Abstract
Bordetella pertussis adenylate cyclase (AC) toxin–hemolysin (Hly) (CyaA, ACT, or AC-Hly) is a cytotoxin of the RTX (repeat in toxin) family. It delivers into target cells an AC domain that catalyzes uncontrolled conversion of ATP to cAMP, a key signaling molecule subverting phagocyte functions. CyaA utilizes a heavily N-glycosylated β2 integrin receptor CD11b/CD18 (αMβ2, Mac-1, or CR3). We show that deglycosylation of cell surface proteins by glycosidase treatment, or inhibition of protein N-glycosylation by tunicamycin, ablates CyaA binding and penetration of CD11b-expressing cells. Furthermore, binding of CyaA to cells was strongly inhibited in the presence of free saccharides occurring as building units of integrin oligosaccharide complex, whereas saccharides absent from integrin oligosaccharide chains failed to inhibit CyaA binding to CD11b/CD18-expressing cells. CyaA, hence, selectively recognized sugar residues of N-linked oligosaccharides of integrins. Moreover, glycosylation of CD11a/CD18, another receptor of the β2 integrin family, was also essential for cytotoxic action of other RTX cytotoxins, the leukotoxin of Aggregatibacter actinomycetemcomitans (LtxA) and the Escherichia coli α-Hly (HlyA). These results show that binding and killing of target cells by CyaA, LtxA, and HlyA depends on recognition of N-linked oligosaccharide chains of β2 integrin receptors. This sets a new paradigm for action of RTX cytotoxins.
Keywords: adenylate cyclase toxin, complement receptor 3
The secreted adenylate cyclase (AC) toxin–hemolysin (Hly) (CyaA, ACT, or AC-Hly) is a key virulence factor of Bordetella pertussis, the etiological agent of whooping cough (1, 2). CyaA is synthesized as a single polypeptide of 1,706 residues and consists of an amino-terminal AC domain of ≈400 residues and a pore-forming hemolysin (Hly) moiety of ≈1,306 residues (3). The Hly, itself, harbors the following: a hydrophobic pore-forming domain (residues 500–700) (4); a calcium-binding glycine and aspartate-rich nonapeptide repeats (last 700 residues); a fatty acylation domain (residues 800–1,000), where the essential posttranslational activation takes place (5); and, finally, a C-terminal secretion signal (6). The AC domain is enzymatically active by itself (7), but the entire toxin is needed for AC delivery into target cells (8), where it is activated by intracellular calmodulin and catalyzes unregulated dissipation of cytosolic ATP to the key signaling molecule, cAMP (9). This results in disruption of cellular signaling and inhibition of bactericidal functions of myeloid phagocytic cells (10). In turn, membrane-insertion and pore-forming (hemolytic) activities of CyaA do not require the AC domain and are located at the Hly portion that exhibits hemolytic activity on erythrocytes and can form small cation-selective membrane pores (4, 11).
CyaA belongs to the RTX (repeat in toxin) family of cytotoxins, which are produced by Gram-negative genera, such as Actinobacillus, Bordetella, Escherichia, Moraxella, Morganella, Pasteurella, Proteus, and Vibrio (12). These proteins are characterized by various numbers of carboxy-proximal repetitions of a nonapeptide motif, L-X-G-G-X-G-(D/N)-D-X. Some RTX cytotoxins, including the Aggregatibacter actinomycetemcomitans leukotoxin (LtxA), Escherichia coli α-Hly (HlyA), Mannheimia haemolytica leukotoxin (LktA), and CyaA of Bordetella pertussis, have recently been shown to bind to different receptors of the β2 integrin family (13–16). The β2 integrins are restricted to leukocytes and include four heterodimeric transmembrane glycoproteins sharing the same β2 subunit pairing with four distinct α subunits (17, 18): αLβ2 (CD11a/CD18, LFA-1), αMβ2 (CD11b/CD18, CR3, Mac1), αXβ2 (CD11c/CD18, p150/195), and αDβ2 (CD11d/CD18). LtxA and HlyA have been found to bind the CD11a/CD18 integrin (13), and CyaA has been found to bind the CD11b/CD18 integrin (16). Contradictory data, however, have been published for HlyA, claiming that HlyA binds nonspecifically to target cells and that no receptor is required for HlyA to provoke hemolysis or to trigger cellular reactions (19). LktA was shown initially to bind most β2 integrins (CD11a/CD18, CD11b/CD18, and CD11c/CD18), very likely through their CD18 subunit (14, 15). Later reports, however, have shown that only CD11a/CD18 is the receptor for LktA and is involved in leukotoxin-induced biological effects (20, 21).
Both α and β integrin subunits of the β2 integrins harbor several potential N-glycosylation sites (Asn-Xaa-Ser/Thr), and at least some of them appear to be modified by oligosaccharide chains linked to asparagine residues. Structural studies have revealed that β2 integrins from human leukocytes contain high mannose-type, high-molecular-weight complex-type, and a small amount of hybrid-type oligosaccharide chains, respectively (22). The complex type of N-linked oligosaccharide chains of β2 integrins is of the mono-, bi-, tri-, and tetra-antennary type, and no subunit-specific glycosylation has been observed among the α and β integrin subunits (22).
Here, we demonstrate that glycosylation of the CD11b/CD18 integrin is crucial for CyaA binding and subsequent efficient intoxication of target cells by cAMP. Competitive inhibition of CyaA binding to the integrin receptor by excess of free sugars showed that CyaA directly recognizes the N-linked oligosaccharides of CD11b/CD18. Moreover, glycosylation also was shown to be involved in cytotoxic action of the RTX leukotoxin of A. actinomycetemcomitans (LtxA) and HlyA of E. coli, which target cells via the CD11a/CD18 integrin receptor.
Results
Pretreatment of CD11b-Expressing Cells with Glycosidases Abolishes Binding of CyaA.
Binding and cytotoxic action of CyaA on myeloid cells depends on toxin interaction with the CD11b/CD18 integrin (16), which is heavily glycosylated (22). To examine the potential importance of CD11b/CD18 glycosylation for CyaA binding, we used three types of selective glycosidases able to remove N-linked oligosaccharide chains from glycoproteins (Fig. 1). Peptide-N-glycosidase F (PNGase F) was used as an amidase that cleaves the amide bond of N-linked glycoproteins between the innermost N-acetylglucosamine and asparagine residue. Endoglycosidase H (Endo H) was used to cleave within the chitobiose core of N-linked glycoproteins, and neuraminidase was used to hydrolyze α-(2→3), α-(2→6), α-(2→8)-glycosidic linkages of terminal sialic residues in glycoproteins and oligosaccharides.
Fig. 1.
Simplified schematic representation of a complex type of N-linked oligosaccharide chain of β2 integrins. The representative complex type of N-linked oligosaccharide chain includes a chitobiose core (two N-acetylglucosamine residues) linked on one side to an asparagine residue of a potential N-glycosylation site (Asn-Xaa-Ser/Thr) and, on the other side, to a trimannosyl core that is linked to an N-acetyllactosamine unit (N-acetylglucosamine and galactose). This can be repeated (n) and be either left unmodified or capped with sugars such as sialic acid. Cleavage sites for the three highly specific glycosidases, PNGase F, Endo H, and neuraminidase, respectively, are depicted by black arrows.
Chinese hamster ovary K1 cells stably transfected with human CD11b/CD18 (CHO-CD11b/CD18) were used as model target cells because their ability to bind efficiently and saturably CyaA and to be susceptible to cAMP elevation by the toxin has been demonstrated previously (16). After a 30-min preincubation of CHO-CD11b/CD18 cells with protease inhibitors and chlorpromazine (inhibitor of clathrin-dependent β2 integrin endocytosis), the cells were treated for 1 h at 37°C with PNGase F, Endo H, or neuraminidase, respectively. Washed cells were incubated with the purified CyaA/233FLAG protein (0.5 μg/ml) at 4°C for 30 min, and binding of the toxin to the cell surface was determined by flow cytometry. As shown in Fig. 2A, binding of CyaA/233FLAG was highest to CHO-CD11b/CD18 cells that were not treated by any glycosidase, whereas toxin binding was considerably reduced when sialic residues of the cell surface glycoproteins were removed by neuraminidase (22% ± 6%; expressed as a percentage of CyaA binding to untreated cells) or when all saccharide units of the cell surface glycoproteins, except of the innermost N-acetylglucosamine linked to asparagine residue, were removed by Endo H (13% ± 4%). Nearly complete loss of CyaA binding to CHO-CD11b/CD18 cells was repeatedly observed when oligosaccharide chains of cell surface glycoproteins were removed by PNGase F (5% ± 3%). To confirm that the decrease of the CyaA/233FLAG binding to deglycosylated CHO-CD11b/CD18 cells was not attributable to a reduction in the amount of surface-exposed CD11b/CD18, aliquots of glycosidase-treated cells were always tested for exposure of the β2 integrin by flow cytometry, by using an anti-CD11b mAb MEM-174. Fig. 2B, indeed, clearly shows that deglycosylation of CHO-CD11b/CD18 cells did not alter the amounts of the CD11b/CD18 receptor molecules on cell surface.
Fig. 2.
Treatment with different N-glycosidases and tunicamycin ablates binding of CyaA to the CD11b/CD18-expressing cells, whereas surface expression of CD11b/CD18 is not affected. CHO-CD11b/CD18, J774A.1, and human neutrophils were treated with neuraminidase, Endo H, PNGase F, or HBSS buffer alone for 1 h at 37°C or with or without 10 μg/ml tunicamycin at 37°C for 24 h in growth medium with 10% FCS. (A) One aliquot of the cells was incubated with 0.5 μg/ml CyaA/233FLAG for 30 min on ice, and the surface-bound toxin was detected with anti-FLAG mAb M2 and revealed by AlexaFluor488-conjugated anti-mouse antibody. (B) The second aliquot of the cells was incubated with anti-CD11b mAb MEM-174 and AlexaFluor488-conjugated anti-mouse antibody. Cells were analyzed by flow cytometry, and binding data were deduced from the MFI and expressed as percentage of CyaA or MEM-174 binding to untreated cells, as described in detail in Materials and Methods. Cells that were incubated without CyaA (A) or without MEM-174 (B) were used as negative controls. The values represent an average of three independent determinations ± SD. The asterisk indicates a statistically significant difference (P < 0.0001; Student's t test) between glycosidase- or tunicamycin-treated and untreated cells.
Because CyaA targets primarily myeloid phagocytic cells, such as macrophages or neutrophils, we verified that also removal of N-linked oligosaccharides from such cells affects CyaA activity. Deglycosylation of cell surface integrin molecules by PNGase F, Endo H, or neuraminidase resulted, indeed, in importantly decreased CyaA binding to J774A.1, a murine macrophage-like cell line expressing the CD11b/CD18 integrin receptor (Fig. 2A), and to CD11b-expressing primary human neutrophils isolated from fresh blood (Fig. 2A). Similarly, CyaA binding was abolished when cells were treated with PNGase F (6% ± 4% for J774A.1 cells and 3% ± 3% for human neutrophils), and toxin binding was significantly reduced when cells were deglycosylated with Endo H (14% ± 4% for J774A.1 cells and 15% ± 3% for human neutrophils) or neuraminidase (29% ± 3% for J774A.1 cells and 26% ± 3% for human neutrophils). Finally, as in the case of CHO-CD11b/CD18 cells, surface expression of the CD11b/CD18 integrin receptor on J774A.1 cells or on human neutrophils was not affected by deglycosylation, as detected by the MEM-174 anti-CD11b mAb (Fig. 2B).
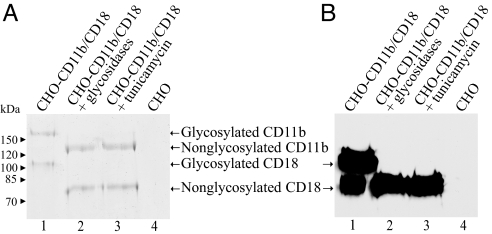
To confirm that CD11b/CD18 was efficiently deglycosylated, the integrin was immunoprecipitated from lysates of glycosidase-treated or untreated CHO-CD11b/CD18 cells by using MEM-174 anti-CD11b mAb covalently linked to agarose beads. As shown in Fig. 3, indeed, the focused protein bands corresponding to integrin subunits isolated from glycosidase-treated CHO-CD11b/CD18 cells were shifted in their molecular mass to ≈125 kDa for CD11b and to 80 kDa for CD18, respectively (lane 2), as compared with migration of integrin subunits isolated from the untreated CHO-CD11b/CD18 cells (≈160 kDa for CD11b and 100 kDa for CD18) (lane 1). This shows that treatment with the glycosidases quite efficiently removed the oligosaccharide chains of the integrin. Moreover, this experiment clearly demonstrated that the deglycosylated CD11b/CD18 heterodimer was stable and did not dissociate into individual CD11b and CD18 subunits upon deglycosylation, because the CD18 subunit coimmunoprecipitated with the CD11b subunit on anti-CD11b MEM-174 beads (Fig. 3). In conclusion, binding of CyaA to CD11b-expressing cells was lost upon deglycosylation of cell surface glycoproteins, suggesting that CyaA binding to the cell surface-expressed CD11b/CD18 integrin fully depends on its glycosylation status.
Fig. 3.
Deglycosylation of CD11b and CD18 does not induce dissociation of the CD11b/CD18 complex into individual subunits. The β2 integrin was immunoprecipitated from CHO-CD11b/CD18 cell lysates by using CD11b-specific mAb MEM-174 covalently linked to agarose beads. Upon elution, the samples were analyzed by SDS/PAGE (7.5%) with Coomassie blue staining (A) or transferred to a nitrocellulose membrane and immunodetected by a mAb MEM-48 selectively recognizing the CD18 subunit (B). Lane 1, untreated cells; lane 2, glycosidase-treated cells; lane 3, tunicamycin-treated cells; lane 4, untransfected CHO cells were used throughout the experiment as a negative control.
Inhibition of Protein Glycosylation by Tunicamycin Ablates Binding of CyaA to CD11b-Expressing Cells.
To corroborate that binding of CyaA to CD11b/CD18 depends on its glycosylation status, CHO-CD11b/CD18 and J774A.1 cells were treated for 24 h before the binding experiments with 10 μg/ml tunicamycin, an antibiotic that blocks N-glycosylation of newly synthesized proteins at asparagine residues. As documented in Fig. 2A, this resulted in loss of CyaA binding both to CHO-CD11b/CD18 (8% ± 5%) and to J774A.1 (6% ± 5%). This was not attributable to decreased expression of CD11b/CD18 in the presence of tunicamycin, because no decrease of surface exposure of CD11b/CD18 was observed in tunicamycin-treated cells (Fig. 2B). Moreover, as also documented in Fig. 3, tunicamycin treatment caused loss of CD11b/CD18 glycosylation without affecting the stability of the integrin, because the CD18 subunit could be coimmunoprecipitated with the CD11b subunit. Hence, both enzymatic and tunicamycin-mediated deglycosylation of CD11b/CD18 caused loss of CyaA binding with a comparable efficacy.
Free Saccharides Inhibit Binding of CyaA to the CD11b/CD18 Integrin Receptor.
To examine whether toxin binding involved a direct recognition of oligosaccharide chains of CD11b/CD18, or whether a conformational change caused by receptor deglycosylation may have accounted for loss of toxin interaction, binding of CyaA to CD11b/CD18 was examined in the presence of excess of free saccharides. We used N,N′-diacetylchitobiose, N,N′,N″-triacetylchitotriose, d-mannose, N-acetyllactosamine, and sialic acid, all of which occur as building units in the representative oligosaccharide complex linked to integrins (Fig. 1). When CyaA was preincubated with different concentrations of d-mannose (from 10 μM to 100 mM) before addition to CHO-CD11b/CD18 cells, a weak inhibition of CyaA binding to the integrin occurred already at concentrations of d-mannose that were >0.1 mM, and the extent of inhibition of toxin binding steeply increased at 7 mM to 10 mM d-mannose, yielding a 40% inhibition of CyaA binding [supporting information (SI) Fig. S1]. At the highest tested concentration of d-mannose (100 mM), the inhibition of CyaA binding reached 47% (SI Fig. S1). Therefore, a saccharide concentration of 10 mM was chosen for inhibition experiments with N,N′-diacetylchitobiose, N,N′,N″-triacetylchitotriose, N-acetyllactosamine, and sialic acid. All of these saccharides effectively inhibited binding of CyaA to CHO-CD11b/CD18 cells, J774A.1 cells, or human neutrophils (Fig. 4), with a potency equivalent to that of d-mannose. On the other hand, identical concentrations of d-arabinose, d-fructose, l-fucose, galacturonic acid, N-acetylgalactosamine, N-acetylmannosamine, and d-xylose, hence of the saccharides that are not found in the oligosaccharide chains of integrins, had no effect on CyaA binding to cells (Fig. 4). Thus, toxin binding to CD11b/CD18-expressing cells was efficiently and specifically inhibited in the presence of only saccharide units that occur in the oligosaccharide chains of integrin molecules. This demonstrates that CyaA directly recognizes the N-linked oligosaccharide chains of its β2 integrin receptor.
Fig. 4.
Binding of CyaA to CD11b/CD18-expressing cells is selectively inhibited by specific free saccharides. At concentrations of 10 mM, N,N′-diacetylchitobiose, N,N′,N″-triacetylchitotriose, d-mannose, N-acetyllactosamine (LacNAc), sialic acid, d-arabinose, d-fructose, l-fucose, galacturonic acid, N-acetylgalactosamine (GalNAc), N-acetylmannosamine (ManNAc), and d-xylose or HBSS medium alone (no saccharide) were preincubated with CyaA/233FLAG (0.5 μg/ml) for 15 min at 4°C, and then the solutions were added to 1 × 106 CHO-CD11b/CD18, J774A.1, or human neutrophils and incubated for the next 30 min at 4°C. The surface-bound toxin was detected as described above. Results are expressed for each saccharide as percentages of CyaA binding, as described in Materials and Methods, and the values represent an average of three independent determinations ± SD. The asterisk indicates a statistically significant difference (P < 0.0001; Student's t test) between saccharide-treated and untreated cells.
Glycosylation of CD11b/CD18 Is the Prerequisite for Cytotoxic Action of CyaA on CD11b-Expressing Cells.
To confirm the physiological relevance of CD11b/CD18 glycosylation for cytotoxic action of CyaA on CD11b+ cells, the amounts of cAMP produced in deglycosylated cells exposed to the toxin were determined. As shown in Fig. 5, when tunicamycin-treated CHO-CD11b/CD18 cells were incubated with various concentrations of CyaA/233FLAG for 30 min at 37°C, the intracellularly accumulated cAMP level was reduced by approximately one order of magnitude, as compared with mock-treated cells. Similarly, a strongly reduced intracellular cAMP level was observed in CHO-CD11b/CD18 cells that were pretreated with a mixture of glycosidases (Fig. 5). Comparable results also were obtained when conversion of cellular ATP to cAMP by CyaA was determined in tunicamycin-treated J774A.1 cells and glycosidase-treated J774A.1 cells or human neutrophils (data not shown). Hence, cytotoxic action of CyaA depended on the glycosylation status of the CD11b/CD18-expressing cells.
Fig. 5.
Cells treated with tunicamycin or glycosidases are less sensitive to CyaA-mediated intoxication by cAMP. CHO-CD11b/CD18 cells were treated with or without 10 μg/ml tunicamycin at 37°C for 24 h. Alternatively, cells were treated with inhibitors of proteases and 5 μg/ml chlorpromazine for 30 min at 4°C and incubated for 1 h at 37°C with or without a mixture of glycosidases (Endo H, PNGase F, and neuraminidase). Pretreated cells (1 × 105 cells/vial) were then incubated with the indicated concentrations of CyaA/233FLAG for 30 min at 37°C, and intracellular cAMP levels were determined by a competitive ELISA as described previously (29). The results represent the average of values obtained in three independent experiments ± SD.
Glycosylated CD11a Is also Required for Cytotoxic Activity of Leukotoxin of A. actinomycetemcomitans and HlyA of E. coli.
To examine whether the cytotoxic action of other RTX toxins also depends on glycosylation of their β2 integrin receptors, we used the leukotoxin of A. actinomycetemcomitans (LtxA) and the HlyA of E. coli, which specifically bind to target cells via another receptor of the β2 integrin family, CD11a/CD18 (13). CD11a-expressing Jurkat T cells treated with a mixture of PNGase F, Endo H, and neuraminidase were exposed to LtxA-containing bacterial lysates or HlyA-containing culture supernatants (see Materials and Methods), and cell viability was monitored by using trypan blue. As shown in Fig. 6, untreated Jurkat cells were significantly more susceptible to the LtxA- or HlyA-mediated killing than cells that were deglycosylated by a mixture of glycosidases. Whereas almost all mock-treated cells were killed by LtxA or HlyA within 1 h of incubation, ≈40% of the glycosidase-treated Jurkat cells survived over that period (Fig. 6). These results show that cytotoxic activity of LtxA or HlyA also depends on the glycosylation of CD11a/CD18-expressing cells.
Fig. 6.
Deglycosylation of CD11a/CD18-expressing Jurkat cells decreases cytotoxic activity of LtxA of A. actinomycetemcomitans and of HlyA of E. coli. Jurkat cells, expressing the CD11a/CD18 integrin complex on the cell surface, were treated or not with a mixture of PNGase F, Endo H, and neuraminidase at 37°C overnight. Next, protein-content-equalized mock or LtxA-containing bacterial lysates (A) and mock or HlyA-containing culture supernatants (B) were added to the cells for the indicated times, and cell viability was determined by using trypan blue dye exclusion assay (see Materials and Methods). The data are representative of three independent experiments ± SD.
Discussion
We show here that action of RTX cytotoxins depends on recognition of oligosaccharide chains linked to their β2 integrin receptors. Oligosaccharide chains of integrin molecules appear, indeed, to play an important role in various integrin-ligand interactions. For example, deglycosylation of the α5β1 integrin with a mixture of selective glycosidases, or upon tunicamycin treatment, inhibits binding of its ligand, fibronectin (23). Similarly, removal of oligosaccharide residues from the α6β1 integrin leads to reduced binding of laminin (24). Recently, N-glycosylation of the α2β1 integrin was reported to be necessary for interaction with subtilase cytotoxin of Stx (shiga-like toxin)-producing enterohaemorrhagic E. coli (25). To clarify the contribution of N-linked oligosaccharide chains of the β2 integrin CD11b/CD18 to the binding of the AC toxin, here, we examined the effect of PNGase F, Endo H, neuraminidase, and tunicamycin treatment of CD11b-expressing cells on CyaA activity. An important reduction of CyaA binding to the cells was, indeed, already observed upon removal of the peripheral sialic acid residues from the cell surface glycoproteins by neuraminidase treatment. Moreover, CyaA binding was completely abolished when sugar chains were entirely removed by PNGase F, and a complete inhibition of CyaA binding to the CD11b/CD18-expressing cells was observed when N-glycosylation of de novo-synthesized proteins was blocked with tunicamycin. Hence, glycosylation of the CD11b/CD18 integrin receptor was essential for its recognition by CyaA.
Previous findings have demonstrated that N-glycosylation of integrin subunits is important not only for optimal ligand binding but also for integrin stability and cell surface expression. For example, the α5β1 integrin deglycosylated with specific glycosidases tends to dissociate into the α and β subunits, and its surface expression is reduced when the cells are treated with tunicamycin (23). Our results, however, clearly demonstrate that the cell surface expression and stability of the heterodimer of the CD11b/CD18 integrin was not significantly affected when CD11b-expressing cells were deglycosylated either by a mixture of specific glycosidases or upon tunicamycin treatment. The deglycosylated integrin was, indeed, efficiently recognized on cell surface by mAbs, and coimmunoprecipitation experiments demonstrated that N-glycosylation of CD11b/CD18 was not essential for stable association of the CD11b and CD18 subunits, which coimmunoprecipitated at a 1:1 molar ratio from lysates of glycosidase or tunicamycin-treated cells.
Hence, it appears plausible to assume that deglycosylation of the integrin did not induce any substantial conformational changes of its subunits. It is, therefore, unlikely that loss of CyaA binding to cells was attributable to loss of recognition of the polypeptide chain of the integrin receptor itself. The most plausible interpretation of our results is that initial toxin binding involves direct recognition of the oligosaccharide chains of CD11b/CD18. This is strongly supported by experiments demonstrating that CyaA binding to the integrin receptor is specifically and efficiently inhibited in the presence of free saccharides that are found as building units of the oligosaccharide chains of CD11b/CD18. Moreover, binding of CyaA to CD11b-expressing cells was approximately five times reduced when the cells were treated with neuraminidase, whereas neuraminidase treatment had a negligible effect on the background CyaA binding to untransfected CHO cells lacking CD11b/CD18 (SI Fig. S2). This shows that the toxin primarily and specifically interacts with the terminal sialic acid residues of the CD11b/CD18 integrin receptor, in the presence of which the interaction of CyaA with sialic acid residues of other surface glycoproteins contributes negligibly to overall toxin binding. The interaction between CyaA and the peripheral negatively charged sialic acid residues of the integrin may well depend on electrostatic interactions. Indeed, we have recently shown that the main CD11b/CD18 interaction domain of CyaA is located between amino acid residues 1,166 and 1,281 of the glycine- and aspartate-rich RTX repeat region of the toxin (26). Based on the structure of the prototype RTX repeat block, the negative charge of aspartate and/or glutamate residues of the RTX domain that chelates bivalent calcium ions would be neutralized by binding of ≈40 Ca2+ per RTX domain. The positively charged arginine and lysine residues of the integrin binding domain of CyaA would, however, remain available for interaction with sialic acid residues of the CD11b/CD18 glycoprotein and may mediate the first contact between CyaA and its receptor. Except for sialic acid residues, CyaA also has to interact with other sugar units of the oligosaccharide complex of the integrin (chitobiose core, trimannosyl core, N-acetyllactosamine unit; see Fig. 1). Indeed, a complete deglycosylation of CD11b/CD18 was needed to fully abrogate CyaA binding.
The ability of CyaA to bind sugar structures offers an attractive hypothesis for explaining previous observations that CyaA also binds and penetrates with reduced, but well detectable, efficiency a variety of eukaryotic cells lacking the CD11b/CD18 receptor. On such cells, gangliosides would be potentially involved in CyaA binding, because incubation of CyaA with gangliosides before addition to target cells has been shown previously to result in inhibition of its capacity to cause intracellular cAMP accumulation (27). Gangliosides are indeed acidic lipids composed of a ceramide linked to an oligosaccharide chain containing one or more sialic acid residues (28). CyaA may thus potentially recognize an oligosaccharide moiety of gangliosides, including negatively charged sialic acid residues, similarly as it recognizes oligosaccharide chains of the β2 integrin CD11b/CD18. However, whereas binding of CyaA to CD11b-expressing cells is highly efficient, dose-dependent, and saturable, binding to cells lacking the integrin receptor occurs with low-affinity and appears to be unsaturable (16). This suggests that after initial interaction of CyaA with oligosaccharide structures, interaction of CyaA with the polypeptide chain of CD11b/CD18 is essential for high-affinity binding of the toxin to target cells.
Recently, three other toxins of the RTX family, LtxA of A. actinomycetemcomitans, HlyA of E. coli, and LktA of M. haemolytica, have been shown to specifically bind to target cells through another receptor of the β2 integrin family, CD11a/CD18 (13, 20). Here, we showed that glycosylation of CD11a/CD18 is required for cytotoxic action of LtxA and HlyA on Jurkat T cells. This shows that although CyaA is not able to bind CD11a/CD18, and LtxA and HlyA do not recognize CD11b/CD18, recognition of oligosaccharide chains of both β2 integrins is crucial for binding of these RTX cytotoxins. After such initial interaction, recognition of the polypeptide chains of the CD11a or CD11b subunits would determine the integrin and target cell specificity of these cytotoxins.
These results provide important insights into the interactions of the RTX cytotoxins with their β2 integrin receptors and set a new paradigm for this whole toxin family.
Materials and Methods
Antibodies, Cell Lines, Isolation of Neutrophils, and Production of CyaA/233FLAG, LtxA, and HlyA.
All details are given in SI Text.
Removal of N-Linked Oligosaccharide Chains from Cellular Receptors.
CHO-CD11b/CD18 cells, J774A.1 cells, or human neutrophils (1 × 106) were preincubated for 30 min at 4°C in Hanks' balanced salt solution (HBSS) buffer containing inhibitors of proteases and 5 μg/ml of chlorpromazine (inhibitor of β2 integrin endocytosis). Next, the cells were incubated in HBSS buffer at 37°C for 1 h with 500 milliunit/ml Endo H (New England Biolabs), PNGase F (New England Biolabs) and 40 milliunits/ml neuraminidase (Sigma-Aldrich), respectively, or in buffer alone (mock control). After treatment, cells were washed three times with HBSS and used in binding experiments.
Immunoprecipitation, SDS/PAGE, and Western Blot Analysis.
Details are given in SI Text.
Inhibition of N-Glycosylation of Cellular Receptors.
Cell monolayers were treated with 10 μg/ml tunicamycin (Sigma-Aldrich) for 24 h in growth medium with 10% FCS and cells were washed with HBSS buffer before addition of CyaA.
Inhibition of CyaA/233FLAG Binding to Cells by Free Saccharides.
l-Fucose, d-arabinose, N-acetylgalactosamine, N-acetylmannosamine, d-mannose, and sialic acid were obtained from Sigma-Aldrich. Galacturonic acid, d-fructose, and d-xylose were purchased from Lachema. N,N′-Diacetylchitobiose and N,N′,N′-triacetylchitotriose were obtained from MP Biomedicals and N-acetyllactosamine from Calbiochem. CyaA/233FLAG (0,5 μg/ml) in HBSS buffer containing 2 mM CaCl2 was preincubated for 15 min at 4°C with a given saccharide (10 mM final concentration) and added to 1 × 106 cells, and toxin binding was determined.
CyaA Binding to Cells.
For binding studies, 1 × 106 cells in 500 μl of HBSS buffer complemented with 2 mM CaCl2 were incubated with purified CyaA/233FLAG protein (0.5 μg/ml) for 30 min at 4°C. Cells were washed three times with HBSS and the cell-bound CyaA/233FLAG was stained with anti-FLAG mAb M2 diluted in 500 μl of PBS/1% BSA buffer (1:1,000 dilution) for 30 min at 4°C. Cells were washed three times with HBSS buffer and incubated for 30 min at 4°C in 500 μl of PBS/1% BSA with AlexaFluor488-conjugated anti-mouse antibody (1:1,000 dilution). Cells were washed three times, resuspended in 150 μl of HBSS, and analyzed by flow cytometry on a FACS LSR II instrument (Becton Dickinson Bioscience) in the presence of 5 μg/ml propidium iodide.
Appropriate gatings were done to exclude cell aggregates and dead cells by propidium iodide staining. Binding data were deduced from the mean fluorescence intensity (MFI) and expressed as percentage of CyaA binding to untreated cells = [(MFI value of glycosidase, tunicamycin, or saccharides-treated cells incubated with CyaA − MFI value of untreated cells incubated without CyaA)/(MFI value of untreated cells incubated with CyaA − MFI value of untreated cells incubated without CyaA)] × 100.
Detection of CD11b/CD18 on the Cell Surface.
For staining of the CD11b/CD18 integrin on the cell surface, incubation with CyaA was omitted and anti-CD11b mAb MEM-174 (1:1,000 dilution) was used instead of the anti-FLAG antibody following an otherwise identical procedure as for detection of CyaA binding. Binding of MEM-174 was calculated by using the same formula as above, by entering MFI values of cells incubated with or without MEM174.
cAMP Assay.
CHO-CD11b/CD18, J774A.1, or human neutrophils (105) in microcentrifuge tubes were preincubated for 20 min in HBSS buffer complemented with 2 mM CaCl2 and 100 μM 3-isobutyl-1-methylxanthin, a phosphodiesterase inhibitor. Various concentrations of CyaA were added and cells were incubated for 30 min at 37°C. Intracellular cAMP levels were determined as described previously (29).
Killing Assay.
Jurkat T cells were treated with glycosidases or mock-treated at 37°C overnight. Afterward, the cells (1 × 105 cells/vial) were incubated at 37°C for different times with 100 μl of mock or LtxA-containing bacterial lysates and mock or HlyA-containing culture supernatants, normalized to the same total bacterial protein content. Treated cells were placed on ice and mixed with 100 μl of trypan blue (0.4%), and the surviving cells were counted. The results were expressed as percentages of live cells = [(number of surviving cells after LtxA or HlyA treatment)/(number of surviving cells in the negative control)] × 100.
Acknowledgments.
We thank E. T. Lally (University of Pennsylvania, Philadelphia, PA) for the pSHH vector for expression of LtxA and J. Vojtova and K. Bezouska for helpful initial input in this work. This work was supported by Grants 204/07/P105 from the Czech Science Foundation; 1M0506 from the Ministry of Education, Youth and Sports; IAA5020406 from the Academy of Sciences of the Czech Republic, European Union Sixth Framework Programme Contract LSHB-CT-2003-503582 THERAVAC; and Institutional Research Concept 50200510 from the Academy of Sciences of the Czech Republic.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0711400105/DCSupplemental.
References
- 1.Goodwin MS, Weiss AA. Adenylate cyclase toxin is critical for colonization and pertussis toxin is critical for lethal infection by Bordetella pertussis in infant mice. Infect Immun. 1990;58:3445–3447. doi: 10.1128/iai.58.10.3445-3447.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Weiss AA, Hewlett EL, Myers GA, Falkow S. Pertussis toxin and extracytoplasmic adenylate cyclase as virulence factors of Bordetella pertussis. J Infect Dis. 1984;150:219–222. doi: 10.1093/infdis/150.2.219. [DOI] [PubMed] [Google Scholar]
- 3.Glaser P, et al. The calmodulin-sensitive adenylate cyclase of Bordetella pertussis: Cloning and expression in Escherichia coli. Mol Microbiol. 1988;2:19–30. [PubMed] [Google Scholar]
- 4.Benz R, Maier E, Ladant D, Ullmann A, Sebo P. Adenylate cyclase toxin (CyaA) of Bordetella pertussis. Evidence for the formation of small ion-permeable channels and comparison with HlyA of Escherichia coli. J Biol Chem. 1994;269:27231–27239. [PubMed] [Google Scholar]
- 5.Hackett M, Guo L, Shabanowitz J, Hunt DF, Hewlett EL. Internal lysine palmitoylation in adenylate cyclase toxin from Bordetella pertussis. Science. 1994;266:433–435. doi: 10.1126/science.7939682. [DOI] [PubMed] [Google Scholar]
- 6.Sebo P, Ladant D. Repeat sequences in the Bordetella pertussis adenylate cyclase toxin can be recognized as alternative carboxy-proximal secretion signals by the Escherichia coli alpha-haemolysin translocator. Mol Microbiol. 1993;9:999–1009. doi: 10.1111/j.1365-2958.1993.tb01229.x. [DOI] [PubMed] [Google Scholar]
- 7.Glaser P, et al. Identification of residues essential for catalysis and binding of calmodulin in Bordetella pertussis adenylate cyclase by site-directed mutagenesis. EMBO J. 1989;8:967–972. doi: 10.1002/j.1460-2075.1989.tb03459.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bellalou J, Sakamoto H, Ladant D, Geoffroy C, Ullmann A. Deletions affecting hemolytic and toxin activities of Bordetella pertussis adenylate cyclase. Infect Immun. 1990;58:3242–3247. doi: 10.1128/iai.58.10.3242-3247.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wolff J, Cook GH, Goldhammer AR, Berkowitz SA. Calmodulin activates prokaryotic adenylate cyclase. Proc Natl Acad Sci USA. 1980;77:3841–3844. doi: 10.1073/pnas.77.7.3841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vojtova J, Kamanova J, Sebo P. Bordetella adenylate cyclase toxin: A swift saboteur of host defense. Curr Opin Microbiol. 2006;9:69–75. doi: 10.1016/j.mib.2005.12.011. [DOI] [PubMed] [Google Scholar]
- 11.Sakamoto H, Bellalou J, Sebo P, Ladant D. Bordetella pertussis adenylate cyclase toxin. Structural and functional independence of the catalytic and hemolytic activities. J Biol Chem. 1992;267:13598–13602. [PubMed] [Google Scholar]
- 12.Welch RA. RTX toxin structure and function: A story of numerous anomalies and few analogies in toxin biology. Curr Top Microbiol Immunol. 2001;257:85–111. doi: 10.1007/978-3-642-56508-3_5. [DOI] [PubMed] [Google Scholar]
- 13.Lally ET, et al. RTX toxins recognize a beta2 integrin on the surface of human target cells. J Biol Chem. 1997;272:30463–30469. doi: 10.1074/jbc.272.48.30463. [DOI] [PubMed] [Google Scholar]
- 14.Ambagala TC, Ambagala A-P, Srikumaran S. The leukotoxin of Pasteurella haemolytica binds to beta(2) integrins on bovine leukocytes. FEMS Microbiol Lett. 1999;179:161–167. doi: 10.1111/j.1574-6968.1999.tb08722.x. [DOI] [PubMed] [Google Scholar]
- 15.Li J, Clinkenbeard KD, Ritchey JW. Bovine CD18 identified as a species specific receptor for Pasteurella haemolytica leukotoxin. Vet Microbiol. 1999;67:91–97. doi: 10.1016/s0378-1135(99)00040-1. [DOI] [PubMed] [Google Scholar]
- 16.Guermonprez P, et al. The adenylate cyclase toxin of Bordetella pertussis binds to target cells via the alpha(M)beta(2) integrin (CD11b/CD18) J Exp Med. 2001;193:1035–1044. doi: 10.1084/jem.193.9.1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Arnaout MA. Structure and function of the leukocyte adhesion molecules CD11/CD18. Blood. 1990;75:1037–1050. [PubMed] [Google Scholar]
- 18.Mazzone A, Ricevuti G. Leukocyte CD11/CD18 integrins: Biological and clinical relevance. Haematologica. 1995;80:161–175. [PubMed] [Google Scholar]
- 19.Valeva A, et al. Binding of Escherichia coli hemolysin and activation of the target cells is not receptor-dependent. J Biol Chem. 2005;280:36657–36663. doi: 10.1074/jbc.M507690200. [DOI] [PubMed] [Google Scholar]
- 20.Jeyaseelan S, et al. Lymphocyte function-associated antigen 1 is a receptor for Pasteurella haemolytica leukotoxin in bovine leukocytes. Infect Immun. 2000;68:72–79. doi: 10.1128/iai.68.1.72-79.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Thumbikat P, Dileepan T, Kannan MS, Maheswaran SK. Characterization of Mannheimia (Pasteurella) haemolytica leukotoxin interaction with bovine alveolar macrophage beta2 integrins. Vet Res. 2005;36:771–786. doi: 10.1051/vetres:2005036. [DOI] [PubMed] [Google Scholar]
- 22.Asada M, Furukawa K, Kantor C, Gahmberg CG, Kobata A. Structural study of the sugar chains of human leukocyte cell adhesion molecules CD11/CD18. Biochemistry. 1991;30:1561–1571. doi: 10.1021/bi00220a017. [DOI] [PubMed] [Google Scholar]
- 23.Zheng M, Fang H, Hakomori S. Functional role of N-glycosylation in alpha 5 beta 1 integrin receptor. De-N-glycosylation induces dissociation or altered association of alpha 5 and beta 1 subunits and concomitant loss of fibronectin binding activity. J Biol Chem. 1994;269:12325–12331. [PubMed] [Google Scholar]
- 24.Chammas R, Veiga SS, Line S, Potocnjak P, Brentani RR. Asn-linked oligosaccharide-dependent interaction between laminin and gp120/140. An alpha 6/beta 1 integrin. J Biol Chem. 1991;266:3349–3355. [PubMed] [Google Scholar]
- 25.Yahiro K, et al. Identification and characterization of receptors for vacuolating activity of subtilase cytotoxin. Mol Microbiol. 2006;62:480–490. doi: 10.1111/j.1365-2958.2006.05379.x. [DOI] [PubMed] [Google Scholar]
- 26.El-Azami-El-Idrissi M, et al. Interaction of Bordetella pertussis adenylate cyclase with CD11b/CD18: Role of toxin acylation and identification of the main integrin interaction domain. J Biol Chem. 2003;278:38514–38521. doi: 10.1074/jbc.M304387200. [DOI] [PubMed] [Google Scholar]
- 27.Gordon VM, et al. Adenylate cyclase toxins from Bacillus anthracis and Bordetella pertussis. Different processes for interaction with and entry into target cells. J Biol Chem. 1989;264:14792–14796. [PubMed] [Google Scholar]
- 28.Hirai M, Iwase H, Hayakawa T, Koizumi M, Takahashi H. Determination of asymmetric structure of ganglioside-DPPC mixed vesicle by using SANS, SAXS, and DLS. Biophys J. 2003;85:1600–1610. doi: 10.1016/S0006-3495(03)74591-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Karimova G, Pidoux J, Ullmann A, Ladant D. A bacterial two-hybrid system based on a reconstituted signal transduction pathway. Proc Natl Acad Sci USA. 1998;95:5752–5756. doi: 10.1073/pnas.95.10.5752. [DOI] [PMC free article] [PubMed] [Google Scholar]