Abstract
A full-factorial study of the effects of rates of temperature change and start temperatures was undertaken for both upper and lower critical thermal limits (CTLs) using the tsetse fly, Glossina pallidipes. Results show that rates of temperature change and start temperatures have highly significant effects on CTLs, although the duration of the experiment also has a major effect. Contrary to a widely held expectation, slower rates of temperature change (i.e. longer experimental duration) resulted in poorer thermal tolerance at both high and low temperatures. Thus, across treatments, a negative relationship existed between duration and upper CTL while a positive relationship existed between duration and lower CTL. Most importantly, for predicting tsetse distribution, G. pallidipes suffer loss of function at less severe temperatures under the most ecologically relevant experimental conditions for upper (0.06°C min−1; 35°C start temperature) and lower CTL (0.06°C min−1; 24°C start temperature). This suggests that the functional thermal range of G. pallidipes in the wild may be much narrower than previously suspected, approximately 20–40°C, and highlights their sensitivity to even moderate temperature variation. These effects are explained by limited plasticity of CTLs in this species over short time scales. The results of the present study have broad implications for understanding temperature tolerance in these and other terrestrial arthropods.
Keywords: temperature tolerance, phenotypic plasticity, rapid cold-hardening, acclimation rate, lethal limits, survival
1. Introduction
The ability of an organism to remain active under extreme conditions is a significant component of fitness (Loeschcke & Hoffmann 2007). Therefore, determining the limits to activity is an important first step in understanding the ways in which environmental variation affects fitness and the dynamics of a given population. Thermal limits have received much attention because their investigation provides insight into the manner in which climate shapes variation in the ecology, distribution and evolution of species (Janzen 1967; Pörtner 2001; Ghalambor et al. 2006; Chown & Terblanche 2007). Furthermore, upper temperature limits are positively related to optimal performance temperatures (Huey & Bennett 1987; Garland et al. 1991) and these limits are relatively simple to measure (Chown & Nicolson 2004). In consequence, factors that affect the assessment of thermal tolerance are significant (Lutterschmidt & Hutchison 1997a; Chown & Nicolson 2004). In insects, these include photoperiod (Lanciani et al. 1992), ontogeny (Hollingsworth & Bowler 1966; Rossolimo 1997), CO2 anaesthesia (Nilson et al. 2006), temperature acclimation (e.g. Terblanche et al. 2005, 2006) and time of day (Sinclair et al. 2003; McMillan et al. 2005). Importantly, they also include elements of basic experimental design, such as the rate of temperature change (Worland 2005) and exposure temperature (David et al. 2003; Rako & Hoffmann 2006).
Typically, limits are assessed using either dynamic or static methods (Lutterschmidt & Hutchison 1997a; Hoffmann et al. 2003). Briefly, the dynamic method involves changing temperature at a constant rate and assessing individual knockdown. By contrast, when using the static method, temperature is held constant and exposure duration varied (or duration can be held constant while temperature is varied) with assessments based on survival of a proportion of a sample. Techniques that assess recovery from coma are dynamic in the sense that recovery time is assessed following exposure to one or more coma-inducing temperatures (David et al. 2003). One dynamic technique that is widely used to resolve both high- and low-temperature thresholds is the determination of critical thermal limits (CTLs; for critique and reviews see Lutterschmidt & Hutchison 1997a,b; Beitinger et al. 2000; Chown & Nicolson 2004). CTL determinations involve heating or cooling an animal from a starting temperature until physiological failure (e.g. knockdown, loss of righting response, onset of muscle spasms). CTLs are considered ecologically relevant because they provide an indication of the activity range for a population under acute exposure conditions (Vannier 1994; Somero 2005), although animals may mediate effects of environmental temperature variation through behavioural adjustments (Huey et al. 2003). However, the extent to which experimental design of CTLs influences their outcome has not been systematically explored (Chown & Nicolson 2004).
The few studies that have been done have mostly concerned critical thermal minima (CTmin), demonstrating that slow cooling rates lower the CTmin, thus improving acute low-temperature tolerance (Kelty & Lee 1999, 2001; Powell & Bale 2004, 2006; Overgaard et al. 2006). The general explanation for this improvement in tolerance is that slower rates provide sufficient time for hardening, a form of phenotypic plasticity (see Hoffmann et al. 2003) that protects cells from subsequent injury (Overgaard et al. 2006). However, despite expectations that plasticity in lower thermal limits is widespread in all organisms (Rako & Hoffmann 2006), theory suggests that acclimation responses should be much less common in tropical or polar species compared with their temperate counterparts (Tsuji 1988; Somero et al. 1996; Ghalambor et al. 2006; Chown & Terblanche 2007). Given that the tropics probably house more insect species than non-tropical regions (Gaston 1996; Hillebrand 2004), and the small number of studies restricted to temperate species, the generality of the rate effect on CTmin cannot be considered well established. Moreover, rate effects on critical thermal maxima (CTmax) have not been widely explored (Chown & Nicolson 2004; Mora & Maya 2006).
A further complication in the determination of CTLs is the question of the start temperature used before cooling or heating commences. Start temperature usually means the constant temperature period during which animals are held before heating or cooling to allow equilibration of body temperature with ambient temperature. Although some studies use the same acclimation and start temperature, these can differ (e.g. Powell & Bale 2006), although the influence of these alternatives has not always been clear. Although start temperature is usually explicitly recorded in descriptions of experimental design (e.g. Kelty & Lee 1999, 2001; Shermin & Levitis 2003), and is typically varied by investigators when species or populations from different thermal environments are examined (e.g. Brattstrom 1965), its effect on CTLs is poorly understood (but see Das et al. 2005). Indeed, an implicit assumption is made that start temperature has little effect on experimental outcome. However, start temperature may have a significant influence on CTL determinations, directly, and through its influence on the overall time (duration) an experimental group of individuals experience conditions that either affect physiological functioning or induce a physiological response (e.g. Terblanche et al. 2005; see also Rako & Hoffmann 2006).
If start temperature and rate of temperature change affect CTLs in similar ways, and predominantly via the effects on duration of exposure, it might be expected that slow rates and start temperatures distant from the CTL in question would result in an improvement of tolerance. Moreover, these experimental effects might be substantial as a consequence of the considerable plasticity in CTLs and other thermal tolerance traits shown by various species (Chen et al. 1990; Hoffmann et al. 2003; Klok & Chown 2003).
Given the above and that among-population and interspecific variation in CTLs have been used to test a variety of macrophysiological hypotheses (e.g. Gaston & Chown 1999; Addo-Bediako et al. 2000; Ghalambor et al. 2006), examination of the extent to which experimental conditions influence CTLs is important. Therefore, here, using the full-factorial design, we dissect the effects of rate and start temperature on CTLs in a widely distributed, economically important tropical insect species, the tsetse fly (Glossina pallidipes; Diptera, Glossinidae). Since the ways in which ambient temperature affect the population dynamics, distribution and abundance of tsetse are of considerable interest (Hargrove 2001, 2004; Rogers & Robinson 2004), we also explore the outcomes of this work in the context of microclimates encountered by G. pallidipes in natural conditions.
2. Material and methods
Adult tsetse flies (G. pallidipes) were collected from South Luangwa National Park, Zambia during October 2006. Odour-baited biconical and Ngu traps were used to collect live flies in the morning (06.00–09.00 hours; as outlined in Terblanche et al. 2006). Flies were kept in an insulated container and returned to the field laboratory within 2 hours of the end of each morning's collection period. All experiments were conducted on freshly collected animals only.
Microhabitat temperature data were logged for 10 days at three sites using calibrated iButton data loggers, with a resolution of 0.5°C (8-bit Model DS1923, Dallas, TX, USA). These sites were located on shaded trees and had been identified by previous inspection as sites used by resting flies during all daylight hours.
Effects of age, gender and feeding status on critical limits in G. pallidipes are negligible (table S1 in the electronic supplementary material; Terblanche et al. 2006). Therefore, these characteristics were not determined prior to the trials. Ten animals were placed individually into chambers in an insulated, water-jacketed isolation chamber system connected to a programmable water bath (as in Terblanche et al. 2006). A thermocouple (type T, 36 SWG) was inserted into a control chamber to monitor chamber temperature. In preliminary trials, we have recorded body temperature (Tb) changes of up to 10°C within 2 min as Tb tracks ambient temperature change prior to a CTL determination. Furthermore, Tb does not lag behind chamber temperatures during CTL experiments at a heating/cooling rate of 0.25°C min−1 (figure S1 in the electronic supplementary material). Starting temperatures were selected on the basis of previous assessments of CTLs in East African populations of this species (Terblanche et al. 2006). A full-factorial 3×3 design was used to assess the effects of rate and start temperature on CTmin and CTmax. Start temperatures of 16, 20 and 24°C and cooling rates of 0.06, 0.12 and 0.25°C min−1 were used for the CTmin experiments. For CTmax experiments, start temperatures of 35, 38 and 41°C and heating rates of 0.06, 0.12 and 0.25°C min−1 were used. After 12 min of equilibration at one of the three starting temperatures (periods of 10 min or less are frequently used in CTL assessments, e.g. Kelty & Lee 2001), flies were heated or cooled at one of the three rates. The point of critical thermal minimum (CTmin) was defined as the temperature of loss of coordinated muscle function, and critical thermal maximum (CTmax) was defined as the temperature of onset of muscle spasms (as in Terblanche et al. 2006). To avoid observer bias and diurnal effects, a single observer (J.A.D.) undertook all of the experimental work between 10.00 and 14.00 hours. Sample sizes varied between 10 and 20 for each of the nine rate×start temperature combinations in the CTmax experiment, and n=20 for each unique combination in the CTmin experiment. Ambient laboratory temperatures ranged from 30 to 35°C between trials and experimental days and were within the range of recorded microclimate temperatures.
Prior to analysis, data were inspected for normality and equality of variances using Shapiro–Wilk and Hartley–Bartlett tests, respectively. In most cases data were normally distributed (15/18 cases) and had similar variance (16/18 cases). Exclusion of outliers corrected these issues in the remainder thereof. Initial analyses were performed using a full-factorial ANOVA implemented in Statistica v. 7 (Statsoft, Tulsa, OK, USA) and normality of residuals confirmed in each ANOVA. The effects of start temperature and rate on upper and lower CTLs were investigated using an ordered-factor ANOVA implemented in SAS (v. 9.0, SAS Institute, Cary, NC, USA).
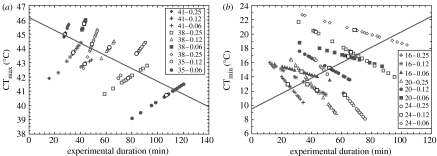
Duration of exposure is thought to be a major proximate cause of variation in CTLs. On the one hand it is thought that increasing exposure duration might compound stress and therefore reduce tolerance (Cossins & Bowler 1987; Sømme 1999). On the other hand, prolonged exposures to sublethal temperatures might improve stress resistance (see §1). Therefore, the role of duration in altering CTLs in the nine different treatments was examined to determine if it might at least partially account for the effects of rate and start temperature. At first, it might appear simple to do so by including duration, as calculated from the start of a given CT trial to the thermal limit identified for each individual, as a covariate in a general linear model. However, owing to the way the CTL is determined, it is colinear with duration for each rate and start temperature group. For example, in an upper CTL experiment, a higher CTL necessarily means that the animal has been warming for a longer period. In consequence, a general linear model including duration as a covariate cannot be used to investigate its effects. In other words, the physiological effects of duration of exposure as a consequence of different rates and start temperatures cannot be separated from the within-treatment or experimental effect of duration. The latter will always be positive for upper CTLs and negative for lower CTLs, as scatter plots of duration versus CTL (figure 1), and a general linear model incorporating duration in this manner (table 1), demonstrate. However, from a physiological consequences perspective, the relationship between duration and CTL might either be positive, negative or non-existent.
Figure 1.
Scatter plot of experimental duration against (a) critical thermal maxima (CTmax) and (b) critical thermal minima (CTmin). Each treatment is plotted as a unique series. In the figure, the first number refers to the start temperature and the second number indicates the rate of temperature change during the experiment. Means of each treatment are indicated as open circles (CTmax) or filled squares (CTmin). Note the colinearity within treatments, but the opposite relationship among treatments for both CTmin and CTmax. The lines shown were fitted by least-squares regression using the means for each treatment, to illustrate the differences in direction of relationship within and among treatments.
Table 1.
Outcome of a type III general linear model showing the confounding effect of experimental duration (duration) on CTmax and CTmin in G. pallidipes. Rate of temperature change (rate) and start temperature (temp) are included as might typically be done using such an approach (see text for detail). (Rate and temp were defined as categorical variables while duration was included as a continuous covariate.)
| effect | SS | d.f. | F | p | estimate ±s.e. |
|---|---|---|---|---|---|
| CTmax | |||||
| intercept | 1901.1 | 1 | 14 101.7 | <0.0001 | |
| duration | 33.3 | 1 | 247.3 | <0.0001 | 0.086±0.006 |
| rate | 119.1 | 2 | 441.6 | <0.0001 | |
| temp | 54.5 | 2 | 202.1 | <0.0001 | |
| rate×temp | 44.5 | 4 | 82.6 | <0.0001 | |
| error | 12.9 | 96 | |||
| CTmin | |||||
| intercept | 5413.8 | 1 | 6139.8 | <0.0001 | |
| duration | 450.0 | 1 | 510.4 | <0.0001 | −0.115±0.005 |
| rate | 1238.1 | 2 | 702.1 | <0.0001 | |
| temp | 703.5 | 2 | 398.9 | <0.0001 | |
| rate×temp | 270.7 | 4 | 76.7 | <0.0001 | |
| error | 158.7 | 180 | |||
One way of disentangling the effects of the experimental duration for each individual from the physiological consequences of longer exposures to stressful temperatures is to examine the relationships between the mean CTL and mean duration for each treatment. In doing so, the colinearity is removed (e.g. upper CTL cannot simultaneously be positively and negatively related to duration). Therefore, we adopted this approach and examined the correlation between mean CTL and mean duration of exposure over the nine trials separately for the CTmax and the CTmin experiments. An additional general linear model including mean duration, mean CTL, and rate and start temperature was also implemented to distinguish the effects of duration from those of rate and start temperature. Here, a type I (additive) sums of squares model was used because the significance of the incremental increase in the sums of squares with each added effect provides an indication of the importance of that effect. The order of effects in the type I model was duration of experiment, rate of temperature change and start temperature.
3. Results
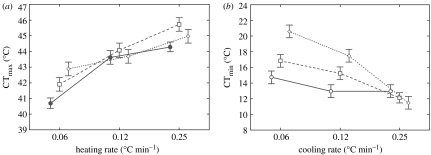
The treatments resulted in greater than 5°C variation in CTmax and greater than 10°C variation in CTmin (figure 2). Rate of temperature change, start temperature and their interaction had significant effects on CTmin and CTmax (table 2). As figure 2 shows, faster rates resulted in greater thermal tolerances. Moreover, when start temperatures were high, upper CTL values were the largest, while when they were low, lower CTLs were the lowest. The significant interaction term indicates that start temperature had a striking influence on the extent to which rate affected CTLs (table 2). Because the start temperatures most distant from the mean CTL appeared to have the largest effects, it seemed probable that exposure duration had a crucial role in altering the CTLs determined in each of the nine treatments.
Figure 2.
(a) Mean critical thermal maxima (CTmax) values for three heating rates (0.06, 0.12 and 0.25°C min−1) using start temperatures of 35 (filled circles), 38 (open squares) and 41°C (open diamonds). (b) Mean critical thermal minima (CTmin) values for three heating rates (0.06, 0.12 and 0.25°C min−1) using start temperatures of 16 (open circles), 20 (open squares) and 24°C (open diamonds).
Table 2.
Outcome of an ordered-factor ANOVA (proc GLM in SAS) investigating the effects of start temperature and rate of temperature change on critical thermal maxima (CTmax) and critical thermal minima (CTmin) in wild G. pallidipes. (Estimates are given only for the linear effects.)
| trait | effect | d.f. | F | p-value | estimate ±s.e. |
|---|---|---|---|---|---|
| CTmax | start temperature | 2 | 187.89 | <0.0001 | 0.977±0.164 |
| heating rate | 2 | 26.30 | <0.0001 | 3.156±0.164 | |
| start×rate | 4 | 8.64 | <0.0001 | 0.919±0.679 | |
| error | 97 | ||||
| CTmin | start temperature | 2 | 129.85 | <0.0001 | 2.957±0.335 |
| cooling rate | 2 | 39.57 | <0.0001 | −5.201±0.325 | |
| start×rate | 4 | 23.35 | <0.0001 | 13.400±1.421 | |
| error | 181 |
Correlation analyses using the means of each treatment demonstrate that this is the case. The relationship between mean CTmax and mean exposure duration was significantly negative (r=−0.759, p=0.011, n=9) whereas it was positive between mean CTmin and mean exposure duration. The latter relationship was marginally non-significant when including the 24°C and 0.25°C min−1 slightly outlying treatment (r=0.598, p=0.068, n=9), and highly significant following its exclusion (r=0.829, p=0.006, n=8). Thus, the longer duration of exposure to experimental conditions results in poorer thermal tolerance at both upper and lower temperatures. The type I general linear model confirmed the significance of duration and rate, but suggested that start temperature added little further explanatory power (table 3).
Table 3.
Outcome of a general linear model using type I sums of squares showing the incremental effects of experimental duration (duration), rate of temperature change (rate) and start temperature on critical thermal minima (CTmin) and maxima (CTmax) in G. pallidipes using mean values for each treatment group.
| effect | SS | d.f. | MS | F | p | estimate ±s.e. |
|---|---|---|---|---|---|---|
| CTmax | ||||||
| intercept | 17 060.4 | 1 | 17 060.4 | 25335.1 | <0.0001 | |
| duration | 10.8 | 1 | 10.8 | 16.1 | 0.010 | −0.032±0.036 |
| rate | 4.6 | 1 | 4.6 | 6.8 | 0.048 | 9.608±7.464 |
| start temp | 0.1 | 1 | 0.1 | 0.1 | 0.811 | −0.073±0.291 |
| error | 3.4 | 5 | 0.7 | |||
| CTmin | ||||||
| intercept | 2008.3 | 1 | 2008.3 | 712.0 | <0.0001 | |
| duration | 25.0 | 1 | 25.0 | 8.9 | 0.031 | −0.125±0.139 |
| rate | 24.8 | 1 | 24.8 | 8.8 | 0.031 | −34.816±11.419 |
| start temp | 6.0 | 1 | 6.0 | 2.1 | 0.203 | 0.905±0.619 |
| error | 14.1 | 5 | 2.8 | |||
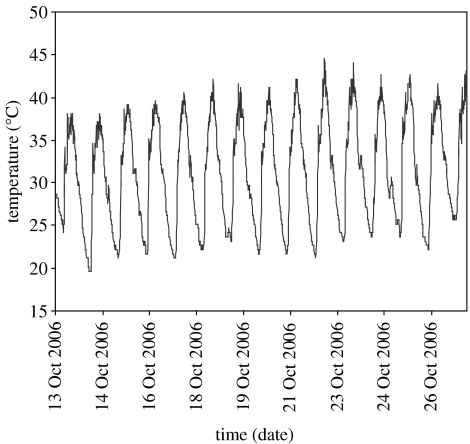
In the field, mean shaded cooling rate was 0.020±0.007°C min−1 (mean±s.d.) while mean shaded heating rate over the same period was 0.050±0.010°C min−1 across the three fly resting sites that were assessed. Thus, the 0.06°C min−1 experimental rate of change is the most ecologically relevant of the three rates used in the laboratory trials. Similarly, inspection of the microclimate data (figure 3) suggested that the 24 and 35°C start temperatures for lower and upper CTL, respectively, are most ecologically relevant to tsetse in their natural habitat.
Figure 3.
Average shade temperature for the habitat representative of G. pallidipes thermal refugia, gathered over two weeks during October 2006 (South Luangwa National Park, Zambia). Data obtained using thermochron iButtons sampling at 10 min frequency.
4. Discussion
Several points, critical for all CTL investigations of ectotherms, have emerged from this study. Perhaps the most obvious outcome is that the range of variation caused by changes in experimental protocol is as wide as that found in many investigations of geographical or acclimation-induced variation in CTLs. For example, in the same species, Terblanche et al. (2006) found approximately 3.3°C variation of CTmin and 0.5°C variation of CTmax induced by acclimation temperatures ranging from 20 to 30°C over a 10-day period. In temperate weevils, Klok & Chown (2003) found approximately 2.6°C variation of CTmin and approximately 2.4°C variation of CTmax induced by 15°C range of acclimation temperatures. The present findings do not mean that the results of these and other past studies are confounded because in these investigations standardized protocols are typically used. However, they do suggest that additional thought needs to be given to distinguishing environmental signal from experimental noise in broader comparisons (see discussion in Chown et al. (2003); Hodkinson (2003)). Nonetheless, many of the conclusions from previous broad scale comparisons are likely to remain unaltered. For example, as has been found in many previous interspecific, intraspecific and acclimation-based investigations (reviewed in Chown (2001) and Chown & Terblanche (2007)), the present work revealed greater plasticity in lower thermal limits (overall range 10°C) than in upper thermal limits (overall range 5°C).
Perhaps more importantly, this study demonstrated that both start temperature and rate of temperature change affect CTLs, and that their effects interact, probably as a consequence of the duration of exposure. Indeed, when mean duration of exposure, rate of temperature change and start temperature were entered sequentially into the type I general linear model, the latter variable was not significant, and in the CTmax trials rate of change was only marginally significant. These findings make it clear that just as recovery from coma experiments have to take the knockdown temperature into account (David et al. 2003; Rako & Hoffmann 2006), so too do CTL experiments have to take rate and start temperature into account. They also confirm the fact, well known from other thermobiological investigations (Cossins & Bowler 1987; Sømme 1999; Ramløv 2000), that exposure duration×temperature interactions ultimately determine mortality rates. Moreover, they emphasize that rates of temperature change affect critical limits, but that the extent of this influence depends on whether upper or lower critical limits are being examined, thus emphasizing the differences between these traits and their underlying mechanisms (see Chown & Nicolson 2004).
While a few previous studies have shown that duration of exposure has an effect on thermal tolerance, this effect is typically the opposite of what was documented in this study (although see Overgaard et al. (2006) for a more complex picture). Here, CTLs range declined as exposure duration increased. That is, CTmin increased and CTmax declined with prolonged exposure duration. By contrast, previous studies of CTmin in Drosophila melanogaster (Kelty & Lee 1999, 2001) and the grain aphid Sitobion avenae (Powell & Bale 2006) have shown that as the duration of exposure increases (rate of temperature change is decreased), so the insects are able to mount an increasingly effective physiological response to the stress and CTmin declines. The mechanisms underlying rapid cold-hardening (RCH; Lee et al. 1987, 2006) are thought to lie at the heart of this response (Kelty & Lee 2001), although clearly too low a rate of change can prove deleterious at least as far as survival is concerned (Overgaard et al. 2006). What influence exposure duration has on CTmax is less clear, largely because very few studies have been undertaken in insects (Chown & Nicolson 2004). In one of the few (and in this case incidental) examinations of this trait, Kay & Whitford (1978) noted that during pilot trials using the honeypot ant Myrmecocystus depilis (Hymenoptera, Formicidae), an increase of heating rate from 1 to 2°C min−1 resulted in a decline in CTmax of approximately 2°C. Once again, G. pallidipes showed the opposite response, with CTmax increasing as exposure duration declined. These latter results are similar to those obtained in studies of fish, where a general trend exists for higher CTmax values at faster heating rates (e.g. Cocking 1959 and see discussions in Beitinger et al. 2000; Mora & Maya 2006).
Why these differences in the response of CTLs to exposure duration should exist between G. pallidipes and the other species studied is not entirely clear. In the case of CTmax, the effects of rate variation might not differ to any large extent among species, but the absence of comparative information makes it difficult to tell whether or not this is probable. In the case of CTmin, the differences may well be a reflection of the absence of RCH in a tropical species from a relatively stable thermal environment. In other words, prolonged exposure to stressful conditions simply increases mortality and does not elicit a hardening response. More generally, tropical species are thought to show less phenotypic plasticity than their temperate counterparts that encounter a wider range of temperatures (Janzen 1967; Ghalambor et al. 2006; Chown & Terblanche 2007). Although few studies have sought RCH in tropical species (Chown & Nicolson 2004), broader investigations of thermal tolerance have shown relatively little scope for short-term change in lower thermal tolerance in tropical insect species versus their temperate counterparts (e.g. Chen et al. 1990). In the case of G. pallidipes, adults are able to mount an acclimation response in CTmin over a week-long period (Terblanche et al. 2006), but it is clear that over a period of hours they are unable to do so (Terblanche et al., in press). Whether or not this is a reflection of a poor acclimation response in tropical species is difficult to determine, especially given the ability of G. pallidipes to respond to acclimation at longer time scales. What may turn out to be more significant than temperature range per se is the timing of the temperature shift. Thus, G. pallidipes encounters low temperatures that are likely to limit activity only at night when it is least active (Brady 1988), but the need for rapid physiological response under these conditions is difficult to construe. Clearly, the significance of potential interactions between behavioural and physiological responses needs to be explored further (see also Huey et al. 2003). Nonetheless, it is apparent that in species which are capable of rapidly cold hardening, cooling rates will typically be positively correlated with CTmin assuming that these cooling rates are ecologically relevant over daily time scales. By contrast, in species that are unable to rapidly cold harden (low levels of phenotypic plasticity) a negative correlation might be expected between CTmin and cooling rates. Presumably similar patterns would be characteristic of CTmax.
The final significant feature of this study is that at ecologically relevant rates of temperature change, CTLs provide a strong indication that physiological limits to activity might mediate the negative effects of environmental temperatures on tsetse population dynamics. Mark-recapture studies have demonstrated a strong relationship between temperature and mortality rate in G. pallidipes (Hargrove 2004) and other tsetse species (Hargrove 2001). However, previous investigations of acute physiological limits to activity, using a 0.25°C min−1 rate of temperature change and 16 or 35°C as start temperatures, revealed CTLs of 10–45°C (Terblanche et al. 2006). The CTmax value (CTmax: 44–45°C) lies well beyond the point at which temperature-dependent mortality occurs in the field (100% mortality: approx. 40°C; Hargrove 2004) suggesting that CTmax plays little part in mediating temperature effects on mortality. By contrast, at the ecologically relevant heating rate of 0.06°C min−1 the CTmax value of approximately 40°C is not only close to the absolute temperature limits experienced in the field in this study (figure 3, see also table S2 in the electronic supplementary material for macroclimatic data), but also closely matches the temperature at which mortality is 100%, determined from field mark-recapture studies in a similar south-central African location (Hargrove 2004). Therefore, these results provide one, but not necessarily the only, causal explanation for why temperature is such an excellent predictor of tsetse population dynamics (Hargrove 2004), abundance and distribution (Rogers & Robinson 2004). The marked sensitivity of tsetse to even moderate temperature variation makes prospects for a bottom-up, mechanistic model of the relationship between climate change and tsetse distribution and abundance (see Crozier & Dwyer (2006) for a similar approach) much better than had previously appeared to be the case.
Acknowledgments
Erika Nortje, the Veterinary and Livestock Department, Wildlife Camp and the Personal Touch provided valuable logistic or field support. Elrike Marais and Sue Jackson provided useful comments on a previous draft. We are especially grateful to Ray Huey, Paul Rainey and an anonymous reviewer for detailed and insightful comments. This work was funded by National Institutes of Health grant AI-52456 to E. S. Krafsur.
Supplementary Material
Supplementary material containing a) experimental data showing a lack of gender, feeding and age effects on critical thermal limits, b) climate data for Mfuwe, Zambia and c) body temperatures recorded during a critical thermal limit trial
References
- Addo-Bediako A, Chown S.L, Gaston K.J. Thermal tolerance, climatic variability and latitude. Proc. R. Soc. B. 2000;267:739–745. doi: 10.1098/rspb.2000.1065. doi:10.1098/rspb.2000.1065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beitinger T.L, Bennett W.A, McCauley R.W. Temperature tolerance of North American freshwater fishes exposed to dynamic changes in temperature. Environ. Biol. Fish. 2000;58:237–275. doi:10.1023/A:1007676325825 [Google Scholar]
- Brady J. Circadian ontogeny in the tsetse fly: a permanent major phase change after the first feed. J. Insect Physiol. 1988;34:743–749. doi:10.1016/0022-1910(88)90147-3 [Google Scholar]
- Brattstrom B.H. Body temperatures of reptiles. Am. Midl. Nat. 1965;73:376–422. doi:10.2307/2423461 [Google Scholar]
- Chen C.-P, Lee R.E, Denlinger D.L. A comparison of the responses of tropical and temperate flies (Diptera: Sarcophagidae) to cold and heat stress. J. Comp. Physiol. B. 1990;160:543–547. doi:10.1007/BF00258982 [Google Scholar]
- Chown S.L. Physiological variation in insects: hierarchical levels and implications. J. Insect Physiol. 2001;47:649–660. doi: 10.1016/s0022-1910(00)00163-3. doi:10.1016/S0022-1910(00)00163-3 [DOI] [PubMed] [Google Scholar]
- Chown S.L, Nicolson S.W. Oxford University Press; Oxford, UK: 2004. Insect physiological ecology. Mechanisms and patterns. [Google Scholar]
- Chown S.L, Terblanche J.S. Physiological diversity in insects: ecological and evolutionary contexts. Adv. Insect Physiol. 2007;33:50–152. doi: 10.1016/S0065-2806(06)33002-0. doi:10.1016/S0065-2806(06)33002-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chown S.L, Addo-Bediako A, Gaston K.J. Physiological diversity: listening to the large-scale signal. Funct. Ecol. 2003;17:568–572. doi:10.1046/j.1365-2435.2003.07622.x [Google Scholar]
- Cocking A.W. The effects of high temperatures on roach. II. The effects of temperature increasing at a known constant rate. J. Exp. Biol. 1959;36:217–226. [Google Scholar]
- Cossins A.R, Bowler K. Chapman and Hall; London, UK: 1987. Temperature biology of animals. [Google Scholar]
- Crozier L, Dwyer G. Combining population-dynamic and ecophysiological models to predict climate-induced insect range shifts. Am. Nat. 2006;167:853–866. doi: 10.1086/504848. doi:10.1086/504848 [DOI] [PubMed] [Google Scholar]
- Das T, Pal A.K, Chakraborty S.K, Manush S.M, Sahu N.P, Mukherjee S.C. Thermal tolerance, growth and oxygen consumption of Labeo rohita fry (Hamilton, 1822) acclimated to four temperatures. J. Therm. Biol. 2005;30:378–383. doi:10.1016/j.jtherbio.2005.03.001 [Google Scholar]
- David J.R, Gibert P, Moreteau B, Gilchrist G.W, Huey R.B. The fly that came in from the cold: geographic variation of recovery time from low-temperature exposure in Drosophila subobscura. Funct. Ecol. 2003;17:425–430. doi:10.1046/j.1365-2435.2003.00750.x [Google Scholar]
- Garland T, Huey R.B, Bennett A.F. Phylogeny and coadaptation of thermal physiology in lizards: a reanalysis. Evolution. 1991;45:1969–1975. doi: 10.1111/j.1558-5646.1991.tb02703.x. doi:10.2307/2409846 [DOI] [PubMed] [Google Scholar]
- Gaston K.J. Biodiversity—latitudinal gradients. Prog. Phys. Geogr. 1996;20:466–476. [Google Scholar]
- Gaston K.J, Chown S.L. Elevation and climatic tolerance: a test using dung beetles. Oikos. 1999;86:584–590. doi:10.2307/3546663 [Google Scholar]
- Ghalambor C.K, Huey R.B, Martin P.R, Tewksbury J.J, Wang G. Are mountain passes higher in the tropics? Janzen's hypothesis revisited. Integr. Comp. Biol. 2006;46:5–17. doi: 10.1093/icb/icj003. doi:10.1093/icb/icj003 [DOI] [PubMed] [Google Scholar]
- Hargrove J.W. The effect of temperature and saturation deficit on mortality in populations of male Glossina morsitans morsitans (Diptera: Glossinidae) in Zimbabwe and Tanzania. Bull. Entomol. Res. 2001;91:79–86. [PubMed] [Google Scholar]
- Hargrove J.W. Tsetse population dynamics. In: Maudlin I, Holmes P.H, Miles M.A, editors. The trypanosomiases. CABI Publishing; Wallingford, UK: 2004. pp. 113–138. [Google Scholar]
- Hillebrand H. On the generality of the latitudinal diversity gradient. Am. Nat. 2004;163:192–211. doi: 10.1086/381004. doi:10.1086/381004 [DOI] [PubMed] [Google Scholar]
- Hodkinson I.D. Metabolic cold adaptation in arthropods: a smaller-scale perspective. Funct. Ecol. 2003;17:562–567. doi:10.1046/j.1365-2435.2003.07431.x [Google Scholar]
- Hoffmann A.A, Sørensen J.G, Loeschcke V. Adaptation of Drosophila to temperature extremes: bringing together quantitative and molecular approaches. J. Therm. Biol. 2003;28:175–216. doi:10.1016/S0306-4565(02)00057-8 [Google Scholar]
- Hollingsworth M.J, Bowler K. The decline in ability to withstand high temperature with increase in age in Drosophila subobscura. Exp. Gerontol. 1966;1:251–257. doi:10.1016/0531-5565(66)90010-6 [Google Scholar]
- Huey R.B, Bennett A.F. Phylogenetic studies of coadaptation: preferred temperatures versus optimal performance temperatures of lizards. Evolution. 1987;41:1098–1115. doi: 10.1111/j.1558-5646.1987.tb05879.x. doi:10.2307/2409194 [DOI] [PubMed] [Google Scholar]
- Huey R.B, Hertz P.E, Sinervo B. Behavioural drive versus behavioural inertia in evolution: a null model approach. Am. Nat. 2003;161:357–366. doi: 10.1086/346135. doi:10.1086/346135 [DOI] [PubMed] [Google Scholar]
- Janzen D.H. Why mountain passes are higher in the tropics. Am. Nat. 1967;101:233–247. doi:10.1086/282487 [Google Scholar]
- Kay C.A.R, Whitford W.G. Critical thermal limits of desert honey ants: possible ecological implications. Physiol. Zool. 1978;51:206–213. [Google Scholar]
- Kelty J.D, Lee R.E. Induction of rapid cold hardening by cooling at ecologically relevant rates in Drosophila melanogaster. J. Insect Physiol. 1999;45:719–726. doi: 10.1016/s0022-1910(99)00040-2. doi:10.1016/S0022-1910(99)00040-2 [DOI] [PubMed] [Google Scholar]
- Kelty J.D, Lee R.E. Rapid cold-hardening of Drosophila melanogaster (Diptera: Drosophilidae) during ecologically based thermoperiodic cycles. J. Exp. Biol. 2001;204:1659–1666. doi: 10.1242/jeb.204.9.1659. [DOI] [PubMed] [Google Scholar]
- Klok C.J, Chown S.L. Resistance to temperature extremes in sub-Antarctic weevils: interspecific variation, population differentiation and acclimation. Biol. J. Linn. Soc. 2003;78:401–414. doi:10.1046/j.1095-8312.2003.00154.x [Google Scholar]
- Lanciani C, Lipp K.E, Giesel J.T. The effect of photoperiod on cold tolerance in Drosophila melanogaster. J. Therm. Biol. 1992;17:147–148. doi:10.1016/0306-4565(92)90025-B [Google Scholar]
- Lee R.E, Chen C.-P, Denlinger D.L. A rapid cold hardening process in insects. Science. 1987;238:1415–1417. doi: 10.1126/science.238.4832.1415. doi:10.1126/science.238.4832.1415 [DOI] [PubMed] [Google Scholar]
- Lee R.E, Damodaran K, Yi S.-X, Lorigan G.A. Rapid cold-hardening increases membrane fluidity and cold tolerance of insect cells. Cryobiology. 2006;52:459–463. doi: 10.1016/j.cryobiol.2006.03.003. doi:10.1016/j.cryobiol.2006.03.003 [DOI] [PubMed] [Google Scholar]
- Loeschcke V, Hoffmann A.A. Consequences of heat hardening on a field fitness component in Drosophila depend on environmental temperature. Am. Nat. 2007;169:175–183. doi: 10.1086/510632. doi:10.1086/510632 [DOI] [PubMed] [Google Scholar]
- Lutterschmidt W.I, Hutchison V.H. The critical thermal maximum: history and critique. Can. J. Zool. 1997a;75:1561–1574. [Google Scholar]
- Lutterschmidt W.I, Hutchison V.H. The critical thermal maximum: data to support the onset of spasms as the definitive end point. Can. J. Zool. 1997b;75:1553–1560. [Google Scholar]
- McMillan D.M, Fearnley S.L, Rank N.E, Dahlhoff E.P. Natural temperature variation affects larval survival, development and Hsp70 expression in a leaf beetle. Funct. Ecol. 2005;19:844–852. doi:10.1111/j.1365-2435.2005.01031.x [Google Scholar]
- Mora C, Maya M.F. Effect of rate of temperature increase of the dynamic method on the heat tolerance of fishes. J. Therm. Biol. 2006;31:337–341. doi:10.1016/j.jtherbio.2006.01.005 [Google Scholar]
- Nilson T.L, Sinclair B.J, Roberts S.P. The effects of carbon dioxide anesthesia and anoxia on rapid cold-hardening and chill coma recovery in Drosophila melanogaster. J. Insect Physiol. 2006;52:1027–1033. doi: 10.1016/j.jinsphys.2006.07.001. doi:10.1016/j.jinsphys.2006.07.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overgaard J, Sørensen J.G, Petersen S.O, Loeschcke V, Holmstrup M. Reorganization of membrane lipids during fast and slow cold hardening in Drosophila melanogaster. Physiol. Entomol. 2006;31:328–335. doi:10.1111/j.1365-3032.2006.00522.x [Google Scholar]
- Pörtner H.O. Climate change and temperature-dependent biogeography: oxygen limitation of thermal tolerance in animals. Naturwissenschaften. 2001;88:137–146. doi: 10.1007/s001140100216. doi:10.1007/s001140100216 [DOI] [PubMed] [Google Scholar]
- Powell S.J, Bale J.S. Cold shock injury and ecological costs of rapid cold hardening in the grain aphid Sitobion avenae (Hemiptera: Aphidae) J. Insect Physiol. 2004;50:277–284. doi: 10.1016/j.jinsphys.2004.01.003. doi:10.1016/j.jinsphys.2004.01.003 [DOI] [PubMed] [Google Scholar]
- Powell S.J, Bale J.S. Effects of long-term and rapid cold hardening on the cold torpor temperature of an aphid. Physiol. Entomol. 2006;31:348–352. doi:10.1111/j.1365-3032.2006.00527.x [Google Scholar]
- Rako L, Hoffmann A.A. Complexity of the cold acclimation response in Drosophila melanogaster. J. Insect Physiol. 2006;52:94–104. doi: 10.1016/j.jinsphys.2005.09.007. doi:10.1016/j.jinsphys.2005.09.007 [DOI] [PubMed] [Google Scholar]
- Ramløv H. Aspects of natural cold tolerance in ectothermic animals. Hum. Reprod. 2000;15:26–46. doi: 10.1093/humrep/15.suppl_5.26. [DOI] [PubMed] [Google Scholar]
- Rogers D.J, Robinson T.P. Tsetse distribution. In: Maudlin I, Holmes P.H, Miles M.A, editors. The trypanosomiases. CABI Publishing; Wallingford, UK: 2004. pp. 139–180. [Google Scholar]
- Rossolimo T. Temperature adaptations of Siberian Pterostichus species (Coleoptera: Carabidae) Eur. J. Entomol. 1997;94:235–242. [Google Scholar]
- Shermin E, Levitis D. Heat hardening as a function of developmental stage in larval and juvenile Bufo americanus and Xenopus laevis. J. Therm. Biol. 2003;28:373–380. doi:10.1016/S0306-4565(03)00014-7 [Google Scholar]
- Sinclair B.J, Klok C.J, Scott M.B, Terblanche J.S, Chown S.L. Diurnal variation in supercooling points of three species of Collembola from Cape Hallett, Antarctica. J. Insect Physiol. 2003;49:1049–1061. doi: 10.1016/j.jinsphys.2003.08.002. doi:10.1016/j.jinsphys.2003.08.002 [DOI] [PubMed] [Google Scholar]
- Somero G.N. Linking biogeography to physiology: evolutionary and acclimatory adjustments of thermal limits. Front. Zool. 2005;2:1. doi: 10.1186/1742-9994-2-1. doi:10.1186/1742-9994-2-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somero G.N, Dahlhoff E, Lin J.J. Stenotherms and eurytherms: mechanisms establishing thermal optima and tolerance ranges. In: Johnston I.A, Bennett A.F, editors. Animals and temperature. Phenotypic and evolutionary adaptation. Cambridge University Press; Cambridge, UK: 1996. pp. 53–78. [Google Scholar]
- Sømme L. The physiology of cold hardiness in terrestrial arthropods. Eur. J. Entomol. 1999;96:1–10. [Google Scholar]
- Terblanche J.S, Sinclair B.J, Klok C.J, McFarlane M, Chown S.L. The effects of acclimation on thermal tolerance, desiccation resistance and metabolic rate in Chirodica chalcoptera (Coleoptera: Chrysomelidae) J. Insect Physiol. 2005;51:1013–1023. doi: 10.1016/j.jinsphys.2005.04.016. doi:10.1016/j.jinsphys.2005.04.016 [DOI] [PubMed] [Google Scholar]
- Terblanche J.S, Klok C.J, Krafsur E.S, Chown S.L. Phenotypic plasticity and geographic variation in thermal tolerance and water loss of the tsetse Glossina pallidipes (Diptera: Glossinidae): implications for distribution modelling. Am. J. Trop. Med. Hyg. 2006;74:786–794. [PMC free article] [PubMed] [Google Scholar]
- Terblanche J.S, Clusella-Trullas S, Deere J.A, Chown S.L. Thermal tolerance in a south-east African population of the tsetse fly Glossina pallidipes (Diptera: Glossinidae): implications for forcasting climate-change impacts. J. Insect Physiol. 2007 doi: 10.1016/j.jinsphys.2007.08.007. doi:10.1016/j.jinsphys.2007.08.007 [DOI] [PubMed] [Google Scholar]
- Tsuji J.S. Thermal acclimation of metabolism in sceloporus lizards from different latitudes. Physiol. Zool. 1988;61:241–253. [Google Scholar]
- Vannier G. The thermobiological limits of some freezing tolerant insects: the supercooling and thermostupor points. Acta Oecol. 1994;15:31–42. [Google Scholar]
- Worland M.R. Factors that influence freezing in the sub-Antarctic springtail Tullbergia antarctica. J. Insect Physiol. 2005;51:881–894. doi: 10.1016/j.jinsphys.2005.04.004. doi:10.1016/j.jinsphys.2005.04.004 [DOI] [PubMed] [Google Scholar]
Notice of correction
The acknowledgements are now presented in the correct form.
20 September 2007
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material containing a) experimental data showing a lack of gender, feeding and age effects on critical thermal limits, b) climate data for Mfuwe, Zambia and c) body temperatures recorded during a critical thermal limit trial