Abstract
Mice subjected to social isolation (3–4 weeks) exhibit enhanced contextual fear responses and impaired fear extinction. These responses are time-related to a decrease of 5α-reductase type I (5α-RI) mRNA expression and allopregnanolone (Allo) levels in selected neurons of the medial prefrontal cortex, hippocampus, and basolateral amygdala. Of note, the cued fear response was not different between group housed and socially isolated mice. In socially isolated mice, S-norfluoxetine, a selective brain steroidogenic stimulant (SBSS), in doses (0.45–1.8 μmol/kg) that increase brain Allo levels but fail to inhibit serotonin reuptake, greatly attenuates enhanced contextual fear response. SKF 105,111 (a potent 5α-RI inhibitor) decreases corticolimbic Allo levels and enhances the contextual fear response in group housed mice, which suggests that social isolation alters emotional responses by reducing the positive allosteric modulation of Allo at GABAA receptors in corticolimbic circuits. Thus, these procedures model emotional hyperreactivity, including enhanced contextual fear and impaired contextual fear extinction, which also is observed in posttraumatic stress disorder (PTSD) patients. A recent clinical study reported that cerebrospinal fluid Allo levels also are down-regulated in PTSD patients and correlate negatively with PTSD symptoms and negative mood. Thus, protracted social isolation of mice combined with tests of fear conditioning may be a suitable model to study emotional behavioral components associated with neurochemical alterations relating to PTSD. Importantly, drugs like SBSSs, which rapidly increase corticolimbic Allo levels, normalize the exaggerated contextual fear responses resulting from social isolation, suggesting that selective activation of neurosteroidogenesis may be useful in PTSD therapy.
Keywords: fear conditioning, selective brain steroidogenic stimulants
In 1969, Valzelli (1) discovered that social isolation induces aggressive behavior in mice. These findings were later confirmed by other researchers (2, 3) who also have revealed that mice socially isolated for 3–4 weeks express reduced corticolimbic levels of allopregnanolone (Allo) (2, 4), a potent endogenous positive allosteric modulator of GABA action at GABAA receptors (5, 6).
This decrease of brain Allo is associated with a down-regulation in the expression of 5α-reductase type I (5α-RI), the rate-limiting step enzyme in brain Allo biosynthesis (7). A 5α-RI mRNA expression reduction occurs selectively in glutamatergic neuronal populations of the olfactory bulb (OB) (i.e., mitral cells), basolateral amygdala (BLA) (i.e., cortical pyramidal-like neurons), hippocampus (i.e., CA3 pyramidal neurons), and medial prefrontal cortex (mPFC) (i.e., layers V/VI pyramidal neurons) (8). In rodents, these corticolimbic structures regulate emotional behavioral responses, including aggression, through the action of neurons projecting from the OB, mPFC, and hippocampus to the BLA, central, and medial amygdala. The central amygdala controls the expression of behavioral responses via projections to the brainstem, accumbens, and hypothalamus (8–10).
The behavioral abnormalities induced by social isolation can be reproduced in group-housed mice by administering the selective 5α-RI inhibitor 17β-(N,N-diisopropylcarbamoyl)-androst-3,5-diene-3-carboxylic acid (SKF 105,111), which down-regulates brain Allo levels (≈80% in 1 h) (3, 4, 7, 11). Treatment with SKF 105,111 also reduces behavioral responses elicited by the administration of agonists or positive allosteric modulators of GABAA receptors (3, 11, 12).
In socially isolated or SKF-treated mice, the systemic administration of Allo or drugs that stimulate Allo biosynthesis [e.g., selective brain steroidogenic stimulants (SBSSs)], including fluoxetine and its congeners, reduces aggression and anxiety-like behaviors (2, 3, 11, 13). Taken together, these findings suggest that a down-regulation of Allo biosynthesis in corticolimbic GABAergic synapses may be an important component in eliciting aggression and anxiety-like behavior (8, 13).
The corticolimbic network responsible for the control of aggression also includes the neuronal system involved in the expression of a number of emotional disorders, such as fear, anxiety disorders, impulsivity, affective instability, and posttraumatic stress disorder (PTSD) (14–17). For example, recent neuroimaging data support the concept that the exaggerated amygdala response due to mPFC and hippocampal functional deficits may be associated with exaggerated fear reexperiencing in PTSD patients (15, 18). Moreover, in Vietnam War veterans who suffered brain injury and emotionally traumatic events, a substantially reduced occurrence of PTSD symptoms was found in individuals with damage to the ventromedial PFC and/or the anterior temporal area that included the amygdala (19). These results further suggest that the mPFC and amygdala are critically involved in the pathogenesis of PTSD (14–19). A recent clinical study reported that, in PTSD patients, a down-regulation of Allo levels in cerebrospinal fluid (CSF) was correlated with increased PTSD reexperiencing and comorbid depressive symptoms (20).
The present study shows that, in socially isolated mice, a down-regulation of Allo expression in corticolimbic circuits is associated with alterations of emotional responses, such as an increase of contextual fear responses and delayed contextual fear extinction after fear conditioning. In addition, the administration of Allo or SBSSs that up-regulate brain Allo levels appeared to normalize these dysfunctional behaviors.
Results
Social Isolation Increases Contextual but Not Cued Fear Responses.
In male mice, social isolation lasting 3–4 weeks caused a 2-fold increase in the duration of contextual freezing time (Fig. 1). The duration in contextual freezing remained elevated at a plateau after 5–8 weeks of social isolation (data not shown). The increased duration of contextual freezing in socially isolated mice was independent of the number of electric foot shocks [unconditioned stimulus (US)] plus acoustic tones [conditioned stimulus (CS)] applied during the training session (Table 1). Importantly, there was no difference in freezing behavior measured during the habituation phase (group housed, 3.6 ± 0.4 s/min; socially isolated, 3.8 ± 0.6 s/min, mean ± SEM; n = 5) or during the training session (Table 1). These findings suggest that socially isolated mice fail to exhibit changes in locomotor activity, US perception, or unconditioned fear-related behaviors.
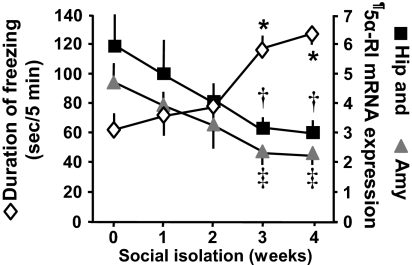
Fig. 1.
In male mice socially isolated for 3–4 weeks, contextual freezing time is increased and 5α-RI mRNA expression in the hippocampus (Hip) and amygdala (Amy) is decreased. Total duration of freezing time was measured 24 h after a training session. The training session consisted of a CS (acoustic tone, 30 s, 85 dB) paired with a US (electric foot shock, 2 s, 0.5 mA) three times every 2 min. Each value is the mean ± SEM of five animals. †, P < 0.01 when hippocampus 5α-RI mRNA content in mice socially isolated for 3–4 weeks is compared with mice group housed (0 week of social isolation); ‡, P < 0.01 when amygdala 5α-RI mRNA content in mice socially isolated for 3–4 weeks is compared with mice group housed (0 week of social isolation); *, P < 0.01 freezing time at a given social isolation time period compared with a social isolation period of 0 week (one-way ANOVA followed by Bonferroni comparison). ¶, 5αRI mRNA values are expressed as fmol 5α-RI mRNA/pmol NSE mRNA.
Table 1.
Increased duration of contextual freezing time in socially isolated mice
| No. electric foot shocks during training session | Freezing during training test, seconds per 5-minute period |
Freezing during contextual test, seconds per 5-minute period |
||
|---|---|---|---|---|
| Group housed | Socially isolated | Group housed | Socially isolated | |
| 1 | 15.0 ± 3.3 | 12.8 ± 1.7 | 37 ± 9.7 | 60 ± 1.11* |
| 3 | 38.3 ± 7.1 | 33.2 ± 1.9 | 68 ± 9.9 | 121 ± 5.6* |
| 6 | 137 ± 20 | 138 ± 14 | 94 ± 9.3 | 161 ± 9.6* |
Freezing involved the absence of all movement, except for respiratory-related movements while the mouse was in a stereotyped crouching posture. Each value is the mean ± SEM of five animals.
*, P < 0.01, freezing time of socially isolated mice compared to group-housed mice (Student's t test).
Fig. 2 shows that, in mice previously conditioned with three tones (CS) paired with three electric foot shocks (US) and exposed 24 h later to the cued fear-conditioning test [11 tones (cue) without electric foot shock], no differences were detected in the duration of the freezing time between group housed and socially isolated mice.
Fig. 2.
Cued fear-conditioning response is not altered in socially isolated mice. The duration of cued freezing time was measured 24 h after a training session. The training session consisted of a CS (acoustic tone, 30 s, 85 dB) paired with a US (electric foot shock, 2 s, 0.5 mA) three times every 2 min. Mice previously trained were exposed after 24 h to a modified context (using a smaller Plexiglas box and a few drops of lemon scent in the contextual chamber) and 11 tones (cue) without footshocks. Each value is the mean ± SEM of five animals.
In the cued fear-conditioning trial, the time of freezing was measured for 1 min after the beginning of each tone and never exceeded 25–30 s, which is significantly below the maximal freezing time that can be reached in 1 min. This finding suggests that the cued fear-conditioning trial was appropriate to detect possible increases in the duration of the freezing time in response to the cue presentation. Of note, both group housed and socially isolated mice receiving electric foot shocks plus tone during the training session and then exposed to a different contextual chamber (different shape and smell) showed levels of immobility virtually identical to mice that were not exposed to foot shock plus tone during the training session (group housed, 3.6 ± 0.4 s/min; socially isolated, 3.7 ± 0.2 s/min, mean ± SEM; n = 5).
Social Isolation Impairs Contextual but Not Cued Fear Extinction.
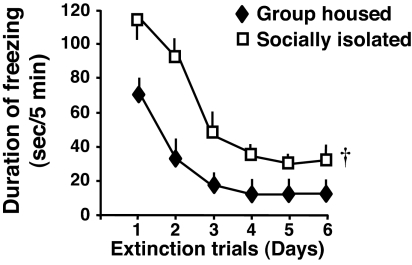
When fear-conditioned mice were subjected to the contextual test (5-min exposure to the context chamber without electric foot shock) for six consecutive days, group-housed mice showed an almost complete extinction of freezing in 3 days. In contrast, the socially isolated mice showed a delayed and incomplete extinction even after six contextual extinction trials (Fig. 3).
Fig. 3.
Extinction of contextual freezing behavior is impaired in socially isolated mice. Contextual freezing time was measured for six consecutive days starting 24 h after the training session. During the extinction trials, mice were reexposed daily to the conditional context without receiving footshocks. Two-way repeated measures ANOVA with factors for housing, time, and their interactions with the duration of freezing time revealed that mice socially isolated for 3 weeks expressed an overall higher duration of freezing time compared with group housed mice. †, P < 0.01 (two-way repeated measures ANOVA, followed by Bonferroni comparison). The slope values for the extinction curve (expressed in percentage of total duration of freezing time) during the first 3 days of the extinction trials were calculated for each mouse. The results show a delayed extinction in socially isolated compared with group housed mice (slope values: group housed, −41.0 ± 2.2; socially isolated, −27.2 ± 5.2, mean ± SEM; n = 5; P = 0.04, Student's t test).
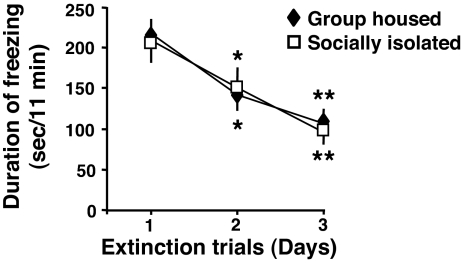
In the cued fear-conditioning test, no significant extinction differences between the socially isolated and group-housed mice were noted when the trials were repeated for three consecutive days (Fig. 4).
Fig. 4.
Extinction of cued freezing behavior is not altered in socially isolated mice. Cued freezing time was measured for 3 consecutive days starting 24 h after the training session. During the cued extinction trials, mice were exposed daily to a modified context (using a smaller Plexiglas box and a few drops of lemon scent in the contextual chamber), and 11 tones without footshocks were delivered. Each value is the mean ± SEM of five animals. *, P < 0.04; **, P < 0.02 when freezing time at a given extinction trial was compared with the freezing time of the extinction trial at day 1 (one-way ANOVA, followed by Bonferroni test).
5α-RI mRNA Expression and Allo Content Down-Regulation in Corticolimbic Structures of Socially Isolated Mice.
In the FC, hippocampus, and amygdala, which are the brain regions reputed to be involved in the regulation of fear-conditioning behavior, we measured 5α-RI mRNA at several time points during social isolation. The social isolation-induced increase of the contextual fear-conditioning response is associated with a marked (≈50%) down-regulation of 5α-RI mRNA expression in the hippocampus, amygdala (Fig. 1), and FC (2).
Allo levels varied in different brain structures; in fact, they were higher in the OB than in the cerebellum or striatum (Fig. 5). We noted that social isolation induced a significant reduction of Allo levels in the OB, FC, amygdala, and hippocampus, but not in the striatum or cerebellum. The social isolation-induced decrease in Allo levels is greater in the amygdala and hippocampus (≈75%) than in the OB and FC (≈40–50%) (Fig. 5).
Fig. 5.
Decrease of Allo content in selected corticolimbic structures of socially isolated mice. Each value is the mean ± SEM of five animals. *, P < 0.05 and †, P < 0.01 Allo content of socially isolated mice compared with group housed mice (Student's t test). OB, olfactory bulb; FC, frontal cortex; Amy, amygdala; Hip, hippocampus; Str, striatum; CB, cerebellum.
Allo and S-NFLX Reduces Fear-Conditioning Responses.
To study whether a corticolimbic Allo content decrease is operative in regulating contextual fear-conditioned responses, Allo or the potent SBSS, S-NFLX, was administrated systemically to socially isolated mice. Allo doses (8–16 μmol/kg, s.c.) that increased OB and FC Allo levels (13, 21) reversed the social isolation-induced increase of contextual conditioned freezing time duration (Table 2). S-NFLX treatment, at doses below those that block serotonin reuptake (0.45–1.8 μmol/kg, s.c.), increased corticolimbic Allo content (2, 22) and reduced freezing time in socially isolated but not group housed mice (Table 2).
Table 2.
Allo or S-NFLX blocks the increased duration of contextual freezing time in socially isolated but not in group-housed mice
| Drug treatment | Freezing time (sec/5 min) |
|
|---|---|---|
| Group housed | Socially isolated | |
| Vehicle | 90 ± 5.1 | 148 ± 4.3* |
| Allo (8 μmol/kg s.c.) | 105 ± 11 | 108 ± 3.0 |
| Allo (16 μmol/kg s.c.) | 91 ± 4.9 | 88 ± 2.8 |
| S-NFLX (0.45 μmol/kg i.p.) | 92 ± 11 | 110 ± 6.6 |
| S-NFLX (0.9 μmol/kg i.p.) | 85 ± 6.1 | 96 ± 9.3 |
| S-NFLX (1.8 μmol/kg i.p.) | 98 ± 8.7 | 80 ± 4.2 |
Allo or S-NFLX was injected 45 min before the training session. Each value is the mean ± SEM of five mice.
*, P < 0.01 in socially isolated compared with their respective group housed mice (Student's t test).
SKF 105,111 Reproduces the Altered Fear-Conditioning Behavior Observed in Socially Isolated Mice.
To assess whether a decrease of telencephalic Allo content reproduces the altered contextual fear responses observed during social isolation, corticolimbic Allo levels were decreased by administering the potent 5α-RI inhibitor SKF 105,111 (dose range, 1–80 μmol/kg, s.c.) to group housed mice. SKF 105,111 induced a marked dose-dependent decrease (≈80% at the highest doses) of cortical, hippocampal, and amygdala Allo content and a dose-dependent increase in the duration of contextual freezing time (Fig. 6). The increased contextual freezing time due to s.c. injection of SKF 105,111 at a dose of 20 μmol/kg was reversed by a single injection of Allo (16 μmol/kg, s.c.) (Fig. 7). At these doses, SKF 105,111 failed to directly change the action of GABA at GABAA receptors (4, 11).
Fig. 6.
SKF 105,111 induces increased contextual freezing behaviors and reduces hippocampal Allo content in group-housed male mice. Total duration of contextual freezing time was measured 24 h after the contextual training session. SKF 105,111 was injected 4 h before the training fear-conditioning session or before measurements of Allo content in the hippocampus (Hip). Each value is the mean ± SEM of five animals. *, P < 0.05 or †, P < 0.01 in SKF 105,111-treated groups compared with the respective vehicle-treated group (one-way ANOVA, followed by Bonferroni test).
Fig. 7.
Allo reverses increased contextual fear conditioning due to SKF 105,111 treatment. SKF 105,111 was injected 4 h before and Allo 1 h before the conditioning training session. Each value is the mean ± SEM of four animals. *, P < 0.05 in SKF 105,111-treated groups compared with control group or SKF plus Allo-treated group (one-way ANOVA, followed by Bonferroni test).
Discussion
Corticolimbic Allo Biosynthesis Down-Regulation Is Associated with Enhanced Fear Behavior.
Male mice subjected to 3–4 weeks of social isolation exhibited (i) a marked decrease of Allo levels in corticolimbic structures, such the hippocampus, amygdala, FC, and OB (Fig. 5), which play a prominent role in the acquisition and expression of conditioned fear (17); (ii) an enhanced contextual fear-conditioning response (Fig. 1); (iii) a delayed and incomplete contextual fear extinction (Fig. 3); and (iv) the absence of changes in the expression and extinction of the cued fear-conditioning response (Figs. 2 and 4).
The changes in contextual fear responses and contextual fear extinction in socially isolated mice are not due to a decrease in motor activity. In fact, socially isolated mice tested in the contextual environment before the application of electric foot shocks or tested in a context different from the one in which conditioned training took place express minimal differences in immobility behaviors comparable with those of group housed mice.
In humans and rodents, the neuronal pathways operative in the expression of contextual or cued fear conditioning include the corticolimbic circuits, which comprise the OB, mPFC, hippocampus, and amygdala (17). Lesions of the hippocampus and of specific nuclei of the amygdala have demonstrated that the normal functioning of both the hippocampal formation and amygdaloid complex are important for the expression of contextual fear conditioning (23). On the contrary, the cued fear-conditioning responses are mediated by the normal function of the amygdala (23). Hence, differences between group housed and socially isolated mice in contextual fear-conditioning responses (hippocampus plus amygdala-dependent process) and the lack of difference in cued fear conditioning (amygdala-dependent process) suggest that the hippocampus, not the amygdala, is critical for the enhancement of contextual fear-conditioning responses in socially isolated mice.
Recently, a prominent role for mPFC–amygdala circuits has been suggested in the contextual modulation of the extinction of fear memory (16, 24). Several lines of evidence suggest that GABAA receptor function in corticolimbic structures plays a primary role in the modulation of fear-conditioning responses and also may mediate learning inhibition during fear-conditioning extinction (16). For example, microinjections of benzodiazepines or GABAA receptor agonists in rat BLA reduce the expression of fear conditioning (25). On the contrary, infusion of bicuculline, a competitive antagonist of GABA action at GABAA receptors, increases fear-conditioning responses in the rat BLA complex, whereas pretreatment with midazolam prevented the bicuculline-induced facilitating influence on fear memory (26). Systemic administration of diazepam and congeners that positively modulate GABAA receptor function increases fear extinction (27).
Moreover, a reduction of α5-containing GABAA receptor expression in the hippocampus and BLA or a decrease in function of these receptors by administering selective α5-containing GABAA receptor antagonists impairs extinction of a conditioned fear response, which leads to persistent conditioned responses (28).
These data indicate that, in socially isolated mice, the increase in fear-conditioning responses and the delayed and incomplete contextual fear-conditioning extinction could be related to an impairment of GABAA receptor neurotransmission, likely related to Allo level down-regulation in selected corticolimbic structures, including the mPFC, hippocampus, and amygdala.
This study shows that in socially isolated mice Allo content is decreased by ≈70% in the hippocampus and amygdala and by a lesser extent (40–50%) in the frontal cortex and OB (Fig. 5). Using an in situ hybridization technique to detect 5α-RI mRNA in neurons, we found that the transcript of this enzyme was dramatically decreased in hippocampal CA3 glutamatergic pyramidal neurons, dentate gyrus granule cells, glutamatergic pyramidal-like neurons of the BLA, and in layers V/VI glutamatergic pyramidal neurons of the mPFC of mice socially isolated for 4 weeks (8). In contrast, 5α-RI mRNA expression failed to change in CA1 pyramidal neurons, pyramidal neurons of layers II/III mPFC, ventromedial thalamic nucleus neurons, or striatal medium spiny and reticular thalamic nucleus neurons (8). Taken together, these data suggest that in socially isolated mice the expression of Allo is specifically down-regulated in glutamatergic pyramidal neurons that converge on the BLA from the mPFC and hippocampus.
Hence, it is plausible to propose that a decrease of Allo biosynthesis in corticolimbic glutamatergic neurons of socially isolated mice might result in a down-regulation of synaptic or extrasynaptic GABAA receptor function with consequent excitation of the hippocampal and amygdaloid formation, increased contextual fear conditioned responses, and decreased contextual fear extinction (Figs. 1 and 3).
Pharmacological Studies of the Regulation of Brain Allo-Level Expression in Corticolimbic Structures.
To confirm the inference that Allo depletion in selected corticolimbic neurons could account for the expression of enhanced contextual fear conditioning in socially isolated mice, at the moment of the training session, we used pharmacological tools to manipulate Allo levels in corticolimbic structures in opposite directions: (i) S-NFLX, a potent SBSS (13, 29) that increases brain Allo levels at doses that do not affect the reuptake of serotonin (13), (ii) SKF 105,111 (a potent inhibitor of 5α-RI) to decrease brain Allo levels in group housed mice (7, 11), and (iii) reversal of the SKF 105,111 and social isolation-induced corticolimbic Allo down-regulation by directly injecting Allo at doses capable of reversing the SKF 105,111- or social isolation-induced brain Allo depletion (11).
We show that in socially isolated mice Allo or S-NFLX, in doses that increase FC and hippocampus Allo levels at the time of the training session (2, 22), also reduces or abolishes the expression of an enhanced contextual fear-conditioning response (Table 2). In contrast, injecting SKF 105,111 into group housed mice facilitates the enhancement of contextual fear responses by decreasing hippocampus, amygdala, and cortex Allo levels (Fig. 6), which strongly suggests that social isolation produces altered emotional responses in mice by reducing the positive allosteric action of Allo at GABAA receptors located on corticolimbic circuits.
The role that corticolimbic Allo content plays in modulating contextual fear responses should be confirmed by locally microinjecting Allo or drugs that increase (e.g., SBSSs) or decrease (e.g., 5α-RI inhibitors) Allo levels in specific corticolimbic structures.
Can the Socially Isolated Mouse Exposed to Fear Conditioning Model the Behavioral and Neurochemical Alterations Observed in PTSD Patients?
Disturbances in behavior that model some aspects of PTSD symptomatology can be reproduced by exposing rodents to severe and protracted stressors (21, 30, 31). Protracted social isolation is a markedly distressing event that induces time-dependent behavioral disturbances, including exaggerated anxiety-like and aggressive reactions (2, 13). This procedure also reproduces several aspects of exaggerated emotional reactivity, including enhanced fear conditioning and impaired fear extinction, which develop in PTSD patients when they are reexposed to events that symbolize an aspect of the triggering traumatic event (15, 32, 33).
A variety of alterations in GABAergic neurotransmission have been identified in patients with PTSD, including: (i) the reduced sedative and anxyolitic action of classical benzodiazepines (34–36), (ii) the decreased frontocortical benzodiazepine recognition site binding detected in Balkan and Vietnam War veterans (37, 38), and (iii) a decrease of Allo levels in the CSF of premenopausal women that negatively correlates with PTSD symptoms, negative moods, and comorbid depressive symptoms (20). Of note, Allo levels were found to be decreased in the whole PTSD group, but were lowest in those patients with PTSD and comorbid depression (20).
Similar to PTSD, the emotional dysfunction induced in mice by social isolation is associated with the down-regulation of GABAergic neurotransmission, presumably maintained by decreased Allo levels in selected neuronal populations of the mPFC, hippocampus, and BLA and additionally documented by a decreased behavioral response to GABAergic drugs (e.g., pentobarbital, muscimol, picrotoxin, and benzodiazepines) (3, 4, 11, 12).
In the mouse cortex and hippocampus, protracted social isolation alters the subunit expression of GABAA receptors, with a decrease in α1, α2, and γ2 subunit expression and an increase in α4 and α5 subunits (39). As a consequence, there also was a decrease of [3H]flumazenil binding to hippocampal synaptic membranes and resistance to the sedative effects of classical benzodiazepines, including diazepam and zolpidem (39).
Although the mechanism whereby social isolation in mice decreases corticolimbic Allo levels may be different from the mechanism that decreases CSF Allo levels in PTSD subjects, protracted corticolimbic Allo level deficiency may result in a GABAergic neurotransmission deficit that appears to produce similar behavioral disturbances in socially isolated mice and in subjects with PTSD.
Conclusion
Protracted social isolation combined with fear-conditioned test exposure could be a suitable mouse model to study the emotional behavioral components and neurochemical alterations that relate to PTSD. Importantly, drugs like the SBSSs fluoxetine and congeners are widely used for the treatment of PTSD (40, 41). We have shown that S-NFLX, which is more potent than fluoxetine in increasing corticolimbic Allo levels (2, 13, 22), rapidly normalizes enhanced contextual fear-conditioned responses related to exposure to social isolation. Selective activation of neurosteroidogenesis may be an appropriate drug-development target for the treatment of PTSD.
Materials and Methods
Animals and Tissue Preparation.
Adult male Swiss–Webster mice (Harlan Breeders) (25- to 30-g body weight) maintained under a 12-h dark/light cycle with food and water ad libitum were used for all experiments. Mice were housed either in groups of five per cage (24 × 17 × 12 cm) or individually (socially isolated) in a cage of the same size for time periods varying from 1 day to 8 weeks preceding our behavioral and biochemical studies (4). The vivarium temperature was kept at ≈24°C and the humidity near 65%. Immediately after decapitation, brains were frozen and cut into 1-mm-thick slices by using a Jacobovitz brain slicer (Zivic Miller). The slices obtained from 0.9–1.9 mm anterior to bregma were mounted on a coverslip kept at −4°C, and disks (1.5-mm diameter) were punched out from these slices, including the striatum and the frontoparietal somatosensory cortex. Similarly, the slices obtained at 1.06–2.06 mm posterior to the bregma were used to punch out disks (1.5-mm diameter), including the dorsal hippocampus and amygdala (comprising basolateral and central amygdala nuclei). A single disk from each area was used for neurochemical assays. All of the animal procedures used in our research were approved by the University of Illinois at Chicago Animal Care Committee.
Fear Conditioning.
The fear-conditioning apparatus consisted of a transparent acrylic chamber measuring 25 cm wide, 18 cm high, and 21 cm deep (San Diego Instruments). The cage floor was composed of stainless-steel rods connected to an electric shock generator. A small fan was located on the top wall of the enclosure. A speaker placed on a side wall of the conditioning chamber delivered the auditory tone (San Diego Instruments). The chamber was surrounded by a frame with 16 infrared photo beams. A computer controlled the delivery of electric foot shocks and auditory stimuli and recorded beam interruptions and latencies to beam interruptions (freezing time).
Training Test.
Mice were placed into a training chamber (San Diego Instruments) and allowed to explore it for 2 min. After this time, they received an acoustic tone (CS) (30 s, 85 dB) coterminated with an US (electric footshock, 2 s, 0.5 mA). The tone (cue) plus the foot shock were repeated three times every 2 min. After the last tone plus shock delivery, mice were allowed to explore the context for an additional minute before removal from the training chamber (total of 8 min).
Contextual Test.
Briefly, 24 h after training, the mice were placed in the contextual cage, and freezing behavior was measured for 5 min (Freeze Monitor System; San Diego Instruments) without tone or footshock presentation.
Cued Test.
Twenty-four hours after training, mice were tested for freezing responses to the cue. For this purpose, the conditioning chamber was modified (by using a smaller Plexiglas box, measuring 18 cm wide, 9 cm high, and 11 cm deep, and placing a few drops of lemon scent in the contextual chamber). Mice were allowed to explore the new environment for 2 min. After this time, mice were exposed to 11 tones (30 s, 85 dB) each presented every minute. Freezing time was measured for 1 min starting at the beginning of each tone for a total of 13 min.
Extinction Test.
For contextual extinction experiments, mice were placed in the contextual cage for 6 consecutive days starting 24 h after the training session. Freezing behavior was measured for 5 min without tone or footshock presentation.
For cued extinction experiments, freezing time was measured for 3 consecutive days starting 24 h after the training session. During the cued extinction trials, mice were exposed daily to a modified contextual chamber, and 11 tones without footshock were delivered.
Freezing was defined by the absence of any movement except for those related to respiration while the animal was in a stereotypical crouching posture (42).
Quantitative RT-PCR of 5α-RI, 3α-HSD, and Neuron-Specific Enolase (NSE) mRNAs.
mRNAs were quantified with competitive RT-PCR as described by Auta et al. (43). Internal standards for 5α-RI, 3α-HSD, and NSE were generated by using PCR overlap extension to introduce a deletion midway between the amplification primers (43). Primers for 5α-RI were forward 685–710 and reverse 1075–1109 (GBAN J05035). Primers for 3α-HSD were forward 522–555 and reverse 843–876 (GBAN S57790). Primers for NSE were forward 382–405 and reverse 769–792 (GBAN M22349.1). Each primer pair yielded a single band of the correct molecular size after amplification of RNA isolated from the mouse brain tissue.
Brain Neurosteroid Content.
Extraction, derivatization, and gas chromatography-mass spectrometry analyses of neurosteroids were performed with minor modifications as described previously (11, 44). The various brain areas were homogenized in 10 volumes of distilled water containing 2–5 fmol/ml [3H]Allo (New England Nuclear) to monitor the HPLC retention profile, and 1 pmol deuterium-labeled Allo (Allo-17,21,21,21-D4) (Cambridge Isotope Laboratories) was used as an internal standard. The supernatants were extracted with ethyl acetate and after lyophilization were purified with HPLC.
The HPLC fraction containing Allo was derivatized with HFBA and subjected to gas chromatography-mass fragmentographic analysis.
Mass fragmentographic analysis of derivatized Allo was performed in the standard electron impact mode. The detection limit for Allo was ≈10 fmol; the standard curve was linear between 5 and 105 fmol. The m/z ion-monitoring mode was 496 for HFBA-Allo and 500 for HFBA-D-Allo.
Statistical Analysis.
Results are expressed as the mean ± SEM. Student's t test, one-way ANOVA, or two-way repeated measures ANOVA, followed by Bon-ferroni comparison, were used as indicated for each figure or table legend. The criterion for significance was P < 0.05.
Acknowledgments.
We thank Drs. Francine M. Benes and Ann M. Rasmusson for their constructive criticisms and suggestions in the preparation of the manuscript. This work was supported by Campus Research Board Award 2-611185 (to G.P.), National Institute of Mental Health Grants MH5680 (to A.G.) and MH062090 (to E.C.), and a Regione Autonoma della Sardegna, Italy, “Master and Back” postdoctoral fellowship (to F.P.).
Footnotes
The authors declare no conflict of interest.
References
- 1.Valzelli L. Aggressive behavior induced by isolation. In: Garattini S, Sigg SB, editors. Aggressive Behavior. Amsterdam: Excerpta Medica Foundation; 1969. pp. 70–76. [Google Scholar]
- 2.Pinna G, Dong E, Matsumoto K, Costa E, Guidotti A. In socially isolated mice, the reversal of brain allopregnanolone down-regulation mediates the anti-aggressive action of fluoxetine. Proc Natl Acad Sci USA. 2003;100:2035–2040. doi: 10.1073/pnas.0337642100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Matsumoto K, Puia G, Dong E, Pinna G. GABAA receptor neurotransmission dysfunction in a mouse model of social isolation-induced stress: Possible insights into a non-serotonergic mechanism of action of SSRIs in mood and anxiety disorders. Stress. 2007;10:3–12. doi: 10.1080/10253890701200997. [DOI] [PubMed] [Google Scholar]
- 4.Matsumoto K, et al. Permissive role of brain allopregnanolone content in the regulation of pentobarbital-induced righting reflex loss. Neuropharmacology. 1999;38:955–963. doi: 10.1016/s0028-3908(99)00018-0. [DOI] [PubMed] [Google Scholar]
- 5.Puia G, et al. Neurosteroids act on recombinant human GABAA receptors. Neuron. 1990;4:759–765. doi: 10.1016/0896-6273(90)90202-q. [DOI] [PubMed] [Google Scholar]
- 6.Belelli D, Lambert JJ. Neurosteroids: Endogenous regulators of the GABAA receptor. Nat Rev Neurosci. 2005;6:565–575. doi: 10.1038/nrn1703. [DOI] [PubMed] [Google Scholar]
- 7.Dong E, et al. Brain 5α-dihydroprogesterone and allopregnanolone synthesis in a mouse model of protracted social isolation. Proc Natl Acad Sci USA. 2001;98:2849–2854. doi: 10.1073/pnas.051628598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Agís-Balboa RC, et al. Down-regulation of neurosteroid biosynthesis in corticolimbic circuits mediates social isolation-induced behavior in mice. Proc Natl Acad Sci USA. 2007;104:18736–18741. doi: 10.1073/pnas.0709419104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nelson RJ, Trainor BC. Neural mechanisms of aggression. Nat Rev Neurosci. 2007;8:536–546. doi: 10.1038/nrn2174. [DOI] [PubMed] [Google Scholar]
- 10.McDonald AJ. Cortical pathways to the mammalian amygdala. Prog Neurobiol. 1998;55:257–332. doi: 10.1016/s0301-0082(98)00003-3. [DOI] [PubMed] [Google Scholar]
- 11.Pinna G, et al. Brain allopregnanolone regulates the potency of the GABAA receptor agonist muscimol. Neuropharmacology. 2000;39:440–448. doi: 10.1016/s0028-3908(99)00149-5. [DOI] [PubMed] [Google Scholar]
- 12.Guidotti A, et al. The socially-isolated mouse: A model to study the putative role of allopregnanolone and 5α-dihydroprogesterone in psychiatric disorders. Brain Res Brain Res Rev. 2001;37:110–115. doi: 10.1016/s0165-0173(01)00129-1. [DOI] [PubMed] [Google Scholar]
- 13.Pinna G, Costa E, Guidotti A. Fluoxetine and norfluoxetine stereospecifically and selectively increase brain neurosteroid content at doses that are inactive on 5-HT reuptake. Psychopharmacology (Berlin) 2006;186:362–372. doi: 10.1007/s00213-005-0213-2. [DOI] [PubMed] [Google Scholar]
- 14.Milad MR, et al. A role for the human dorsal anterior cingulate cortex in fear expression. Biol Psychiatry. 2007;62:1191–1194. doi: 10.1016/j.biopsych.2007.04.032. [DOI] [PubMed] [Google Scholar]
- 15.Rauch SL, Shin LM, Phelps EA. Neurocircuitry models of posttraumatic stress disorder and extinction: Human neuroimaging research–past, present, and future. Biol Psychiatry. 2006;60:376–382. doi: 10.1016/j.biopsych.2006.06.004. [DOI] [PubMed] [Google Scholar]
- 16.Akirav I, Maroun M. The role of the medial prefrontal cortex-amygdala circuit in stress effects on the extinction of fear. Neural Plast. 2007 doi: 10.1155/2007/30873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.LeDoux JE. Emotion circuits in the brain. Annu Rev Neurosci. 2000;23:155–184. doi: 10.1146/annurev.neuro.23.1.155. [DOI] [PubMed] [Google Scholar]
- 18.Orr SP, et al. De novo conditioning in trauma-exposed individuals with and without posttraumatic stress disorder. J Abnorm Psychol. 2000;109:290–298. [PubMed] [Google Scholar]
- 19.Koenigs M, et al. Focal brain damage protects against post-traumatic stress disorder in combat veterans. Nat Neurosci. 2007;11:232–237. doi: 10.1038/nn2032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rasmusson AM, et al. Decreased cerebrospinal fluid allopregnanolone levels in women with posttraumatic stress disorder. Biol Psychiatry. 2006;60:704–713. doi: 10.1016/j.biopsych.2006.03.026. [DOI] [PubMed] [Google Scholar]
- 21.Pibiri F, Nelson M, Carboni G, Pinna G. Neurosteroids regulate mouse aggression induced by anabolic androgenic steroids. Neuroreport. 2006;17:1537–1541. doi: 10.1097/01.wnr.0000234752.03808.b2. [DOI] [PubMed] [Google Scholar]
- 22.Pinna G, Costa E, Guidotti A. Fluoxetine and norfluoxetine stereospecifically facilitate pentobarbital sedation by increasing neurosteroids. Proc Natl Acad Sci USA. 2004;101:6222–6225. doi: 10.1073/pnas.0401479101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Phillips RG, LeDoux JE. Differential contribution of amygdala and hippocampus to cued and contextual fear conditioning. Behav Neurosci. 1992;106:274–285. doi: 10.1037//0735-7044.106.2.274. [DOI] [PubMed] [Google Scholar]
- 24.Myers KM, Davis M. Mechanisms of fear extinction. Mol Psychiatry. 2007;12:120–150. doi: 10.1038/sj.mp.4001939. [DOI] [PubMed] [Google Scholar]
- 25.Jasnow AM, Huhman KL. Activation of GABAA receptors in the amygdala blocks the acquisition and expression of conditioned defeat in Syrian hamsters. Brain Res. 2001;920:142–150. doi: 10.1016/s0006-8993(01)03054-2. [DOI] [PubMed] [Google Scholar]
- 26.Rodríguez Manzanares PA, Isoardi NA, Carrer HF, Molina VA. Previous stress facilitates fear memory, attenuates GABAergic inhibition, and increases synaptic plasticity in the rat basolateral amygdala. J Neurosci. 2005;25:8725–8734. doi: 10.1523/JNEUROSCI.2260-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li S, Murakami Y, Wang M, Maeda K, Matsumoto K. The effects of chronic valproate and diazepam in a mouse model of posttraumatic stress disorder. Pharmacol Biochem Behav. 2006;85:324–331. doi: 10.1016/j.pbb.2006.08.015. [DOI] [PubMed] [Google Scholar]
- 28.Yee BK, et al. GABA receptors containing the α5 subunit mediate the trace effect in aversive and appetitive conditioning and extinction of conditioned fear. Eur J Neurosci. 2004;20:1928–1936. doi: 10.1111/j.1460-9568.2004.03642.x. [DOI] [PubMed] [Google Scholar]
- 29.Daniels RN, Lindsley CW. A new, non-SSRI mechanism of action for Prozac. Curr Top Med Chem. 2007;7:1039. doi: 10.2174/156802607780906807. [DOI] [PubMed] [Google Scholar]
- 30.Rau V, DeCola JP, Fanselow MS. Stress-induced enhancement of fear learning: an animal model of posttraumatic stress disorder. Neurosci Biobehav Rev. 2005;29:1207–1223. doi: 10.1016/j.neubiorev.2005.04.010. [DOI] [PubMed] [Google Scholar]
- 31.Morrow BA, Elsworth JD, Rasmusson AM, Roth RH. The role of mesoprefrontal dopamine neurons in the acquisition and expression of conditioned fear in the rat. Neuroscience. 1999;92:553–564. doi: 10.1016/s0306-4522(99)00014-7. [DOI] [PubMed] [Google Scholar]
- 32.Ameli R, Ip C, Grillon C. Contextual fear-potentiated startle conditioning in humans: Replication and extension. Psychophysiology. 2001;38:383–390. [PubMed] [Google Scholar]
- 33.Grillon C, Morgan CA., III Fear-potentiated startle conditioning to explicit and contextual cues in Gulf War veterans with posttraumatic stress disorder. J Abnorm Psychol. 1999;108:134–142. doi: 10.1037//0021-843x.108.1.134. [DOI] [PubMed] [Google Scholar]
- 34.Davidson JR. Use of benzodiazepines in social anxiety disorder, generalized anxiety disorder, and posttraumatic stress disorder. J Clin Psychiatry. 2004;65:29–33. [PubMed] [Google Scholar]
- 35.Gelpin E, Bonne O, Peri T, Brandes D, Shalev AY. Treatment of recent trauma survivors with benzodiazepines: A prospective study. J Clin Psychiatry. 1996;57:390–394. [PubMed] [Google Scholar]
- 36.Viola J, et al. Pharmacological management of post-traumatic stress disorder: Clinical summary of a five-year retrospective study, 1990–1995. Mil Med. 1997;162:616–619. [PubMed] [Google Scholar]
- 37.Geuze E, et al. Reduced GABAA benzodiazepine receptor binding in veterans with post-traumatic stress disorder. Molecular Psychiatry. 2008;13:74–83. doi: 10.1038/sj.mp.4002054. [DOI] [PubMed] [Google Scholar]
- 38.Bremner JD, et al. Decreased benzodiazepine receptor binding in prefrontal cortex in combat-related posttraumatic stress disorder. Am J Psychiatry. 2000;157:1120–1126. doi: 10.1176/appi.ajp.157.7.1120. [DOI] [PubMed] [Google Scholar]
- 39.Pinna G, et al. Imidazenil and diazepam increase locomotor activity in mice exposed to protracted social isolation. Proc Natl Acad Sci USA. 2006;103:4275–4280. doi: 10.1073/pnas.0600329103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Davidson JR, Landerman LR, Farfel GM, Clary CM. Characterizing the effects of sertraline in post-traumatic stress disorder. Psychol Med. 2002;32:661–670. doi: 10.1017/s0033291702005469. [DOI] [PubMed] [Google Scholar]
- 41.Stein DJ, Seedat S, Van der Linden GJ, Zungu-Dirwayi N. Selective serotonin reuptake inhibitors in the treatment of post-traumatic stress disorder: A meta-analysis of randomized controlled trials. Int Clin Psychopharmacol. 2000;15:S31–S39. doi: 10.1097/00004850-200008002-00006. [DOI] [PubMed] [Google Scholar]
- 42.Blanchard RJ, Blanchard DC. Passive and active reactions to fear-eliciting stimuli. J Comp Physiol Psychol. 1969;68:129–135. doi: 10.1037/h0027676. [DOI] [PubMed] [Google Scholar]
- 43.Auta J, Chen Y, Ruzicka WB, Grayson DR. In: Handbook of Neurochemistry and Mol Neurobiol–Practical Neurochemistry Methods. 3rd Ed. Backer G, Dunn S, Holt A, Lajtha A, editors. New York: Springer; 2006. pp. 341–361. [Google Scholar]
- 44.Uzunov DP, Cooper TB, Costa E, Guidotti A. Fluoxetine-elicited changes in brain neurosteroid content measured by negative ion mass fragmentography. Proc Natl Acad Sci USA. 1996;93:12599–12604. doi: 10.1073/pnas.93.22.12599. [DOI] [PMC free article] [PubMed] [Google Scholar]