Abstract
Stress is known to induce dendritic hypertrophy in the basolateral amygdala (BLA) and to enhance anxiety. Stress also leads to secretion of glucocorticoids (GC), and the BLA has a high concentration of glucocorticoid receptors. This raises the possibility that stress-induced elevation in GC secretion might directly affect amygdaloid neurons. To address the possible effects of GC on neurons of amygdala and on anxiety, we used rats treated either acutely with a single dose or chronically with 10 daily doses of high physiological levels of corticosterone (the rat-specific glucocorticoid). Behavior and morphological changes in neurons of BLA were measured 12 days after the initiation of treatment in both groups. A single acute dose of corticosterone was sufficient to induce dendritic hypertrophy in the BLA and heightened anxiety, as measured on an elevated plus maze. Moreover, this form of dendritic hypertrophy after acute treatment was of a magnitude similar to that caused by chronic treatment. Thus, plasticity of BLA neurons is sufficiently sensitive so as to be saturated by a single day of stress. The effects of corticosterone were specific to anxiety, as neither acute nor chronic treatment caused any change in conditioned fear or in general locomotor activity in these animals.
Keywords: amygdala, glucocorticoid, neuron
Stress is known to cause structural alterations in neurons of the central nervous system including changes in dendritic architecture and density of spines. For example, chronic restraint stress reduces dendritic length and number of branch points of hippocampal neurons (1–3). This atrophy of hippocampal neurons is known to be correlated with behavioral deficits in hippocampal-dependent spatial memory tasks, such as the Morris water maze (2–7). In contrast, chronic immobilization stress enhances dendritic length, branch points, and spines in neurons of basolateral amygdala (BLA) (8, 9). In addition to such dendritic hypertrophy in the amygdala, animals treated with chronic immobilization stress show enhanced anxiety (8–10). Thus, structural alterations in the hippocampus and amygdala are associated with concomitant behavioral alterations.
Stressful stimuli activate the hypothalamus–pituitary–adrenal (HPA) axis, leading to secretion of stress hormones, including glucocorticoids (GCs) (11–13). GCs play important roles in organizing the stress response by binding to glucocorticoid receptors in peripheral tissue and in brain. Both the hippocampus (14) and the BLA (15) have high concentration of GC receptors. Chronic GC treatment is known to cause the neuronal atrophy in the hippocampus and spatial memory deficits (16–18) similar to that seen in stress, leading to suggestions that GC secretion is critical in stress-induced hippocampal damage (5). However, the effects of GC on amygdaloid neurons remain unknown. Specifically it is not known whether high GC concentrations alone are sufficient to induce dendritic hypertrophy of BLA neurons and accompanying anxiety. Interactions between GC and the BLA might be important for understanding the high levels of GCs and enhanced anxiety observed in several neuropsychiatric disorders.
In the present article, we investigate whether acute and chronic treatment with corticosterone (CORT), the predominant GC of rats, can influence amygdaloid neuronal structure and anxiety. We specifically address whether acute GC treatment is sufficient to induce robust effects on neuroarchitecture and behavior.
Results
Effects of CORT on Cellular Morphology of the Amygdala Neurons.
The effect of vehicle or CORT treatment for various lengths of administration was studied (Fig. 1Inset). Dendritic arborization of BLA neurons was measured in terms of dendritic length and number of branch points. In terms of dendritic length, a two-way ANOVA revealed significant main effects of duration [chronic > acute; P < 0.001; supporting information (SI) Table S1], of treatment (CORT > vehicle; P < 0.001), and of their interaction (P < 0.01). Differences in number of branch points followed similar contours (P < 0.001 for main effect of treatment and interaction), except that main effect of duration did not reach statistical significance (P = 0.09).
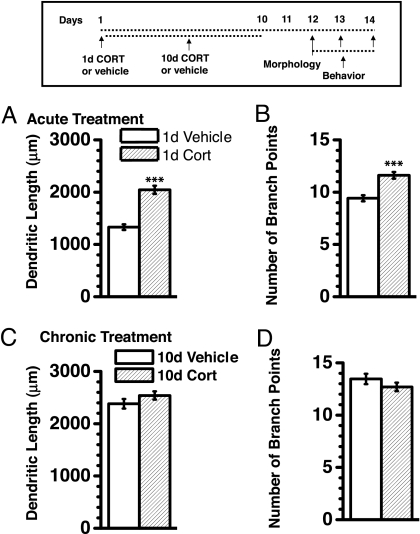
Fig. 1.
Acute CORT treatment caused an increase in dendritic arborization when quantified after 12 days, comparable in magnitude to that of chronic CORT treatment. (Inset) Time profile for the experimental design. The increase in dendritic arborization after 1 day of CORT treatment was reflected in both total dendritic length (A) and total number of branch points (B). n = 38 neurons for vehicle and 49 neurons for CORT. ***, P < 0.001 (Student's t test). Chronic CORT treatment caused a change in dendritic length (C) and branch points (D) equivalent to that of chronic vehicle treatment. n = 50 neurons for vehicle and 40 neurons for CORT. Values are mean ± SEM.
When compared with acute vehicle treatment, acute CORT treatment increased total dendritic length (Fig. 1A; 54% increase; P < 0.00001) and total number of branch points (Fig. 1B; 23%; P < 0.0001) in spiny principle BLA neurons 12 days later. Such treatment also increased the farthest radial distance from soma where the presence of dendrites could be detected (radial extent from soma; CORT-treated = 275 ± 8 μm, vehicle-treated = 229 ± 7 μm; P < 0.001) and enlarged BLA volume (Table 1). In contrast, these CORT-induced effects were not demonstrable 1 day after acute treatment (P > 0.75), suggesting that a temporal delay was necessary.
Table 1.
BLA volume in animals treated with acute or chronic CORT
| Treatment | BLA volume, mm3 |
|
|---|---|---|
| Vehicle | CORT | |
| Acute | 1.1 ± 0.2 | 1.7 ± 0.1* |
| Chronic | 1.8 ± 0.1 | 1.6 ± 0.1 |
*P < 0.05 compared with respective vehicle-treated group.
Both chronic vehicle and CORT treatment caused dendritic expansion as compared with acute vehicle treatment (Fig. 1 C and D; > 79% increase in total dendritic length; P < 0.0001) and to equivalent extents (P > 0.15). Representative camera lucida drawings of neuron from the different treatment groups are depicted in Fig. 2 for qualitative comparison. Moreover, both treatments increased BLA volume (Table 1) and to equivalent extents.
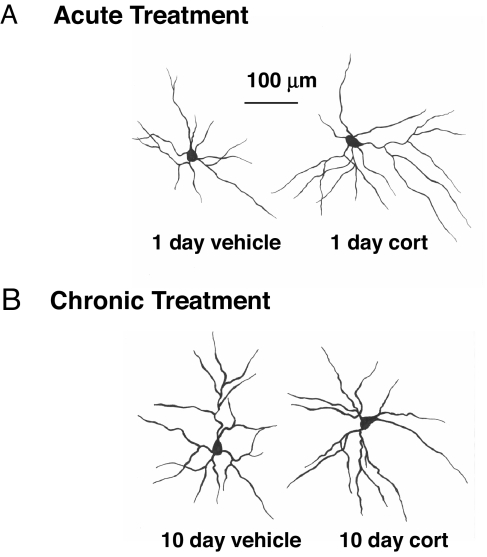
Fig. 2.
Representative camera lucida drawing of neurons from animals treated either acutely (A) or chronically (B) with CORT (Right) compared with their respective vehicle-treated controls (Left).
A detailed segmental analysis was performed by measuring dendritic arborization as a function of radial distance from the soma (Sholl analysis; Fig. 3B Inset). The values for the initial nine segments was analyzed by using two-way ANOVA with repeated measures (treatment and duration as intersubject source of variance; segments as intrasubject variance; Table S1). Intersubject analysis revealed significant main effects of duration (chronic > acute; P < 0.01), of treatment (CORT > vehicle; P < 0.001), and of their interaction (P < 0.01); the only exception was a nonsignificant main effect of treatment in terms of branch point numbers. Similarly, intrasubject analysis revealed significant main effect of segments and of their interaction with group and duration (P < 0.01).
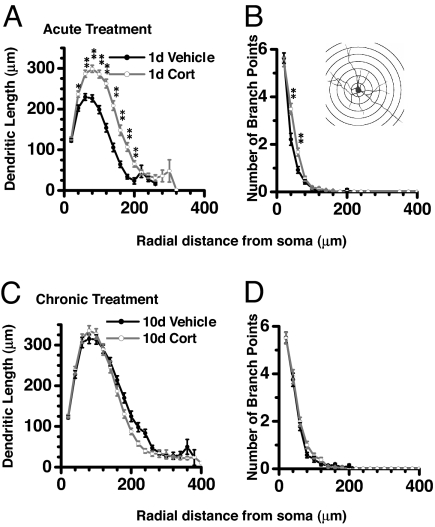
Fig. 3.
Acute CORT treatment caused an increase in dendritic arborization along a wide range of dendritic segments (A and B) in contrast to chronic CORT treatment, which did not show any difference with respective control (C and D). Ordinates depict number of dendritic length or number of branch points as a function of radial distance from soma depicted in abscissa. (Inset) A representative camera lucida drawing with various radial segments used for the analysis. *, P < 0.05; **, P < 0.01 (Student's t test).
Segmental analysis showed that, in the case of acute CORT treatment, dendritic expansion was distributed along a wide range of dendritic segments (Fig. 3 A and B). For example, acute CORT significantly increased dendritic length at radial distances between 25 μm and 225 μm from the soma (P < 0.05; Fig. 3A).
Unlike acute treatment, segmental dendritic measurements were comparable between neurons from animals treated chronically with either CORT or vehicle (Fig. 3 C and D).
Effects of CORT on Anxiety.
Anxiety in the elevated plus maze was measured in terms of reduction in open-arm exploration. A two-way ANOVA revealed significant interaction (P ≤ 0.05) between treatment and duration for both percentage open-arm entries and percentage open-arm time (Table S1). The number of enclosed-arm entries did not show significant main effects of treatment and duration or of their interaction (P > 0.2).
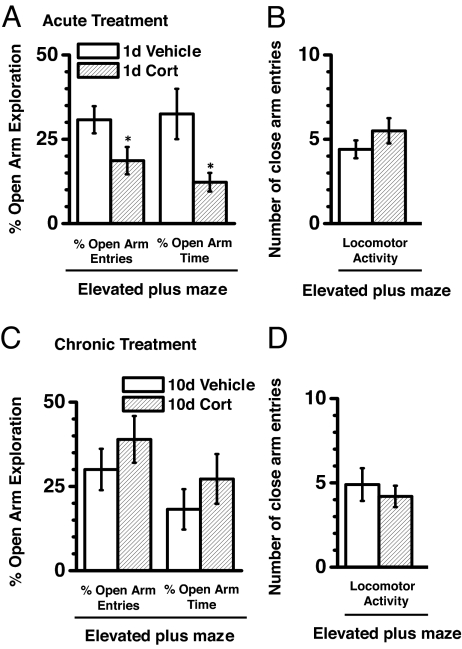
As compared with acute vehicle-treated rats, acute CORT treatment reduced open-arm exploration, an indication of enhanced anxiety (Fig. 4A; 39% reduction in percentage open-arm entries and 63% reduction in percentage open-arm time; P < 0.05) amid no change in locomotor activity (Fig. 4B; P > 0.2). In contrast, these CORT effects on anxiety were not demonstrable 1 day after acute treatment (P > 0.35), suggesting that a temporal delay was necessary for the effect of CORT treatment on anxiety.
Fig. 4.
Acute CORT treatment increased anxiety in elevated plus maze. (A) CORT-treated animals (n = 18) exhibited reduced open-arm exploration in terms of both open-arm entries (left set of bars) and open-arm time (right set of bars) compared with vehicle-treated animals (n = 18). *, P < 0.05 (Student's t test). Both vehicle- and CORT-treated animals exhibited similar locomotor activity as measured in closed-arm entries of elevated plus maze (B). Chronic CORT (n = 17) and vehicle (n = 11) treatment resulted in similar open-arm exploration as evident from both the number of open arm entries (C, left bars) and open arm time (C, right bars) along with similar locomotion (D). Values are mean ± SEM.
Consistent with the observed similarities in dendritic arborization, chronic, vehicle, or CORT treatment did not alter open-arm exploration (one-way ANOVA, P > 0.5; Fig. 4C) or locomotor activity (number of closed-arm entries: vehicle-treated: 4.9 ± 0.97; CORT-treated: 4.2 ± 0.63; P > 0.6; Fig. 4D). Additionally the open-arm exploration in chronically treated groups was comparable to untreated naïve control animals (one-way ANOVA, P > 0.5).
Effects of CORT on Fear Conditioning and Extinction.
Performance of animals during training, testing, and extinction of fear conditioning was analyzed by using two-way ANOVA with repeated measures. None of the parameters exhibited significant interaction between treatment and duration (Table S1).
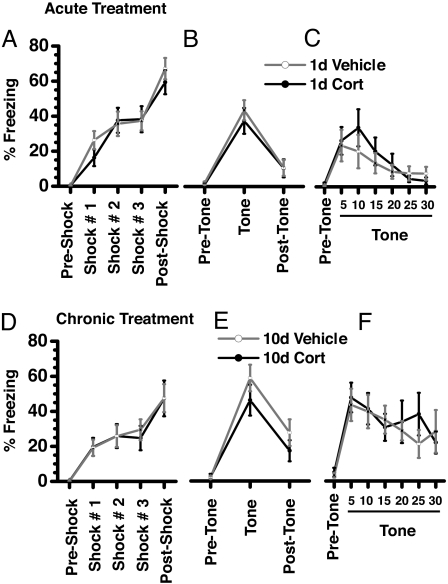
Foot shocks delivered during the training caused a substantial and similar increase in freezing in animals acutely treated with either vehicle or CORT (Fig. 5A). Both experimental groups demonstrated conditioning comparable to the auditory cue, as determined by a similar amount of time freezing (Fig. 5B). During the exposure to conditioned tone, all experimental groups exhibited similar temporal profiles of freezing (two-way ANOVA with repeated measures, P > 0.05). When presented with 30 successive tones 1 day after testing (Fig. 5C), time spent in freezing by both groups dropped steadily, showing no significant difference in extinction of fear memory.
Fig. 5.
Neither acute (A–C) nor chronic (D–F) CORT treatment affected fear conditioning of an auditory tone to foot shock. The ordinate depicts the percentage of time spent in freezing. Both vehicle-treated (n = 12 for acute and chronic treatment) and CORT-treated (n = 15 for acute and n = 12 for chronic treatment) animals exhibited similar levels of freezing during training (A and D), testing for strength of conditioning to auditory cue (B and E), and extinction of cued conditioning (C and F).
Similar to acute treatment, animals chronically treated with either vehicle or CORT were comparable in terms of fear conditioning (Fig. 5D), freezing to auditory tone (Fig. 5E), and extinction of auditory fear conditioning (Fig. 5F).
Effect of CORT on Physiological Parameters.
Among the various groups, there were no differences in relative body weight gain during the experiment, adrenal weight, or thymus weight, as revealed by insignificant main effects and interaction in two-way ANOVA (Table S1).
The initial CORT injection raised plasma CORT concentrations 30 min after injection (median baseline before injection = 2.7 ng/ml; after CORT = 229; P < 0.05, Mann–Whitney test; n = 5). The initial vehicle injection, on the other hand, did not raise CORT levels 30 min later (P > 0.05). However, vehicle injection increased plasma CORT concentration beginning on the third day of daily injections, having habituated back to the baseline by the ninth day (Table S2). The peak CORT recorded during repeated injections was significantly different from the baseline (median baseline CORT = 2.1, peak CORT = 9.7; P < 0.05).
Plasma CORT concentrations were also determined at a time point when behavior and morphological experiments were initially conducted (i.e., 12 days after the single CORT injection in the acute group and 2 days after the final injection in the chronic group; Table 2). It is expected that these measurements were independent of exogenously applied CORT by virtue of being at least 48 h after the last exogenous CORT of vehicle treatment. There was no significant difference among any of the groups (two-way ANOVA, Table S1).
Table 2.
Basal CORT levels in animals treated with acute or chronic CORT
| Treatment | Basal CORT level, ng/ml of plasma |
|
|---|---|---|
| Vehicle | CORT | |
| Acute | 4.08 ± 1.3 | 4.4 ± 1.5 |
| Chronic | 4.7 ± 1.3 | 5.3 ± 1.3 |
Discussion
This study explored dendritic and behavioral changes brought about by acute (1-day) and chronic (10-day) CORT treatment. Acute CORT treatment caused dendritic expansion in the BLA and enhanced anxiety 12 days after treatment; chronic CORT treatment caused no further changes in either of these endpoints. Chronic vehicle treatment produced significant dendritic expansion relative to acute vehicle treatment, such that the magnitude of dendritic arborization was similar to that in chronic CORT animals. Neither acute nor chronic CORT treatment altered the learned fear response.
CORT-Induced BLA Hypertrophy.
The ability of glucocorticoids to induce structural plasticity in the BLA has not been previously investigated despite the crucial role of the amygdala in regulation of stress hormone secretion (11–13) and the presence of a high concentration of glucocorticoid receptors in the BLA (15). Data presented here support the notion that acute exposure to stress hormones is sufficient to cause long-term plasticity in the BLA. In fact, the magnitude of hypertrophy observed in this study appears greater than that previously observed during chronic stress (8, 9). The robustness and induction of a long-lasting effect by an acute manipulation makes these results relevant to the long-term response to trauma, gene manipulation of amygdala plasticity, and emotional regulation (19, 20).
Rapid and persistent expression of plasticity induced by aversive experience can be crucial for survival. Such plasticity occurs in the amygdala. For example, a single exposure to a cat can cause long-lasting plasticity in the amygdala and amygdala-dependent behaviors (19). Similarly, a single acute session of stress is sufficient to cause spine changes in the BLA (8), even though this manipulation does not cause dendritic hypertrophy. Another important feature of the data reported here is the temporal delay, in that dendritic expansion and behavioral changes were present 12 days, but not 1 day, after a single CORT treatment. This indicates that BLA expansion is a delayed consequence of CORT exposure.
Possible Cellular Mechanisms Underlying Dendritic Expansion.
Glucocorticoids increase excitability of amygdala neurons by reducing inhibitory GABA currents (21). Acute glucocorticoid application in BLA slices can also enhance the magnitude of Ca2+ currents and expression of Ca2+ channel subunits (22). Such greater intake of Ca2+ could trigger cytoarchitectural changes and result in neuronal reorganization. For example, activation of Ca2+ permeable NMDA channels in Xenopus tectal neurons promotes dendritic outgrowth (23, 24). In rat motorneurons, in vivo delivery of DNA coding for GluR1, a subunit of glutamatergic AMPA channel, results in enhanced branching of the neurons (25). Similarly, neuronal activity induced by seizures is known to influence neuronal structure, plausibly through increased Ca2+ entry (26). Thus, the GC effects on neuronal excitability and on cytosolic calcium levels might plausibly influence neuronal structure of BLA neurons. Furthermore, activation of glucocorticoid receptors can directly influence gene transcription by binding to their specific promoter leading to long-term changes in expression of genes related to cytoarchitectural reorganization (27–31).
Functional Consequences of BLA Hypertrophy.
Dendritic expansion can directly influence electrical properties of the neuron ranging from altered passive electrotonic properties to greater surface area for receiving synaptic inputs (32). In addition, electrical properties of neurons can substantially change if active voltage-dependant channels are being added on the newly generated dendritic surface. Such enhanced synaptic connectivity and hyperexcitability of the amygdala can potentially have a variety of consequences. Given the stimulating role of amygdala on the HPA axis (12, 13), amygdaloid expansion could potentially enhance glucocorticoid secretion. A related consequence concerns the fact that the amygdala modulates hippocampal functions (3, 33–35), thus influencing mnemonic functions related to stress. For example, the BLA is essential for memory-enhancing effects of glucocorticoids in hippocampus. The amygdala plays an integral stimulatory role in the central autonomic network through which the brain controls several sympathetic visceromotor responses (36, 37). Thus, amygdaloid expansion could augment such response.
We report enhanced anxiety in response to acute CORT administration, in agreement with prior reports. For example, exposure of a rat to a cat causes a long-lasting increase in anxiety, a phenomenon known to depend on the BLA (19) and on the stress-induced secretion of CORT (38, 39).
We report that acute CORT is sufficient to induce a long-lasting increase in anxiety and dendritic architecture of BLA neurons. Based on co-occurrence reported here and previous reports (9, 40), it can be suggested that dendritic expansion in BLA is related to anxiety. This is consistent with the idea that activation of BLA is crucial for emotional regulation and that BLA plasticity is important for emotional change (20, 41–45). The relationship between dendritic architecture and behavioral changes has been studied extensively in the context of stress-induced hippocampal atrophy and spatial memory deficits (1, 7). In the recent past, several studies have focused on a similar relationship between behavioral change and cellular plasticity in the amygdala. This study adds to this emerging body of literature. Nonetheless, it should be noted that evidence for relationship between structural and behavioral changes in the amygdala is based on a small number of studies until now and is purely correlational in nature.
Glucocorticoids are important endocrine mediators of stress reaction. Additionally, their role in emotions, anxiety, and aversive memories has also been investigated independent of stress paradigms. For example, baseline CORT levels can predict predisposition to posttraumatic stress disorder in animal models (46, 47). Similarly, CORT has also been studied as an important component of associative learning in rat pups, inhibitory avoidance learning in rats, and modulation by the amygdala of hippocampus-dependent tasks (48–52). Data presented in this article could have relevance to these paradigms.
Despite having a robust effect on amygdaloid neurons and anxiety, acute CORT treatment did not influence conditioned fear. The BLA is known to be critical for both fear conditioning and anxiety. Previous studies have shown that glucocorticoids influence both conditioning to the aversive stimuli and extinction of fear conditioning (53, 54). An important difference between these and the present study is the time elapsed between CORT treatment and behavioral measurements. Whereas previous studies measured fear conditioning and extinction acutely (within 24–48 h), in our experiment 12 days elapsed before behavioral endpoints (53, 54). Our results suggest that effects of CORT on fear conditioning are not as long-lasting as those on anxiety. Long-term effects of acute amygdala manipulation on anxiety, on the other hand, have been reported several times in the literature (19, 20, 40).
As shown, acute CORT treatment caused dendritic expansion, relative to acute vehicle treatment. Chronic CORT treatment caused the same degree of expansion, suggesting that the single injection of the acute group was sufficient to produce a ceiling effect. Interestingly, chronic treatment with vehicle itself caused a dendritic expansion comparable to animals undergoing acute or chronic CORT treatments. Furthermore, the examination revealed that chronic injections with vehicle enhanced CORT secretion. Such activation habituated by the ninth day of injections, in agreement with previous reports (55, 56). Although not comparable in magnitude to the increase in plasma CORT in the CORT-treated animals, activation of endogenous CORT during chronic vehicle treatment was cumulatively sufficient to cause dendritic expansion.
In conclusion, this study demonstrates sustained structural and behavioral plasticity in the BLA in response to acute CORT treatment. This could have relevance in understanding long-lasting neurobiological effects of glucocorticoids and of acute stress.
Materials and Methods
Experimental Animals.
Adult male Wistar rats (10–11 weeks of age at the beginning of experiments) were used. All animals were housed in groups of three with access to food and water ad libitum. Vehicle-treated and CORT-treated animals were housed separately. Animals were maintained in a temperature-controlled room, with a light/dark cycle of 14:10 h (lights on at 0700 hours). All procedures related to animal maintenance and experimentation were approved by the Stanford University Administrative Panel on Laboratory Animal Care (APLAC) and were in accordance with animal care standards outlined in National Institutes of Health guidelines.
CORT Treatment.
Rats, randomly assigned to experimental groups, were subjected to either acute or chronic CORT treatment. Acute treatment consisted of a single s.c. injection of CORT (10 mg/kg of body weight) dissolved in peanut oil. This dose is known to result in a CORT level comparable to that of several hours of high physiological stress (57). Morphological and behavioral endpoints were measured 12 days after the acute injection. Chronic treatment consisted of 10 successive daily s.c. injections of CORT (10 mg/kg), a dose resulting in physiological levels of CORT comparable to that of chronic exposure to major stressors. Morphological and behavioral parameters were quantified 2 days after the last injection. Animals treated with either acute or chronic vehicle (peanut oil) served as respective controls. Separate sets of animals were used for morphological and behavioral studies to dissociate any influence of behavioral testing on morphological parameters measured.
Tissue Preparation.
After completion of CORT treatment, animals were killed under deep anesthesia. Brain tissue was removed quickly, and blocks of tissue containing the amygdala were dissected out and processed for rapid Golgi staining. Coronal sections (120 μm thick) were prepared by using a rotary microtome (Leica RM2155). Sections were collected serially, dehydrated in absolute alcohol, cleared in xylene, and coverslipped. Slides were coded before quantitative analysis, and the code was broken only after the analysis was completed.
Quantification of Dendritic Arborization.
To be selected for analysis, Golgi-impregnated neurons (10 neurons per animal) had to satisfy the following criteria, which have been applied in similar morphometric studies (8, 9): (i) the presence of untruncated dendrites, (ii) consistent and dark impregnation along the entire extent of all dendrites, and (iii) relative isolation from neighboring impregnated neurons to avoid interfering with analysis. Both spiny pyramidal-like and stellate neurons from the BLA were selected for analysis on the basis of morphological criteria described in the literature (9). Our analysis of BLA neurons was restricted to those located between bregma −2.3 mm (medial/lateral, 4.8 to 5.1 mm; dorsal/ventral, 6.7 to 7.5 mm from dura) and bregma −3.6 mm (medial/lateral, 4.8 to 5.2 mm; dorsal/ventral, 5.6 to 7.6 mm from dura).
Camera lucida tracings (×500X were obtained (Nikon Phase Contrast) from selected neurons and then scanned (eight-bit grayscale TIFF images with 1,200-dpi resolution; Canon MultiPass MP360) along with a calibrated scale for subsequent computerized image analysis. Custom-designed macros embedded in NIH Image software were used for morphometric analysis of digitized images. Total dendritic length and total number of branch points were calculated. In addition, a detailed segmental analysis was performed. By using the center of the soma as a reference point, dendritic length and branch points were measured as a function of radial distance from the soma by adding up all values in each successive concentric segment (Sholl's analysis; segment diameter, 25 μm; Fig. 3B Inset).
Estimation of Amygdala Volume.
Brains were harvested after transcardial perfusion with PBS (100 ml) followed by 4% paraformaldehyde in PBS (200 ml). Coronal sections (50 μm thick) were collected serially and stained with Nissl stain. A systematic random series of one-in-six section was used for volume measurement of BLA using the Cavelieri principle.
Elevated Plus Maze.
The elevated plus maze consisted of two opposite open arms (60 × 15 cm) and two enclosed arms (60 × 15 cm, surrounded by a 15-cm-high black wall) elevated 75 cm from the ground. Individual trials lasted 5 min each. At the beginning of each trial, animals were placed at the center of the maze, facing an enclosed arm. The maze was cleaned with 70% (vol/vol) ethanol solution after each trial. The number of entries and the time spent in open arms were measured in addition to the number of entries in enclosed arms. Open-arm exploration was measured by normalizing open-arm exploration (entries and time spent) to total exploration (entries in open plus enclosed arms and total duration of trial, respectively). In this paradigm, anxiety is measured as a function of decreased open-arm exploration (58).
Fear Conditioning.
Rats were conditioned in two modified observation chambers (30 × 24 × 40 cm; MedAssociates). A load-cell platform, which was located beneath chambers, recorded locomotor activity of rats by measuring chamber displacement. Freezing was quantified as the endpoint and was defined as the cessation of all movements except breathing. Conditioning consisted of three successive presentations of auditory tones (5 KHz, 80 dB, 10 s, intertrial duration = 90 s) coterminating with footshock (1 mA, 1 s). The next day, the strength of conditioning to auditory cue was measured as freezing in response to a continuous tone (3 min, 5 KHz, 80 dB) in a different context. The next day, rats were presented with 30 successive auditory tones (5 KHz, 80 dB, 10 s, intertrial duration = 50 s) to measure the extinction of cued fear conditioning. Both fear to tone and extinction of fear to tone were tested to address whether glucocorticoid treatment influenced any of these independent of influencing other generalized fear, like anxiety.
Plasma CORT Concentration.
Concentration of CORT in plasma was quantified by using enzyme-linked immunoassay. A separate set of animals was used for these measurements to avoid the effect of blood collection on anatomical or behavioral endpoints. Tail vein blood was collected in heparinized microcapillary tubes and centrifuged (centrifuge model 5415C; Eppendorf) at 10,000 rpm for 10 min to obtain plasma, and CORT titers were assessed by using a competitive enzyme immunoassay kit (Assay Design). To assess the effects of vehicle or CORT injection, blood was collected 30 min after treatment. To assess the effects of chronic vehicle injection, blood was collected on alternate days during 10 days of treatment; peak CORT was defined as the maximum level of CORT obtained. Additionally, in a separate set of animals, blood was collected at a time point when behavior and morphological experiments were initially conducted.
Statistical Analysis.
Values are reported as mean ± SEM, and percentage changes are calculated with respect to corresponding control values. Statistical analysis was performed by using two-way ANOVA with treatment (CORT or vehicle) and duration (acute or chronic) as intersubject sources of variance. In circumstances where an intrasubject source of variance was present, analysis was performed by using two-way ANOVA with repeated measures. Significant effects of ANOVA were further analyzed post hoc with Student's t test. Effects of treatment on CORT levels were assessed by using the nonparametric Mann–Whitney U test.
Acknowledgments.
This study was funded by National Institutes of Health Grant RO1 AGO20633.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0705615105/DCSupplemental.
References
- 1.Watanabe Y, Gould E, McEwen BS. Stress induces atrophy of apical dendrites of hippocampal CA3 pyramidal neurons. Brain Res. 1992;588:341–345. doi: 10.1016/0006-8993(92)91597-8. [DOI] [PubMed] [Google Scholar]
- 2.McEwen BS. Effects of adverse experiences for brain structure and function. Biol Psychiatry. 2000;48:721–731. doi: 10.1016/s0006-3223(00)00964-1. [DOI] [PubMed] [Google Scholar]
- 3.Kim JJ, Diamond DM. The stressed hippocampus, synaptic plasticity and lost memories. Nat Rev Neurosci. 2002;3:453–462. doi: 10.1038/nrn849. [DOI] [PubMed] [Google Scholar]
- 4.Conrad CD, Galea LA, Kuroda Y, McEwen BS. Chronic stress impairs rat spatial memory on the Y maze, and this effect is blocked by tianeptine pretreatment. Behav Neurosci. 1996;110:1321–1334. doi: 10.1037//0735-7044.110.6.1321. [DOI] [PubMed] [Google Scholar]
- 5.Conrad CD. What is the functional significance of chronic stress-induced CA3 dendritic retraction within the hippocampus? Behav Cognit Neurosci Rev. 2006;5:41–60. doi: 10.1177/1534582306289043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sousa N, Lukoyanov NV, Madeira MD, Almeida OF, Paula-Barbosa MM. Reorganization of the morphology of hippocampal neurites and synapses after stress-induced damage correlates with behavioral improvement. Neuroscience. 2000;97:253–266. doi: 10.1016/s0306-4522(00)00050-6. [DOI] [PubMed] [Google Scholar]
- 7.Luine V, Villegas M, Martinez C, McEwen BS. Repeated stress causes reversible impairments of spatial memory performance. Brain Res. 1994;639:167–170. doi: 10.1016/0006-8993(94)91778-7. [DOI] [PubMed] [Google Scholar]
- 8.Mitra R, Jadhav S, McEwen BS, Vyas A, Chattarji S. Stress duration modulates the spatiotemporal patterns of spine formation in the basolateral amygdala. Proc Natl Acad Sci USA. 2005;102:9371–9376. doi: 10.1073/pnas.0504011102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vyas A, Mitra R, Shankaranarayana Rao BS, Chattarji S. Chronic stress induces contrasting patterns of dendritic remodeling in hippocampal and amygdaloid neurons. J Neurosci. 2002;22:6810–6818. doi: 10.1523/JNEUROSCI.22-15-06810.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vyas A, Chattarji S. Modulation of different states of anxiety-like behavior by chronic stress. Behav Neurosci. 2004;118:1450–1454. doi: 10.1037/0735-7044.118.6.1450. [DOI] [PubMed] [Google Scholar]
- 11.Smith SM, Vale WW. The role of the hypothalamic-pituitary-adrenal axis in neuroendocrine responses to stress. Dialogues Clin Neurosci. 2006;8:383–395. doi: 10.31887/DCNS.2006.8.4/ssmith. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Herman JP, Prewitt CM, Cullinan WE. Neuronal circuit regulation of the hypothalamo-pituitary-adrenocortical stress axis. Crit Rev Neurobiol. 1996;10:371–394. doi: 10.1615/critrevneurobiol.v10.i3-4.50. [DOI] [PubMed] [Google Scholar]
- 13.Herman JP, et al. Central mechanisms of stress integration: Hierarchical circuitry controlling hypothalamo-pituitary-adrenocortical responsiveness. Front Neuroendocrinol. 2003;24:151–180. doi: 10.1016/j.yfrne.2003.07.001. [DOI] [PubMed] [Google Scholar]
- 14.Joels M. Corticosteroid actions in the hippocampus. J Neuroendocrinol. 2001;13:657–669. doi: 10.1046/j.1365-2826.2001.00688.x. [DOI] [PubMed] [Google Scholar]
- 15.Gray TS, Bingaman EW. The amygdala: Corticotropin-releasing factor, steroids, and stress. Crit Rev Neurobiol. 1996;10:155–168. doi: 10.1615/critrevneurobiol.v10.i2.10. [DOI] [PubMed] [Google Scholar]
- 16.McEwen BS. Corticosteroids and hippocampal plasticity. Ann NY Acad Sci. 1994;746:134–142. doi: 10.1111/j.1749-6632.1994.tb39223.x. discussion 142–134 and 178–139. [DOI] [PubMed] [Google Scholar]
- 17.Watanabe Y, Gould E, Cameron HA, Daniels DC, McEwen BS. Phenytoin prevents stress- and corticosterone-induced atrophy of CA3 pyramidal neurons. Hippocampus. 1992;2:431–435. doi: 10.1002/hipo.450020410. [DOI] [PubMed] [Google Scholar]
- 18.Magarinos AM, McEwen BS. Stress-induced atrophy of apical dendrites of hippocampal CA3c neurons: Involvement of glucocorticoid secretion and excitatory amino acid receptors. Neuroscience. 1995;69:89–98. doi: 10.1016/0306-4522(95)00259-l. [DOI] [PubMed] [Google Scholar]
- 19.Adamec RE, Burton P, Shallow T, Budgell J. Unilateral block of NMDA receptors in the amygdala prevents predator stress-induced lasting increases in anxiety-like behavior and unconditioned startle—effective hemisphere depends on the behavior. Physiol Behav. 1999;65:739–751. doi: 10.1016/s0031-9384(98)00225-x. [DOI] [PubMed] [Google Scholar]
- 20.Rainnie DG, et al. Corticotrophin releasing factor-induced synaptic plasticity in the amygdala translates stress into emotional disorders. J Neurosci. 2004;24:3471–3479. doi: 10.1523/JNEUROSCI.5740-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Duvarci S, Pare D. Glucocorticoids enhance the excitability of principal basolateral amygdala neurons. J Neurosci. 2007;27:4482–4491. doi: 10.1523/JNEUROSCI.0680-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Karst H, et al. Glucocorticoids alter calcium conductances and calcium channel subunit expression in basolateral amygdala neurons. Eur J Neurosci. 2002;16:1083–1089. doi: 10.1046/j.1460-9568.2002.02172.x. [DOI] [PubMed] [Google Scholar]
- 23.Rajan I, Cline HT. Glutamate receptor activity is required for normal development of tectal cell dendrites in vivo. J Neurosci. 1998;18:7836–7846. doi: 10.1523/JNEUROSCI.18-19-07836.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lohmann C, Wong RO. Regulation of dendritic growth and plasticity by local and global calcium dynamics. Cell Calcium. 2005;37:403–409. doi: 10.1016/j.ceca.2005.01.008. [DOI] [PubMed] [Google Scholar]
- 25.Inglis FM, et al. The AMPA receptor subunit GluR1 regulates dendritic architecture of motor neurons. J Neurosci. 2002;22:8042–8051. doi: 10.1523/JNEUROSCI.22-18-08042.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wong M. Modulation of dendritic spines in epilepsy: Cellular mechanisms and functional implications. Epilepsy Behav. 2005;7:569–577. doi: 10.1016/j.yebeh.2005.08.007. [DOI] [PubMed] [Google Scholar]
- 27.Brandt PC, Vanaman TC. Elevated glucocorticoid receptor transactivation and down-regulation of alpha 1 integrin are associated with loss of plasma membrane Ca2+-ATPase isoform 1. J Biol Chem. 2000;275:24534–24539. doi: 10.1074/jbc.M003388200. [DOI] [PubMed] [Google Scholar]
- 28.Kye MJ, Spiess J, Blank T. Transcriptional regulation of intronic calcium-activated potassium channel SK2 promoters by nuclear factor-kappa B and glucocorticoids. Mol Cell Biochem. 2007;300:9–17. doi: 10.1007/s11010-006-9320-6. [DOI] [PubMed] [Google Scholar]
- 29.Baumann H, Jahreis GP, Morella KK. Interaction of cytokine- and glucocorticoid-response elements of acute-phase plasma protein genes. Importance of glucocorticoid receptor level and cell type for regulation of the elements from rat alpha 1-acid glycoprotein and beta-fibrinogen genes. J Biol Chem. 1990;265:22275–22281. [PubMed] [Google Scholar]
- 30.Li G, Wang S, Gelehrter TD. Identification of glucocorticoid receptor domains involved in transrepression of transforming growth factor-beta action. J Biol Chem. 2003;278:41779–41788. doi: 10.1074/jbc.M305350200. [DOI] [PubMed] [Google Scholar]
- 31.Schaaf MJ, De Kloet ER, Vreugdenhil E. Corticosterone effects on BDNF expression in the hippocampus. Implications for memory formation. Stress. 2000;3:201–208. doi: 10.3109/10253890009001124. [DOI] [PubMed] [Google Scholar]
- 32.Spruston N, Jaffe DB, Johnston D. Dendritic attenuation of synaptic potentials and currents: The role of passive membrane properties. Trends Neurosci. 1994;17:161–166. doi: 10.1016/0166-2236(94)90094-9. [DOI] [PubMed] [Google Scholar]
- 33.Roozendaal B, Portillo-Marquez G, McGaugh JL. Basolateral amygdala lesions block glucocorticoid-induced modulation of memory for spatial learning. Behav Neurosci. 1996;110:1074–1083. doi: 10.1037//0735-7044.110.5.1074. [DOI] [PubMed] [Google Scholar]
- 34.Vouimba RM, Yaniv D, Richter-Levin G. Glucocorticoid receptors and beta-adrenoceptors in basolateral amygdala modulate synaptic plasticity in hippocampal dentate gyrus, but not in area CA1. Neuropharmacology. 2007;52:244–252. doi: 10.1016/j.neuropharm.2006.07.007. [DOI] [PubMed] [Google Scholar]
- 35.Kim JJ, Koo JW, Lee HJ, Han JS. Amygdalar inactivation blocks stress-induced impairments in hippocampal long-term potentiation and spatial memory. J Neurosci. 2005;25:1532–1539. doi: 10.1523/JNEUROSCI.4623-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Peper M, Herpers M, Spreer J, Hennig J, Zentner J. Functional neuroimaging of emotional learning and autonomic reactions. J Physiol Paris. 2006;99:342–354. doi: 10.1016/j.jphysparis.2006.03.006. [DOI] [PubMed] [Google Scholar]
- 37.Zald DH. The human amygdala and the emotional evaluation of sensory stimuli. Brain Res Brain Res Rev. 2003;41:88–123. doi: 10.1016/s0165-0173(02)00248-5. [DOI] [PubMed] [Google Scholar]
- 38.Adamec RE, Blundell J, Burton P. Relationship of the predatory attack experience to neural plasticity, pCREB expression and neuroendocrine response. Neurosci Biobehav Rev. 2006;30:356–375. doi: 10.1016/j.neubiorev.2005.04.004. [DOI] [PubMed] [Google Scholar]
- 39.Adamec R, Muir C, Grimes M, Pearcey K. Involvement of noradrenergic and corticoid receptors in the consolidation of the lasting anxiogenic effects of predator stress. Behav Brain Res. 2007;179:192–207. doi: 10.1016/j.bbr.2007.02.001. [DOI] [PubMed] [Google Scholar]
- 40.Vyas A, Pillai AG, Chattarji S. Recovery after chronic stress fails to reverse amygdaloid neuronal hypertrophy and enhanced anxiety-like behavior. Neuroscience. 2004;128:667–673. doi: 10.1016/j.neuroscience.2004.07.013. [DOI] [PubMed] [Google Scholar]
- 41.Anand A, Shekhar A. Brain imaging studies in mood and anxiety disorders: Special emphasis on the amygdala. Ann NY Acad Sci. 2003;985:370–388. doi: 10.1111/j.1749-6632.2003.tb07095.x. [DOI] [PubMed] [Google Scholar]
- 42.Evans KC, et al. A functional MRI study of amygdala responses to angry schematic faces in social anxiety disorder. Depress Anxiety. 2007 Jun 26; doi: 10.1002/da.20347. [DOI] [PubMed] [Google Scholar]
- 43.Lesscher HM, et al. Amygdala protein kinase C epsilon regulates corticotropin-releasing factor and anxiety-like behavior. Genes Brain Behav. 2007 Oct 1; doi: 10.1111/j.1601-183X.2007.00356.x. [DOI] [PubMed] [Google Scholar]
- 44.Walker DL, Toufexis DJ, Davis M. Role of the bed nucleus of the stria terminalis versus the amygdala in fear, stress, and anxiety. Eur J Pharmacol. 2003;463:199–216. doi: 10.1016/s0014-2999(03)01282-2. [DOI] [PubMed] [Google Scholar]
- 45.Wu LJ, et al. Increased anxiety-like behavior and enhanced synaptic efficacy in the amygdala of GluR5 knockout mice. PLoS ONE. 2007;2:e167. doi: 10.1371/journal.pone.0000167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cohen H, et al. Decreased circulatory levels of neuroactive steroids in behaviourally more extremely affected rats subsequent to exposure to a potentially traumatic experience. Int J Neuropsychopharmacol. 2007;10:203–209. doi: 10.1017/S146114570600664X. [DOI] [PubMed] [Google Scholar]
- 47.Cohen H, et al. Blunted HPA axis response to stress influences susceptibility to posttraumatic stress response in rats. Biol Psychiatry. 2006;59:1208–1218. doi: 10.1016/j.biopsych.2005.12.003. [DOI] [PubMed] [Google Scholar]
- 48.Roozendaal B, Griffith QK, Buranday J, De Quervain DJ, McGaugh JL. The hippocampus mediates glucocorticoid-induced impairment of spatial memory retrieval: Dependence on the basolateral amygdala. Proc Natl Acad Sci USA. 2003;100:1328–1333. doi: 10.1073/pnas.0337480100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Roozendaal B, Hahn EL, Nathan SV, de Quervain DJ, McGaugh JL. Glucocorticoid effects on memory retrieval require concurrent noradrenergic activity in the hippocampus and basolateral amygdala. J Neurosci. 2004;24:8161–8169. doi: 10.1523/JNEUROSCI.2574-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Roozendaal B, McReynolds JR, McGaugh JL. The basolateral amygdala interacts with the medial prefrontal cortex in regulating glucocorticoid effects on working memory impairment. J Neurosci. 2004;24:1385–1392. doi: 10.1523/JNEUROSCI.4664-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Moriceau S, Sullivan RM. Corticosterone influences on mammalian neonatal sensitive-period learning. Behav Neurosci. 2004;118:274–281. doi: 10.1037/0735-7044.118.2.274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Moriceau S, Wilson DA, Levine S, Sullivan RM. Dual circuitry for odor-shock conditioning during infancy: Corticosterone switches between fear and attraction via amygdala. J Neurosci. 2006;26:6737–6748. doi: 10.1523/JNEUROSCI.0499-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Roozendaal B, et al. Basolateral amygdala noradrenergic activity mediates corticosterone-induced enhancement of auditory fear conditioning. Neurobiol Learn Mem. 2006;86:249–255. doi: 10.1016/j.nlm.2006.03.003. [DOI] [PubMed] [Google Scholar]
- 54.Yang YL, Chao PK, Lu KT. Systemic and intra-amygdala administration of glucocorticoid agonist and antagonist modulate extinction of conditioned fear. Neuropsychopharmacology. 2006;31:912–924. doi: 10.1038/sj.npp.1300899. [DOI] [PubMed] [Google Scholar]
- 55.Pitman DL, Ottenweller JE, Natelson BH. Plasma corticosterone levels during repeated presentation of two intensities of restraint stress: Chronic stress and habituation. Physiol Behav. 1988;43:47–55. doi: 10.1016/0031-9384(88)90097-2. [DOI] [PubMed] [Google Scholar]
- 56.Kant GJ, et al. Habituation to repeated stress is stressor specific. Pharmacol Biochem Behav. 1985;22:631–634. doi: 10.1016/0091-3057(85)90286-2. [DOI] [PubMed] [Google Scholar]
- 57.Stein-Behrens B, Mattson MP, Chang I, Yeh M, Sapolsky R. Stress exacerbates neuron loss and cytoskeletal pathology in the hippocampus. J Neurosci. 1994;14:5373–5380. doi: 10.1523/JNEUROSCI.14-09-05373.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Mechiel Korte S, De Boer SF. A robust animal model of state anxiety: Fear-potentiated behaviour in the elevated plus-maze. Eur J Pharmacol. 2003;463:163–175. doi: 10.1016/s0014-2999(03)01279-2. [DOI] [PubMed] [Google Scholar]