Abstract
In the absence of an effective vaccine, there is an urgent need for safe and effective antiviral agents to prevent transmission of HIV. Here, we report that an amphipathic α-helical peptide derived from the hepatitis C virus NS5A anchor domain (designated C5A in this article) that has been shown to be virocidal for the hepatitis C virus (HCV) also has potent antiviral activity against HIV. C5A exhibits a broad range of antiviral activity against HIV isolates, and it prevents infection of the three in vivo targets of HIV: CD4+ T lymphocytes, macrophages, and dendritic cells by disrupting the integrity of the viral membrane and capsid core while preserving the integrity of host membranes. C5A can interrupt an ongoing T cell infection, and it can prevent transmigration of HIV through primary genital epithelial cells, infection of mucosal target cells and transfer from dendritic cells to T cells ex vivo, justifying future experiments to determine whether C5A can prevent HIV transmission in vivo.
Keywords: antiviral, C5A, HIV, microbicide
Of the 33 million people living with HIV/AIDS, almost 25 million live in sub-Saharan Africa. In the absence of a vaccine, there is an urgent need for the development of alternative prevention approaches. To date, there are 22 anti-HIV drugs that have been licensed for clinical use. A combination of these drugs forms the basis of highly active antiretroviral therapy (HAART). Despite successful HAART therapy, the emergence of antiretroviral drug resistance in patients has prompted efforts to develop new antiretrovirals that differ from existing agents with regard to mechanism of action and resistance profiles (1). Therefore, it is of concern that existing and future therapies will have to be effective against newly evolving (including drug-resistant) HIV variants in patients who currently face many years, if not decades, of chronic anti-HIV drug treatment.
Thus, the identification of new agents to treat and prevent HIV transmission with different mechanisms of action is clearly important. An amphipathic α-helical peptide (C5A) derived from the hepatitis C virus (HCV) NS5A membrane anchor domain has been recently shown by Cheng et al. (2) to have antiviral activity in vitro against HCV, other members of the Flaviviridae, and HIV. In the current work, we investigated the antiviral potential of C5A against multiple HIV isolates, demonstrated its ability to protect multiple cell types from HIV infection, defined its mechanism of action, and explored its ability to prevent HIV transmission. Based on its virocidal mechanism of action, its ability to prevent infection of CD4+ T lymphocytes, macrophages, and dendritic cells (DC) by multiple HIV clade representatives and multidrug-resistant viruses, its activity at low pH, and its ability to prevent genital epithelial transmigration and mucosal Langerhans cells (LC) infection ex vivo, we suggest that C5A may represent an effective strategy for the treatment and prevention of HIV infection.
Results
Broad Anti-HIV Spectrum of C5A.
We began our experiments by testing the ability of C5A to inhibit the ability of four distinct categories of viruses to infect CD4+ HeLa cells (TZM cells) that produce β-galactosidase upon HIV infection (3). The first category includes HIV-1 isolates representative of various HIV subtypes with different coreceptor usage, either CCR5 (R5 viruses) or CXCR4 (X4 viruses). The second category includes HIV-1 isolates that are resistant to reverse transcriptase (RT), protease (PR) or fusion (T20) inhibitors. The third category is other retroviruses such as HIV-2 and simian immunodeficiency virus (SIV) isolates, and the fourth is nonretroviruses such as adeno- and vesicular stomatitis (VSV) viruses. Viruses were added to CD4+ HeLa TZM cells together with C5A (SWLRDIWDWICEVLSDFK) for 4 h and washed, and infection was measured 48 h after infection as β-galactosidase activity. A C5A variant (SWRLDIWDWICESVLDFK) that is known to have no antiviral activity against HCV (2) was used as a negative control. The antiviral activity of C5A against the nonretroviruses was tested with 293 cells as targets and adenovirus and VSV encoding the GFP reporter gene. As above, viruses were added to 293 cells together with C5A or the control peptide for 4 h, washed, and infection was scored 48 h after infection by GFP cellular content. As shown in supporting information (SI) Table S1, C5A prevents infection of all HIV and SIV isolates tested at submicromolar to low-micromolar concentrations. In contrast, C5A did not block the infectivity of adenovirus or VSV.
C5A Prevents HIV Infection of CD4+ T Lymphocytes, Macrophages, and DC.
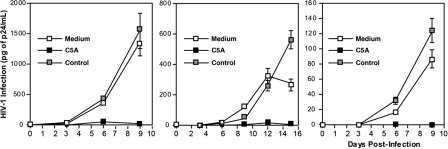
Having shown that C5A can prevent the infection of CD4+ HeLa cells, we asked whether C5A could block HIV infection in human primary CD4+ T lymphocytes, macrophages, and DC. Cells were exposed to HIV-1 (R5 NL4.3-BaL) (4) together with C5A or the control peptide (5 μM). Twenty-four hours after infection, cells were washed, and viral replication was monitored by measuring the amount of HIV capsid protein present in the cell culture supernatant every 3 days for up to 15 days thereafter. As shown in Fig. 1, C5A blocked HIV infection of all three in vivo cell targets of HIV. These results suggest that C5A has the potential to prevent infection of T cells, macrophages, and DC during the early steps of HIV transmission.
Fig. 1.
C5A prevents HIV infection of primary T lymphocytes (Left), macrophages (Center), and DC (Right). Blood-derived CD4+ T lymphocytes, macrophages, or DC (0.5 × 106 cells) were exposed to NL4.3-BaL (1 ng of p24) and C5A or control peptide (5 μM) for 1 day at 37°C and washed. The amount of virus released was monitored by quantitating particulate p24 capsid protein in cell supernatants by ELISA. Error bars represent standard errors of duplicates.
Antiviral Mechanism of Action of C5A.
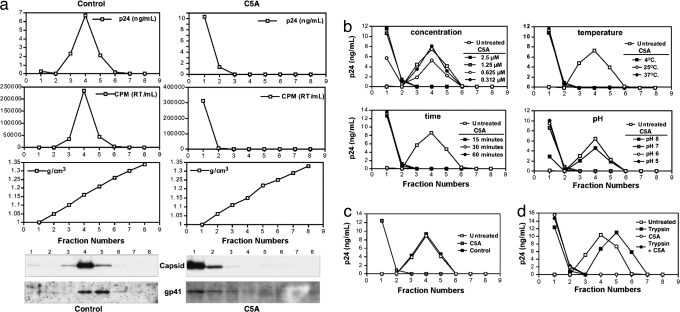
As shown by Cheng et al. (2), C5A is virocidal for HCV, destabilizing it at the level of the viral membrane. Thus, we used a virus sedimentation assay to determine the impact of C5A on the structural integrity of HIV. Purified viruses (X4 NL4.3) (4) (20 ng of p24 in PBS) were incubated with or without C5A (5 μM) for 30 min at 37°C and loaded onto a 20–70% sucrose gradient, and each fraction was analyzed for HIV protein content including capsid (by p24 ELISA and/or by immunoblot) (4), RT (by exo-RT assay) (5), and gp41 (by immunoblot) (4). The density of each fraction was determined by measuring the refractive index. Untreated HIV sediments at a density of 1.16 g/cm3 as demonstrated by the presence of viral capsid, gp41, and RT (Fig. 2a Left). In contrast, upon C5A treatment, all viral components, including capsid, RT, and membrane-associated gp41, relocated to the top of the gradient (Fig. 2a Right), suggesting that C5A destroys the integrity of viral particles. Even submicromolar concentrations (0.6 μM) of C5A suffice to destabilize HIV (Fig. 2b). The antiviral effect is not temperature-dependent because C5A (5 μM) destabilized HIV integrity after a 30-min incubation at 4, 25, and 37°C (Fig. 2b). The antiviral effect is rapid because C5A disrupted the integrity of HIV after only a 15-min exposure (Fig. 2b). HIV destabilization by C5A is pH-dependent because it disrupted viral particles after a 30-min exposure at pH 7, 6, and 5, but not at pH 8 (Fig. 2b). The control peptide (5 μM) did not destroy HIV (Fig. 2c), supporting the notion that the amphipathicity of C5A is crucial for its antiviral activity against HIV, as it is for HCV (2). To determine whether C5A needs to interact with proteins at the surface of HIV to disrupt the virus, we asked whether preincubation of HIV with trypsin would reduce its susceptibility to destruction by C5A. Trypsin treatment of the virus did not prevent C5A from destabilizing HIV (Fig. 2d), suggesting that C5A does not require trypsin-sensitive molecules on the surface of the virus to mediate its virocidal activity.
Fig. 2.
C5A destabilizes HIV particles. (a) NL4.3 virus (20 ng of p24) was incubated with or without C5A for 30 min at 37°C and loaded over a sucrose density gradient (see Materials and Methods). Each gradient fraction was analyzed for capsid by p24 ELISA (Top) and immunoblot, RT by exoRT assay (Middle), and gp41 content by immunoblot. (b) Same as a except that the virus was treated with decreasing concentrations of C5A for 30 min at 37°C (Upper Left), with C5A for 15, 30, and 60 min at 37°C (Lower Left), for 30 min at 4, 25, or 37°C (Upper Right), and for 30 min at pH 8, 7, 6, and 5 (Lower Right). Gradient fractions were analyzed for HIV capsid content by p24 ELISA. (c) Same as a except that the virus was treated either with C5A or control peptide. (d) Same as a except that the virus was first trypsinized for 15 min at 37°C, incubated in 10% FCS to neutralize trypsin, microcentrifuged for 90 min at 4°C, resuspended, and loaded over a sucrose gradient for virus integrity evaluation by p24 ELISA.
Antiviral Effect of C5A Before, During, or After Viral Exposure.
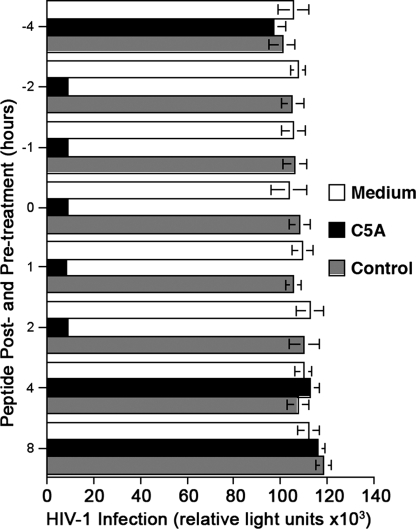
To determine the likely duration of antiviral activity of C5A and whether it can inactivate cell-bound virus, we asked whether C5A inhibits HIV infection when added to cells for varying lengths of time before or after adding the virus. Initially, we added C5A or its nonamphipathic variant (5 μM) to TZM cells 1 (t = −1), 2 (t = −2) or 4 h (t = −4) before adding HIV-1 (X4 NL4.3) (1 ng of p24) or together with the virus (t = 0), and it was maintained in the culture until infection was monitored 48 h later. C5A blocked infection when it was added together with the virus (t = 0) (Fig. 3) or 1–2 h before the virus, but not when it was added 4 h before adding the virus to cells (Fig. 3), indicating that C5A is potent in vitro at 37°C for 2 h but becomes inactivated after 4 h. Next, we asked whether C5A can inhibit HIV infection when it is added after the virus is added to target cells. C5A prevents HIV infection when it is added to cells up to 2 h (t = +2) after virus inoculation but not 4 h later (t = +4) (Fig. 3). Because HIV attaches to and starts entering into HeLa cells in 2 h (6), the data suggest that C5A neutralizes both free and cell-bound viruses before their entry into the cell.
Fig. 3.
Antiviral effect of C5A before, during, or after viral exposure. C5A or control peptide (5 μM) was added to TZM cells 1, 2, or 4 h before (t = −1, −2, and −4) or after (t = +1, +2, + 4, and + 8) adding HIV-1 (X4 NL4.3) (1 ng of p24) or together (t = 0) with the virus. Infection was measured 48 h after infection as β-galactosidase activity. Error bars represent standard errors of duplicates.
C5A Prevents Transmission of HIV in Vitro.
There are three methods by which HIV is known to be transmitted: HIV epithelial transmigration, DC-mediated transmission to T cells, and direct infection of mucosal target cells (7).
Primary genital epithelial cells (PGEC).
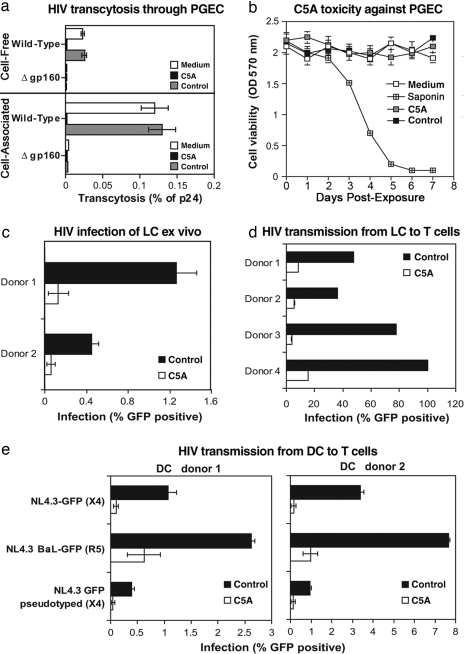
To examine the antiviral effect of C5A on HIV transcytosis, we developed an in vitro transwell chamber assay that mimics HIV transmigration through PGEC (8). Either cell-free viruses or HIV-infected Jurkat T cells were added to the apical surface of PGEC in the upper chamber of a transwell system for 8 h at 37°C, and the amount of transcytosed virus was quantified by p24 ELISA in the lower chamber medium in contact with the basal PGEC surface. C5A or control peptide (5 μM) was added to the apical surface of the PGEC monolayer 30 min after adding the virus. As shown in Fig. 4a, HIV efficiently transmigrated through PGEC, in contrast to a virus lacking gp160, suggesting that the viral glycoprotein is required for transcytosis and that the PGEC layer does not allow nonspecific transmigration of viruses. Amounts of transcytosed viruses were quantified by measuring amounts of particulate capsid in the basal chamber. C5A prevents cell-free HIV transmigration through PGEC (Fig. 4a). We obtained similar results when we applied C5A 30 min before virus addition (data not shown). C5A also prevents the accumulation of HIV in the basal PGEC chamber when the virus is added to the upper chamber as cell-associated HIV (Fig. 4a). As expected, C5A likely inactivates the virus before it penetrates into the genital epithelial monolayer.
Fig. 4.
C5A inhibits both HIV transmigration and transmission. (a) Either cell-free (20 ng of p24) or cell-associated NL4.3 (100,000 HIV-infected Jurkat T cells washed twice just before being added to the upper chamber) was added to the apical surface of PGEC. C5A or the control peptide (5 μM) was added to PGEC 30 min after adding the virus. Amounts of transcytosed viruses in the basal chamber were quantified by p24 ELISA. Results are expressed in percentage of p24 of the original inoculum. Error bars represent standard errors of duplicates. (b) PGEC were treated twice daily with 200 μM C5A or control peptide or 0.01% saponin for a week. CellQuanti-MTTTM reagent was added, and cell viability was quantified by A570 reading. (c–e) Epidermal sheets were infected at 37°C with NL4.3-BaL-eGFP (100 ng of p24). After 4 h, C5A, control peptide (10 μM) or medium (0.1% DMSO) was added to sheets. After 3 days, migrated cells, which exfoliated from the epidermal sheets, were isolated, washed, and incubated with Jurkat T cells (100,000 cells). Infection of migrated cells (day 3) or T cells in LC-T coculture (day 5) were analyzed for GFP expression by FACS. (c) Percentage of HIV-infected migrated cells (mainly LC) at day 3. (d) Percentage of HIV-infected Jurkat T cells (gated by using an anti-CD3 antibody) in LC-T coculture was analyzed by GFP expression on day 5. Error bars represent standard errors of duplicates. (e) DC (100,000 cells) were incubated for 2 h at 37°C with wild-type NL4.3-eGFP (X4 virus) and NL4.3-BaL-eGFP (R5 virus) viruses or with the pseudotyped NL4.3ΔEnv-eGFP/gp160 X4 Env virus (25 ng of p24). C5A or control peptide (10 μM) was added 2 h later. DC were washed 2 h after adding the peptide, Jurkat T cells (100,000 cells) were added for 3 days, and the percentage of infected Jurkat T cells was analyzed as described in d. Error bars represent standard errors of duplicates.
To examine the possibility that C5A prevented HIV transcytosis because it is toxic for PGEC, cells were exposed twice daily to high concentrations (200 μM) of C5A for a week. To maintain a continuous exposure of cells to the peptide, no washes were performed. Cell viability was evaluated by methyl thiazol tetrazolium (MTT)-based colorimetric assessment. As a control, cells were exposed to the detergent saponin. In contrast to 0.01% saponin, C5A applied to cells at a concentration of 10- to 100-fold greater than that which blocks HIV infection (Table 1) is not toxic to cervical epithelial cells (Fig. 4b). The fact that the peptide disrupts the HIV membrane (Fig. 2a) without PGEC toxicity (Fig. 4b) suggests that C5A prevents HIV transmigration without interfering with epithelial integrity.
Langerhans cells.
DC amplify HIV infection by rapidly transferring infectious particles to T cells (9–14). The formation of an intimate contact between the virus-presenting DC and a T cell, the virological synapse, ensures efficient cell–cell transmission of infectious HIV particles (14) that is not inhibited by neutralizing anti-HIV antibody (15). Transmission of HIV from DC to T cells can occur irrespective of infection of the DC (9–14). Uninfected DC can capture viruses and direct them rapidly to the DC-T cell synapse (in trans transmission), and infected DC can transfer de novo produced virus to T cells (de novo transmission). We thus examined the potential of C5A to prevent HIV infection of and transmission by DC and by LC, a DC subset present in the epidermal and epithelial tissues (9, 10). We examined the capacity of C5A to prevent LC infection and LC-mediated transmission of HIV as previously described (10). NL4.3-BaL-eGFP (100 ng of p24) was added to human epidermal sheets floating in complete medium. Four hours later, at which time virus entry into the tissue is complete (10), C5A or control peptide (10 μM) was added. After 3 days, epidermal sheets were removed, and migrated cells that had exfoliated from the epidermal sheets were isolated, washed to remove free virus and peptide, and cultured as described in ref. 10. The majority of infected cells in the migrated population are known to be LC (ref. 10 and data not shown). Importantly, C5A, but not the control peptide (5 μM), blocks tissue LC infection (Fig. 4c). We obtained similar results when C5A was added to epidermal sheets 1 h before the addition of the virus (data not shown). These results suggest that C5A prevents LC infection.
To eliminate the possibility that, despite the presence of C5A, the virus that has attached to the surface of LC can be transmitted to T cells, naïve Jurkat T cells were added to the LC and analyzed for infection (GFP expression) by FACS 2 (day 5) days later. No T cell infection was observed (Fig. 4d), suggesting that C5A blocks transmission of HIV to T cells by infected LC and by LC that carry HIV attached to their cell surface (10), presumably by virtue of its virocidal activity.
Dendritic Cells.
We then examined the capacity of C5A to prevent DC-mediated transmission of HIV. We took advantage of a replication-defective virus (NL4.3ΔEnv-eGFP) (4), which does not encode Env, but which has been pseudotyped with HIV Env. Pseudotyped viruses can infect cells because they contain Env. However, cells that have been infected by pseudotyped viruses cannot produce infectious viruses because de novo viruses do not encode Env. Thus, the use of pseudotyped viruses allowed the analysis of the effect of C5A on the transmission of infectious particles from DC to T cells independently of DC infection. DC were incubated with wild-type NL4.3-eGFP and NL4.3-BaL-eGFP or pseudotyped NL4.3ΔEnv-eGFP/gp160 Env viruses (25 ng of p24). Two hours later, at which time the entry of the virus into DC is completed (11), C5A or control peptide (10 μM) was added. After 2 h, DC were washed to remove both free virus and peptide. To measure DC–T cell transmission, Jurkat T cells were added for 3 days, and the percentage of infected T cells (gated with an anti-CD3 antibody) was analyzed by FACS. Only pseudotyped viruses that have been rapidly transferred from DC to T cells through the virological synapse (independently of DC infection) can infect T cells. Indeed, pseudotyped viruses that infect DC can no longer infect T cells because they do not encode Env. Because DC were washed before adding T cells, the T cell infection by the pseudotyped virus observed in Fig. 4e could only arise from pseudotyped particles that were transferred from DC to T cells. Importantly, C5A added to DC prevents subsequent T cell infection with the pseudotyped virus. We obtained similar results when C5A was added to DC 1 h before adding virus (data not shown). This finding suggests that C5A inactivates cell-free or cell-bound virus either before DC infection or during DC transfer to T cells. Similarly, C5A blocks T cell infection by wild-type viruses transmitted from DC (Fig. 4e). These results suggest that, unlike neutralizing antibodies, C5A can block cell-to-cell transfer of HIV even when transmission occurs via the virological synapse. Thus, C5A interferes with HIV transmission at three levels: PGEC transmigration, DC and LC infection, and subsequent T cell infection.
C5A Inhibits an Ongoing HIV Infection of T Cells.
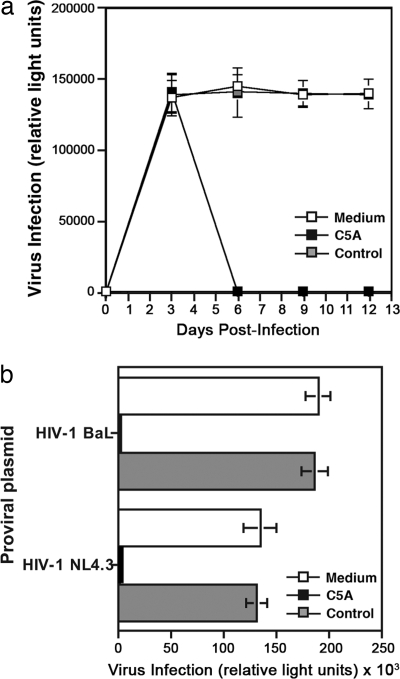
To determine whether C5A can block an ongoing HIV infection, primary CD4+ T lymphocytes were exposed to NL4.3-BaL (1 ng of p24) in the absence of peptide for 3 days at 37°C to establish infection. Three days after infection, C5A or the control peptide (5 μM) was added to infected cells for 2 h at 37°C, at which point the cells were washed to remove peptides and, over the next 12 days, the infectivity of the treated and control cell culture supernatants was tested on TZM reporter cells. In the presence of the control peptide, the infection persisted over the 12-day observation period, whereas viral infectivity was abrogated in C5A-treated infected T cells (Fig. 5a).
Fig. 5.
C5A blocks an ongoing HIV infection. (a) Primary CD4+ T lymphocytes (0.5 × 106 cells) were exposed to NL4.3-BaL (1 ng of p24) for 1 day at 37°C and washed. Three days after infection, C5A or control peptide (5 μM) was added to infected cells for 2 h and washed. Infected cell culture supernatants were collected every 3 days, normalized for particulate p24 content, and tested for infectivity (1 ng of p24) on TZM reporter cells. Error bars represent standard errors of duplicates. (b) 293T cells (1 × 106 cells) transfected with 1 μg of proviral NL4.3 or NL4.3-BaL DNA for 24 h at 37°C were treated with or without C5A or control peptide (5 μM) for 1 h at 37°C and washed. Twenty-four hours after peptide treatment, supernatants of transfected 293T cells containing newly formed viruses were tested for infectivity as described in a. Error bars represent standard errors of duplicates.
To determine whether C5A can neutralize budding viruses by destabilizing their viral membrane, we analyzed the effect of the peptide on the ability of cells transfected with HIV proviral DNA to produce infectious viruses. 293T cells transfected with HIV (NL4.3 or NL4.3-BaL) for 24 h were treated with C5A or the control peptide (5 μM) for 1 h and then washed to remove both previously produced and released viruses and peptides. Twenty-four hours after peptide treatment, the infectivity of newly formed viruses released into the supernatant was tested on TZM reporter cells. As shown in Fig. 5b, C5A completely inactivated virus infectivity. Collectively, these results suggest that C5A disrupts the integrity both of cell-free (Figs. 2 and 5a) and of budding virus (Fig. 5b).
Discussion
In this work, we report that an amphipathic α-helical peptide designated C5A that has been shown to be virocidal for HCV infection (2) also has potent antiviral activity against HIV. C5A exhibits a broad range of antiviral activity against HIV primary isolates. Its wide spectrum of antiviral activity correlates with its unique mechanism of action. C5A is unique because it does not function like other anti-HIV amphipathic peptides, such as the T20 peptide, that target the HIV-1 viral glycoprotein (16). Moreover, several well defined antimicrobial amphipathic peptides that are known to disrupt bacterial membrane integrity (i.e., cathelicidin, buforin, hecate, piscidin, etc.), do not affect HIV infection (data not shown). We obtained several lines of evidence that suggest that C5A disrupts the integrity of the membrane of HIV. The C5A-mediated viral membrane disruption renders HIV particles noninfectious likely by either destabilizing the physical linkage between the mature conical capsid core and the viral membrane, i.e., the core-membrane linkage (17) or by provoking the shedding of the viral glycoprotein gp120 responsible for HIV entry into cells.
Trypsin treatment of the virus did not prevent C5A from destabilizing HIV, suggesting that it does not require trypsin-sensitive molecules on the surface of the viral membrane for its virocidal activity. Thus, C5A uses either trypsin-resistant proteinaceous receptors or nonproteinaceous receptors (e.g., lipids) to bind HIV. The resistance of VSV and many other viruses to C5A (2) may reflect either of these alternatives. Because HCV (18) and HIV (19, 20) bud from lipid rafts, whereas VSV does not (21), it is possible that the protein and/or lipid composition of the VSV membrane differs from that of HIV. The enrichment of a specific proteinaceous or lipid “receptor” for the NS5A anchor domain in the HIV membrane might explain why it has no effect on VSV and is relatively nontoxic for host cells.
The remarkable durability of the antiviral effect of C5A on primary T cell infection (Fig. 5a) could be explained by inactivation of both cell-free and budding viruses if the rate of apoptosis of infected cells is higher than the rate of virus production. Because massive apoptotic fragmentation of both host and viral (integrated) DNA occurs in <24 h in HIV-infected primary T cells (22), this finding may explain the persistent antiviral effect of C5A on T cell infection.
Interestingly, many antiviral properties of C5A satisfy important preconditions for the use of C5A as a potential microbicide. Specifically, C5A is active against a broad spectrum of HIV isolates; it prevents transmission to the major in vivo HIV cellular targets and prevents transcytosis across primary genital epithelial cells; it exhibits no apparent cell toxicity at inhibitory doses; it is active at low pH; it is potent for 2 h before and after exposure; and most importantly, its rapid virocidal mode of action is unique compared with that of drugs that are currently used in HIV-infected patients. In addition, if it targets the lipid composition of HIV membranes (2), C5A should not select for viral escape variants, and, if used in combination with agents that do select for resistance variants, it could prevent their spread. Thus, C5A appears to represent the prototype of a new generation of antiviral agents that may have promise for the prevention and treatment of HIV.
Materials and Methods
Detailed methods and procedures are provided in SI Methods.
Cells and Viruses.
Blood-derived primary DC, CD4+ T lymphocytes, macrophages, and PGEC were isolated and characterized as described in SI Methods. TZM cells express CD4, CXCR4, and CCR5 and contain an integrated lacZ gene driven by the HIV-1 LTR (4). Wild-type pNL4.3 (X4), pNL4.3-BaL (R5), pNL4.3ΔEnv, pNL4.3-eGFP (X4), and pNL4.3-BaL-eGFP (R5) viruses were produced as described in SI Methods.
Transmigration and Infection Assays.
The HIV transmigration assay was conducted as described in SI Methods. The PGEC monolayer divides the transwell into an apical and a basolateral compartment. HIV was added to the upper chamber, and after 8 h at 37°C HIV, transcytosis was monitored by measuring the amount of particulate capsid in the basal chamber by p24 ELISA. Peptide toxicity to PGEC was examined as described in SI Methods.
LC and DC Transmission Assays.
Human epidermal sheets were isolated as described in SI Methods and incubated with NL4.3-BaL-eGFP (100 ng of p24) for 4 h. Sheets were then exposed to peptides (10 μM) and cultured for 3 days. Infection of migrated cells, which exfoliated from the epidermal sheets, was quantified by GFP expression by FACS. To measure HIV transmission to T cells, migrated cells were mixed with Jurkat T cells (100,000 cells), and the percentage of infected Jurkat T cells was quantified by GFP expression of anti-CD3 gated T cells by FACS at day 5 as described in SI Methods. To analyze DC transmission, DC (50,000 cells) were incubated with NL4.3-eGFP (X4), NL4.3-BaL-eGFP (R5), or NL4.3ΔEnv-eGFP-pseudotyped with NL4.3 gp160 (X4) (25 ng of p24) for 2 h at 37°C. Cells were washed, exposed to peptide for 2 h, washed again, and added to Jurkat T cells (100,000 cells). The percentage of infected T cells was measured by FACS as described in SI Methods. Infection of TZM reporter cells was conducted as described in ref. 4.
Virus Sedimentation Assay.
Virus (20 ng of p24 of NL4.3) was purified, treated with peptides under various conditions, and immediately analyzed for rupture in a 20–70% sucrose gradient as described in detail in SI Methods.
Acknowledgments.
This is publication 18935-IMM from The Scripps Research Institute, La Jolla, CA. We thank A. Montero and R. Ghadiri for peptide synthesis; F. Penin, D. Moradpour, and T. Tellinghuisen for helpful discussion; Stefan Wieland for graphic art; Drs. J. Bukh and R. Whitley for helpful critical comments on the manuscript. P.A.G was supported by National Institutes of Health Grant R21 AI071952. G.C. and F.V.C. were supported by National Institutes of Health Grant R01 CA108304 and a generous gift from Mr. Clifford Evans. L.d.W. was supported by Dutch Scientific Research Program Grant (NWO 917-46-367 and the Saal van Zwanenbergstichting.
Footnotes
Conflict of interest statement: F.V.C. has a financial interest in Viriome, Inc., which has licensing rights to the information provided in this article.
This article contains supporting information online at www.pnas.org/cgi/content/full/0801388105/DCSupplemental.
References
- 1.Klasse PJ, Shattock R, Moore JP. Antiretroviral drug-based microbicides to prevent HIV-1 sexual transmission. Annu Rev Med. 2008;59:291–307. doi: 10.1146/annurev.med.59.061206.112737. [DOI] [PubMed] [Google Scholar]
- 2.Cheng G, et al. A virocidal amphipathic α-helical peptide that inhibits hepatitis C virus infection in vitro. Proc Natl Acad Sci USA. 2008;105:3088–3093. doi: 10.1073/pnas.0712380105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wei X, et al. Emergence of resistant human immunodeficiency virus type 1 in patients receiving fusion inhibitor (T-20) monotherapy. Antimicrob Agents Chemother. 2002;46:1896–1905. doi: 10.1128/AAC.46.6.1896-1905.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chatterji U, et al. Naturally occurring capsid substitutions render HIV-1 cyclophilin A independent in human cells and TRIM-cyclophilin-resistant in Owl monkey cells. J Biol Chem. 2005;280:40293–40300. doi: 10.1074/jbc.M506314200. [DOI] [PubMed] [Google Scholar]
- 5.Aiken C, Trono D. Nef stimulates human immunodeficiency virus type 1 proviral DNA synthesis. J Virol. 1995;69:5048–5056. doi: 10.1128/jvi.69.8.5048-5056.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Saphire AC, Bobardt MD, Zhang Z, David G, Gallay PA. Syndecans serve as attachment receptors for human immunodeficiency virus type 1 on macrophages. J Virol. 2001;75:9187–9200. doi: 10.1128/JVI.75.19.9187-9200.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hladik F, et al. Initial events in establishing vaginal entry and infection by human immunodeficiency virus type 1. Immunity. 2007;26:257–270. doi: 10.1016/j.immuni.2007.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bobardt MD, et al. Cell-free human immunodeficiency virus type 1 transcytosis through primary genital epithelial cells. J Virol. 2007;81:395–405. doi: 10.1128/JVI.01303-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Miller CJ. HIV transmission: Migratory Langerhans cells are primary targets in vaginal HIV transmission. Immunol Cell Biol. 2007;85:269–270. doi: 10.1038/sj.icb.7100058. [DOI] [PubMed] [Google Scholar]
- 10.de Witte L, et al. Langerin is a natural barrier to HIV-1 transmission by Langerhans cells. Nat Med. 2007;13:367–371. doi: 10.1038/nm1541. [DOI] [PubMed] [Google Scholar]
- 11.Geijtenbeek TBH, et al. DC-SIGN, a dendritic cell-specific HIV-1-binding protein that enhances trans-infection of T cells. Cell. 2000;100:587–597. doi: 10.1016/s0092-8674(00)80694-7. [DOI] [PubMed] [Google Scholar]
- 12.Turville SG, et al. Immunodeficiency virus uptake, turnover, and two-phase transfer in human dendritic cells. Blood. 2004;103:2170–2179. doi: 10.1182/blood-2003-09-3129. [DOI] [PubMed] [Google Scholar]
- 13.Wiley RD, Gummuluru S. Immature dendritic cell-derived exosomes can mediate HIV-1 trans infection. Proc Natl Acad Sci USA. 2006;103:738–743. doi: 10.1073/pnas.0507995103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McDonald D, et al. Recruitment of HIV and its receptors to dendritic cell–T cell junctions. Science. 2003;300:1295–1297. doi: 10.1126/science.1084238. [DOI] [PubMed] [Google Scholar]
- 15.Ganesh L, et al. Infection of specific dendritic cells by CCR5-tropic human immunodeficiency virus type 1 promotes cell-mediated transmission of virus resistant to broadly neutralizing antibodies. J Virol. 2004;78:11980–11987. doi: 10.1128/JVI.78.21.11980-11987.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kilby JM, et al. Potent suppression of HIV-1 replication in humans by T-20, a peptide inhibitor of gp41-mediated virus entry. Nat Med. 1998;4:1302–1307. doi: 10.1038/3293. [DOI] [PubMed] [Google Scholar]
- 17.Höglund S, et al. Spatial visualization of the maturing HIV-1 core and its linkage to the envelope. AIDS Res Hum Retroviruses. 1992;8:1–7. doi: 10.1089/aid.1992.8.1. [DOI] [PubMed] [Google Scholar]
- 18.Aizaki H, Lee KJ, Sung VM, Ishiko H, Lai MM. Characterization of the hepatitis C virus RNA replication complex associated with lipid rafts. Virology. 2004;324:450–461. doi: 10.1016/j.virol.2004.03.034. [DOI] [PubMed] [Google Scholar]
- 19.Nguyen DH, Hildreth JE. Evidence for budding of human immunodeficiency virus type 1 selectively from glycolipid-enriched membrane lipid rafts. J Virol. 2000;74:3264–3272. doi: 10.1128/jvi.74.7.3264-3272.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Brügger B, et al. The HIV lipidome: A raft with an unusual composition. Proc Natl Acad Sci USA. 2006;103:2641–2646. doi: 10.1073/pnas.0511136103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Scheiffele P, Rietveld A, Wilk T, Simons K. Influenza viruses select ordered lipid domains during budding from the plasma membrane. J Biol Chem. 1999;274:2038–2044. doi: 10.1074/jbc.274.4.2038. [DOI] [PubMed] [Google Scholar]
- 22.Terai C, Kornbluth RS, Pauza CD, Richman DD, Carson DA. Apoptosis as a mechanism of cell death in cultured T lymphoblasts acutely infected with HIV-1. J Clin Invest. 1991;87:1710–1715. doi: 10.1172/JCI115188. [DOI] [PMC free article] [PubMed] [Google Scholar]