Abstract
Ligation between glucocorticoid-induced tumor necrosis factor receptor (GITR) and its ligand (GITRL) provides an undefined signal that renders CD4+CD25− effector T cells resistant to the inhibitory effects of CD4+CD25+ regulatory T cells. To understand the structural basis of GITRL function, we have expressed and purified the extracellular domain of human GITR ligand in Escherichia coli. Chromotography and cross-linking studies indicate that human GITRL (hGITRL) exists as dimers and trimers in solution and also can form a supercluster. To gain insight into the nature of GITRL oligomerization, we determined the crystallographic structures of hGITRL, which revealed a loosely associated open trimer with a deep cavity at the molecular center and a flexible C-terminal tail bent for trimerization. Moreover, a tetramer of trimers (i.e., supercluster) has also been observed in the crystal, consistent with the cross-linking analysis. Deletion of the C-terminal distal three residues disrupts the loosely assembled trimer and favors the formation of a dimer that has compromised receptor binding and signaling activity. Collectively, our studies identify multiple oligomeric species of hGITRL that possess distinct kinetics of ERK activation. The studies address the functional implications and structural models for a process by which hGITRL utilizes multiple oligomerization states to regulate GITR-mediated signaling during T cell costimulation.
Keywords: dimer, equilibrium, supercluster, crystal structure, mechanism
Members of the tumor necrosis factor superfamily (TNFSF) and their cognate receptors (TNFRs) mediate important roles in diverse cellular events involved in the immune system. Glucocorticoid-induced tumor necrosis factor receptor (GITR) belongs to the TNFR family (member 18) and is constitutively expressed on CD4+CD25+ regulatory T cells and certain activated T cells (1–5). GITR is involved in controlling T cell-mediated responses including organ-specific autoimmunity, chronic infection, and antitumor immunity (6, 7).
The natural ligand of GITR (GITRL) belongs to TNF family and is a type II transmembrane protein. GITRL is expressed on endothelial cells, dendritic cells, macrophages, and B cells but not in T cells (8–10), and this distribution supports its functional role in the process of T cell activation. GITRL expression is transiently up-regulated and then down-regulated by triggering of T cell receptor (11). A recent study revealed that GITRL influences immune regulation of tryptophan catabolism through reverse signaling (12).
GITRL–GITR interactions are involved in the interplay among regulatory T cells, effector T cells and antigen-presenting cells (13). GITR stimulation by GITRL or agonist anti-GITR antibodies reverses regulatory T cell suppression, leading to enhanced immunity to tumors and viral pathogens, and to exacerbated autoimmune diseases (3, 4, 6, 7, 14–17). For example, expression of GITRL on tumor cells delays tumor growth and increases T cell infiltration (18). In other models, treatment with GITR agonistic antibody may break self-tolerance and generate therapeutic immunity (19). These studies indicate that GITRL–GITR interactions represent a pharmacological target for the treatment of certain diseases or conditions.
Among TNF/TNFR superfamily members that are involved in cell regulation, a group of costimulatory ligands/receptors including GITRL/GITR, CD40L/CD40, and OX40L/OX40 mediate T cell survival and activation. Soluble forms of GITRL have been detected and suggested to represent regulatory molecules (12, 20). A recent study of HIV vaccine adjuvants revealed that plasmids expressing multimeric soluble CD40L or GITRL induced stronger T cell responses than trimer-only species (21). To better understand the signaling mechanism of these costimulatory molecules, we and others have recently determined the structural basis by which mouse GITRL (mGITRL) exists as a dimeric TNF species (22, 23). Here, we show that human GITRL (hGITRL) exists in an equilibrium of dimeric and trimeric forms and may also organize into higher-order superclusters that are functional.
Results
Recombinant Soluble hGITRL Exists As an Equilibrium of Dimer and Trimer in Solution.
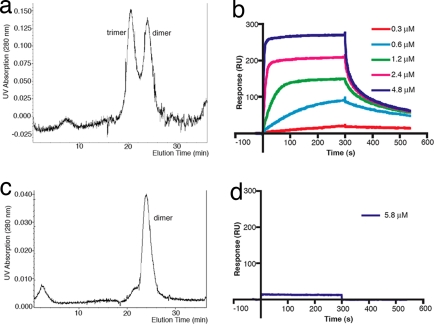
We expressed the extracellular domain (residues 50–177) of hGITRL and purified the soluble protein, which was visualized as a single band on SDS/PAGE. Gel filtration experiments using the purified protein indicate that hGITRL exists as a mixture of dimers and trimers in solution (Fig. 1a). When the protein isolated from either the dimer or the trimer peak was subjected to another round of chromatography, both the dimer and trimer peaks reappeared in the same ratio, indicating a dynamic equilibrium between these oligomeric forms. It appears that the dimer is more stable than the trimer because the dimer species could be observed on SDS/PAGE and Western blot by using fresh samples without boiling, whereas no discernible amounts of trimer species could be detected under the condition of these methods [supporting information (SI) Fig. S1]. Interestingly, we did not observe significant amounts of monomeric species existing in solution by gel filtration (Fig. 1a) using hGITRL samples that were either treated or not treated with cross-linking reagent (see below and Discussion).
Fig. 1.
Biophysical characterization of wild-type and C-terminal deletion mutant hGITRL. (a) Wild-type hGITRL was eluted from Superdex 75 column as two peaks, with calculated molecular masses of ≈27 and ≈43 kDa, corresponding to dimer and trimer, respectively, whereas mutant hGITRL appeared as a single peak of ≈27 kDa, corresponding to a dimer (b). Surface plasmon resonance analysis shows that wild-type hGITRL has modest receptor binding activity (c), whereas mutant hGITRL does not have significant receptor binding at the concentration tested (d).
Surface plasmon resonance (SPR) analysis confirmed the receptor binding ability of the recombinant hGITRL (Fig. 1c). Different shapes of the sensorgram curves as a function of the ligand concentration are indicative of changes in the binding kinetics due to the heterogeneity of the sample. Although low concentration curves fit to a simple 1:1 Langmuirian model with the dissociation constant values between 0.4 and 0.7 μM, the binding becomes more complex at higher concentrations at which it can no longer be described as a one-step interaction. Apparently, self-association of hGITRL leading to the formation of rapidly dissociating complexes occurs at higher ligand concentrations and contributes to the receptor–ligand interaction signal. The receptor binding affinity observed for hGITRL is lower than typical affinities observed for receptor–ligand interactions in other members of TNFR superfamily.
hGITRL Forms a Loosely Assembled Open Trimer in Crystals.
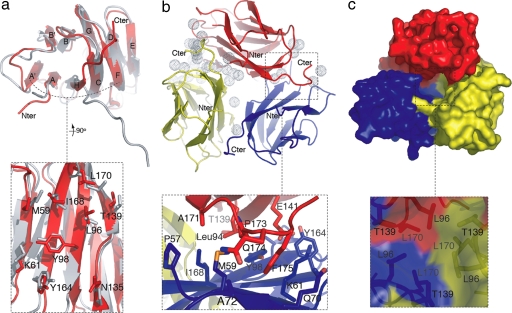
Despite the failure of mouse GITRL to interact with human GITR (hGITR) (data not shown), we have noted that human and mouse GITRL orthologs share 55% sequence homology. As expected, their overall monomer structures are very similar with an rmsd of 1.7 Å when we superimposed all of the Cα atoms except for those in the C terminus (Fig. 2a). For instance, the β-strand F exists similarly as two discontinuous fragments in both structures. However, in contrast to the mGITRL dimer, hGITRL associates as an open trimer with either pseudo or crystallographic threefold symmetry in two crystal forms (with space group C2 and P63) that we obtained (Fig. 2b).
Fig. 2.
Crystal structures of hGITRL. Residues of particular interest were labeled. (a) Comparison of human and mouse GITRL monomer structures shows that they share a conserved A′AHCF β-sheet but have different C-terminal orientations. The zoom image shows the molecular surface of the A′AHCF β-sheet. (b) The asymmetric assembly of hGITRL open trimer. The dotted spheres represent solvent molecules that are unequally distributed on the trimeric interface. The zoom image shows that the hook-shaped C terminus is involved in the trimeric packing. (c) The cavity in the trimeric center of hGITRL. The zoom image shows the weakened hydrophobic packing around the threefold.
There are three distinct copies of hGITRL trimers in the two crystal forms, namely, one symmetric trimer and two quasisymmetric trimers. The overall assemblies of all three hGITRL trimers are similar. Compared with the intimately intertwined hydrophobic tiling around the threefold axis characteristically observed for TNFSF members (24–26), the trimeric core packing of hGITRL is significantly weakened by the reduced number and size of hydrophobic residues involved in the interactions (L42, L118, and T87) (Fig. 2c). A total of ≈3,500 Å2 accessible molecular surface was buried, which is considerably less than the average of 12,000 Å2 for TNFSF trimers. As a result, a cavity with a large opening is formed at the hGITRL trimer interface, which has not been observed for other TNFSF members. The cavity is large enough to accommodate compounds that may help to stabilize or destabilize the hGITRL trimer.
Molecular profile analysis of the asymmetric trimer revealed that only one of the three protomer–protomer interfaces forms a regular crevice that is considered to represent the receptor-binding site. The other two interfaces form grooves with varied depths. These grooves break down when the two neighboring protomers progressively lose contact and separate with each other at the ends of the molecules. Accordingly, unequal amounts of molecular surface are buried for each protomer, 1,314, 1,227, and 1,033 Å2, respectively. Distinct numbers of solvent molecules are distributed on the three protomer–protomer interfaces (Fig. 2b). In addition, one of the three protomers in hGITRL asymmetric trimer displays relatively higher B-factors.
hGITRL C Terminus Is Flexible yet Critical for Trimer Formation and Signaling.
In contrast to the high overall structural similarity between human and mouse GITRLs, the orientations of their C termini are different. The C terminus of mGITRL is highly extended as a protruding arm to join the β-sheet A′AHCF of the other protomer, leading to an intermolecular β-sheet stabilized dimer (22, 23). However, in hGITRL, the C terminus (L118–S125) is bent into a hook-shaped loop, traversing the surface of the β-sheet A′AHCF of the next protomer (Fig. 2b and Fig. S2). Notably, the apex (residues P121–I124) of the C terminus hook slightly knocks into the surface of the β-sheet through packing the side chains of P121, F123, and I124 against the β-strand A and A′ from that β-sheet.
Superimposition of all hGITRL trimers, as well as B-factor analysis indicated that the C terminus is flexible although it is involved in the trimeric interactions as described. In fact, most of the C termini observed in P63 space group were partially disordered, also reflecting its flexible nature. However, removal of the C termini from the hGITRL trimer decreased the total buried molecular surface by 34% (from 3,500 to 2,300 Å2). These observations indicate that hGITRL C termini play an important role in stabilizing the trimer, although at the same time the flexibility of this region may also introduce conformational changes affecting the oligomerization.
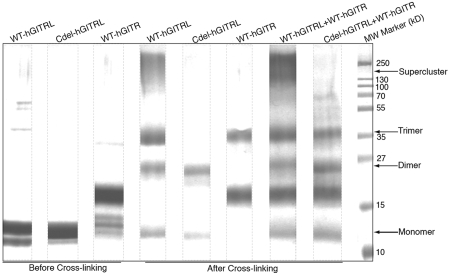
In keeping with the structural analysis, gel filtration and cross-linking studies revealed that deletion of the C terminus distal three residues (Phe-Ile-Ser) disrupts the open trimer, rendering hGITRL exclusively as a dimer in solution (Figs. 1b and 3). We reasoned that the oligomerization change that resulted from the C terminus removal might also lead to modified receptor-binding and signaling behavior. Indeed, SPR analysis showed that the receptor binding activity of the C-terminal deletion mutant hGITRL is substantially lower than that of the wild-type ligand (Fig. 1d). Moreover, cross-linking did not capture a detectable amount of putative complex formed between the mutant ligand and the wild-type receptor, confirming the SPR results.
Fig. 3.
Cross-linking analysis of wild-type and mutant hGITRL. After cross-linking using BS3, equal amounts of samples were loaded for SDS/PAGE analysis. Sample names were labeled on the upper side. Note that wild-type hGITRL can form high-molecular-weight superclusters binding to hGITR, whereas mutant hGITRL forms can only form dimer and shows weak receptor binding. Different oligomeric states of hGITRL were labeled. WT-hGITRL, wild-type hGITRL; Cdel-hGITRL, C-terminal deletion mutant hGITRL; WT-hGITR, wild-type hGITR.
hGITRL May Form Higher-Order Clusters in Solution and in Crystals.
hGITRL was detected as a mixture of dimers and trimers by gel filtration, and was reduced to monomers under the condition of SDS/PAGE. However, cross-linking analysis revealed that hGITRL may also form clusters of higher molecular weight (Fig. 3). These clusters seem to exist transiently in solution and covalent bonding is required to stabilize them.
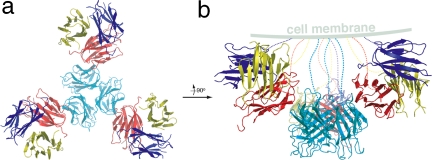
Interestingly, in the crystal lattice of the P63 space group, 12 hGITRL monomers associate together, forming a tetramer of trimers (Fig. 4). The overall organization of the hGITRL tetramer of trimers appears similar to that observed for TALL-1, which is also capable of forming functional superclusters (27). Interactions between the neighboring trimers are mediated by residues clustering at the molecular surface of A′B′, BC, and FG loops, and B′, B, and G β-strands. Specifically, residues P76, P77, N80, V82, E88, L90, Q91, and V144 from both interacting subunits are involved in the crystal defined tetramer of hGITRL trimers. It is worth noting that all 12 subunits in hGITRL tetramer of trimers are orientated toward the same putative membrane (Fig. 4b). As such, the N termini of all subunits in the cluster may be anchored to the surface of a single cell. The spatial organization of the ligand molecules also allows multiple receptor molecules to simultaneously approach all of the binding sites in the cluster without steric hindrance.
Fig. 4.
Tetramer of trimers formed in hGITRL crystals. (a) The cluster viewed along the threefold axis. (b) Image obtained by rotating a 90° along the y axis. Notably, the organization of the cluster allows all of the subunits to be anchored on a same putative membrane.
Oligomerization Status of hGITRL Affects Its Biological Activity.
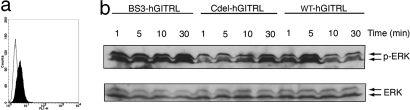
The MAP kinase pathway is activated by a wide variety of external signals leading to cell proliferation or differentiation (28). GITRL–GITR signaling activates MAPKs and NF-κB (29–31). We have confirmed that human macrophage cell line THP-1 expresses GITR (Fig. 5a). To compare the biological activity of different forms of hGITRL, we treated THP-1 cells with purified proteins of wild-type hGITRL, C-terminal deletion mutant hGITRL and cross-linked wild-type hGITRL, and examined the phosphorylation states of ERK (Fig. 5b).
Fig. 5.
hGITRL stimulation leads to activation of ERK. (a) Flow cytometry analysis of human THP-1 cells to confirm GITR expression. (b) Western blot analysis of ERK phosphorylation. BS-hGITRL, Cdel-hGITRL, and wt-hGITRL represent BS3-treated wild-type hGITRL, mutant hGITRL, and wild-type hGITRL, respectively. Phosphorylated forms and total ERK are indicated with arrows.
Our data showed that wild-type hGITRL (dimer plus trimer plus transient supercluster) induced significant ERK phosphorylation within 5 min, whereas the phosphorylation level was attenuated afterward. The mutant hGITRL (dimer) failed to induce strong ERK phosphorylation initially at 5 min although an increased phospho-ERK level was observed at a later time point (30 min). The cross-linked hGITRL (dimer plus trimer plus stabilized supercluster) leads to a constant and even stronger ERK activation than does wild-type hGITRL alone. These observations support the SPR analysis and cross-linking studies showing that mutation forced dimerization of hGITRL substantially reduced the receptor-binding activity, whereas stabilized superclusters have better receptor binding.
Discussion
hGITRL Trimer Has High Plasticity and May Convert into a Dimer by Losing One Protomer.
Our data indicate the existence of dimeric forms of hGITRL. However, it is unclear how this species is generated and what its role would be in T cell function. We found that the dimeric form may originate from the unusual plasticity of the hGITRL open trimer. The strongest indicator of the trimer's flexibility is its capacity to convert from a symmetric assembly to an asymmetric one in which the three protomers are unequally packed against one another.
As the concentration of hGITRL decreases in solution, the trimer can convert into a dimer by losing one of its three protomers, a process that is likely facilitated by structural aspects of the C terminus. In fact, multimeric assembly analysis of the asymmetric trimer indicates that the subcomplex formed by only two protomers stably exists in solution. This is consistent with our solution studies showing that the oligomeric species of hGITRL include significant amounts of dimer. However, we did not observe significant amount of monomers in solution under the condition of gel filtration experiment, suggesting the interconversion between hGITRL trimer and dimer is a rather quick process.
Furthermore, the molecular surface of the β-sheet A′AHCF used for oligomerization is highly conserved between human and mouse GITRLs (Fig. 2a). This feature indicates that hGITRL may form a mGITRL-like dimer, which is presumably more stable than the monomer in solution because the entire hydrophobic surface of the β-sheet A′AHCF would have to be exposed to solvent in monomer species (Fig. S3). Finally, despite a conserved overall structure, the C-terminal orientations are different in human and mouse GITRLs, reflecting a dominant role of the C terminus conformation as a determinant of distinct oligomerization states.
hGITRL Dimer May Act As a Regulatory Intermediate Form of the Ligand Functional Units.
As mentioned, the recombinant wild-type hGITRL protein is heterogeneous and shows lower receptor-binding activity compared with other TNFSF members. The lower affinity observed for the soluble hGITRL can be explained by less active molecular species that exists in the heterogeneous sample. Considering its specific conformation and geometry, the loosely assembled open trimer may not be very active, and perhaps not sufficient for effective signaling before further clustering. As a reduced intermediate of this open trimer, the hGITRL dimer is likely to represent an even less active species as indicated by mutagenesis. However, the high-order supercluster of hGITRL that we observed may prove to be an active functional unit that is able to form and that is used together with dimer and trimer to regulate the GITR-mediated signaling.
We propose that the hGITRL dimer is a self-inhibitory form involved in negative modulation of downstream signaling. A recent structural study of a small molecule TNF-α inhibitor has revealed it acts by promoting dissociation of a single subunit from the preassembled TNF trimer, thereby rendering the inhibitor-bound TNF dimeric species incapable of signaling through the TNF receptor (32). In the case of hGITRL, the open trimer may naturally lose one subunit and then undergo conformational readjustment to form an inhibitory dimer. The experimental C-terminal deletion as expected promotes all other forms of the GITR ligands to form dimers.
The mutation-forced dimeric species of hGITRL did not form high-order complexes (Fig. 3) and failed to show significant receptor binding activity as judged by SPR (Fig. 1d). Moreover, the cell-based assay indicated that it induces delayed ERK phosphorylation (Fig. 5). Compromised signaling leading to delayed ERK activation has been previously defined. For example, a study of the mechanism of estrogen-induced human thyroid tumor growth has revealed that a specific estrogen antagonist ICI 182780 prevented the rapid temporary ERK activation induced by E2, leading to delayed ERK phosphorylation (33). The altered ERK activation may have important biological consequences because kinetic differences (“slow and sustained” versus “fast and transient”) in ERK signaling determine cell fate as life or death during T cell selection (34, 35).
Functional hGITRL Superclusters Explain Signal Amplification.
Both solution and crystallographic studies revealed that hGITRL may form high-order superclusters. Cross-linking stabilized superclusters corresponding to ligand–receptor complexes in the case of wild-type hGITRL, suggesting that wild-type hGITRL, but not the dimeric mutant hGITRL can form a functional cluster able to bind receptors (Fig. 3). Notably, the cross-linking treated wild-type hGITRL leads to more persistent ERK activation that is stronger than that induced by untreated wild-type hGITRL (Fig. 5), indicating an enhanced signaling activity of the high-order oligomers. These observations support our contention that the supercluster, i.e., tetramer of trimers we observed in crystals, is not an artifact but is physiologically relevant. hGITRL may form such functional clusters under physiological conditions in which the ligand molecules could be locally enriched. It is also conceivable that membrane restriction as well as receptor binding may act to induce and stabilize the clustering.
Superclustering of TNF ligands has been suggested before as a means of signal amplification (27). Ligand superclusters may engage multiple receptor molecules to form a two-dimensional signaling network (36). Increasing biochemical and structural data suggest that the high-order clustering of TNF family ligands could play an essential role in signal transduction initiation for this superfamily (37). For example, the membrane-bound form of Fas ligand (FasL) induces apoptosis whereas the soluble trimeric FasL is not able to unless clustered by cross-linking or engineering to form at least hexamer (38). Similarly, cross-linked or multimeric CD40L is required to efficiently trigger CD40-mediated signaling (21, 39). Other studies suggested that soluble EDA-A1 and EDA-A2 are activated by forming hexamers or high-order clusters (40, 41). It has been argued by Holler et al. (38) that, for TNFSF ligands that have limited or no activity in soluble form, the interactions of two or more trimeric molecules to create hexamers or higher-order clusters, render the soluble species biologically active.
Interestingly, soluble GITR ligand has also been shown to be stronger costimulatory molecules when engineered to form multiple trimers (21). Therefore, clustering of hGITRL may also represent a functional form of the cytokine to activate the trimeric ligands, as suggested for TALL-1, another TNF family member that also forms high-order oligomers (42). As previously argued, this feature reflects a more universal mechanism by which soluble forms of TNF ligands that are inactive gain activity by forming high-order oligomers that are able to trigger efficient signaling at an active threshold (37, 38). Our data with hGITRL oligomerization and MAPK activation support this notion.
The existence of hGITRL supercluster in solution is transient and unstable. This feature suggests its formation may be conditional for certain physiological events. Considering its potent signaling effects, the supercluster species is probably required to only maintain a relatively low concentration, because massive ERK activation induced by hGITRL (Fig. 5) would eventually lead to deregulation of many other cellular events.
Biological Implications of the Multiple hGITRL Oligomeric Species.
In addition to an open trimer that is atypical for TNF family members, we have identified two oligomeric forms of hGITRL, namely, the dimer and supercluster species that potentially resemble mouse GITRL (22) and TALL-1 (27), respectively. Of note, these oligomeric species may result in ERK activation with distinct kinetics, which in turn determines cell fate as life or death. Our cross-linking of full-length hGITRL on the surface of 293 cells (data not shown) revealed similar results as did the recombinant hGITRL in vitro (Fig. 3), suggesting that the bacterial expressed hGITRL was properly folded and that N-glycosylation does not affect its oligomerization significantly.
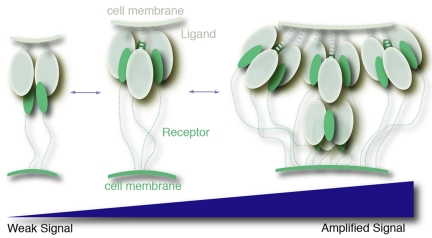
Interactions between GITRL and GITR are involved in T cell immunity in which context-dependent responses are required to differentially control and fine-tune signaling. Perhaps multiple oligomerization levels of the ligand molecules provide a natural means to regulate its activity. In this process, we hypothesize that the dimer and the supercluster represent an inhibitory state and an amplified signaling state, respectively (Fig. 6). Our data and previous studies (21) indicate that soluble GITRL gains activity by forming superclusters. However, simple dimerization of the ligands compromises its activity by preventing clustering. The hGITRL open trimer may represent a major molecular species in vivo. Assisted by the membrane restriction and receptor interactions, this dimer–trimer–supercluster equilibrium of hGITRL may be shifted bidirectionally in response to varied stimulations. Interestingly, it has been reported that soluble trimeric OX40L, whose structure resembles hGITRL open trimer, also shows low receptor binding activity (43). Thus, the multiple oligomerization features of hGITRL may represent a general structural principle used by members of the costimulatory subfamily in which soluble trimeric forms are not sufficiently active for minimal signaling.
Fig. 6.
A hypothetical model for the regulation of hGITRL activity. In this model, hGITRL adopts multiple oligomerization states to progressively control GITR-mediated signaling intensity during T cell costimulation. The hGITRL dimer and the supercluster are shown representing inhibitory and hyperstimulatory species, respectively.
The interconversion between different levels of the oligomerization emerges as a general principle important for the ligands that function to modulate the receptor-mediated T cell responses. Consequently, manipulation of the oligomeric states of the ligands may have therapeutic value in the treatments of diseases caused by immune deregulation. Relevant in this regard, is the cavity we have now identified on the trimeric interface of hGITRL, which may permit a structure-based search for small molecules that either stabilize or disrupt the trimeric conformation of the ligand.
Methods
Protein Expression and Purification.
Human GITRL and GITR extracellular domains were cloned, expressed, and purified as described in SI Text.
Structure Determination and Analysis.
The crystal structures of hGITRL were determined in two crystal forms as described in SI Text. Statistics for structure determination are summarized in Table S1.
Cross-Linking Analysis.
Cross-linking analysis using bis(sulfosuccinimidyl)suberate (BS3) (Pierce) was carried out according to the product manual. See details in SI Text.
Surface Plasmon Resonance.
Binding experiments were performed with the surface plasmon resonance as described in SI Text.
Flow Cytometry.
Flow cytometry was performed as described in SI Text.
MAPK Activation Assay.
MAPK activation assay was performed as described in SI Text.
Acknowledgments.
We thank Dr. David S. Waugh (National Cancer Institute, Frederick, MD) for providing Addgene plasmid 8827 and Dr. Jaclyn Freudenberg for her review of this manuscript. This work was carried out in part at National Synchrotron Light Source X6A beam line, funded by the National Institute of General Medical Sciences, National Institutes of Health under Agreement GM-0080. The National Synchrotron Light Source Brookhaven National Laboratory is supported by the U.S. Department of Energy under Contract DE-AC02-98CH10886. This work was supported in part by grants from the National Institutes of Health (to M.I.G.) and The Abramson Family Cancer Research Institute (to M.I.G.).
Note.
During the preparation of this manuscript, another group independently performed structural studies of hGITRL (44). Their study also defined an atypical loosely assembled trimer in which the flexible C termini were disordered, supporting our hypothesis. However, they observed a trimer–monomer equilibrium, different from our observation of hGITRL ensemble that includes dimer, trimer, and supercluster. The discrepancy possibly originates from the truncation of the hGITRL extracellular fragment, as well as from specific experimental methods.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
Data deposition: The atomic coordinates and structure factors have been deposited in the Protein Data Bank, www.pdb.org (PDB ID codes 3B93 and 3B94).
This article contains supporting information online at www.pnas.org/cgi/content/full/0711350105/DCSupplemental.
References
- 1.Nocentini G, et al. A new member of the tumor necrosis factor/nerve growth factor receptor family inhibits T cell receptor-induced apoptosis. Proc Natl Acad Sci USA. 1997;94:6216–6221. doi: 10.1073/pnas.94.12.6216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zelenika D, et al. Regulatory T cells overexpress a subset of Th2 gene transcripts. J Immunol. 2002;168:1069–1079. doi: 10.4049/jimmunol.168.3.1069. [DOI] [PubMed] [Google Scholar]
- 3.Shimizu J, Yamazaki S, Takahashi T, Ishida Y, Sakaguchi S. Stimulation of CD25+CD4+ regulatory T cells through GITR breaks immunological self-tolerance. Nat Immunol. 2002;3:135–142. doi: 10.1038/ni759. [DOI] [PubMed] [Google Scholar]
- 4.McHugh RS, et al. CD4+CD25+ immunoregulatory T cells: Gene expression analysis reveals a functional role for the glucocorticoid-induced TNF receptor. Immunity. 2002;16:311–323. doi: 10.1016/s1074-7613(02)00280-7. [DOI] [PubMed] [Google Scholar]
- 5.Cobbold SP, et al. Regulatory T cells and dendritic cells in transplantation tolerance: Molecular markers and mechanisms. Immunol Rev. 2003;196:109–124. doi: 10.1046/j.1600-065x.2003.00078.x. [DOI] [PubMed] [Google Scholar]
- 6.Muriglan SJ, et al. GITR activation induces an opposite effect on alloreactive CD4+ and CD8+ T cells in graft-versus-host disease. J Exp Med. 2004;200:149–157. doi: 10.1084/jem.20040116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Suri A, et al. Regulation of autoimmune diabetes by non-islet-specific T cells—a role for the glucocorticoid-induced TNF receptor. Eur J Immunol. 2004;34:447–454. doi: 10.1002/eji.200324599. [DOI] [PubMed] [Google Scholar]
- 8.Tone M, et al. Mouse glucocorticoid-induced tumor necrosis factor receptor ligand is costimulatory for T cells. Proc Natl Acad Sci USA. 2003;100:15059–15064. doi: 10.1073/pnas.2334901100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kim JD, et al. Cloning and characterization of GITR ligand. Genes Immun. 2003;4:564–569. doi: 10.1038/sj.gene.6364026. [DOI] [PubMed] [Google Scholar]
- 10.Yu KY, et al. Identification of a ligand for glucocorticoid-induced tumor necrosis factor receptor constitutively expressed in dendritic cells. Biochem Biophys Res Commun. 2003;310:433–438. doi: 10.1016/j.bbrc.2003.09.024. [DOI] [PubMed] [Google Scholar]
- 11.Stephens GL, et al. Engagement of glucocorticoid-induced TNFR family-related receptor on effector T cells by its ligand mediates resistance to suppression by CD4+CD25+ T cells. J Immunol. 2004;173:5008–5020. doi: 10.4049/jimmunol.173.8.5008. [DOI] [PubMed] [Google Scholar]
- 12.Grohmann U, et al. Reverse signaling through GITR ligand enables dexamethasone to activate IDO in allergy. Nat Med. 2007;13:579–586. doi: 10.1038/nm1563. [DOI] [PubMed] [Google Scholar]
- 13.Nocentini G, Ronchetti S, Cuzzocrea S, Riccardi C. GITR/GITRL: More than an effector T cell co-stimulatory system. Eur J Immunol. 2007;37:1165–1169. doi: 10.1002/eji.200636933. [DOI] [PubMed] [Google Scholar]
- 14.Kohm AP, Williams JS, Miller SD. Cutting edge: Ligation of the glucocorticoid-induced TNF receptor enhances autoreactive CD4+ T cell activation and experimental autoimmune encephalomyelitis. J Immunol. 2004;172:4686–4690. doi: 10.4049/jimmunol.172.8.4686. [DOI] [PubMed] [Google Scholar]
- 15.Cohen AD, et al. Agonist anti-GITR antibody enhances vaccine-induced CD8+ T-cell responses and tumor immunity. Cancer Res. 2006;66:4904–4912. doi: 10.1158/0008-5472.CAN-05-2813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ramirez-Montagut T, et al. Glucocorticoid-induced TNF receptor family related gene activation overcomes tolerance/ignorance to melanoma differentiation antigens and enhances antitumor immunity. J Immunol. 2006;176:6434–6442. doi: 10.4049/jimmunol.176.11.6434. [DOI] [PubMed] [Google Scholar]
- 17.Ko K, et al. Treatment of advanced tumors with agonistic anti-GITR mAb and its effects on tumor-infiltrating Foxp3+CD25+CD4+ regulatory T cells. J Exp Med. 2005;202:885–891. doi: 10.1084/jem.20050940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Calmels B, et al. Bypassing tumor-associated immune suppression with recombinant adenovirus constructs expressing membrane bound or secreted GITR-L. Cancer Gene Ther. 2005;12:198–205. doi: 10.1038/sj.cgt.7700781. [DOI] [PubMed] [Google Scholar]
- 19.Santucci L, et al. GITR modulates innate and adaptive mucosal immunity during the development of experimental colitis in mice. Gut. 2007;56:52–60. doi: 10.1136/gut.2006.091181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mahesh SP, Li Z, Liu B, Fariss RN, Nussenblatt RB. Expression of GITR ligand abrogates immunosuppressive function of ocular tissue and differentially modulates inflammatory cytokines and chemokines. Eur J Immunol. 2006;36:2128–2138. doi: 10.1002/eji.200635893. [DOI] [PubMed] [Google Scholar]
- 21.Stone GW, et al. Multimeric soluble CD40 ligand and GITR ligand as adjuvants for human immunodeficiency virus DNA vaccines. J Virol. 2006;80:1762–1772. doi: 10.1128/JVI.80.4.1762-1772.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhou Z, et al. Structural basis for ligand-mediated mouse GITR activation. Proc Natl Acad Sci USA. 2008;105:641–645. doi: 10.1073/pnas.0711206105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chattopadhyay K, Ramagopal UA, Brenowitz M, Nathenson SG, Almo SC. Evolution of GITRL immune function: Murine GITRL exhibits unique structural and biochemical properties within the TNF superfamily. Proc Natl Acad Sci USA. 2008;105:635–640. doi: 10.1073/pnas.0710529105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bodmer JL, Schneider P, Tschopp J. The molecular architecture of the TNF superfamily. Trends Biochem Sci. 2002;27:19–26. doi: 10.1016/s0968-0004(01)01995-8. [DOI] [PubMed] [Google Scholar]
- 25.Locksley RM, Killeen N, Lenardo MJ. The TNF and TNF receptor superfamilies: Integrating mammalian biology. Cell. 2001;104:487–501. doi: 10.1016/s0092-8674(01)00237-9. [DOI] [PubMed] [Google Scholar]
- 26.Watts TH. TNF/TNFR family members in costimulation of T cell responses. Annu Rev Immunol. 2005;23:23–68. doi: 10.1146/annurev.immunol.23.021704.115839. [DOI] [PubMed] [Google Scholar]
- 27.Liu Y, et al. Crystal structure of sTALL-1 reveals a virus-like assembly of TNF family ligands. Cell. 2002;108:383–394. doi: 10.1016/s0092-8674(02)00631-1. [DOI] [PubMed] [Google Scholar]
- 28.Cowley S, Paterson H, Kemp P, Marshall CJ. Activation of MAP kinase kinase is necessary and sufficient for PC12 differentiation and for transformation of NIH 3T3 cells. Cell. 1994;77:841–852. doi: 10.1016/0092-8674(94)90133-3. [DOI] [PubMed] [Google Scholar]
- 29.Esparza EM, Lindsten T, Stockhausen JM, Arch RH. Tumor necrosis factor receptor (TNFR)-associated factor 5 is a critical intermediate of costimulatory signaling pathways triggered by glucocorticoid-induced TNFR in T cells. J Biol Chem. 2006;281:8559–8564. doi: 10.1074/jbc.M512915200. [DOI] [PubMed] [Google Scholar]
- 30.Ronchetti S, et al. GITR, a member of the TNF receptor superfamily, is costimulatory to mouse T lymphocyte subpopulations. Eur J Immunol. 2004;34:613–622. doi: 10.1002/eji.200324804. [DOI] [PubMed] [Google Scholar]
- 31.Bae EM, et al. Reverse signaling initiated from GITRL induces NF-κB activation through ERK in the inflammatory activation of macrophages. Mol Immunol. 2008;45:523–533. doi: 10.1016/j.molimm.2007.05.013. [DOI] [PubMed] [Google Scholar]
- 32.He MM, et al. Small-molecule inhibition of TNF-α. Science. 2005;310:1022–1025. doi: 10.1126/science.1116304. [DOI] [PubMed] [Google Scholar]
- 33.Manole D, Schildknecht B, Gosnell B, Adams E, Derwahl M. Estrogen promotes growth of human thyroid tumor cells by different molecular mechanisms. J Clin Endocrinol Metab. 2001;86:1072–1077. doi: 10.1210/jcem.86.3.7283. [DOI] [PubMed] [Google Scholar]
- 34.Daniels MA, et al. Thymic selection threshold defined by compartmentalization of Ras/MAPK signalling. Nature. 2006;444:724–729. doi: 10.1038/nature05269. [DOI] [PubMed] [Google Scholar]
- 35.Werlen G, Hausmann B, Naeher D, Palmer E. Signaling life and death in the thymus: Timing is everything. Science. 2003;299:1859–1863. doi: 10.1126/science.1067833. [DOI] [PubMed] [Google Scholar]
- 36.Siegel RM, Chan FK, Chun HJ, Lenardo MJ. The multifaceted role of Fas signaling in immune cell homeostasis and autoimmunity. Nat Immunol. 2000;1:469–474. doi: 10.1038/82712. [DOI] [PubMed] [Google Scholar]
- 37.Zhang G. Tumor necrosis factor family ligand-receptor binding. Curr Opin Struct Biol. 2004;14:154–160. doi: 10.1016/j.sbi.2004.03.003. [DOI] [PubMed] [Google Scholar]
- 38.Holler N, et al. Two adjacent trimeric Fas ligands are required for Fas signaling and formation of a death-inducing signaling complex. Mol Cell Biol. 2003;23:1428–1440. doi: 10.1128/MCB.23.4.1428-1440.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Haswell LE, Glennie MJ, Al-Shamkhani A. Analysis of the oligomeric requirement for signaling by CD40 using soluble multimeric forms of its ligand, CD154. Eur J Immunol. 2001;31:3094–3100. doi: 10.1002/1521-4141(2001010)31:10<3094::aid-immu3094>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 40.Bayes M, et al. The anhidrotic ectodermal dysplasia gene (EDA) undergoes alternative splicing and encodes ectodysplasin-A with deletion mutations in collagenous repeats. Hum Mol Genet. 1998;7:1661–1669. doi: 10.1093/hmg/7.11.1661. [DOI] [PubMed] [Google Scholar]
- 41.Srivastava AK, et al. The Tabby phenotype is caused by mutation in a mouse homologue of the EDA gene that reveals novel mouse and human exons and encodes a protein (ectodysplasin-A) with collagenous domains. Proc Natl Acad Sci USA. 1997;94:13069–13074. doi: 10.1073/pnas.94.24.13069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Liu Y, et al. Ligand-receptor binding revealed by the TNF family member TALL-1. Nature. 2003;423:49–56. doi: 10.1038/nature01543. [DOI] [PubMed] [Google Scholar]
- 43.Compaan DM, Hymowitz SG. The crystal structure of the costimulatory OX40-OX40L complex. Structure (London) 2006;14:1321–1330. doi: 10.1016/j.str.2006.06.015. [DOI] [PubMed] [Google Scholar]
- 44.Chattopadhyay K, et al. Assembly and structural properties of glucocorticoid-induced TNF receptor ligand: Implications for function. Proc Natl Acad Sci USA. 2007;104:19452–19457. doi: 10.1073/pnas.0709264104. [DOI] [PMC free article] [PubMed] [Google Scholar]