Abstract
MicroRNAs (miRNAs) are small noncoding RNAs that can act to repress target mRNAs by suppressing translation and/or reducing mRNA stability. Although it is clear that miRNAs and Dicer, an RNase III enzyme that is central to the production of mature miRNAs, have a role in the early development of neurons, their roles in the postmitotic neuron in vivo are largely unknown. To determine the roles of Dicer in neurons, we ablated Dicer in dopaminoceptive neurons. Mice that have lost Dicer in these cells display a range of phenotypes including ataxia, front and hind limb clasping, reduced brain size, and smaller neurons. Surprisingly, dopaminoceptive neurons without Dicer survive over the life of the animal. The lack of profound cell death contrasts with other mouse models in which Dicer has been ablated. These studies highlight the complicated nature of Dicer ablation in the brain and provide a useful mouse model for studying dopaminoceptive neuron function.
Keywords: dopamine receptor-1, microRNAs, striatum, Rett syndrome, clasping
MicroRNAs (miRNAs) are ≈22-nt noncoding small RNAs that can negatively regulate gene expression via the repression of target mRNAs (reviewed in ref. 1). Their canonical mechanism of action includes base-pairing of the miRNA to sites within the 3′ UTR of target genes, mediating translational repression. Evidence is accumulating that small RNAs, such as miRNAs, have important and diverse roles in neurons, from defining neuronal stem cell fate to modulating learning and memory (reviewed in ref. 2).
To study the importance of miRNAs in a tissue or developmental process, many groups have used tissue-specific recombination approaches to ablate enzymes important for miRNA biogenesis. In most approaches, the RNase III enzyme Dicer is ablated, resulting in the loss of miRNAs. However, removal of this enzyme is embryonic lethal; thus, to study Dicer in contexts outside of early development, conditional mouse models are used (3, 4). In almost every published Dicer knockout study, the mouse phenotype is hallmarked by a striking proliferation defect and/or apoptosis, suggesting an importance of miRNAs for cell survival (4–7).
Thus far, there have been a few studies that examined the in vivo roles of Dicer and miRNAs in the brain. One recent study determined that loss of Dicer in postmitotic Purkinje neurons results in profound neurodegeneration that becomes readily apparent between 13 and 17 weeks of age (8). Thus, Dicer loss in Purkinje neurons leads to cell death similar to that found in other published Dicer loss studies.
In these studies, we created a conditional mouse model to ablate Dicer in dopaminoceptive neurons by using a dopamine receptor-1 (DR-1) Cre. Drd1a (DR-1) is broadly expressed in the basal ganglia of the postnatal brain but is most highly expressed in the GABAergic, medium spiny neurons of the striatum (9). These neurons are involved in mediating numerous functions including initiation of movement, cognition, and feeding behavior. Dysfunction of dopaminoceptive neurons has been implicated in several human disorders such as Parkinson's disease, drug addiction, schizophrenia, obsessive–compulsive disorder, and Rett syndrome (10–13).
To date, there is not much known about what roles that Dicer and small RNAs may have in the pathology of human neurological disorders. A recent study demonstrated that disruption of Dicer with a dopamine transporter (DAT) Cre in postmitotic midbrain dopaminergic neurons leads to the loss of 90% of the cells in the substantia nigra and ventral tegmental area by 8 weeks of age, the class of neurons affected in Parkinson's disease (7). In our study, we examined a class of neurons that receive inputs from DAT neurons, and we found that removal of Dicer in these cells leads to distinct phenotypes from those seen in the DAT Cre mice or any other mouse model in which Dicer has been ablated. Despite the observed phenotypes, Dicer knockout dopaminoceptive neurons survive over the life of the animal, raising the possibility that these lines could be used to study human neurological disorders.
Results
Loss of Dicer in DR-1 Neurons Leads to Behavioral Defects and Decreased Lifespan.
To investigate the role of Dicer in postmitotic DR-1 neurons, we crossed mice conditional for Dicer (Dcr flox/flox) to animals expressing a DR-1 Cre (Fig. 1A) (4, 14). Dcr flox/flox;DR-1 Cre animals appear to be normal at birth, exhibiting normal weights and weaning behaviors as compared with Dcr flox/wt; DR-1 Cre controls (data not shown). At ≈6 weeks of age, the animals begin to undergo wasting and continue to lose weight until their death, which occurs between 10 and 12 weeks of age. Females exhibit a median lifespan shorter than males (median lifespan females: 69 days, n = 22; males: 78 days, n = 21), which may be caused by their smaller size and body mass (for weights: n = 13 for each female genotype and n = 14 for each male genotype; Student's t test was performed and P < 0.0001 for both males and females) (Fig. 1 C and D). During this period of wasting, the animals develop elongated and uneven wearing of their teeth and display an ungroomed appearance (data not shown).
Fig. 1.
Behavioral deficits and decreased lifespan in Dcrflox/flox;DR-1 cre mice. (A) Schematic of Dcr conditional targeting construct. (B) Clasping phenotype observed in Dcrflox/flox;DR-1 Cre animals. Dcrflox/wt;DR-1 Cre animals were used as controls for all experiments. (C) Kaplan-Meier survival curves of Dcrflox/flox;DR-1 Cre animals as compared with controls. Females have a median lifespan of 69 days (n = 22) and males have a median lifespan of 78 days (n = 21). (D) Body weights at death (between 10 and 12 weeks of age); Dcrflox/flox;DR-1 Cre animals exhibit wasting and loss of body mass as compared with controls. ***, P < 0.0001 for females (n = 13) and males (n = 14), Student's t test. SEM is shown. (E) Footprint analysis of Dcrflox/flox;DR-1 Cre animals reveals abnormal gait. (F) Reduced stride length in Dcrflox/flox;DR-1 Cre animals as compared with controls (n = 8; **, P = 0.0004, Student's t test). SEM is shown.
Because DR-1-expressing neurons are fundamental afferents within the basal ganglia, which play a central role in the initiation of movement, we sought to determine whether these animals displayed defects in movement. At ≈4 weeks of age, allDcr flox/flox; DR-1 Cre animals develop a strong front and hind limb clasping phenotype, as determined by a tail-suspension assay (Fig. 1B). This phenotype is indicative of motor deficits and is commonly seen in mouse models of neurodegenerative disorders, such as Huntington's disease (15). By ≈6 weeks of age, Dcr flox/flox; DR-1 Cre animals exhibit profound gait abnormalities, taking short, wobbly strides, as revealed by footprint analysis (Fig. 1 D and E) (n = 8 for each genotype; Student's t test was performed, P = 0.0004). These data indicate a perturbation of basal ganglia function caused by Dicer loss in dopaminoceptive neurons. Given that loss of Dicer in other tissues results in cell death, we anticipated profound cell loss in the striatum of these mice.
Loss of Dicer Results in Smaller Brain Size and Mass.
Overall examination of the brains of Dcr flox/flox; DR-1 Cre mice at death revealed no severe structural changes as determined by gross histological analysis. However, Dcr flox/flox; DR-1 Cre brains were significantly smaller and exhibited a reduction in mass, as compared with Dcr flox/wt; DR-1 Cre controls (Fig. 2). This smaller size and decrease in mass occurs before animals undergo wasting and is readily apparent by 5 weeks of age, and thus is not a result of weight loss but instead probably reflects perturbed brain development (mean brain mass at 5 weeks of age is 0.4933 g for controls, and 0.4367 g for Dcr flox/flox; DR-1 Cre animals, n = 3 for each genotype; Student's t test was performed, P = 0.0039).
Fig. 2.
Reduced brain size and weight in Dcr flox/flox;DR-1 Cre mice. (A) A representative brain from a control Dcr flox/wt; DR-1 Cre mouse (Left) as compared with a brain from a Dcr flox/flox; DR-1 Cre animal (Right) (littermates, 10 weeks old). (B) Brain weights of dying Dcr flox/flox; DR-1 Cre animals (empty bars) and controls (filled bars) from males (n = 7) and females (n = 6). Student's t test, ***, P = 0.0004 and 0.0007, respectively, SEM is shown. Brains were obtained from animals between 10 and 12 weeks of age.
To determine when and where DR-1 Cre recominbation occurs, we crossed Dicer conditional; DR-1 Cre mice to mice carrying an R26R allele (16). The R26R allele contains a lox-stop-lox cassette upstream of the lacZ gene and is under the control of the Rosa26 promoter. Thus, only cells in which successful Cre recombination has occurred should express LacZ and be visualized by X-gal staining. Cre recombinase mapping data for the DR-1 Cre allele revealed that the Cre expression pattern recapitulates the endogenous expression of the DR-1 receptor and the previously reported expression pattern for this line, including strong expression in the striatum and deeper layers of the cortex [supporting information (SI) Fig. S1 and ref. 14]. In this work, we have focused on the striatal class of neurons, which encompass the majority of DR-1 cells in the brain. Although data suggest that the Cre is expressed in postmitotic DR-1 neurons, it should be noted that the Cre is also expressed in a small number of neurons in the tectum at embryonic day 10.5 (14).
Loss of Dicer Results in a Reduction of miRNAs in the Striatum.
To confirm that loss of Dicer results in the loss of mature miRNAs, miRNA microarray analysis was performed on dissected striata from dying (10–12 weeks old) Dcr flox/flox; DR-1 Cre brains and controls to obtain candidates (data not shown). Both Northern blotting and Taqman analysis was performed on several candidates including miR-124a, miR-132, and miR-134 (Fig. 3 A and B). These analyses demonstrate a strong reduction of miRNAs in dying (10–12 weeks old) animals. To confirm the loss of miRNAs in neurons, locked nuclei acid (LNA) in situ hybridization analysis was performed on miR-124a (Fig. 3C). In situ analysis demonstrates nearly 90% reduction of miR-124a in the striatum in dying animals (10–12 weeks old). This strong reduction is consistent with efficient removal of Dicer. The ≈10% of neurons still positive for miR-124a are not unexpected, because this Cre is not expressed in all striatal neurons, including interneurons (14).
Fig. 3.
Reduced levels of miRNAs in the striatum of Dcr flox/flox; DR-1 Cre mice. (A) Northern blotting for miR-124a, miR-132, and miR-134 miRNAs in the striatum of Dcr flox/flox; DR-1 Cre mouse and a control mouse (lane 1, Dcr flox/wt; DR-1 Cre; lane 2, Dcr flox/flox; DR-1 Cre). RNA was obtained from 10- to 12-week-old mice. (B) Taqman qRT-PCR analysis for miR-124a, miR-132, and miR-134 in Dcr flox/flox; DR-1 Cre (empty bars) mice as compared with Dcrflox/wt; DR-1 Cre controls (filled bars). RNA was obtained from 10- to 12-week-old mice. (C) miR-124a LNAish in the striatum of a representative dying (10 weeks old) Dcr flox/flox; DR-1 Cre mouse and a control. (Scale bar: 100 μM.)
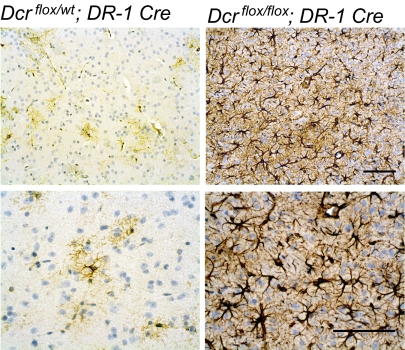
Loss of Dicer Leads to Astrogliosis, but Not Neurodegeneration.
As Dcr flox/flox; DR-1 Cre brains are smaller, mice are ataxic, and because previous studies of Dicer loss in the brain resulted in the degeneration of neurons, we hypothesized that neurodegeneration may be occurring in the striatum of these brains. We first performed staining for glial fibrilary acidic protein (GFAP), a marker for astrocytes. Increases in GFAP signal can indicate neuronal toxicity or neuronal death. Astrogliosis can be induced in response to a variety of neuronal insults or trauma (reviewed in ref. 17). Staining for GFAP demonstrated a profound increase in the level of this marker in Dcr flox/flox; DR-1 Cre mutant animals (Fig. 4A). Thus, we sought to determine whether neurons were undergoing apoptosis or were lost in these brains. TUNEL staining demonstrated no significant increases in apoptotic cells in dying animals (Fig. S2). It is possible that apoptotic cells are undetectable, as degenerating neurons are often cleared rapidly and/or because cells have undergone apoptosis at an earlier age. Therefore, to determine whether cells were lost, we performed rigorous cell counts throughout the entire striatum across five different Bregma coordinates (+0.74, +0.38, +0, −0.82, and −1.0 mm). Spacing between Bregma coordinates was set in such a way as to avoid counting the same neurons twice. Only neurons that were both NeuN positive and had a discernable nucleolus by DAPI staining were included in the analysis. The counts revealed a slightly greater density of neurons but no neuronal loss in Dcr lox/flox; DR-1 Cre animals compared with control animals (Fig. 5 A and B). Consistent with the idea that there is no or little loss of these neurons is that the expression level of tyrosine hydroxylase appears to be similar to that of control animals (Fig. S3).
Fig. 4.
Astrogliosis in the striatum of Dcr flox/flox; DR-1 Cre mice. GFAP immunostaining from a representative Dcr flox/flox; DR-1 Cre mouse and control at 10 weeks of age (n = 4 for all genotypes). (Scale bars: 100 μM.)
Fig. 5.
NeuN staining of neurons in the striatum demonstrates smaller neuronal size but no loss of neurons in Dcr flox/flox; DR-1 Cre mice. (A) (Upper) Schematic of striatal regions stained for NeuN/DAPI. Regions 1–5 correspond to approximate Bregma coordinates: +074, +0.38, +0, −0.82, and −1.0 mm. For each coordinate, five images were taken representing most of the striatal region of each image, and all NeuN/DAPI cells were counted. (Lower) Average NeuN/DAPI-positive neurons counted per image in Dcr flox/wt; DR-1 Cre animals (filled bars) and Dcr flox/flox; DR-1 Cre animals (empty bars) for each coordinate shown above. (B) Representative images of NeuN/DAPI-stained sections from region 3 of a Dcr flox/flox; DR-1 Cre mouse and a control. n = 3 for all genotypes. (C) LacZ staining demonstrates no loss of DR-1 neurons in the striatum of Dcr flox/flox; DR-1 Cre animals, as compared with controls. (D) Average number of LacZ-positive neurons counted per image in Dcr flox/wt; DR-1 Cre animals (filled bar) and Dcr flox/flox; DR-1 Cre animals (empty bar). (Scale bars: B and C, 100 μM.)
If DR-1 cells survive after Dicer ablation, they might also be visualized via fate-mapping with the R26R Cre reporter allele used to determine the recombination pattern of this Cre. To test this and further confirm the NeuN cell counts, brains from mice carrying the R26R reporter were subjected to X-gal staining, and striatal cells were counted as described for the NeuN experiments, but only across Bregma +0.74 (Fig. 5 C and D). These data showed similar numbers of LacZ-positive cells present in 10- to 12-week-old Dcr flox/flox; DR-1 Cre animals and controls, although the LacZ staining was less intense in the Dcr flox/flox; DR-1 Cre sections.
Loss of Dicer Results in Smaller Striatal Medium Spiny Neurons.
Because NeuN counting analysis demonstrated no apparent loss of neurons in the striatum, we sought to determine whether there were changes in neuronal size attributing to the reduction in brain size. The area of the soma of the neurons was quantified from the NeuN/DAPI-stained neurons used for the counting analysis (Fig. 5B), NeuN staining demonstrates a ≈20–30% reduction in cell body area in Dcr flox/flox; DR-1 Cre striatal neurons (Student's t test, P = 0.0153). LacZ staining also demonstrates decreases in the overall size of the neurons as compared with controls (Fig. 5C).
Discussion
Dicer and miRNAs have been demonstrated to have important roles in the early development of animals and mammalian neuronal cell cultures. However, to date, the roles of Dicer, and by extension miRNAs, in mammalian postmitotic neurons in vivo are largely unknown. Furthermore, nothing is known about the roles of Dicer in postmitotic dopaminoceptive neurons, which are perturbed in a variety of devastating neurodevelopmental, neurodegenerative, and addictive disorders.
In this study, we demonstrate that removal of Dicer in postmitotic dopaminoceptive neurons leads to variety of anatomical and behavioral deficits including: clasping, ataxia, reduction in brain size and neuronal cell size, astrogliosis, and death of these animals at ≈10–12 weeks of age. The animals also undergo wasting beginning at ≈6 weeks of age. These animals continue to waste and, at the time of death, most animals have lost ≈50% of their body mass. It is well known that dopamine function is centrally linked to motivation behaviors, including feeding and drinking (18); these results are therefore consistent with dysfunction of dopaminergic neurons.
The phenotypes we observed in these mice are associated with the loss of miRNAs. We believe that we are obtaining efficient removal of Dicer and miRNAs in these neurons, as evidenced by R26R LacZ recombination, our initial miRNA array analysis (data not shown), miRNA Northerns, quantitative RT-PCR (qRT-PCR) analysis, and most convincingly the LNA in situ hybridization of miR-124a, which demonstrated efficient loss of this miRNA in nearly all striatal DR-1 neurons. In future studies, it will be crucial to unravel the roles and contributions of individual DR-1 miRNAs. However, it is possible that not all of the observed phenotypes are attributed to the loss of mature miRNAs. It is conceivable that Dicer is processing other dsRNAs within these neurons and or may have nuclear roles as suggested by loss of centromeric and pericentromeric silencing in Dicer null ES cells (19, 20). To support this idea, the reduction of DNA methylation has also been associated with Dicer loss (20). Although no one has discovered additional RNAs processed by Dicer in mammals, recent studies by Wang et al. (21) demonstrate that removal of another miRNA processing enzyme, DGCR8, has less pronounced phenotypes than removal of Dicer in ES cells, suggesting that Dicer has additional functions outside of miRNA processing in these cells or that DGCR8 does not process all miRNAs in these cells, such as miRtrons (22).
The herein described DR-1 Dicer mouse model could be a useful reagent to explore these questions in the adult mouse brain.
Interestingly, we observe that dopaminoceptive neurons within the striatum survive in the absence of Dicer and miRNAs. Although we cannot discount the possibility that there is neuronal loss, the neuronal cell counts suggest that such loss is minimal. We originally suspected neuronal cell death in our mice; however, our data suggest the smaller brain size is caused by smaller neuronal soma size and increased packing. This phenotype, along with the others described, are rather compelling, as they are reminiscent of mouse models of the neurodevelopmental disorder, Rett syndrome. In this model, the MeCP2 protein, mutations of which underlie Rett syndrome, is disrupted, leading to brains and neurons that are smaller without neuron loss, but have increased neuron and glial cell density and animals that have clasping, elongated teeth, and gait impairments (23, 24). In line with our observations, it was recently demonstrated that miR-132 can regulate levels of MeCP2 in cultured rat neurons, and that loss of MeCP2, using a Rett mouse model, leads to the reduction of BDNF and miR-132 levels in vivo (25).
The finding that neurons survive in absence of Dicer is quite distinct from what has been observed in mouse models in which Dicer was removed in postmitotic Purkinje neurons and postmitotic DAT-expressing neurons, both of which resulted in the degeneration and dramatic loss of most of those cells (7, 8). Given that Dicer has a known critical role in cell survival, we were surprised to determine that the striatal neurons in these mice do not degenerate, highlighting roles for Dicer outside the context of cell survival and proliferation in postmitotic neurons. This system could provide an attractive model for studies to determine more precise roles for Dicer outside of the context of cell viability. Alternatively, future studies might explore how these neurons are able to escape cell death, how they might react in cell cultures, and if possible, measure the extent of their survival in animals coaxed to live longer. In the appropriate contexts, this mouse model might be used to study neurodegenerative and/or addictive disorders, and importantly, it could aid studies addressing which miRNAs are responsible for the described phenotypes.
Materials and Methods
Animals.
Animals were kept in a pathogen-free barrier facility and maintained in accordance with Institutional Animal Care and Use Committee standards and conducted in accordance with appropriate national regulations concerning animal welfare. Mice were housed under a standard 12 h light/dark cycle with access to food and water ad libitum. Dicerflox/flox mice (4) were bred to mice expressing DR-1 Cre (characterization of the line was reported in ref. 14) as well as R26R mice (16). Mice were maintained on a C57BL/sv129 mixed background.
Footprint Analysis and Stride-Length Measurements.
Footprint analysis was performed as described (26, 27). Briefly, mice were allowed to run across a paper-lined runway (50 cm long, 10 cm wide). A bright desk lamp was lit from above. Mice were allowed to acclimate to the testing room for 60 min before experimentation. Mice were allowed one practice run, followed by three trials. Stride lengths were measured from all usable trials and averaged. Student's t test was performed to determine statistical significance.
Histological Analysis.
For lacZ staining and NeuN immunofluorescence, mice were transcardially perfused with 4% paraformaldehyde (PFA). Brains were removed and postfixed overnight in 4% PFA + 10% sucrose and then either cryoprotected in 30% sucrose before freezing in OCT or sectioned by using a vibratome at 100 μM. Cryosections were cut at 12 microns and stored at −80°C. For X-gal staining, sections were washed 3 × 15 min with sodium phosphate buffer containing .01% deoxycholate and 0.02% Nonidet P-40. Sections were then stained in wash buffer including 5 mM potassium ferrocyanide, 5 mM potassium ferricyanide, and 1 mg/ml X-gal at 37°C. After staining, sections were washed, dehydrated, mounted in permount, and imaged on a Zeiss bright-field microscope. For NeuN staining, sodium citrate antigen retrieval was performed, followed by incubation with a mAb from Chemicon (MAB377) at 1:200 overnight at 4°C. Alexa Fluor 594 goat anti-mouse secondary was used at 1:200, and slides were mounted in Vectashield with DAPI and visualized on a Leica SP2 confocal microscope using identical capturing parameters for all images. For GFAP analysis, formalin-fixed, paraffin-embedded tissue after antigen retrieveal with 0.1 M Citrate pH 6.0 was probed with a GFAP mAb from Visionbiosystems Novacastra at 1:100.
Quantification of Soma Size.
NeuN-stained sections were processed in imageJ software. The soma of 25 neurons from each section was selected by using the magic wand tool and area was calculated. n = 2 for all genotypes.
LNAish.
In situ hybridizations were performed as described (28), with a few modifications. Prehybridization was carried out in hybridization buffer without heparin for 3 h at 48°C, followed by the addition of 10 μl of 3 μM miR-124a probe to 600 μl of prehybridization buffer overnight at 48°C. The next day, sections were rinsed once in prewarmed (48°C) 5× SSC (quick washes with 1-ml micropipette), followed by two 5-min washes with 5× SSC at 48°C. Then sections were washed three times for 20 min with 50% formamide/2× SSC at 48°C. Sections were then washed five times with PBST for 3 min each and incubated with anti- digoxigenin antibody as described. Detection was carried out in staining solution for 2 h at 4°C, then at room temperature for 2 h, replacing the staining solution between the two incubations.
miRNA Analysis.
Brains were rapidly removed from mice and sectioned with a rodent brain matrix, followed by striatal dissection. Striata were then snap-frozen and immersed in TRIZOL for RNA isolation. For small Northern blotting, 20 μg of total RNA was run on 10% or 15% acrylamide denaturing gels and then transferred to Hybond N+ membranes at 250 mA for 2 h. After transfer, membranes were cross-linked at 1,200 × 100 μJoles. Oligo probes were end-labeled with γ-32P by T4 kinase, cleaned up with Amersham microspin g-25 columns, and hybridized to membranes overnight at 37°C in Ambion ULTRAhyb-oligo buffer. Membranes were washed twice with 2× SSC/0.1% SDS at 37°C for 30 min each. They were then washed three times at room temperature. Images were obtained with a Storm phosphorimager. RNA was normalized to U6 snRNA. Taqman qRT-PCR analysis was performed on 10 ng of total striatal RNA following the manufacturer's protocol (Applied Biosystems) and normalized to U6. Analysis was performed by using 2−ΔΔCt.
Acknowledgments.
We thank members of the laboratories of M.T.M. and E.U. for advice, reagents, and critical reading of the manuscript, Bernard W. Rajeev in P.T.N.'s laboratory for help with LNA in situ analysis, and Gunther Schutz's laboratory (German Cancer Research Center, Heidelberg) for providing the DR-1 Cre mouse line. T.L.C. was funded by Genentech Inc. in association with the Sandler family. This work was supported by Autism Speaks and National Institutes of Health Grants R21-MH083090 (to E.U.) and RO3-DA022201 (to M.T.M.).
Note Added in Proof.
At the time of publication, two additional Dicer conditional brain studies were completed to which the authors would like to refer readers: Davis et al. (29) and Damiani et al. (30).
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/cgi/content/full/0801689105/DCSupplemental.
References
- 1.Valencia-Sanchez MA, Liu J, Hannon GJ, Parker R. Control of translation and mRNA degradation by miRNAs and siRNAs. Genes Dev. 2006;20:515–524. doi: 10.1101/gad.1399806. [DOI] [PubMed] [Google Scholar]
- 2.Kosik KS. The neuronal microRNA system. Nat Rev. 2006;7:911–920. doi: 10.1038/nrn2037. [DOI] [PubMed] [Google Scholar]
- 3.Bernstein E, et al. Dicer is essential for mouse development. Nat Genet. 2003;35:215–217. doi: 10.1038/ng1253. [DOI] [PubMed] [Google Scholar]
- 4.Harfe BD, McManus MT, Mansfield JH, Hornstein E, Tabin CJ. The RNaseIII enzyme Dicer is required for morphogenesis but not patterning of the vertebrate limb. Proc Natl Acad Sci USA. 2005;102:10898–10903. doi: 10.1073/pnas.0504834102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cobb BS, et al. T cell lineage choice and differentiation in the absence of the RNase III enzyme Dicer. J Exp Med. 2005;201:1367–1373. doi: 10.1084/jem.20050572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Andl T, et al. The miRNA-processing enzyme dicer is essential for the morphogenesis and maintenance of hair follicles. Curr Biol. 2006;16:1041–1049. doi: 10.1016/j.cub.2006.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kim J, et al. A microRNA feedback circuit in midbrain dopamine neurons. Science. 2007;317:1220–1224. doi: 10.1126/science.1140481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schaefer A, et al. Cerebellar neurodegeneration in the absence of microRNAs. J Exp Med. 2007;204:1553–1558. doi: 10.1084/jem.20070823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gerfen CR, et al. D1 and D2 dopamine receptor-regulated gene expression of striatonigral and striatopallidal neurons. Science. 1990;250:1429–1432. doi: 10.1126/science.2147780. [DOI] [PubMed] [Google Scholar]
- 10.Graybiel AM, Rauch SL. Toward a neurobiology of obsessive-compulsive disorder. Neuron. 2000;28:343–347. doi: 10.1016/s0896-6273(00)00113-6. [DOI] [PubMed] [Google Scholar]
- 11.Evans AH, Lees AJ. Dopamine dysregulation syndrome in Parkinson's disease. Curr Opin Neurol. 2004;17:393–398. doi: 10.1097/01.wco.0000137528.23126.41. [DOI] [PubMed] [Google Scholar]
- 12.Nieoullon A, Coquerel A. Dopamine: A key regulator to adapt action, emotion, motivation and cognition. Curr Opin Neurol. 2003;16(Suppl 2):S3–S9. [PubMed] [Google Scholar]
- 13.Gerdeman GL, Partridge JG, Lupica CR, Lovinger DM. It could be habit forming: Drugs of abuse and striatal synaptic plasticity. Trends Neurosci. 2003;26:184–192. doi: 10.1016/S0166-2236(03)00065-1. [DOI] [PubMed] [Google Scholar]
- 14.Lemberger T, et al. Expression of Cre recombinase in dopaminoceptive neurons. BMC Neurosci. 2007;8:4. doi: 10.1186/1471-2202-8-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mangiarini L, et al. Exon 1 of the HD gene with an expanded CAG repeat is sufficient to cause a progressive neurological phenotype in transgenic mice. Cell. 1996;87:493–506. doi: 10.1016/s0092-8674(00)81369-0. [DOI] [PubMed] [Google Scholar]
- 16.Soriano P. Generalized lacZ expression with the ROSA26 Cre reporter strain. Nat Genet. 1999;21:70–71. doi: 10.1038/5007. [DOI] [PubMed] [Google Scholar]
- 17.Correa-Cerro LS, Mandell JW. Molecular mechanisms of astrogliosis: New approaches with mouse genetics. J Neuropathol Exp Neurol. 2007;66:169–176. doi: 10.1097/01.jnen.0000248555.53079.d5. [DOI] [PubMed] [Google Scholar]
- 18.Drago J, et al. Altered striatal function in a mutant mouse lacking D1A dopamine receptors. Proc Natl Acad Sci USA. 1994;91:12564–12568. doi: 10.1073/pnas.91.26.12564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Murchison EP, Partridge JF, Tam OH, Cheloufi S, Hannon GJ. Characterization of Dicer-deficient murine embryonic stem cells. Proc Natl Acad Sci USA. 2005;102:12135–12140. doi: 10.1073/pnas.0505479102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kanellopoulou C, et al. Dicer-deficient mouse embryonic stem cells are defective in differentiation and centromeric silencing. Genes Dev. 2005;19:489–501. doi: 10.1101/gad.1248505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang Y, Medvid R, Melton C, Jaenisch R, Blelloch R. DGCR8 is essential for microRNA biogenesis and silencing of embryonic stem cell self-renewal. Nat Genet. 2007;39:380–385. doi: 10.1038/ng1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Berezikov E, Chung WJ, Willis J, Cuppen E, Lai EC. Mammalian mirtron genes. Mol Cell. 2007;28:328–336. doi: 10.1016/j.molcel.2007.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Guy J, Hendrich B, Holmes M, Martin JE, Bird A. A mouse Mecp2-null mutation causes neurological symptoms that mimic Rett syndrome. Nat Genet. 2001;27:322–326. doi: 10.1038/85899. [DOI] [PubMed] [Google Scholar]
- 24.Chen RZ, Akbarian S, Tudor M, Jaenisch R. Deficiency of methyl-CpG binding protein-2 in CNS neurons results in a Rett-like phenotype in mice. Nat Genet. 2001;27:327–331. doi: 10.1038/85906. [DOI] [PubMed] [Google Scholar]
- 25.Klein ME, et al. Homeostatic regulation of MeCP2 expression by a CREB-induced microRNA. Nat Neurosci. 2007;10:1513–1514. doi: 10.1038/nn2010. [DOI] [PubMed] [Google Scholar]
- 26.Harper SQ, et al. RNA interference improves motor and neuropathological abnormalities in a Huntington's disease mouse model. Proc Natl Acad Sci USA. 2005;102:5820–5825. doi: 10.1073/pnas.0501507102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Crawley JN. Current Protocols in Neuroscience. New York: Wiley; 2001. [Google Scholar]
- 28.Nelson PT, et al. RAKE and LNA-ISH reveal microRNA expression and localization in archival human brain. RNA. 2006;12:187–191. doi: 10.1261/rna.2258506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Davis TH, et al. Conditional loss of Dicer disrupts cellular and tissue morphogenesis in the cortex and hippocampus. J Neurosci. 2008 doi: 10.1523/JNEUROSCI.4815-07.2008. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Damiani D, et al. Dicer inactivation leads to progressive functional and structural degeneration of the mouse retina. J Neurosci. 2008 doi: 10.1523/JNEUROSCI.0828-08.2008. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]