Abstract
Autoantibodies to glutamate decarboxylase 65 (GAD65Ab) are commonly believed to be a major characteristic for type 1 diabetes (T1D). We investigated the presence of GAD65Ab in healthy individuals (n = 238) and first-degree relatives (FDRs) of T1D patients (n = 27) who tested negative for GAD65Ab in conventional RIAs. Sera were applied to affinity columns coated with GAD65-specific mAbs to absorb anti-idiotypic antibodies (anti-Ids). The absorbed sera were analyzed for binding to GAD65 by RIAs. Both healthy individuals and FDRs present GAD65Ab that are inhibited by anti-Id, masking them in conventional detection methods. The presence of GAD65Ab-specific anti-Ids was confirmed by competitive ELISA. Remarkably, T1D patients (n = 54) and Stiff Person Syndrome patients (n = 8) show a specific lack of anti-Ids to disease-associated GAD65Ab epitopes. Purified anti-Ids from healthy individuals and FDRs inhibited the binding of GAD65Ab from T1D patients to GAD65. We conclude that masked GAD65Ab are present in the healthy population and that a lack of particular anti-Ids, rather than GAD65Ab per se, is a characteristic of T1D. The lack of these inhibitory antibodies may contribute to T cell activation by GAD65Ab.
Type 1 diabetes (T1D) is an autoimmune disease characterized by the presence of serologically detectable autoantibodies to multiple islet cell autoantigens. Autoantibodies to glutamate decarboxylase 65 (GAD65Ab) can be detected in the majority of new-onset T1D patients (1), in patients with latent autoimmune diabetes in adults (for review see ref. 2), diabetes-related polyendocrine diseases (for review see ref. 3), and in some rare neurologic diseases, notably Stiff Person Syndrome (SPS) (4), but rarely in the general population. GAD65Ab often herald the onset of T1D by months or years and are used to predict disease together with other islet cell autoantibodies (5, 6). The function of these autoantibodies and their B cells in the pathogenesis of T1D is not clear, especially as a patient with severe B cell deficiency and diabetes was reported (7). Although GAD65Ab are often considered to be an epiphenomenon resulting from the autoimmune destruction of the pancreatic β cells, some studies suggest that they may be involved in antigen processing and presentation and thus modulate the immune response (8–10). This hypothesis is supported by a recent study demonstrating a pathogenic role of autoantibodies by enhancing islet cell antigen presentation to autoreactive T cells (11). Harbers et al. (11) showed that both antigen-specific CD8+ T cells and antigen-specific antibodies were necessary for the development of autoimmune diabetes in transgenic mice that express a membrane-bound form of ovalbumin in pancreatic β cells.
To investigate the possible role of GAD65Ab in T1D pathogenesis, we previously injected young nonobese diabetic (NOD) mice with the GAD65-specific mAb b96.11 (12). This antibody specificity was shown earlier to be predictive of the development of T1D in humans (13). We found that this treatment induced b96.11-specific anti-idiotypic antibodies (anti-Ids) that efficiently blocked the binding of b96.11 to GAD65 and was accompanied by a significant reduction of incidence of insulitis and diabetes in the mice. Moreover, injections with the GAD65-specific mAb b78, that reacts with an epitope that is important in SPS, but rarely recognized in T1D (14), had no significant effect on the development of diabetes in NOD mice, suggesting that the effect was epitope-specific. We hypothesized that the anti-Ids could affect the disease progression by preventing GAD65Ab binding to its antigen.
The major aim of this study was to investigate whether GAD65Ab could be detected in healthy individuals. We found that while healthy individuals and first-degree relatives (FDRs) of T1D patients tested GAD65Ab-negative in conventional RIAs, they presented GAD65Ab after preabsorption with various GAD65-reactive mAbs, suggesting that these GAD65Ab were present, but masked by an epitope-specific anti-Id. Moreover, GAD65Ab-positive T1D and SPS patients show a specific lack of anti-Ids to disease-associated GAD65Ab, b96.11, or b78, possibly releasing these epitope GAD65Ab specificities to the circulation.
Results
Sera from Healthy Individuals and FDR Contain GAD65Ab That Are Masked, Whereas T1D Patients and SPS Patients Lack Inhibitors to Disease-Specific GAD65Ab.
We investigated the presence of masked GAD65Ab in human serum samples. Samples were incubated at 55°C either in the presence or absence of monoclonal GAD65Ab immobilized to protein A Sepharose (PAS). After the reaction was allowed to cool to room temperature the flow-through was tested in an RIA for binding to GAD65 (Fig. 1a). We analyzed sera of healthy individuals (n = 238) and FDR (n = 27) that had tested negative for GAD65Ab in conventional RIAs, GAD65Ab-positive T1D patients (n = 54), and GAD65Ab-positive SPS patients (n = 8).
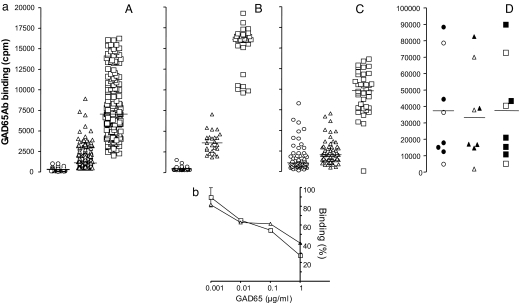
Fig. 1.
GAD65Ab in healthy individuals and FDRs are revealed upon removal of inhibitor. (a) Sera of healthy individuals and FDRs contain GAD65Ab/inhibitor complexes, whereas T1D patients and SPS patients lack inhibitors to disease-specific GAD65Ab. Sera of healthy individuals (A) and FDRs (B) that tested negative for GAD65Ab in conventional RIA and sera from GAD65Ab-positive T1D patients (C) and SPS patients (D) were absorbed on immobilized b96.11-PAS and b78-PAS. The serum samples and the flow-through of beads were tested for the presence of GAD65Ab in an RIA. Binding to GAD65 before (circles) and after absorption to b96.11 (triangles) or b78 (squares) is shown in cpm. SPS patients that are also diagnosed with T1D are represented by the filled symbols. Median binding is indicated. (b) ELISA of isolated inhibitor from a healthy individual. Binding of inhibitor isolated from b96.11-PAS or b78-PAS after absorption with serum from a healthy individual to b96.11-HRP (triangles) and b78-HRP (squares) was analyzed in the presence of the indicated concentrations of human recombinant GAD65. Binding is shown as percent binding with binding in the absence of GAD65 set as 100%
For the healthy individuals we observed a significant increase in binding to GAD65 after absorption to b96.11-PAS or b78-PAS [median binding before absorption: 292 (112–1,055) cpm, after absorption to b96.11-PAS: 1,088 (401–8,881) cpm, after absorption to b78-PAS: 6,519 (2,056–1,6278) cpm (P < 0.001)]. The GAD65Ab titer of all samples after absorption to both b96.11-PAS and b78-PAS was higher than the median before absorption and 123/238 (52%) of the b96.11-absorbed samples had a GAD65Ab titer above the maximum GAD65Ab titer before absorption. All samples absorbed to b78-PAS tested above the maximum GAD65Ab titer before absorption. Increases in GAD65 binding were seen when sera were absorbed with biotinylated GAD65Ab coupled to streptavidin-agarose, demonstrating that the revealed GAD65 binding is not consequence of release of coupled IgG from beads (data not shown).
Similar results were obtained when absorbing the sera without previous heat dissociation (data not shown). However, the effect was less pronounced, indicating that the moderate temperature increase catalyzed the dissociation of the GAD65Ab/inhibitor complexes and allowed the absorption of the released inhibitor to the immobilized GAD65Ab. Dissociation of antibody complexes by heat has been used previously (15), and we confirmed that the binding capacity of purified GAD65Ab remains stable under these conditions (data not shown).
Binding of the isolated inhibitor to GAD65Ab was confirmed in an ELISA (Fig. 1b). The inhibitor was eluted from b96.11-PAS or b78-PAS after absorption of serum obtained from a healthy individual and detected by HRP-labeled b96.11 or b78, respectively. Addition of human recombinant GAD65 diminished the signal in a dose-dependent manner, suggesting that the inhibitor binds to the antigen-binding site of GAD65Ab. Addition of BSA had no effect on the binding (data not shown).
A significant increase in GAD65Ab levels after absorption to b96.11-PAS and b78-PAS was also observed for FDR (P < 0.001) (Fig. 1a). In the analysis of the T1D patients we found a significant increase from 1,094 to 9,830 cpm in the level of GAD65Ab after absorption on b78-PAS (P < 0.001); however, no difference was detected between the level of GAD65Ab in nonabsorbed and b96.11-PAS absorbed sera. In the analysis of the SPS patients we found no increase in the level of GAD65Ab after absorption on either b96.11-PAS or b78-PAS.
We tested the possible release of GAD65Ab from the GAD65Ab-PAS by analyzing the flow-through of GAD65Ab-PAS that underwent the same procedure without incubation with serum. No binding to GAD65 could be detected under these conditions.
The observed binding was specific to GAD65, because competition with unlabeled human recombinant GAD65, but not BSA reduced the binding to radiolabeled GAD65 (P < 0.001) (Fig. 2a). Moreover, no increase in binding to the tyrosine phosphatase-like protein IA-2 was observed when testing sera that had been heated and absorbed (data not shown).
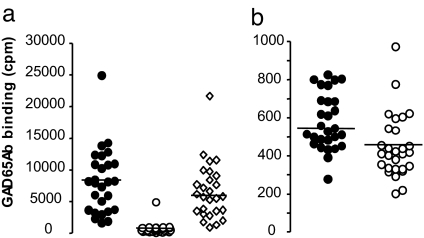
Fig. 2.
The inhibitor is specific to GAD65Ab. (a) GAD65 binding by unmasked sera is competed by recombinant human GAD65 and not by BSA. Sera of FDR absorbed by b96.11-PAS were tested for binding to [35]S-GAD65 in the presence of recombinant human GAD65 (150 mM) (○), or BSA (150 nM) (◇). Median binding is indicated. (b) Absorption of inhibitors that reduce GAD65Ab binding is specific to GAD65Ab and cannot be achieved by an irrelevant human mAb. Sera of healthy individuals (n = 15) and FDR (n = 15) were absorbed to immobilized HAA1. The flow-through was tested for binding to GAD65. GAD65Ab titer before (○) and after (●) absorption is shown in cpm. Median binding is indicated.
We investigated whether the effect was specific to GAD65Ab by absorbing serum samples to an irrelevant human mAb HAA1 immobilized to PAS. This antibody recognizes blood group A antigen. We tested 14 healthy individuals and 14 FDRs. No significant increase in GAD65Ab binding was observed (Fig. 2b).
Characterization of the Inhibitor.
We identified the inhibitor as human Ig by preparing purified Ig and Ig-depleted fractions from sera of four FDRs. The purified Ig and the Ig-depleted sera were incubated with b96.11-PAS to identify the fraction that contained the inhibitor and GAD65Ab (Fig. 3a). As expected, absorption of the purified Ig fraction on b96.11-PAS resulted in a significant increase in binding to GAD65, whereas absorption of the Ig-depleted fraction on b96.11-PAS had no effect on GAD65 binding. These data suggest that (i) the Ig fraction contains both GAD65Ab and the inhibitor and (ii) the observed effect is not caused by release of b96.11 from b96.11-PAS by serum proteases.
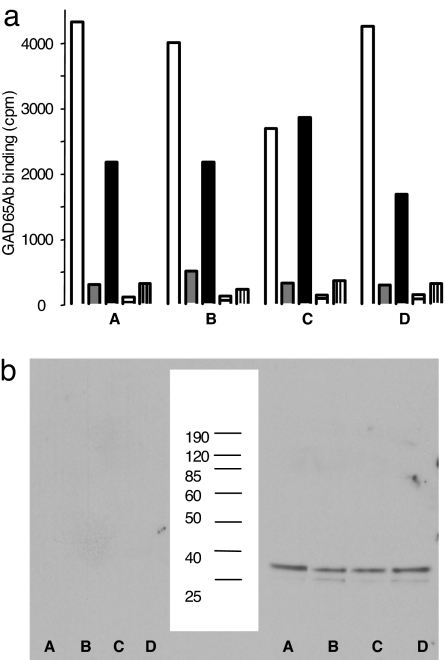
Fig. 3.
GAD65Ab and inhibitors are present only in the sera's Ig fraction. (a) Purified Ig and depleted serum were prepared from four FDRs (A–D). Absorbed sera (empty bars), purified Ig before (gray bars) and after absorption (black bars), Ig-depleted sera before (bars with horizontal lines) and after absorption (bars with vertical lines) were tested for binding to GAD65. The data show that affinity purification of GAD65Ab-negative sera on b96.11-PAS leads to the dissociation of the immune complexes to reveal the presence of hitherto undetectable GAD65Ab. (b) The above-described Ig-depleted serum fractions (Left) and purified Ig fractions (Right) were tested with goat-anti human κ chain. (Center) Molecular weight markers are shown.
The inhibiting Ig was further analyzed by eluting the serum fraction absorbed to b96.11-PAS and testing the eluted fractions for the presence of human Ig by Western blot analysis (Fig. 3b). In addition to both human κ and λ light chains, Western blot analysis showed the inhibiting fractions to contain human IgG, whereas testing with human IgM showed no signal (data not shown). As an additional control, we found no signal in Western blot analysis using fractions eluted from b96.11-PAS beads that had not been incubated with human serum, indicating that the coupled b96.11 was not released from the b96.11-PAS. These results led to our characterization of the inhibitor as anti-Id.
Anti-Ids Show Cross-Reactivity to GAD65Ab from Different Individuals.
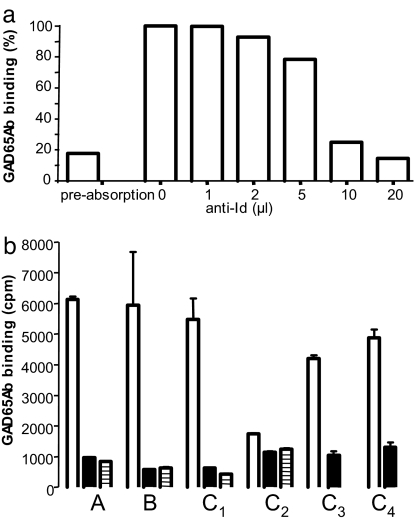
Anti-Ids were eluted from b96.11-PAS after absorption with serum from a FDR as described above. Dose-dependent inhibition of GAD65 binding was observed when the anti-Id was added to the absorbed sample (Fig. 4a). Following the same protocol, we prepared anti-Ids from a healthy individual and carried out cross-inhibition experiments between the groups using the optimal conditions (20 μl) as determined above (Fig. 4b). The data suggest that anti-Ids isolated from healthy individuals efficiently inhibit antigen binding by absorbed sera from FDR and vice versa. Moreover, anti-Ids obtained from a healthy individual and a FDR also inhibited GAD65 binding by four T1D patients' sera (Fig. 4b), although this inhibition was weaker (74% and 79%, respectively) as compared with that in the healthy individual and the FDR (≈90%).
Fig. 4.
Anti-Ids are cross-inhibitory between healthy individuals and FDRs and inhibit GAD65 binding by T1D patients' sera. (a) Anti-Ids were isolated from a FDR as described and added to the unmasked serum at the indicated concentrations. GAD65 binding is shown as percent binding (binding of absorbed, uninhibited serum is set to 100%). A dose-dependent inhibition of GAD65 binding was observed. (b) Anti-Ids were isolated from a healthy individual as described. Anti-Ids (20 μl) from the healthy individual (filled bars) and the FDR (bars with horizontal lines) were added to unmasked sera of a healthy individual (A), FDR (B), and unabsorbed sera of four T1D patients (C1–4). GAD65 binding is reported in cpm.
Discussion
In the present study, we demonstrate anti-Ids specific to GAD65Ab. Immune depletion of these anti-Ids reveals the presence of masked GAD65Ab in the majority of sera from healthy individuals and FDRs that otherwise test negative for GAD65Ab in conventional screening assays. Moreover, we show that GAD65Ab-specific anti-Ids are severely reduced in T1D patients. In particular, anti-Ids reactive to mAb b96.11 that is associated with progression to T1D (13) show a marked reduction in T1D patients. Our study suggests that GAD65Ab are found in the majority of healthy, nondiabetic individuals, but that their binding capacity is inhibited by GAD65Ab-specific anti-Ids. We conclude that it is the absence of these anti-Ids, rather than the presence of GAD65Ab, that is characteristic for T1D. Screening of sera for anti-Ids specific to disease-associated GAD65Ab may be valuable in the characterization and risk assessment for T1D.
Anti-Ids have been described in the normal human immune response, where they are believed to play a protective role, as they can block the binding of pathogenic autoantibodies (16). They have also been described in several autoimmune diseases (17–20). Autoantibodies to ribosomal P proteins (anti-P) are regarded as specific for patients with systemic lupus erythematosus. Stafford and colleagues (21–23) demonstrated that in healthy individuals who tested negative for anti-P antibodies by conventional serologic screenings anti-P antibodies were in fact readily detectable after removal of the inhibitory antibodies. Similarly, autoantibodies to the E2 subunit of the pyruvate dehydrogenase complex, thought to be specific to patients with primary biliary cirrhosis, are present but inhibited by anti-Ids in healthy individuals (24). In T1D, anti-Ids to insulin autoantibodies have been reported in both humans (25) and the BB rat model (26). These anti-Ids appear to resemble insulin and may affect the utilization of insulin (27). A balance between autoantibodies and anti-Ids is proposed to contribute to the homeostasis of the adaptive immune response in the “network hypothesis” (28). A disturbance of this balance, by a decline in protective factors or an increase in autoimmune elements, could precipitate autoimmune disease. We speculate that GAD65Ab-specific anti-Ids are formed in the following cascade of events: death of pancreatic β cells leads to the release of islet cell antigens, including GAD65. This β cell death can be the result of a β cell injury or apoptosis during normal development (29, 30). The islet cell antigens are presented in the pancreatic lymph nodes (31) and lead to the formation of GAD65Ab. Indeed, the presence of a wide range of autoantibodies, including GAD65Ab, shortly after birth has been described (32). According to the network hypothesis antibody idiotypes will induce the production of anti-Ids (28, 33). These anti-Ids can then block the initial antibodies, preventing them from binding to the antigen.
However, the actual mechanisms involved in the lack of anti-Ids in autoimmune diseases have not been well studied, and most studies of anti-Ids address autoimmune diseases with a major B cell component to the immune response, whereas in T1D, although autoantibodies are readily demonstrable, the pathogenesis is considered to be T cell-mediated.
In light of recent evidence that autoantibodies may play a role in modulating the T cell response (11), it is notable that antibody specificity, rather than antibody titer, is associated with T1D and disease progression (13, 34, 35). The GAD65Ab response in T1D patients is characterized by GAD65Ab specificities similar to that of mAb b96.11 (13), whereas GAD65Ab in patients with SPS often recognize GAD65Ab epitopes similar to that bound by b78 (14). These two antibodies recognize epitopes located at independent clusters (ctc1 and ctc2), positioned on opposing faces of the C-terminal domain of the GAD65 molecule (36). Recognition of ctc2 by b96.11-like GAD65Ab appears to be associated with an aggressive islet cell autoimmune reaction leading to T1D, whereas binding to the ctc1 epitope by b78-like GAD65Ab appears to be characteristic of an islet cell autoimmunity either unassociated with diabetes (e.g., SPS) or associated with a milder diabetes phenotype. Similarly, it is a lack of anti-Ids specific to b96.11, and not b78, that is associated with T1D.
In the context of our previous findings, the lack of anti-Id specific to b96.11 in T1D patients may suggest that GAD65Ab are functionally inhibited by epitope-specific anti-Ids in the healthy immune system. In the absence of these specific inhibitory anti-Ids, overt GAD65Ab are exposed and consequently are able to modulate the immune response (8–10) and break T cell tolerance as recently demonstrated (11). Perhaps antibodies to ctc2 epitope(s) play a role in antibody-driven effector T cell activation in T1D equivalent to the cooperative role played by antibodies to ovalbumin in the development of diabetes by T cells in RIP-mOVA mice (11), and such activation is blocked by the presence of anti-Id of appropriate specificity.
Although anti-Ids are used in the treatment of allergy (for review see ref. 37), they are currently not used in the treatment of autoimmune diseases. However, the successful treatment of autoimmune disorders with i.v. immune globulin has partially been explained by the presence of anti-Id (for review see refs. 38 and 39), and the potential of recombinant anti-Ids has been suggested in the treatment of some autoimmune diseases (40). The rationale for using GAD65Ab-specific anti-Ids in treatment is to provide enough anti-Id immunoglobulins to neutralize circulating GAD65Ab of the b96.11-idiotype.
The presence of anti-Ids may provide a mechanism for some previously unexplained phenomena. For example, these GAD65Ab-specific anti-Id may explain the persistence of GAD65Ab for years after the onset of T1D (41, 42), despite the loss of β cells as the antigen source after disease onset. Continuous β cell regeneration, protein mimicry, incomplete destruction of the β cells, release of GAD65 from other sources, or cross-reactivity of GAD65Ab with other proteins have also been proposed to explain this phenomenon (43). We speculate that an antigen-independent memory could be generated by the interaction of GAD65Ab-specific B cells with complementary B cells that produce anti-Id (for review see ref. 44). Moreover, the anti-Ids explain previous evidence of a serum factor preventing the binding of autoantibodies to GAD65 observed by Brown and Christie (45).
In conclusion, our findings demonstrate that GAD65Ab are not confined to T1D, but are masked in healthy individuals by specific anti-Ids that are absent in T1D. We propose that anti-Ids may play a protective role in the immune response, by preventing GAD65Ab to bind to their antigen and potentially modulate T cell responses to GAD65.
Materials and Methods
Serum Samples.
Healthy GAD65Ab-negative FDRs of T1D patients (n = 27) (mean age 39 years, range 17 to 61 years) were identified by screening individuals from families with at least two siblings with diabetes to a total of 1,170 probands with T1D enrolled in the Diabetes Incidence Study in Sweden and the Swedish Childhood Diabetes registry. The samples in this study were chosen at random from the GAD65Ab-negative sera.
Sera of healthy individuals (n = 238) (15–34 years old) were randomly selected control samples matched to newly diagnosed T1D patients. These samples were collected in 1992–1993 in the Diabetes Incidence Study in Sweden.
All serum samples tested repeatedly negative for antibodies to GAD65 in our conventional RIA.
GAD65Ab-positive T1D patients (n = 54) were randomly selected from 15- to 35-year-old newly diagnosed Swedish T1D patients. These patients were registered in 1992–1993 in the Diabetes Incidence Study in Sweden and were previously reported to be positive for GAD65Ab.
GAD65Ab-positive SPS patients (n = 8) were collected in Seattle from six females and one male. Another male SPS patient was evaluated at King's College Hospital. All subjects or their legal guardians gave informed consent.
Local institutional ethics committee approval and subjects' consent was obtained before collection of all serum samples.
Antibodies.
Human mAbs b96.11 and b78 specific to GAD65 were derived from a patient with autoimmune polyendocrine syndrome type 2 (46). B96.11 recognizes an epitope that is specifically bound by patients with T1D (13). B78 recognizes an epitope that is specifically bound by patients with SPS (14). The epitope specificities of these antibodies have been described in detail (36). Briefly, they recognize epitopes located at independent clusters that are positioned on opposing faces of the molecule's C-terminal domain.
HAA1 is a human mAb antibody (ATCC) specific to blood group A antigen (47) and shows no binding to GAD65.
The antibodies were purified from supernatants of the respective B cell line by using protein G Sepharose (PGS) (Invitrogen).
RIA.
GAD65Ab were determined by using the RIA as described (48). Briefly, recombinant [35]S-GAD65 was produced in an in vitro-coupled transcription and translation system with SP6 RNA polymerase and nuclease-treated rabbit reticulocyte lysate (Promega). Sera or IgG at the indicated concentrations were incubated with [35]S-GAD65 (25,000 of trichloroacetic acid-precipitable radioactivity). Sera were initially analyzed at 2.5 μl. If the GAD65Ab titer exceeded 9,000 cpm, the sample was diluted and reanalyzed. The reported titer was adjusted accordingly. After an overnight incubation at 4°C, antibody-bound [35]S-GAD65 was separated from unbound antigen by precipitation with PAS (Invitrogen). The immunoprecipitated radioactivity was counted on a Wallac Microbeta Liquid Scintillation Counter (Perkin–Elmer).
Western Blot Analysis.
Protein samples were electrophoresed on 15% SDS/PAGE under reducing conditions. Proteins were electrotransferred from the gel to Immobilon-P membranes (Millipore). Membranes were blocked in TBS buffer containing 1% BSA and incubated with goat anti-human κ light chain-HRP (Serotec), goat anti-human λ light chain-HRP (Serotec), goat anti human IgG-HRP (Bethyl Laboratories), or mouse anti-human IgM-HRP (Invitrogen) at the concentration recommended by the manufacturer. The immunocomplexes were detected with an Enhanced Chemiluminescence Detection system (GE Healthcare).
ELISA.
Binding of anti-Ids to GAD65Ab b96.11 and b78 was measured by ELISA following standard methods. Anti-Ids were isolated from a healthy individual and absorbed to 96-well microtiter plates (Nunc) (50 μl per well). The antibodies were detected by purified b96.11 and b78 that had been labeled with HRP according to the manufacturer's instructions (Pierce). Binding was competed by recombinant human GAD65 (Diamyd Medical AB) or BSA.
Cross-Linking of Antibody to PAS.
Purified antibodies were cross-linked to PAS by using the dimethylpimelimidate method (49). One milligram of antibody was cross-linked to 1 ml of PAS beads (Invitrogen). The efficiency of the coupling was 50% (0.5 mg/ml). Any uncoupled antibody was removed by washing beads with 0.05M glycine (pH 11). There was no significant release of the coupled protein from PAS as tested by RIA and Western blot analysis.
Absorption on Antibody-Coupled PAS Beads.
Serum samples (100 μl) were incubated with antibody-PAS beads (25 μl of 50% slurry) for 1 h. The bead volume was previously titrated for optimal assay conditions (data not shown). The bound fraction was separated from the unbound serum by gravity flow. The flow-through of the column was analyzed for GAD65Ab in an RIA. Change in volume was accounted for.
Heat Dissociation.
To dissociate complexes of GAD65Ab and their bound inhibitors in serum samples, we followed the heat dissociation method as described (15). Briefly, samples (100 μl) were heated to 55°C for 10 min in the absence or presence of antibody-PAS beads (25 μl of 50% slurry). The mixture was then incubated at 37°C for 30 min and at room temperature for a final 10 min and analyzed for GAD65Ab in an RIA. In absorption experiments the flow-through was assayed for GAD65Ab in an RIA.
Competition Experiment.
To evaluate the antibody binding specificity, 150 nmol/liter of unlabeled recombinant human GAD65 (Diamyd Medical AB) was used to block the binding of [35]S-GAD65 to GAD65Ab. Unlabeled BSA (150 nmol/liter) was used as a control.
Elution of Bound Inhibitor.
Bound protein was eluted from b96.11-PAS with 0.05 M glycine buffer (pH 11). One-milliliter fractions were collected, and the pH was immediately adjusted to pH 7.
Preparation of Ig and Ig-Depleted Fractions of Sera.
Ig was purified from sera by successive affinity chromatography on PAS and PGS (Invitrogen). Western blot analysis confirmed the presence of Ig in the purified fraction, whereas the Ig-depleted fraction contained no Ig.
Statistical Analysis.
All samples were analyzed in duplicate determinations. The mean intraassay coefficient of variation was 5% (13–0.04%). Our assay for GAD65Ab showed good sensitivity (86%) and high specificity (93%) in the 2007 Diabetes Antibody Standardization Program Workshop. Positive and negative controls were included for each assay. Binding levels between different treatments within one serum group were adjusted to the untreated positive and negative controls to correct for interassay variations. Binding levels between different serum groups were adjusted to the same serum derived from a healthy individual that was included on each plate and underwent identical procedures with the other samples. Median GAD65Ab levels between groups were analyzed by using the nonparametric ANOVA (Kruskall-Wallis test) followed by Dunn's multiple comparisons test. Significance was defined by P < 0.05.
Acknowledgments.
This work was supported by National Institutes of Health Grants DK53456, DK53004, DK26190 (to Åke Lernmark), and DK17047.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
References
- 1.Baekkeskov S, et al. Identification of the 64K autoantigen in insulin-dependent diabetes as the GABA-synthesizing enzyme glutamic acid decarboxylase. Nature. 1990;347:151–156. doi: 10.1038/347151a0. [DOI] [PubMed] [Google Scholar]
- 2.Falorni A, Brozzetti A. Diabetes-related antibodies in adult diabetic patients. Best Pract Res Clin Endocrinol Metab. 2005;19:119–133. doi: 10.1016/j.beem.2004.11.010. [DOI] [PubMed] [Google Scholar]
- 3.Falorni A, Laureti S, Santeusanio F. Autoantibodies in autoimmune polyendocrine syndrome type II. Endocrinol Metab Clin North Am. 2002;31:369–389. doi: 10.1016/s0889-8529(01)00010-x. [DOI] [PubMed] [Google Scholar]
- 4.Solimena M, De Camilli P. Autoimmunity to glutamic acid decarboxylase (GAD) in Stiff Man syndrome and insulin-dependent diabetes mellitus. Trends Neurosci. 1991;14:452–457. doi: 10.1016/0166-2236(91)90044-u. [DOI] [PubMed] [Google Scholar]
- 5.Verge CF, et al. Prediction of type I diabetes in first-degree relatives using a combination of insulin, GAD, and ICA512bdc/IA-2 autoantibodies. Diabetes. 1996;45:926–933. doi: 10.2337/diab.45.7.926. [DOI] [PubMed] [Google Scholar]
- 6.Bingley PJ, et al. Prediction of IDDM in the general population: Strategies based on combinations of autoantibody markers. Diabetes. 1997;46:1701–1710. doi: 10.2337/diab.46.11.1701. [DOI] [PubMed] [Google Scholar]
- 7.Martin S, et al. Development of type 1 diabetes despite severe hereditary B lymphocyte deficiency. N Engl J Med. 2001;345:1036–1040. doi: 10.1056/NEJMoa010465. [DOI] [PubMed] [Google Scholar]
- 8.Jaume JC, et al. Suppressive effect of glutamic acid decarboxylase 65-specific autoimmune B lymphocytes on processing of T cell determinants located within the antibody epitope. J Immunol. 2002;169:665–672. doi: 10.4049/jimmunol.169.2.665. [DOI] [PubMed] [Google Scholar]
- 9.Banga JP, et al. Modulation of antigen presentation by autoreactive B cell clones specific for GAD65 from a type I diabetic patient. Clin Exp Immunol. 2004;135:74–84. doi: 10.1111/j.1365-2249.2004.02343.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Reijonen H, Daniels TL, Lernmark A, Nepom GT. GAD65-specific autoantibodies enhance the presentation of an immunodominant T cell epitope from GAD65. Diabetes. 2000;49:1621–1626. doi: 10.2337/diabetes.49.10.1621. [DOI] [PubMed] [Google Scholar]
- 11.Harbers SO, et al. Antibody-enhanced cross-presentation of self-antigen breaks T cell tolerance. J Clin Invest. 2007;117:1361–1369. doi: 10.1172/JCI29470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hall TR, et al. Modulation of diabetes in NOD mice by GAD65-specific monoclonal antibodies is epitope specific and accompanied by anti-idiotypic antibodies. Immunology. 2008;123:547–554. doi: 10.1111/j.1365-2567.2007.02724.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Padoa CJ, et al. Recombinant Fabs of human monoclonal antibodies specific to the middle epitope of GAD65 inhibit type 1 diabetes-specific GAD65Abs. Diabetes. 2003;52:2689–2695. doi: 10.2337/diabetes.52.11.2689. [DOI] [PubMed] [Google Scholar]
- 14.Raju R, et al. Analysis of GAD65 autoantibodies in Stiff Person Syndrome patients. J Immunol. 2005;175:7755–7762. doi: 10.4049/jimmunol.175.11.7755. [DOI] [PubMed] [Google Scholar]
- 15.Routsias JG, et al. Idiotype-anti-idiotype circuit in nonautoimmune mice after immunization with the epitope and complementary epitope 289–308aa of La/SSB: Implications for the maintenance and perpetuation of the anti-La/SSB response. J Autoimmun. 2003;21:17–26. doi: 10.1016/s0896-8411(03)00063-5. [DOI] [PubMed] [Google Scholar]
- 16.Geha RS. Idiotypic-antiidiotypic interactions in man. Ric Clin Lab. 1985;15:1–8. doi: 10.1007/BF03029155. [DOI] [PubMed] [Google Scholar]
- 17.Dwyer DS, Bradley RJ, Urquhart CK, Kearney JF. Naturally occurring anti-idiotypic antibodies in myasthenia gravis patients. Nature. 1983;301:611–614. doi: 10.1038/301611a0. [DOI] [PubMed] [Google Scholar]
- 18.Lefvert AK, Fulpius BW. Receptor-like activity of a monoclonal anti-idiotypic antibody against an anti-acetylcholine receptor antibody. Scand J Immunol. 1984;19:485–489. doi: 10.1111/j.1365-3083.1984.tb00958.x. [DOI] [PubMed] [Google Scholar]
- 19.Taniguchi O, Chia DS, Barnett EV. Auto-anti-anti-DNA antibodies from SLE patients and normals. J Rheumatol. 1984;11:291–297. [PubMed] [Google Scholar]
- 20.Birdsall HH, Rossen RD. Characterization of anti-Fab' antibodies in human sera: Identification of soluble immune complexes that contain hidden anti-KLH and blocking anti-immunoglobulins following immunization with keyhole limpet hemocyanin. Clin Exp Immunol. 1983;53:497–504. [PMC free article] [PubMed] [Google Scholar]
- 21.Pan ZJ, Anderson CJ, Stafford HA. Anti-idiotypic antibodies prevent the serologic detection of antiribosomal P autoantibodies in healthy adults. J Clin Invest. 1998;102:215–222. doi: 10.1172/JCI1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stafford HA, Anderson CJ, Reichlin M. Unmasking of anti-ribosomal P autoantibodies in healthy individuals. J Immunol. 1995;155:2754–2761. [PubMed] [Google Scholar]
- 23.Anderson CJ, et al. The presence of masked antiribosomal P autoantibodies in healthy children. Arthritis Rheum. 1998;41:33–40. doi: 10.1002/1529-0131(199801)41:1<33::AID-ART5>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 24.Chen QY, Rowley MJ, Mackay IR. Anti-idiotypic antibodies to anti-PDC-E2 in primary biliary cirrhosis and normal subjects. Hepatology. 1999;29:624–631. doi: 10.1002/hep.510290344. [DOI] [PubMed] [Google Scholar]
- 25.Casiglia D, Giardina E, Triolo G. IgG auto-anti-idiotype antibodies against antibody to insulin in insulin-dependent (type 1) diabetes mellitus: Detection by capture enzyme-linked immunosorbent assay (ELISA) and relationship with anti-insulin antibody levels. Diabetes Res. 1991;16:181–184. [PubMed] [Google Scholar]
- 26.Elias D, et al. Insulin-mimicking anti-idiotypic antibodies in development of spontaneous autoimmune diabetes in BB/E rats. Diabetes. 1990;39:1467–1471. doi: 10.2337/diab.39.12.1467. [DOI] [PubMed] [Google Scholar]
- 27.Elias D, Maron R, Cohen IR, Schechter Y. Mouse antibodies to the insulin receptor developing spontaneously as anti-idiotypes. II. Effects on glucose homeostasis and the insulin receptor. J Biol Chem. 1984;259:6416–6419. [PubMed] [Google Scholar]
- 28.Jerne NK. Towards a network theory of the immune system. Ann Immunol. 1974;125:373–389. [PubMed] [Google Scholar]
- 29.Kassem SA, et al. β-cell proliferation and apoptosis in the developing normal human pancreas and in hyperinsulinism of infancy. Diabetes. 2000;49:1325–1333. doi: 10.2337/diabetes.49.8.1325. [DOI] [PubMed] [Google Scholar]
- 30.Finegood DT, Scaglia L, Bonner-Weir S. Dynamics of β cell mass in the growing rat pancreas: Estimation with a simple mathematical model. Diabetes. 1995;44:249–256. doi: 10.2337/diab.44.3.249. [DOI] [PubMed] [Google Scholar]
- 31.Turley S, et al. Physiological β cell death triggers priming of self-reactive T cells by dendritic cells in a type-1 diabetes model. J Exp Med. 2003;198:1527–1537. doi: 10.1084/jem.20030966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Merbl Y, Zucker-Toledano M, Quintana FJ, Cohen IR. Newborn humans manifest autoantibodies to defined self molecules detected by antigen microarray informatics. J Clin Invest. 2007;117:712–718. doi: 10.1172/JCI29943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shoenfeld Y. The idiotypic network in autoimmunity: Antibodies that bind antibodies that bind antibodies. Nat Med. 2004;10:17–18. doi: 10.1038/nm0104-17. [DOI] [PubMed] [Google Scholar]
- 34.Schlosser M, et al. Dynamic changes of GAD65 autoantibody epitope specificities in individuals at risk of developing type 1 diabetes. Diabetologia. 2005;48:922–930. doi: 10.1007/s00125-005-1719-1. [DOI] [PubMed] [Google Scholar]
- 35.Gilliam LK, et al. Multiplicity of the antibody response to GAD65 in type I diabetes. Clin Exp Immunol. 2004;138:337–341. doi: 10.1111/j.1365-2249.2004.02610.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fenalti G., et al. C-terminal clustering of autoantibody and T cell determinants on the structure of GAD65 provide insights into the molecular basis of autoreactivity. Diabetes. 2008 doi: 10.2337/db07–1461. [DOI] [PubMed] [Google Scholar]
- 37.Kuhn R. Immunoglobulin E blockade in the treatment of asthma. Pharmacotherapy. 2007;27:1412–1424. doi: 10.1592/phco.27.10.1412. [DOI] [PubMed] [Google Scholar]
- 38.Kazatchkine MD, Kaveri SV. Immunomodulation of autoimmune and inflammatory diseases with intravenous immune globulin. N Engl J Med. 2001;345:747–755. doi: 10.1056/NEJMra993360. [DOI] [PubMed] [Google Scholar]
- 39.Sapir T, Shoenfeld Y. Facing the enigma of immunomodulatory effects of intravenous immunoglobulin. Clin Rev Allergy Immunol. 2005;29:185–199. doi: 10.1385/CRIAI:29:3:185. [DOI] [PubMed] [Google Scholar]
- 40.Escher R, et al. Recombinant anti-idiotypic antibodies inhibit human natural anti-glycoprotein (GP)IIb/IIIa autoantibodies. J Autoimmun. 2002;18:71–81. doi: 10.1006/jaut.2001.0560. [DOI] [PubMed] [Google Scholar]
- 41.Borg H, Gottsater A, Fernlund P, Sundkvist G. A 12-year prospective study of the relationship between islet antibodies and β cell function at and after the diagnosis in patients with adult-onset diabetes. Diabetes. 2002;51:1754–1762. doi: 10.2337/diabetes.51.6.1754. [DOI] [PubMed] [Google Scholar]
- 42.Decochez K, et al. High frequency of persisting or increasing islet-specific autoantibody levels after diagnosis of type 1 diabetes presenting before 40 years of age: The Belgian Diabetes Registry. Diabetes Care. 2000;23:838–844. doi: 10.2337/diacare.23.6.838. [DOI] [PubMed] [Google Scholar]
- 43.Savola K, et al. Autoantibodies associated with type I diabetes mellitus persist after diagnosis in children. Diabetologia. 1998;41:1293–1297. doi: 10.1007/s001250051067. [DOI] [PubMed] [Google Scholar]
- 44.Nayak R, Mitra-Kaushik S, Shaila MS. Perpetuation of immunological memory: A relay hypothesis. Immunology. 2001;102:387–395. doi: 10.1046/j.1365-2567.2001.01205.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Brown TJ, Christie MR. Detection of a serum factor that blocks antibody binding to glutamate decarboxylase. Diabetologia. 1993;36(Suppl 1):A92. [Google Scholar]
- 46.Tremble J, et al. Human B cells secreting immunoglobulin G to glutamic acid decarboxylase- 65 from a nondiabetic patient with multiple autoantibodies and Graves' disease: A comparison with those present in type 1 diabetes. J Clin Endocrinol Metab. 1997;82:2664–2670. doi: 10.1210/jcem.82.8.4171. [DOI] [PubMed] [Google Scholar]
- 47.Larrick JW. U.S. Patent 4. 1986;624:921. [Google Scholar]
- 48.Hampe CS, et al. Recognition of glutamic acid decarboxylase (GAD) by autoantibodies from different GAD antibody-positive phenotypes. J Clin Endocrinol Metab. 2000;85:4671–4679. doi: 10.1210/jcem.85.12.7070. [DOI] [PubMed] [Google Scholar]
- 49.Harlow E, Lane D. Antibodies: A Laboratory Manual. Cold Spring Harbor, NY: Cold Spring Harbor Lab Press; 1988. [Google Scholar]