Abstract
An enduring puzzle of human life history is why women cease reproduction midway through life. Selection can favor postreproductive survival because older females can help their offspring to reproduce. But the kin-selected fitness gains of helping appear insufficient to outweigh the potential benefits of continued reproduction. Why then do women cease reproduction in the first place? Here, we suggest that early reproductive cessation in humans is the outcome of reproductive competition between generations, and we present a simple candidate model of how this competition will be resolved. We show that among primates exhibiting a postreproductive life span, humans exhibit an extraordinarily low degree of reproductive overlap between generations. The rapid senescence of the human female reproductive system coincides with the age at which, in natural fertility populations, women are expected to encounter reproductive competition from breeding females of the next generation. Several lines of evidence suggest that in ancestral hominids, this younger generation typically comprised immigrant females. In these circumstances, relatedness asymmetries within families are predicted to give younger females a decisive advantage in reproductive conflict with older females. A model incorporating both the costs of reproductive competition and the benefits of grandmothering can account for the timing of reproductive cessation in humans and so offers an improved understanding of the evolution of menopause.
Keywords: fertility, grandmother hypothesis, human evolution, life history, menopause
Whereas other long-lived mammals can continue to breed until the end of life [elephants, for instance, into their 60s (1) and baleen whales into their 90s (2)], the mean ages at last birth in natural-fertility, human populations cluster around 38 (3). After this age, the female reproductive system undergoes a phase of rapid senescence, culminating in menopause (the permanent loss of fertility) ≈10 years later. Yet, even in hunter–gatherer societies without access to modern medicine or technology, women who reach menopausal age can expect to live well into their 60s (4). This disparity between reproductive and total life span is puzzling because classical life history theory predicts that there will be no selection for nonreproductive survival.
The “mother” and “grandmother” hypotheses suggest that selection has favored postreproductive survival because older women, even if they do not bear more children themselves, can nevertheless gain inclusive fitness by helping their existing offspring to survive and reproduce (5–7). There is, indeed, evidence that postreproductive grandmothers can boost the fitness of their children (8–10). But quantitative analyses [at least those based on calculations of individual inclusive fitness rather than maximization of population growth rate (11, 12)] suggest that these kin-selected fitness gains are insufficient to outweigh the potential benefits of continued reproduction (13–15). Hill and Hurtado (13, 15), for example, use data from the Ache to calculate the inclusive fitness payoffs of grandmothering versus continued reproduction for older women. They conclude that menopause cannot be favored by helping effects in this population because there are few close kin alive for an older woman to help and because her help has too little impact on the survival or reproduction of these kin. Rogers (14) uses a different model and dataset but shows a similar result, namely that the costs of breeding and the benefits of helping must be greater than have so far been documented to favor reproductive cessation around the age of 50. Thus, although current hypotheses can account for the continued survival of postreproductive females, they have difficulty explaining why they should cease reproducing in the first place (16).
Part of the problem is that current analyses focus solely on the personal-fitness consequences of reproduction and compare these with the kin-selected fitness consequences of helping (13–15). Helping is assumed to affect the fitness of other group members, whereas breeding is not. This approach is one-sided because it ignores the potential social costs of reproduction. In other cooperatively breeding vertebrates, females attempt to monopolize reproduction and access to helpers because cobreeding with other females in the group involves costs (17–20). In humans, reproductive competition among cobreeders is expected because groups share food to a degree unmatched among other primates, both within and between families, and because offspring are reliant on the investment of adult helpers (7, 21, 22). The offspring of cobreeding females will draw on the same communal resource pool and compete for the care of helpers for many years (23). As in other cooperative species, therefore, we should expect human females to experience competition when other females in the social unit reproduce. Unlike other cooperative species, the possibility of reproductive conflict in the evolution of the human life history has to date been overlooked.
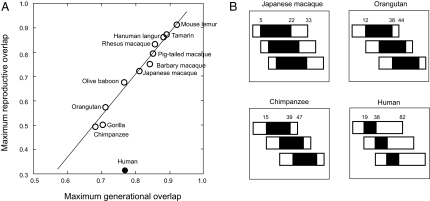
We suggest that the human fertility trajectory has been shaped, in substantial part, by selection to minimize reproductive competition between generations within the same social unit. That humans are unique among primates in their low degree of overlap between reproductive generations is clear from Fig. 1A. For those primates recently classified as exhibiting a postreproductive life span (24) [including humans, represented by the two hunter–gatherer societies for which the relevant demographic information is available (15, 25)], we plot the relationship between maximum generational overlap (calculated as the proportional overlap between the maximum life spans of mother and daughter) and maximum reproductive overlap (calculated as the proportional overlap between the maximum reproductive spans of mother and daughter). Humans exhibit an extraordinarily low level of reproductive overlap, both absolutely and in relation to their level of generational overlap. In other words, although human mothers may survive for the majority of their daughters' life span, they will continue to reproduce for at most a small fraction of their daughters' reproductive span (much smaller than is the case for other primates). Humans stand out because the maximum age at last birth (MALB) observed in hunter–gatherers [47 years; mean of the Ache/!Kung data (15, 25)] is much less than the MALB predicted from the regression (70 years). For four of these species, published data are sufficiently detailed to calculate the mean level of reproductive overlap between generations. As shown in Fig. 1B, the mean reproductive overlap for humans is close to zero.
Figure. 1.
Reproductive overlap in humans and other primates. (A) Maximum reproductive versus maximum generational overlap in 12 primate species recently classed as exhibiting a postreproductive life span (24). Maximum generational overlap, G, is defined as (MLS − AFB)/MLS, where AFB is average age at first birth and MLS is maximum recorded life span. Maximum reproductive overlap, R, is defined as (MRS − AFB)/MRS, where MRS is the maximum reproductive span, calculated as maximum age at last birth (MALB) minus AFB. For species without a postreproductive life span, in which MRS ≈ MLS, it would follow that R = 2 − (1/G). For our sample species (excluding humans), the least-squares linear regression of R on G is shown (y = −0.74 + 1.78x, r2 = 0.98). This regression would predict a MALB for humans of 70 years on the basis of generational overlap. We used MLS and MALB because these are the parameters typically reported in the literature. For four species (chimpanzees, orangutans, Japanese macaques, and humans), published data were sufficiently detailed to calculate mean reproductive overlap, defined as (ARS − AFB)/ARS, where ARS is the average (or mean) reproductive span (i.e., mean ALB minus AFB). (B) Pattern of overlap for these four species. For each species, horizontal bars represent the maximum life spans of three successive generations, scaled to a standard length and offset in accordance with the value of AFB relative to MLS, with mean reproductive spans shaded. The mean reproductive overlap values for Japanese macaques, orangutans, and chimpanzees were 0.71, 0.52, and 0.39, respectively, compared with a mean reproductive overlap for humans of 0.00. Values (in years) and reference sources used to plot the figure are as follows. Common chimpanzee (Pan troglodytes): MLS = 47, AFB = 14.7, MALB = 43.8 [means of Mahale/Tai/Bossou/Gombe populations (42–44, 65–67)]), mean ALB = 38.9 (mean of Mahale/Tai/Gombe values for females that survived to the MALB of their respective populations). Gorilla (Gorilla gorilla): MLS = 35, AFB = 10, MALB = 30 (68, 69). Orangutan (Pongo pygmaeus): MLS = 44, AFB = 12.3, MALB = 41; mean ALB = 38 (70). Human (Homo sapiens): MLS = 82.5, AFB = 19.1, MALB = 47 [Ache/!Kung mean values (15, 25)], mean ALB = 38.2 [mean for Ache/!Kung females surviving to age 50 (15, 25)]. Olive baboon (Papio cynocephalus anubis): MLS = 27, AFB = 6, MALB = 25 (71). Japanese macaque (Macaca fuscata): MLS = 32.7, AFB = 5, MALB = 25.4 (72), mean ALB = 22.5 [mean for females surviving to MALB (72)]. Barbary macaque (Macaca sylvanus): MLS = 28, AFB = 4, MALB = 23 (73). Pig-tailed macaque (Macaca nemestrina): MLS = 28, ALB = 22, AFB = 5 (74). Rhesus macaque (Macaca mulatto): MLS = 19, MALB = 18 (69), AFB = 2.6 (75). Hanuman langur (Presbytis entellus): MLS = 35, AFB = 4, MALB = 32 (76). Tamarins (Sanguinus spp. (2 spp.): MLS = 20, MALB = 17 (77), AFB = 2 (75). Mouse lemur (Microcebus murinus): MLS = 14, MALB = 11 (78), AFB = 0.95 (75).
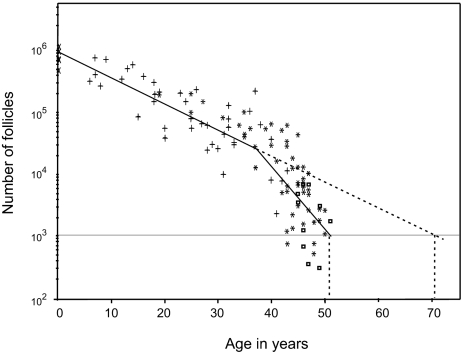
At a proximate level, menopause in humans is caused by attrition of the primordial ovarian follicle stock (26, 27). Across species, primordial follicle stocks are evolutionarily labile and are adjusted to life span and body weight (28). The initial oocyte stock and rate of follicular attrition in human females are commensurate with a longer reproductive life span: specifically, an age at menopause of ≈70 years (ref. 29; Fig. 2), which offers an intriguing match with the predicted age at menopause based on reproductive versus generational overlap in other primates (Fig. 1A). Around the age of 38, however, there is a marked increase in the follicular hazard rate, so that by age 50, follicle stocks have dropped below a minimum number required to sustain menstrual activity (29–31). The onset of the accelerated phase of reproductive senescence that leads to menopause coincides with the age at which, in natural-fertility populations, human females can first expect to encounter reproductive competition from the next generation (Fig. 1B). For comparison, there is no indication of an increase in the rate of follicular attrition late in life in chimpanzees (32), rhesus macaques (33), or laboratory rodents (34), the other species for which such data are available. The pattern of attrition in humans, therefore, appears to fit quite well with our hypothesis concerning the timing of intergenerational reproductive conflict. Information on the schedule of attrition in other species (particularly cooperative breeders) would help to evaluate this pattern further.
Fig. 2.
Number of follicles in pairs of human ovaries from neonatal age to 51 years. Note the logarithmic scale on the y axis. The solid line shows a fitted biphasic regression model (29, 30), which gives the best fit to the data compared with a range of alternative models [including those that assume a smooth acceleration in the rate of decline (29, 30, 79)]. This model assumes a constant exponential rate of follicular decline from birth to ≈38 years, after which the exponential rate parameter approximately doubles. The threshold minimum number of follicles required to maintain regular menstrual cycles (assumed to be ≈1,000) is reached at approximately age 50. Data are pooled from four autopsy studies, denoted by different symbols. [Redrawn with permission from ref. 29 (copyright 1992, Oxford University Press).]
Reproductive competition has led to the evolutionary separation of reproductive generations in many other cooperative vertebrates, but in these species it is almost always the older generation that retains breeding status and the younger generation that is reproductively suppressed (35–39). What might account for a reversal of this pattern in humans? One answer may lie in the unusual demography of humans and, in particular, the pattern of sex-biased dispersal. The pattern of dispersal is important to consider in a model of reproductive conflict because it determines which females will compete for reproduction within groups. Unlike most mammals, in which dispersal is male-biased (40, 41), several lines of evidence suggest that female-biased dispersal may be ancestral to the genus Homo. First, our closest primate relatives, chimpanzees, bonobos, and gorillas, are unusual among primates because they exhibit strongly female-biased dispersal and male philopatry (42–47). Second, patterns of variation in mitochondrial DNA and the Y chromosome are consistent with substantially greater rates of female than male dispersal (48, 49), at least on the relevant, local scale (50). Third, among modern human foraging societies, female-biased transfer is considerably more common than the reverse pattern [(51, 52) although, as in other mammals, the level of bias is usually not absolute (52, 53)]. Taken together, this evidence suggests that mutations affecting female reproductive life span are likely to have arisen in a social environment in which dispersal was female-biased.
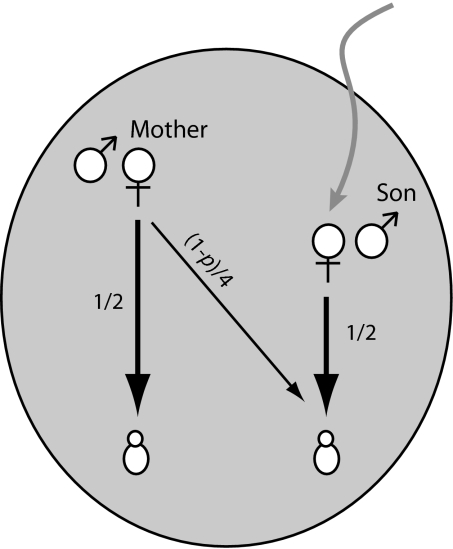
The effect of female-biased dispersal on reproductive conflict can be explored by using a simple model of an ancestral human social unit (Fig. 3). We assume that males and females are socially monogamous, but we allow for a proportion p of offspring to be fathered by unrelated males. For simplicity, we assume that only females disperse, although qualitatively similar results are obtained where dispersal is merely biased toward females [supporting information (SI) Text]. Females leave their natal groups at maturity, pair with a male of similar age, and join his natal social group. Consequently, when a young female first arrives in the group, she has no other genetic relatives present. This female can choose to breed herself and produce offspring to whom she is related by 1/2 or to refrain from breeding and assist the breeding attempts of the older female (i.e., the mother of her mate), who produces offspring to whom the younger female is unrelated. The difference in relatedness to her own offspring versus those produced by helping is therefore 1/2. The older female, by contrast, can choose to breed and produce offspring of relatedness 1/2 or refrain from breeding and help to rear grandoffspring, to whom she is related by (1 − p)/4. The difference in relatedness to offspring produced by breeding versus helping for the older female is therefore 1/2 − (1 − p)/4, or (1 + p)/4, which means that as long as there is any chance that her son fathered her putative grandchildren (i.e., p < 1), the difference in relatedness to offspring produced by breeding rather than helping is lower for the older female than for the younger female. As a result, a younger female will have an advantage in reproductive competition with older females because she is insensitive to the costs she inflicts on an older female by breeding and because older females have more to gain by helping. By contrast, where dispersal is male-biased [as in most social mammals (54)], relatedness asymmetries within the group will favor older females in reproductive conflict with their daughters.
Figure. 3.
Relatedness asymmetry within families assuming female dispersal. Male and female symbols represent parents. A mother is related to the offspring of an immigrant female paired to her son by (1 − p)/4, where p is the probability of extra-pair paternity. The younger female, by contrast, is unrelated to the mother's offspring.
A formal, game-theoretical treatment of the model yields an evolutionarily stable solution in which the older female commits irreversibly to zero reproduction when the younger female starts to reproduce (see SI Text and Figs. S1–S3). This result holds even in the absence of any kin-selected fitness benefits that older females might gain as postreproductive helpers. Thus, the result does not require us to assume grandmothering benefits, although any such benefits will only strengthen the selection to cease reproduction. Moreover, an initial shift in the female life history to redistribute reproductive investment from later to earlier ages is likely to augment the advantage of young females in reproductive conflict with older females, reinforcing selection for early reproductive cessation and a separation of reproductive generations. Given a pattern of female-biased dispersal during the period of lengthening human life span [most likely between the Middle and Upper Pleistocene (55)], our analysis suggests that reproductive competition would generate stabilizing selection against the extension of the reproductive span to match longevity. The predicted outcome is a mismatch between the rates of reproductive and somatic senescence and the separation of reproductive generations that we observe. The intensity of reproductive competition and the magnitude of the benefits that can be conferred by helping must also be important, however, because chimpanzees and bonobos exhibit strongly female-biased dispersal but are not unusual in their degree of reproductive overlap (Fig. 1).
How might the model be tested? A distinction can be drawn between our general hypothesis concerning reproductive competition and our specific model based on female-biased dispersal: in particular, falsification of the model does not necessarily imply falsification of the general hypothesis. Nevertheless, both are useful because they make testable predictions. Our hypothesis that early reproductive cessation reflects “the ghost of reproductive competition past” predicts that there will be detectable costs to females of breeding alongside a reproductive grandmother, similar to the documented costs of cobreeding within generations in polygamous families (56–58). Second, variation between individuals or populations in factors that alter the intensity or timing of reproductive competition from the next generation (for example, individual variation in the number and birth order of sons versus daughters; or variation across societies in the mean age ratio of male to female partners) is predicted to correlate with reproductive overlap and the age at last reproduction. The female dispersal model might be tested in a number of ways. Genetic or genealogical analyses would help to determine whether relatedness asymmetries do indeed exist within family units of natural fertility populations. The wide variation in dispersal systems exhibited by extant human populations could be used to test whether reproductive spans vary with dispersal pattern in the manner we predict. For example, in forager societies in which dispersal has been male-biased for an extended period (i.e., many generations), both age at last birth and the degree of reproductive overlap are predicted to be higher than in female-dispersing societies, and cultural proscriptions on reproduction by grandmothers (e.g., 59–62) are expected to be less strict in the former than the latter. The stability of the dispersal system over time might be inferred from genetic data (49).
The model does not imply that older nonbreeding females should not help daughters if the dispersal system subsequently becomes less female-biased or mothers are able to maintain kin ties to their daughters. The variety of social systems exhibited by modern humans illustrates the potential flexibility of behavior compared with the physiological processes underlying the species-wide trait of rapid reproductive senescence that leads to menopause. If women can choose to help offspring of either sex, it may indeed pay grandmothers to direct care preferentially toward daughters because grandchildren through sons may have been fathered by extra-pair males. Studies showing a benefit of maternal rather than paternal grandmothers in extant or historical populations (8, 9, 63) are not, therefore, incompatible with our model. Indeed, in our model, the option to assist daughters outside the family unit increases the relatedness asymmetry favoring younger females in within-family conflict (because, in this case, older females can help to produce grandoffspring related by 1/4 rather than (1 − p)/4; Fig. 3). However, given that paternity uncertainty is widely accepted as a factor favoring maternal over paternal grandmothering, it is instructive to compare the magnitude of this effect with the magnitude of the relatedness asymmetry between older and younger females that drives our results. If p is the rate of extra-pair paternity, then from a grandmother's perspective, the difference in relatedness to grandchildren through her daughters versus her sons is 1/4 − (1 − p)/4 = p/4, whereas the difference in relatedness of older and younger females to each others' offspring is (1 − p)/4 − 0 = (1 − p)/4. A common best estimate for p in humans is 0.1 (64), in which case the magnitude of the relatedness asymmetry favoring younger females in reproductive conflict with older females is almost 10 times greater than the relatedness asymmetry favoring maternal over paternal grandmothering. This observation explains why the relatedness asymmetry arising from female dispersal has such a decisive effect on the resolution of reproductive conflict in our model, and why the model's qualitative predictions hold when dispersal is merely biased toward, rather than restricted to, females (see SI Text).
To conclude, we emphasize that our hypothesis should be seen as complementary, rather than as an alternative, to the grandmother hypothesis as an explanation for the divergence of rates of somatic and reproductive senescence. The kin-selected benefits of helping can explain postreproductive survival, but not why women cease reproduction so early in the first place. A model incorporating reproductive competition can help to account for this trait and for the particular timing of reproductive cessation in human females. We suggest, therefore, that a combined model that takes into account both the potential inclusive fitness costs of reproduction and the inclusive fitness benefits of helping offers an improved understanding of the evolution of menopause.
Acknowledgments.
We are grateful to A. Russell for initiating our interest in this topic and for many useful comments and discussions. Thanks also to R. Chicot, T. Clutton-Brock, N. Davies, P. Ellison, J. Field, K. Foster, R. Hager, Ø. Holen, S. Hrdy, C. Jones, R. Kilner, N. Kutsukake, K. McAuliffe, F. Ratnieks, J. Silk, S. West, R. Wrangham, and two anonymous referees for comments on the manuscript. M.A.C. was funded by a Royal Society University Research Fellowship.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0711911105/DCSupplemental.
References
- 1.Moss CJ. The demography of an African elephant (Loxodonta africana) population in Amboseli, Kenya. J Zool. 2001;255:145–156. [Google Scholar]
- 2.Mizroch SA. Analyses of some biological parameters of the Antarcticfin whale (Balaenopter physalus) Rep Int Whaling Comm. 1981;31:425–434. [Google Scholar]
- 3.Wood JW. Dynamics of Human Reproduction. New York: de Gruyter; 1994. [Google Scholar]
- 4.Blurton-Jones NG, Hawkes K, O'Connell JF. Antiquity of postreproductive life: Are there modern impacts on hunter–gatherer postreproductive life spans? Am J Hum Biol. 2002;14:184–205. doi: 10.1002/ajhb.10038. [DOI] [PubMed] [Google Scholar]
- 5.Williams GC. Pleiotropy, natural selection, and the evolution of senescence. Evolution (Lawrence, Kans) 1957;11:398–411. [Google Scholar]
- 6.Hamilton WD. The moulding of senescence by natural selection. J Theor Biol. 1966;12:12–45. doi: 10.1016/0022-5193(66)90184-6. [DOI] [PubMed] [Google Scholar]
- 7.Hawkes K, O'Connell JF, Blurton Jones NG, Alvarez H, Charnov EL. Grandmothering, menopause, and the evolution of human life histories. Proc Natl Acad Sci USA. 1998;95:1336–1339. doi: 10.1073/pnas.95.3.1336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sear R, Mace R, McGregor IA. Maternal grandmothers improve nutritional status and survival of children in rural Gambia. Proc R Soc London Ser B. 2000;267:1641–1647. doi: 10.1098/rspb.2000.1190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Voland E, Beise J. Opposite effects of maternal and paternal grandmothers on infant survival in rural Krummhörn. Behav Ecol Sociobiol. 2002;52:435–443. [Google Scholar]
- 10.Lahdenperä M, Lummaa V, Helle S, Tremblay M, Russell AF. Fitness benefits of prolonged postreproductive life span in women. Nature. 2004;428:178–181. doi: 10.1038/nature02367. [DOI] [PubMed] [Google Scholar]
- 11.Shanley DP, Kirkwood TBL. Evolution of the human menopause. Bioessays. 2001;23:282–287. doi: 10.1002/1521-1878(200103)23:3<282::AID-BIES1038>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 12.Shanley DP, Sear R, Mace R, Kirkwood TBL. Testing evolutionary theories of menopause. Proc R Soc London Ser B. 2007;274:2943–2949. doi: 10.1098/rspb.2007.1028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hill K, Hurtado AM. The evolution of premature reproductive senescence and menopause in human females. Hum Nat. 1991;2:315–350. doi: 10.1007/BF02692196. [DOI] [PubMed] [Google Scholar]
- 14.Rogers AR. Why menopause? Evol Ecol. 1993;7:406–426. [Google Scholar]
- 15.Hill K, Hurtado AM. Ache Life History: The Ecology and Demography of a Foraging People. New York: de Gruyter; 1996. [Google Scholar]
- 16.Peccei JS. Menopause: Adaptation or epiphenomenon? Evol Anthropol. 2001;10:43–57. [Google Scholar]
- 17.Keller L, Reeve HK. Partitioning of reproduction in animal societies. Trends Ecol Evol. 1994;9:98–102. doi: 10.1016/0169-5347(94)90204-6. [DOI] [PubMed] [Google Scholar]
- 18.Emlen ST. An evolutionary theory of the family. Proc Natl Acad Sci USA. 1995;92:8092–8099. doi: 10.1073/pnas.92.18.8092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Magrath RD, Johnstone RA, Heinsohn RG. In: Ecology and Evolution of Cooperative Breeding in Birds. Koenig WD, Dickinson JL, editors. Cambridge, UK: Cambridge Univ Press; 2004. pp. 157–176. [Google Scholar]
- 20.Clutton-Brock TH, et al. Intrasexual competition and sexual selection in cooperative mammals. Nature. 2007;444:1065–1068. doi: 10.1038/nature05386. [DOI] [PubMed] [Google Scholar]
- 21.Gurven M. To give or to give not: The behavioral ecology of human food transfers. Behav Brain Sci. 2004;27:543–583. [Google Scholar]
- 22.Hrdy SB. Mother Nature: A History of Mothers, Infants, and Natural Selection. New York: Pantheon; 1999. [DOI] [PubMed] [Google Scholar]
- 23.Kaplan H, Hill K, Lancaster J, Hurtado AM. A theory of human life history evolution: Diet, intelligence, and longevity. Evol Anth. 2000;9:156–185. [Google Scholar]
- 24.Cohen AA. Female post-reproductive life span: A general mammalian trait. Biol Rev. 2004;79:733–750. doi: 10.1017/s1464793103006432. [DOI] [PubMed] [Google Scholar]
- 25.Howells N. Demography of the Dobe !Kung. New York: Academic; 1979. [Google Scholar]
- 26.Faddy MJ. Follicular dynamics during ovarian ageing. Mol Cell Endocrinol. 2000;163:43–48. doi: 10.1016/s0303-7207(99)00238-5. [DOI] [PubMed] [Google Scholar]
- 27.Gosden RG, Faddy MJ. Ovarian aging, follicular depletion, and steroidogenesis. Exp Gerontol. 1994;29:265–274. doi: 10.1016/0531-5565(94)90006-x. [DOI] [PubMed] [Google Scholar]
- 28.Gosden RG, Telfer E. Numbers of follicles in mammalian ovaries, and their allometric relationships. J Zool. 1987;211:169–175. [Google Scholar]
- 29.Faddy MJ, Gosden RG, Gougeon A, Richardson SJ, Nelson JF. Accelerated disappearance of ovarian follicles in midlife: Implications for forecasting menopause. Hum Reprod. 1992;7:1342–1346. doi: 10.1093/oxfordjournals.humrep.a137570. [DOI] [PubMed] [Google Scholar]
- 30.Gougeon A, Ecochard R, Thalabard JC. Age-related changes of the population of human ovarian follicles: Increase in the disappearance rate of nongrowing and early growing follicles in aging women. Biol Reprod. 1994;50:653–663. doi: 10.1095/biolreprod50.3.653. [DOI] [PubMed] [Google Scholar]
- 31.Richardson SJ, Senilkas V, Nelson JF. Follicular depletion during the menopausal transition: Evidence for accelerated loss and ultimate exhaustion. J Clin Endocrinol Metab. 1987;65:1231–1237. doi: 10.1210/jcem-65-6-1231. [DOI] [PubMed] [Google Scholar]
- 32.Jones KP, et al. Depletion of ovarian follicles with age in chimpanzees: Similarities to humans. Biol Reprod. 2007;77:247–251. doi: 10.1095/biolreprod.106.059634. [DOI] [PubMed] [Google Scholar]
- 33.Nichols SM, et al. Ovarian senescence in the rhesus monkey (Macaca mulatta) Hum Reprod. 2005;20:79–83. doi: 10.1093/humrep/deh576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jones EC, Krohn PL. The relationships between age, numbers of oocytes, and fertility in virgin and multiparous mice. J Endocrinol. 1961;21:469–496. doi: 10.1677/joe.0.0210469. [DOI] [PubMed] [Google Scholar]
- 35.Macdonald DW, Moehlman PD. In: Perspectives in Ethology. Bateson P, Klopfer P, editors. Vol 5. New York: Plenum; 1983. pp. 433–467. [Google Scholar]
- 36.Wasser SK, Barash DP. Reproductive suppression among female mammals: Implications for biomedicine and sexual selection theory. Q Rev Biol. 1983;58:513–538. doi: 10.1086/413545. [DOI] [PubMed] [Google Scholar]
- 37.Brown JL. Helping and Communal Breeding in Birds. Princeton: Princeton Univ Press; 1987. [Google Scholar]
- 38.Moehlman PD. In: Behavior and Ecology of Carnivores. Gittleman J, editor. Ithaca, NY: Cornell Univ Press; 1989. pp. 143–163. [Google Scholar]
- 39.French JA. In: Cooperative Breeding in Mammals. Solomon NG, French JA, editors. Cambridge, UK: Cambridge Univ Press; 1997. pp. 34–75. [Google Scholar]
- 40.Greenwood PJ. Mating systems, philopatry, and dispersal in birds and mammals. Anim Behav. 1980;28:1140–1162. [Google Scholar]
- 41.Lawson Handley LJ, Perrin N. Advances in our understanding of mammalian sex-biased dispersal. Mol Ecol. 2007;16:1559–1578. doi: 10.1111/j.1365-294X.2006.03152.x. [DOI] [PubMed] [Google Scholar]
- 42.Boesch C, Boesch-Achermann H. The Chimpanzees of the Tai Forest. Oxford: Oxford Univ Press; 2000. [Google Scholar]
- 43.Pusey AE, Williams J, Goodall J. The influence of dominance rank on the reproductive success of female chimpanzees. Science. 1997;277:828–831. doi: 10.1126/science.277.5327.828. [DOI] [PubMed] [Google Scholar]
- 44.Nishida T, et al. Demography, female life history, and reproductive profiles among the chimpanzees of Mahale. Am J Primatol. 2003;59:99–121. doi: 10.1002/ajp.10068. [DOI] [PubMed] [Google Scholar]
- 45.Eriksson J, et al. Y chromosome analysis confirms highly sex-biased dispersal and suggests a low male effective population size in bonobos (Pan paniscus) Mol Ecol. 2006;15:939–949. doi: 10.1111/j.1365-294X.2006.02845.x. [DOI] [PubMed] [Google Scholar]
- 46.Stokes EJ, Parnell RJ, Olejniczak C. Female dispersal and reproductive success in wild western lowland gorilla (Gorilla gorilla gorilla) Behav Ecol Sociobiol. 2003;54:329–339. [Google Scholar]
- 47.Yamagiwa J, Kahekwa J. In: Mountain Gorillas: Three Decades of Research at Karisoke. Robbins MM, Sicotte P, Stewart KJ, editors. Cambridge, UK: Cambridge Univ Press; 2004. pp. 89–122. [Google Scholar]
- 48.Seielstad MT, Minch E, Cavalli-Sforza LL. Genetic evidence for a higher female migration rate in humans. Nat Genet. 1998;20:278–280. doi: 10.1038/3088. [DOI] [PubMed] [Google Scholar]
- 49.Oota H, Settheetham-Ishida W, Tiwawech D, Ishida T, Stoneking M. Human mtDNA and Y chromosome variation is correlated with matrilocal versus patrilocal residence. Nat Genet. 2001;29:20–21. doi: 10.1038/ng711. [DOI] [PubMed] [Google Scholar]
- 50.Wilder JA, Kingan SB, Mobasher Z, Pilkington MM, Hammer MF. Global patterns of human mitochondrial DNA and Y chromosome structure are not influenced by higher migration rates of females versus males. Nat Genet. 2004;36:1122–1125. doi: 10.1038/ng1428. [DOI] [PubMed] [Google Scholar]
- 51.Ember CR. Myths about hunter–gatherers. Ethnology. 1978;17:439–448. [Google Scholar]
- 52.Marlowe FW. Marital residence among foragers. Curr Anthropol. 2004;45:277–284. [Google Scholar]
- 53.Alvarez H. In: Kinship and Behavior in Primates. Chapais B, Berman CM, editors. New York: Oxford Univ Press; 2004. pp. 420–442. [Google Scholar]
- 54.Clutton-Brock TH. Female transfer and inbreeding avoidance in social mammals. Nature. 1989;337:70–72. doi: 10.1038/337070a0. [DOI] [PubMed] [Google Scholar]
- 55.Caspari R, Lee SH. Older age becomes common late in human evolution. Proc Natl Acad Sci USA. 2004;101:10895–10900. doi: 10.1073/pnas.0402857101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Isaac BL, Feinberg WE. Marital form and infant survival among the Mende of rural Upper Bambarta Chiefdom, Sierra Leone. Hum Biol. 1982;54:627–634. [PubMed] [Google Scholar]
- 57.Pebley AR, Mbuga W. In: Reproduction and Social Organization in Sub-Saharan Africa. Lesthaeghe RJ, editor. Berkeley: Univ California Press; 1989. pp. 339–365. [Google Scholar]
- 58.Amey FK. Polygamous marital structure and child survivorship in Ghana: Age-dependent effect? Social Biol. 2002;49:74–89. [Google Scholar]
- 59.Wilson M. Rituals of Kinship among the Nyakusa. London: Oxford Univ Press; 1957. [Google Scholar]
- 60.Cavalli-Sforza LL. In: How Humans Adapt: A Biocultural Odyssey. Ortner DJ, editor. Washington, DC: Smithsonian Institute Press; 1983. pp. 103–126. [Google Scholar]
- 61.Patel T. Fertility Behaviour: Population and Society in a Rajasthan Village. Oxford, UK: Oxford Univ Press; 1994. [Google Scholar]
- 62.Skinner GW. Grandparental effects on reproductive strategizing: Nôbi villagers in Early Modern Japan. Demogr Res. 2004;11:111–148. [Google Scholar]
- 63.Bereczkei T, Dunbar RIM. Female-biased reproductive strategies in a Hungarian Gypsy population. Proc R Soc London Ser B. 1997;264:17–22. [Google Scholar]
- 64.Mace R. Evolutionary ecology of human life history. Anim Behav. 2000;59:1–10. doi: 10.1006/anbe.1999.1287. [DOI] [PubMed] [Google Scholar]
- 65.Sugiyama Y. Age-specific birth rate and lifetime reproductive success of chimpanzees at Bossou, Guinea. Am J Primatol. 1994;32:311–318. doi: 10.1002/ajp.1350320408. [DOI] [PubMed] [Google Scholar]
- 66.Hill K, et al. Chimpanzee mortality in the wild. J Hum Evol. 2001;40:437–450. doi: 10.1006/jhev.2001.0469. [DOI] [PubMed] [Google Scholar]
- 67.Goodall J. The Chimpanzees of Gombe: Patterns of Behaviour. Boston: Harvard Univ Press; 1984. [Google Scholar]
- 68.Stewart KJ, Harcourt AH, Watts DP. In: Natural Human Fertility: Social and Biological Determinants. Diggory P, Potts M, Teper S, editors. New York: Macmillan; 1988. pp. 22–38. [Google Scholar]
- 69.Caro TM, et al. Termination of reproduction in nonhuman and human female primates. Int J Prim. 1995;16:205–220. [Google Scholar]
- 70.Wich SA, et al. Life history of wild Sumatran orangutans (Pongo abelii) J Hum Evol. 2004;47:385–398. doi: 10.1016/j.jhevol.2004.08.006. [DOI] [PubMed] [Google Scholar]
- 71.Packer C, Tatar M, Collins A. Reproductive cessation in female mammals. Nature. 1998;392:807–811. doi: 10.1038/33910. [DOI] [PubMed] [Google Scholar]
- 72.Pavelka MS, Fedigan LM. Reproductive termination in female Japanese monkeys: A comparative life history perspective. Am J Phys Anthropol. 1999;109:455–464. doi: 10.1002/(SICI)1096-8644(199908)109:4<455::AID-AJPA3>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 73.Paul A, Kuester I, Pdzuweit D. Reproductive senescence and terminal investment in female Barbary macaques (Macaca sylvanus) at Salem. Int J Primatol. 1993;14:105–124. [Google Scholar]
- 74.Ha JC, Robinette RL, Sackett GP. Demographic analysis of the Washington Regional Primate Research Center pigtailed macaque colony, 1967–1996. Am J Primat. 2000;52:187–198. doi: 10.1002/1098-2345(200012)52:4<187::AID-AJP3>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 75.Harvey PH, Martin RD, Clutton-Brock TH. In: Primate Societies. Smuts BB, Cheney DL, Seyfarth RM, Wrangham RW, editors. Chicago: Univ of Chicago Press; 1987. pp. 181–196. [Google Scholar]
- 76.Borries C, Sommer V, Srivastava A. Dominance, age, and reproductive success in female Hanuman langurs (Presbytis entellus) Int J Primatol. 1991;12:231–257. [Google Scholar]
- 77.Tardif SD, Ziegler TE. Features of female reproductive senescence in tamarins, Saguinus sppm: A new world primate. J Reprod Fertil. 1992;94:411–421. doi: 10.1530/jrf.0.0940411. [DOI] [PubMed] [Google Scholar]
- 78.Finch CE, Sapolsky RM. The evolution of Alzheimer's disease, the reproductive schedule, and apoE isoforms. Neurobiol Aging. 1999;20:407–428. doi: 10.1016/s0197-4580(99)00053-6. [DOI] [PubMed] [Google Scholar]
- 79.Faddy MJ, Gosden RG. A model conforming the decline in follicle numbers to the age at menopause in women. Hum Reprod. 1996;11:1484–1486. doi: 10.1093/oxfordjournals.humrep.a019422. [DOI] [PubMed] [Google Scholar]