, but rather each new model may represent a subpopulation of schizophrenia or a particular aspect of pathophysiology (27, 28). Our comprehensive behavioral analyses indicate that BACE1−/− animals exhibit a variety of abnormalities reminiscent of those identified in schizophrenia, including prepulse inhibition impairments, novelty-induced hyperactivity, supersensitivity to the psychostimulant, alterations in social recognition, and cognitive deficits. Along with schizophrenia-relevant behaviors, BACE1−/− mice show altered processing of NRG1 that results in significant reduction of the PSD95-associated pool of the ErbB4 receptor. Synaptically enriched ErBb4 has been shown to have multiple roles in functional and structural glutamatergic and GABAergic synaptic plasticity, and both of these neuromediator systems have been implicated in the etiology of schizophrenia (16). Consistent with the role of synaptic ErbB4 in maintenance and maturation of excitatory spines in hippocampus (13), BACE1−/− mice exhibit significant decreases in both spine density and in the number of mature spines in the CA1 field of the hippocampus. Our findings identify BACE1−/− mice as a rodent model that exhibits schizophrenia-like behavioral abnormalities and suggest that genetic or epigenetic alteration of BACE1 may participate in the development of some schizophrenic symptoms in individuals with this complex psychiatric disorder.
Although it is important to recognize that BACE1 has numerous substrates whose biology could participate in the phenotypes observed in BACE1−/− mice, current evidence supports the view that altered BACE1-dependent NRG1 signaling is one likely mechanism underlying the schizophrenic-like behavioral traits. Given that BACE1 impacts on proteolytic processing of NRG1 (4, 5), behavioral phenotypes of the BACE1−/− mice are consistent with similar traits observed in NRG1+/− mice, including impaired prepulse inhibition, spontaneous hyperactivity, reversal by clozapine of such hyperactivity (8), and deficits in response to social novelty (29). In addition, ErbB4+/−, but not ErbB2 or ErbB3 mutant mice, exhibit some schizophrenia-like traits (8) although to a much lesser extent than in NRG1+/− mice (7, 8, 12). Comparison of phenotypes between NRG1+/− and ErbB4+/− mice indicates that in mice with altered NRG1/ErbB4 signaling the NRG1 ligand is a major determinant for the expression of features relevant to schizophrenia (8). BACE1, by virtue of its role in NRG1 proteolysis, has a high potential for modifying the levels of ligands in the NRG1/ErbB4 pathway and inhibition of BACE1 activity, as seen in BACE1−/− mice, and is sufficient to result in schizophrenia-like phenotypes.
The recognition site for BACE1 has been shown to reside in the stalk region of NRG1-β1 isoforms (5), present in NRG1 types I and III (7). That BACE1 influences myelination of central and peripheral axons (4, 5), a process that depends on NRG1 type III (6), raises the questions as to whether alterations in myelination of central axons play a role in the schizophrenia-like phenotypes in BACE1−/− mice and whether these effects are developmentally regulated or persist throughout adulthood. Conditional deletion of BACE1 will be instructive in clarifying this issue. A possible role of hypomyelination in BACE1-related schizophrenic-like phenotypes would be consistent with the concept that myelin-related dysfunction may be one of the pathologic mechanisms underlying alterations in neuronal connectivity thought to occur in schizophrenia (30, 31). Apart from its role in proteolysis of NRG1 type III, BACE1 may process other NRG1 isoforms, particularly NRG1 type I, which has been linked to schizophrenia (8, 10). Recently, a brain-specific NRG1 isoform, NRG1 type IV, has been identified and linked to schizophrenia (9). NRG1 type IV possesses an Ig-like domain similar to that of NRG1 type I and a β-stalk and transmembrane domains similar to those of NRG1 types I and III (32). The presence of a β-stalk domain in adult and some of the fetal isoforms of NRG1 type IV (32) suggests that it too could be a substrate for BACE1 (5).
In addition to the genetic linkage of NRG1 to schizophrenia (8, 10), ErbB4 has also been linked to this psychiatric disorder (33), indicating that the NRG1/ErbB4 pathway may be involved in the pathophysiology of schizophrenia through multiple mechanisms (10). In schizophrenic patients, the levels of ErbB4 receptors appear to be unaltered; however, the binding of ErbB4 to PSD95 was significantly increased, findings that are interpreted to suggest that enhanced NRG1 signaling contributes to NMDA hypofunction in schizophrenia (14). In contrast, studies in animal models and cultured cells indicated a deficit in NRG1/ErbB4 signaling as a cause of glutamatergic hypofunction (13, 34). Our finding of decreased ErbB4–PSD95 interaction in BACE1−/− mice is consistent with results from studies of NRG1 deficiency in animal models (13, 34) but not with those from postmortem human tissues (14). As noted by Fischbach (35), the differences in outcomes may depend on the duration of modification of NRG1 signaling (acute vs. chronic) and emphasize the importance of understanding the functional links between NRG1 and glutamatergic pathways.
Changes in NRG1/ErbB4 signaling have been shown to affect a number of key mechanisms modulating the activity of glutamatergic and GABAergic functions, including phosphorylation of the NR2B (36) and NR2A (14) subunits of the NMDA receptor, stabilization of the α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptor, maturation of excitatory spines (13), and regulation of GABAergic transmission (15). Importantly, activity-dependent ErbB4–PSD95 interactions have emerged as critical factors modulating the effects of NRG1 and NMDA and AMPA receptors (13, 34). The significant down-regulation of the ErbB4–PSD95 interactions occurring in BACE1−/− mice suggests that similar mechanisms could be at play in this model. Behavioral hypersensitivity to an NMDA receptor antagonist and the decrease in spine density and maturation found in BACE1−/− mice support the glutamatergic hypofunction mechanism in this mouse model. It is plausible that the changes in structural plasticity correspond to functional abnormalities, i.e., the deficit in reversal of the long-term depression that was observed in the same area of the hippocampus (2). It is interesting to note that defects in the fine structure of dendrites, involving loss of spines, also occur in pyramidal neurons of individuals with schizophrenia (37). Nevertheless, BACE1 knockout mice represent an excellent model to clarify further the relationship between alterations in NRG1/ErbB4 signaling and changes in glutamatergic and GABAergic functions implicated in pathophysiology of schizophrenia (16).
In conclusion, our findings offer the possibility that polymorphism in BACE1 could be responsible for an increased risk of schizophrenia in subsets of patients. As an animal model, BACE1−/− mice will be important for the identification of BACE1-related molecular pathways and neural circuits that are involved in endophenotypes resembling features of schizophrenia and offering opportunities for testing therapeutic strategies for this psychiatric illness.
Materials and Methods
Animals.
BACE1−/− mice were generated as described in ref. 38. Male mice from 4 to 8 months of age were used in all procedures that were under the guidelines of The Johns Hopkins University Institutional Animal Care and Use Committee.
Behavioral Testing.
Behaviors in the plus maze, Y maze, and social recognition task were videotaped and scored by trained observers blind to genotype by using a computer-assisted data acquisition system (Stopwatch+; www.cbn-atl.org). In the radial water maze, open-field, and corner tasks, performance was recorded by a computer-based video tracking system (HVS Image). PPI tests were conducted in a startle chamber, and inhibitory avoidance was tested in a freeze monitor (San Diego Instruments). All tests have been described in detail elsewhere (2) and SI Materials and Methods.
Sensitivity to MK-801.
Each mouse was placed in a standard mouse cage, and after 20 min of habituation, locomotor activity was recorded by a computer-based video tracking system (HVS Image) in 3-min samples at −5, 15, 45, 75, and 105 min after the i.p. injection (PBS or MK-801).
Treatment with Clozapine.
The effect of clozapine on motor activity, working memory, and PPI was analyzed by using Y maze and PPI tests 20 and 30 min after the injection (PBS or clozapine), respectively.
Western Blotting and Immunoprecipitation.
Western blotting and immunoprecipitation were performed as described (2, 38). Cortex and hippocampus were dissected for Western blot analysis. The following antibodies were used: ErbB4, sc-283 (1:500; Santa Cruz Biotechnology); NRG1, sc-348 (1:1,000; Santa Cruz Biotechnology); NRG1, sc-28916 (1:1,500; Santa Cruz Biotechnology); PSD95 (1:1,000; Sigma); MBP (SMI-99) (1:2,000; Covance); SOD1 (1:4,000; Abcam). For immunoprecipitation, samples were incubated with the anti-ErbB4 antibody (2 μg of antibody per 500 μg of total protein).
Spine Morphology.
Brains from WT and BACE1−/− mice (n = 2 per genotype) were processed for Golgi staining (FD Rapid GolgiStain kit; FD Neurotechnologies). Pyramidal cells from coronal sections (100 μm; three per animal) in the CA1 region were traced, and spines were counted at ×100 by using Neurolucida software (MicroBrightField). Dendritic complexity, length, cell body area, and spine density were calculated for a total pool of ≈420 neurons and ≈15,000 spines.
Statistical Analyses.
The data were analyzed by using ANOVA with the statistical package STATISTICA 6.0 (StatSoft) and a minimal level of significance (P < 0.05). Newman–Keuls post hoc tests were applied to significant main effects or interactions. For the analysis of differences in spine proportions, neurons were divided into groups (clusters) by K-means cluster analysis of the number of spines per neuron (see SI Materials and Methods).
Acknowledgments.
We thank S. Snyder and A. Sawa for helpful discussions, M. Plentnikov for access to Behavior Care facilities, V. Nehus and D. Prasad for technical support; G. Rudow and J. Troncoso for advice on morphometry methods; J. Kim, S.-W. Kang, H. Kim, I. Kim, M. Boccitto, E. Mok, O. Kim, X. Bi, and B. Ülgen for assistance with behavioral testing; and H.-S. Kim for text editing. This work was supported by National Institute of Neurological Disorders and Stroke, National Institutes of Health Grants R01 NS41438 and P01 NS047308 (to P.C.W.), National Institute on Aging, National Institutes of Health Grant P50 AG05146 (to D.L.P.), and by the Alzheimer's Association (to A.V.S.), the Adler Foundation (to A.V.S.), the Ilanna Starr Scholar Fund (to A.V.S.), and the Bristol–Myers Squibb Foundation (to D.L.P.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0710373105/DCSupplemental.
References
- 1.Citron M. Strategies for disease modification in Alzheimer's disease. Nat Rev Neurosci. 2004;5:677–685. doi: 10.1038/nrn1495. [DOI] [PubMed] [Google Scholar]
- 2.Laird FM, et al. BACE1, a major determinant of selective vulnerability of the brain to Aβ amyloidogenesis, is essential for cognitive, emotional, and synaptic functions. J Neurosci. 2005;25:11693–11709. doi: 10.1523/JNEUROSCI.2766-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ohno M, et al. BACE1 deficiency rescues memory deficits and cholinergic dysfunction in a mouse model of Alzheimer's disease. Neuron. 2004;41:27–33. doi: 10.1016/s0896-6273(03)00810-9. [DOI] [PubMed] [Google Scholar]
- 4.Hu X, et al. Bace1 modulates myelination in the central and peripheral nervous system. Nat Neurosci. 2006;9:1520–1525. doi: 10.1038/nn1797. [DOI] [PubMed] [Google Scholar]
- 5.Willem M, et al. Control of peripheral nerve myelination by the β-secretase BACE1. Science. 2006;314:664–666. doi: 10.1126/science.1132341. [DOI] [PubMed] [Google Scholar]
- 6.Michailov GV, et al. Axonal neuregulin-1 regulates myelin sheath thickness. Science. 2004;304:700–703. doi: 10.1126/science.1095862. [DOI] [PubMed] [Google Scholar]
- 7.Falls DL. Neuregulins: Functions, forms, and signaling strategies. Exp Cell Res. 2003;284:14–30. doi: 10.1016/s0014-4827(02)00102-7. [DOI] [PubMed] [Google Scholar]
- 8.Stefansson H, et al. Neuregulin 1 and susceptibility to schizophrenia. Am J Hum Genet. 2002;71:877–892. doi: 10.1086/342734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Law AJ, et al. Neuregulin 1 transcripts are differentially expressed in schizophrenia and regulated by 5′ SNPs associated with the disease. Proc Natl Acad Sci USA. 2006;103:6747–6752. doi: 10.1073/pnas.0602002103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Harrison PJ, Law AJ. Neuregulin 1 and schizophrenia: Genetics, gene expression, and neurobiology. Biol Psychiatry. 2006;60:132–140. doi: 10.1016/j.biopsych.2005.11.002. [DOI] [PubMed] [Google Scholar]
- 11.Hall J, et al. A neuregulin 1 variant associated with abnormal cortical function and psychotic symptoms. Nat Neurosci. 2006;9:1477–1478. doi: 10.1038/nn1795. [DOI] [PubMed] [Google Scholar]
- 12.Gerlai R, Pisacane P, Erickson S. Heregulin, but not ErbB2 or ErbB3, heterozygous mutant mice exhibit hyperactivity in multiple behavioral tasks. Behav Brain Res. 2000;109:219–227. doi: 10.1016/s0166-4328(99)00175-8. [DOI] [PubMed] [Google Scholar]
- 13.Li B, Woo RS, Mei L, Malinow R. The neuregulin-1 receptor ErbB4 controls glutamatergic synapse maturation and plasticity. Neuron. 2007;54:583–597. doi: 10.1016/j.neuron.2007.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hahn CG, et al. Altered neuregulin 1-ErbB4 signaling contributes to NMDA receptor hypofunction in schizophrenia. Nat Med. 2006;12:824–828. doi: 10.1038/nm1418. [DOI] [PubMed] [Google Scholar]
- 15.Woo RS, et al. Neuregulin-1 enhances depolarization-induced GABA release. Neuron. 2007;54:599–610. doi: 10.1016/j.neuron.2007.04.009. [DOI] [PubMed] [Google Scholar]
- 16.Coyle JT. Glutamate and schizophrenia: Beyond the dopamine hypothesis. Cell Mol Neurobiol. 2006;26:365–384. doi: 10.1007/s10571-006-9062-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Arguello PA, Gogos JA. Modeling madness in mice: One piece at a time. Neuron. 2006;52:179–196. doi: 10.1016/j.neuron.2006.09.023. [DOI] [PubMed] [Google Scholar]
- 18.Leumann L, Feldon J, Vollenweider FX, Ludewig K. Effects of typical and atypical antipsychotics on prepulse inhibition and latent inhibition in chronic schizophrenia. Biol Psychiatry. 2002;52:729–739. doi: 10.1016/s0006-3223(02)01344-6. [DOI] [PubMed] [Google Scholar]
- 19.Chen S, et al. Neuregulin 1–ErbB signaling is necessary for normal myelination and sensory function. J Neurosci. 2006;26:3079–3086. doi: 10.1523/JNEUROSCI.3785-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ferguson JN, Young LJ, Insel TR. The neuroendocrine basis of social recognition. Front Neuroendocrinol. 2002;23:200–224. doi: 10.1006/frne.2002.0229. [DOI] [PubMed] [Google Scholar]
- 21.Malhotra AK, et al. Clozapine blunts N-methyl-d-aspartate antagonist-induced psychosis: A study with ketamine. Biol Psychiatry. 1997;42:664–668. doi: 10.1016/s0006-3223(96)00546-x. [DOI] [PubMed] [Google Scholar]
- 22.Goldberg TE, et al. The effect of clozapine on cognition and psychiatric symptoms in patients with schizophrenia. Br J Psychiatry. 1993;162:43–48. doi: 10.1192/bjp.162.1.43. [DOI] [PubMed] [Google Scholar]
- 23.Buonanno A, Fischbach GD. Neuregulin and ErbB receptor signaling pathways in the nervous system. Curr Opin Neurobiol. 2001;11:287–296. doi: 10.1016/s0959-4388(00)00210-5. [DOI] [PubMed] [Google Scholar]
- 24.Gerecke KM, Wyss JM, Karavanova I, Buonanno A, Carroll SL. ErbB transmembrane tyrosine kinase receptors are differentially expressed throughout the adult rat central nervous system. J Comp Neurol. 2001;433:86–100. doi: 10.1002/cne.1127. [DOI] [PubMed] [Google Scholar]
- 25.Losonczy A, Magee JC. Integrative properties of radial oblique dendrites in hippocampal CA1 pyramidal neurons. Neuron. 2006;50:291–307. doi: 10.1016/j.neuron.2006.03.016. [DOI] [PubMed] [Google Scholar]
- 26.De Simoni A, Edwards FA. Pathway specificity of dendritic spine morphology in identified synapses onto rat hippocampal CA1 neurons in organotypic slices. Hippocampus. 2006;16:1111–1124. doi: 10.1002/hipo.20236. [DOI] [PubMed] [Google Scholar]
- 27.Ross CA, Margolis RL, Reading SA, Pletnikov M, Coyle JT. Neurobiology of schizophrenia. Neuron. 2006;52:139–153. doi: 10.1016/j.neuron.2006.09.015. [DOI] [PubMed] [Google Scholar]
- 28.Chen J, Lipska BK, Weinberger DR. Genetic mouse models of schizophrenia: From hypothesis-based to susceptibility gene-based models. Biol Psychiatry. 2006;59:1180–1188. doi: 10.1016/j.biopsych.2006.02.024. [DOI] [PubMed] [Google Scholar]
- 29.O'Tuathaigh CM, et al. Phenotypic characterization of spatial cognition and social behavior in mice with “knockout” of the schizophrenia risk gene neuregulin 1. Neuroscience. 2007;147:18–27. doi: 10.1016/j.neuroscience.2007.03.051. [DOI] [PubMed] [Google Scholar]
- 30.Corfas G, Roy K, Buxbaum JD. Neuregulin 1–ErbB signaling and the molecular/cellular basis of schizophrenia. Nat Neurosci. 2004;7:575–580. doi: 10.1038/nn1258. [DOI] [PubMed] [Google Scholar]
- 31.Uranova NA, Vostrikov VM, Orlovskaya DD, Rachmanova VI. Oligodendroglial density in the prefrontal cortex in schizophrenia and mood disorders: A study from the Stanley Neuropathology Consortium. Schizophr Res. 2004;67:269–275. doi: 10.1016/S0920-9964(03)00181-6. [DOI] [PubMed] [Google Scholar]
- 32.Tan W, et al. Molecular cloning of a brain-specific, developmentally regulated neuregulin 1 (NRG1) isoform and identification of a functional promoter variant associated with schizophrenia. J Biol Chem. 2007;282:24343–24351. doi: 10.1074/jbc.M702953200. [DOI] [PubMed] [Google Scholar]
- 33.Law AJ, Kleinman JE, Weinberger DR, Weickert CS. Disease-associated intronic variants in the ErbB4 gene are related to altered ErbB4 splice-variant expression in the brain in schizophrenia. Hum Mol Genet. 2007;16:129–141. doi: 10.1093/hmg/ddl449. [DOI] [PubMed] [Google Scholar]
- 34.Huang YZ, et al. Regulation of neuregulin signaling by PSD95 interacting with ErbB4 at CNS synapses. Neuron. 2000;26:443–455. doi: 10.1016/s0896-6273(00)81176-9. [DOI] [PubMed] [Google Scholar]
- 35.Fischbach GD. NRG1 and synaptic function in the CNS. Neuron. 2007;54:495–497. doi: 10.1016/j.neuron.2007.05.009. [DOI] [PubMed] [Google Scholar]
- 36.Bjarnadottir M, et al. Neuregulin1 (NRG1) signaling through Fyn modulates NMDA receptor phosphorylation: Differential synaptic function in NRG1+/− knockouts compared with wild-type mice. J Neurosci. 2007;27:4519–4529. doi: 10.1523/JNEUROSCI.4314-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kolomeets NS, Orlovskaya DD, Rachmanova VI, Uranova NA. Ultrastructural alterations in hippocampal mossy fiber synapses in schizophrenia: A postmortem morphometric study. Synapse. 2005;57:47–55. doi: 10.1002/syn.20153. [DOI] [PubMed] [Google Scholar]
- 38.Cai H, et al. BACE1 is the major β-secretase for generation of Aβ peptides by neurons. Nat Neurosci. 2001;4:233–234. doi: 10.1038/85064. [DOI] [PubMed] [Google Scholar]
- 39.Maatta JA, et al. Proteolytic cleavage and phosphorylation of a tumor-associated ErbB4 isoform promote ligand-independent survival and cancer cell growth. Mol Biol Cell. 2006;17:67–79. doi: 10.1091/mbc.E05-05-0402. [DOI] [PMC free article] [PubMed] [Google Scholar]