Abstract
The existence of a specialized imitation module in humans is hotly debated. Studies suggesting a specific imitation impairment in individuals with autism spectrum disorders (ASD) support a modular view. However, the voluntary imitation tasks used in these studies (which require socio-cognitive abilities in addition to imitation for successful performance) cannot support claims of a specific impairment. Accordingly, an automatic imitation paradigm (a ‘cleaner’ measure of imitative ability) was used to assess the imitative ability of 16 adults with ASD and 16 non-autistic matched control participants. Participants performed a prespecified hand action in response to observed hand actions performed either by a human or a robotic hand. On compatible trials the stimulus and response actions matched, while on incompatible trials the two actions did not match. Replicating previous findings, the Control group showed an automatic imitation effect: responses on compatible trials were faster than those on incompatible trials. This effect was greater when responses were made to human than to robotic actions (‘animacy bias’). The ASD group also showed an automatic imitation effect and a larger animacy bias than the Control group. We discuss these findings with reference to the literature on imitation in ASD and theories of imitation.
Keywords: imitation, autism, mirror neuron, mirror system, animacy
1. Introduction
Theories that address imitation fall into two categories: specialist and generalist theories (Brass & Heyes 2005). Specialist theories propose that imitation is mediated by a special-purpose imitation module, whereas generalist theories suggest that it is mediated by task-general learning and motor control mechanisms. Studies of imitation in individuals with autism spectrum disorders (ASD) appear to provide strong support for specialist theories as they have been claimed to demonstrate a specific imitation impairment in the absence of general motor control and learning disability. Such selective impairment is more consistent with a modular view of imitation than with a generalist perspective (Fodor 1983).
ASD are neurodevelopmental disorders with a heritability rate of over 90% (Yang & Gill 2007), which are characterized by abnormalities of social interaction, impairments in verbal and non-verbal communication and a restricted repertoire of interests and activities (American Psychiatric Association 1994). It has been known for some time that children and adults with ASD perform poorly in a variety of imitation tasks (see Williams et al. (2004) for a review). However, it is not clear whether their weak imitative performance is due to specific or non-specific factors. Abnormal performance in imitation tasks could be due to either impairment of the mechanisms that translate observed into executed actions (specific factors) or impairment of mechanisms that are recruited by both the imitative and the non-imitative tasks (non-specific factors).
Most of the imitation tasks used in the studies of ASD make substantial demands on non-specific mechanisms because they assess intentional or ‘voluntary’ imitation. In tests of voluntary imitation, the experimenter asks the participant to copy an action that has many temporal and spatial features, and does not specify exactly which features of the action are to be reproduced. For example, Rogers et al. (2003) instructed participants simply to ‘do this’. Determining the appropriate action dimensions for imitation, and therefore what constitutes successful performance, is accomplished through the interpretation of subtle cues relating to the social context and the experimenter's mental states. The ability to focus on the selected action dimensions, so that performance is not impaired by imitation of task-irrelevant action dimensions, relies on good executive function and attentional control. Interpretation of social cues, theory of mind, executive functions and attentional control have all been shown to be impaired in autism (Russell 1997; Bird et al. 2006; Frith & Frith 2006). Therefore, they are all candidate non-specific mechanisms that could account for poor performance on voluntary imitation tasks.
Neurological evidence in support of the specific factors hypothesis has come from studies suggesting that ASD are characterized by dysfunction of the mirror system (e.g. Dapretto et al. 2006; Williams et al. 2006). The mirror system, comprising bilateral inferior frontal gyrus and parietal cortex, active when actions are both executed and observed, is maximally activated during imitation (Iacoboni et al. 1999). This characteristic makes it plausible that the mirror system translates observed into executed actions, and is consistent with evidence that lesions to the mirror system result in poor performance on imitation tasks (Heilman et al. 1982). Therefore, reports of abnormal mirror system activity in individuals with ASD support the view that their difficulties in imitation tasks are due, at least in part, to specific factors.
However, studies of mirror system function in ASD have yielded inconsistent findings. Avikainen et al. (1999) studied motor cortex excitability using magnetoence-phalography MEG and found no difference in activity between ASD and control participants when observing simple hand movements, suggesting typical mirror system activity in the ASD group. Also, different studies have localized the mirror system deficit in ASD to different neurological areas. Dapretto et al. (2006) found that individuals with ASD show normal activity in the parietal mirror area but reduced activity in the inferior frontal gyrus, whereas Williams et al. (2006) reported the opposite pattern of results, i.e. normal activity in the inferior frontal gyrus and reduced activity in the parietal mirror area. Until the results of studies investigating mirror system activity in ASD show a more consistent pattern, they cannot support strong claims about the specificity of any imitation impairment in this group.
The present study assessed imitation in high-functioning adults with ASD using an automatic imitation procedure. We chose an automatic, rather than a voluntary, imitation test in order to minimize the demands that it would make on non-specific mechanisms. In tests of automatic imitation, participants are not asked, and do not intend, to imitate modelled movements. Instead, they are required merely to observe actions, either passively or with a simple movement task, while the experimenter measures involuntary muscular responses (passive observation tasks) or involuntary differences in speed to execute prespecified actions (simple movement tasks).
As far as we are aware, only one previous study has tried to investigate automatic imitation in ASD. McIntosh et al. (2006) used electromyography (EMG) to measure muscular activity in the face while participants were presented with emotional facial expressions. Compared with controls, individuals with ASD showed less expression-compatible muscular activation. However, this study did not distinguish automatic imitation from emotional contagion. It is not clear whether, in the controls, observation of a smiling face promoted smiling directly, or by inducing positive affect. The results are also difficult to interpret because face stimuli were presented, and there is a growing body of evidence that gaze patterns to faces are abnormal in autism (Klin et al. 2002). Specifically, individuals with ASD spend less time looking at the eye region of the face, which has been shown to be crucial in emotion recognition (Spezio et al. 2007).
To overcome these problems, we used affectively neutral hand movements in our automatic imitation task. Participants were required to perform a prespecified hand movement (opening or closing) as soon as they saw a hand stimulus begin to move. The movement of the hand stimulus was either the same as the prespecified response (compatible trials), or the opposite of the prespecified response (incompatible trials). Thus, although voluntary actions were performed, any effect of imitation on these actions is automatic in the sense that (i) participants are neither instructed nor intend to imitate, and (ii) in half of the trials (incompatible trials) imitation causes poor task performance.
Previous studies using this paradigm have found two effects. First, a basic automatic imitation effect: responding is faster on compatible than on incompatible trials (Heyes et al. 2005). Second, an ‘animacy effect’: the automatic imitation effect is greater when the observed action is performed by a human effector than when it is performed by a human-like mechanical device, or ‘robot’ (Kilner et al. 2003; Press et al. 2005). It has been argued that the latter effect is a direct consequence of increased mirror system activity in response to observation of human, compared with robotic, action (Tai et al. 2004). Thus, this study sought to investigate automatic imitation and the animacy effect in both a group of high-functioning adults with ASD and typically developing matched controls.
2. Material and methods
(a) Participants
Thirty-two individuals participated in the study: 16 participants with ASD (15 males and 1 female) and 16 typically developing control participants (15 males and 1 female). Groups were matched on sex, age (ASD mean: 34.9 years, s.d.: 13.2; control mean: 33.2 years, s.d.: 11.4) and IQ (ASD mean: 110.3, s.d.: 15.2; control mean: 112.6, s.d.: 13.0). Full-scale IQ was measured using the Wechsler Adult Intelligence Scale—3rd UK Edition (Wechsler 1999). All participants in the ASD group had previously received a diagnosis from an independent clinician according to standard criteria. The Autism Diagnostic Observational Schedule-G (Lord et al. 2000) was used in order to characterize the participants. On this measure, nine participants met criteria for autism, while seven participants met criteria for ASD. All participants were right handed, had normal or corrected-to-normal vision and were naive with respect to the purpose of the experiment. The experiment was performed with local ethical committee approval and in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki.
(b) Stimuli
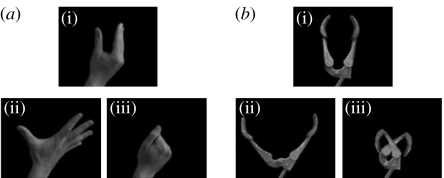
All stimuli were presented on a screen in colour on a black background (figure 1). Each imperative stimulus was a photograph of a human or a robotic hand in an opened or a closed posture. It was preceded by a warning stimulus representing a neutral posture of the same hand type (human or robotic). The transition from the warning to the imperative stimulus induced apparent motion so that the hand appeared to start in the neutral position and then to open or to close.
Figure 1.
(i) Warning stimuli and (ii, iii) opening and closing stimuli for the (a) human and (b) robot stimulus types.
(c) Data recording and analysis
For both open and close responses, response onset was measured by recording the electromyogram (EMG) from the first dorsal interosseous muscle. Details of the signal preprocessing procedure are given in Press et al. (2005). To define a baseline, EMG activity was registered for 100 ms when the participant was not moving at the beginning of each trial. A window of 20 ms was then shifted progressively over the raw data in 1 ms steps. Response onset was defined by the beginning of the first 20 ms window after the imperative stimulus in which the standard deviation for that window, and for the following 20 ms epoch, was greater than 2.75 times the standard deviation of the baseline. Whether the criterion correctly defined movement onset was verified by sight for every trial performed by each participant by an experimenter who was blind to the trial type. Stimulus onset marked the beginning, and EMG onset marked the end, of the response time (RT) interval.
(d) Procedure
The participant's right forearm lay in a horizontal position. It was supported by an armrest which allowed the hand to move. The fingers moved upwards during opening responses and downwards when closing. Stimulus postures were presented in the lateral plane (left–right), and therefore response movements were orthogonal to stimulus postures. This feature of the design allows automatic imitation to be isolated from spatial compatibility. After each response, participants returned their hand to a neutral starting position. In each block, participants were instructed to make a prespecified response (open or close) as soon as possible after the movement stimulus appeared. Participants were instructed to refrain from moving their hand in catch trials, when the imperative stimulus was not presented. Participants were not given feedback concerning the accuracy of their responses.
All trials began with presentation of the warning stimulus. In stimulus trials, this was replaced 800–1500 ms later by the movement stimulus, which was of 480 ms duration. The stimulus onset asynchrony varied randomly between 800 and 1500 ms. After the movement stimulus, a blank screen was presented (3000 ms) before the next trial. In catch trials, the warning stimulus remained on the screen for 1980 ms. Each block presented, in random order, 15 trials in which the hand opened, 15 trials in which the hand closed and 6 catch trials. Thus, in each block, there were 15 trials in which the response and stimulus movements matched (‘compatible trials’) and 15 in which the stimulus and response movements did not match (‘incompatible trials’).
Human and robotic stimuli were presented in separate blocks. Details of stimulus control can be found in Press et al. (2005). Participants therefore completed four blocks in total, two in which closing was the required response and two in which opening was the required response. Response order (open or close first) and stimulus type (human or robotic) were balanced across participants and within groups. Before each block, participants completed five practice trials with the response, and the stimuli, to be used in that block.
3. Results
Incorrect responses (e.g. hand opening when closing was required, 0.05%) were excluded from the analysis, as were all RTs smaller than 100 ms and greater than 1000 ms (0.05%). On each trial, the stimulus movement was either the same as (compatible) or different from (incompatible) the prespecified response. RT data are shown in figure 2.
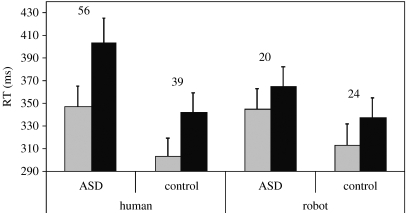
Figure 2.
Mean RT on compatible (grey bars) and incompatible (black bars) trials in response to human and robotic stimuli in both the ASD and Control groups. Error bars indicate s.e.m. Numbers refer to the difference in RT between compatible and incompatible trials (‘automatic imitation effect’) for each condition and group.
RT data were analysed using ANOVA with within-subjects factors of trial type (compatible and incompatible), stimulus type (human and robotic) and a between-subjects factor of group (ASD and Control). This analysis revealed a significant main effect of trial type (F(1,30)=79.0, p<0.001, ) due to faster responses on compatible trials than incompatible trials. The interaction between trial type and stimulus type was also significant (‘animacy bias’, F(1,30)=29.6, p<0.001, ); the compatibility effect was greater when responding to a human compared with a robot stimulus. The three-way interaction between group, trial type and stimulus type was also significant (F(1,30)=4.6, p=0.04, ), indicating that the difference between the human and robotic compatibility effect was larger in the ASD group than in the Control group. No other main effects or interactions were significant (all p-values greater than or equal to 0.10); there was no main effect of group (F(1,30)=2.9, p=0.10, ), or any two-way interaction between group and trial type (F(1,30)<1, p=0.4, ). Furthermore, when mean RT was entered as a covariate into the analysis, there was still no two-way interaction between group and trial type (F(1,30)<1, p=0.6, ), indicating that the trend towards a main effect of group did not mask any group by trial type interaction.
Simple effects analysis was used to examine further the three-way interaction between group, trial type and stimulus type. This revealed that both the compatibility effect (Control: F(1,15)=57.9, p<0.001, ; ASD: F(1,15)=32.9, p<0.001, ) and the animacy bias (Control: F(1,15)=5.6, p=0.03, ; ASD: F(1,15)=27.8, p<0.001, ) were significant in each group. The ASD group exhibited a trend towards a greater compatibility effect in response to observed human actions (F(1,30)=3.0, p=0.09, ) than the Control group. The groups did not differ in the magnitude of their compatibility effect in response to observed robotic actions (F(1,30)<1, p=0.69, ).
4. Discussion
This study tested automatic imitation of affectively neutral hand actions in ASD. In comparison with matched, typically developing controls, the ASD group showed an equivalent automatic imitation effect, and signs of an increased animacy bias, namely, a greater difference in automatic imitation of human and robot actions.
The principal finding of the present study was that individuals with ASD did not show an impairment of automatic imitation of affectively neutral hand actions. This finding contrasts with reports of an imitation impairment in this group (Williams et al. 2004), but it is not wholly anomalous with respect to studies of imitation in ASD. Several studies have found imitative performance to be unimpaired (e.g. Carpenter et al. 2001; Hamilton et al. 2007; see also Sebanz et al. 2005) and, as noted in §1, performance in tests of voluntary imitation is vulnerable to the effects of non-specific factors such as theory of mind and executive function impairments. Therefore, whether or not a particular voluntary imitation task presents a challenge to individuals with ASD may depend upon the interaction between two factors: the extent to which the task requires non-specific abilities and the degree to which these abilities are impaired in the particular sample of individuals recruited for the study.
Given the conflicted findings in the literature, it is necessary to determine that the equivalent performance shown by the ASD and Control groups is not an artefactual ‘null result’ due to insufficient statistical power. Several factors suggest that this is not the case. First, the ASD group was significantly faster to make a prespecified hand movement when it was imitative, than when it was non-imitative, and thus demonstrated automatic imitation. Second, the ASD group showed a greater degree of automatic imitation in response to human than robotic actions, and thus showed the animacy bias typically seen in these experiments. Third, the ASD group showed a significantly greater animacy bias than the Control group, i.e. the extent to which human actions were imitated more than robotic actions was significantly greater in the ASD group than the Control group.
The increased animacy bias in the ASD group was largely due to enhanced automatic imitation of human actions (although this finding should be interpreted with caution as the simple interaction between the group and trial type factors with human stimuli only approached statistical significance). This is a surprising finding, and any explanation is therefore speculative. However, it is consistent with recent evidence that there is a link between theory of mind and the ability to inhibit automatic imitation. It has been shown that imitation inhibition and theory of mind depend on similar neural substrates (Brass et al. 2005), and a positive correlation between the ability to inhibit imitation and performance on theory of mind tasks has been found in patients with both frontal and posterior brain lesions (Brass et al. 2003). The authors of these studies argue that distinguishing the self from others, which relies on the theory of mind system, is a crucial component of imitation inhibition. Theories of mind deficits are well documented in ASD (for a review see Frith & Frith 2003). Therefore, this hypothesis suggests that the ASD group showed a greater compatibility effect because they had problems inhibiting imitation of human actions. Such a suggestion is consonant with two clinical features of autism which indicate problems with imitation inhibition: echolalia (involuntary imitation of the speech patterns of others) and echopraxia (involuntary imitation of observed actions; Russell 1997).
These results undermine an important strand of evidence in favour of specialist theories of imitation which posit that imitation is mediated by a special-purpose imitation module. Previous studies purporting to demonstrate a specific imitation impairment in ASD were consistent with this view, but hard to reconcile with generalist theories which claim that imitation is mediated by task-general mechanisms of learning and motor control. The present results, using a test of imitation which requires fewer imitation-non-specific mechanisms than those used in previous studies, find no evidence for an imitation impairment in autism. If true, then the imitative abilities of individuals with autism provide no firm support for either specialist or generalist theories of imitation.
In summary, the specificity of reported imitation impairments in ASD was investigated using an automatic imitation task. Rather than an impairment, participants with ASD showed typical automatic imitation of robotic actions and equivalent, if not greater, automatic imitation of human actions. This suggests that previous findings of poor performance on tests of imitation may have been due to impairment of non-specific mechanisms, such as those mediating theory of mind and executive functions, which are recruited by both the imitative and the non-imitative tasks. Our findings imply that the core mechanisms of imitation, those that translate observed into executed actions, are intact in individuals with ASD.
Acknowledgments
The experiment was performed with local ethical committee approval and in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki.
We are grateful to Uta Frith for practical support and helpful discussion at all stages of the study, and to Tony Charman for comments on an earlier version of the manuscript. G.B. was supported by the Economic and Social Research Council and by a project grant (no. G9617036) awarded by the Medical Research Council to Prof. Uta Frith. C.H. was supported by the Economic and Social Research Council and the European Community's Sixth Framework Programme under contract no. NEST 012929. J.L. and C.P. were supported by research studentships from the Medical Research Council and the Biotechnology and Biological Sciences Research Council, respectively.
References
- American Psychiatric Association. American Psychiatric Association; Washington, DC: 1994. Diagnostic and statistical manual of mental disorders. [Google Scholar]
- Avikainen S, Kulomaki T, Hari R. Normal movement reading in Asperger subjects. Neuroreport. 1999;10:3467–3470. doi: 10.1097/00001756-199911260-00001. doi:10.1097/00001756-199911260-00001 [DOI] [PubMed] [Google Scholar]
- Bird G, Catmur C, Silani G, Frith C, Frith U. Attention does not modulate neural responses to social stimuli in autism spectrum disorders. Neuroimage. 2006;31:1614–1624. doi: 10.1016/j.neuroimage.2006.02.037. doi:10.1016/j.neuroimage.2006.02.037 [DOI] [PubMed] [Google Scholar]
- Brass M, Heyes C.M. Imitation: is cognitive neuroscience solving the correspondence problem? Trends Cogn. Sci. 2005;9:489–495. doi: 10.1016/j.tics.2005.08.007. doi:10.1016/j.tics.2005.08.007 [DOI] [PubMed] [Google Scholar]
- Brass M, Derrfuss J, Matthes-von Cramon G, von Cramon D.Y. Imitative response tendencies in patients with frontal brain lesions. Neuropsychology. 2003;17:265–271. doi: 10.1037/0894-4105.17.2.265. doi:10.1037/0894-4105.17.2.265 [DOI] [PubMed] [Google Scholar]
- Brass M, Derrfuss J, von Cramon D.Y. The inhibition of imitative and overlearned responses: a functional double dissociation. Neuropsychologia. 2005;43:89–98. doi: 10.1016/j.neuropsychologia.2004.06.018. doi:10.1016/j.neuropsychologia.2004.06.018 [DOI] [PubMed] [Google Scholar]
- Carpenter M, Pennington B.F, Rogers S.J. Understanding of others' intentions in children with autism. J. Autism Dev. Disord. 2001;31:589–599. doi: 10.1023/a:1013251112392. doi:10.1023/A:1013251112392 [DOI] [PubMed] [Google Scholar]
- Dapretto M, Davies M.S, Pfeifer J.H, Scott A.A, Sigman M, Bookheimer S.Y, Iacoboni M. Understanding emotions in others: mirror neuron dysfunction in children with autism spectrum disorders. Nat. Neurosci. 2006;9:28–30. doi: 10.1038/nn1611. doi:10.1038/nn1611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fodor J.A. MIT Press; Cambridge, MA: 1983. The modularity of mind. [Google Scholar]
- Frith C.D, Frith U. The neural basis of mentalizing. Neuron. 2006;50:531–534. doi: 10.1016/j.neuron.2006.05.001. doi:10.1016/j.neuron.2006.05.001 [DOI] [PubMed] [Google Scholar]
- Frith U, Frith C.D. Development and neurophysiology of mentalizing. Phil. Trans. R. Soc. B. 2003;358:459–473. doi: 10.1098/rstb.2002.1218. doi:10.1098/rstb.2002.1218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton A.F, Brindley R.M, Frith U. Imitation and action understanding in autistic spectrum disorders: how valid is the hypothesis of a deficit in the mirror neuron system? Neuropsychologia. 2007;45:1859–1868. doi: 10.1016/j.neuropsychologia.2006.11.022. doi:10.1016/j.neuropsychologia.2006.11.022 [DOI] [PubMed] [Google Scholar]
- Heilman K.M, Rothi L.J, Valenstein E. Two forms of ideomotor apraxia. Neurology. 1982;32:342–346. doi: 10.1212/wnl.32.4.342. [DOI] [PubMed] [Google Scholar]
- Heyes C.M, Bird G, Johnson H, Haggard P. Experience modulates automatic imitation. Brain Res. Cogn. Brain Res. 2005;22:233–240. doi: 10.1016/j.cogbrainres.2004.09.009. doi:10.1016/j.cogbrainres.2004.09.009 [DOI] [PubMed] [Google Scholar]
- Iacoboni M, Woods R.P, Brass M, Bekkering H, Mazziotta J.C, Rizzolatti G. Cortical mechanisms of human imitation. Science. 1999;286:2526–2528. doi: 10.1126/science.286.5449.2526. doi:10.1126/science.286.5449.2526 [DOI] [PubMed] [Google Scholar]
- Kilner J.M, Paulignan Y, Blakemore S.J. An interference effect of observed biological movement on action. Curr. Biol. 2003;13:522–525. doi: 10.1016/s0960-9822(03)00165-9. doi:10.1016/S0960-9822(03)00165-9 [DOI] [PubMed] [Google Scholar]
- Klin A, Jones W, Schultz R, Volkmar F, Cohen D. Visual fixation patterns during viewing of naturalistic social situations as predictors of social competence in individuals with autism. Arch. Gen. Psychiatry. 2002;59:809–816. doi: 10.1001/archpsyc.59.9.809. doi:10.1001/archpsyc.59.9.809 [DOI] [PubMed] [Google Scholar]
- Lord C, Risi S, Lambrecht L, Cook E.J, Levanthal B, DiLavore P.C, Pickles A, Rutter M. The autism diagnostic observation schedule-generic: a standard measure of social and communication deficits associated with the spectrum of autism. J. Autism Dev. Disord. 2000;30:205–223. doi:10.1023/A:1005592401947 [PubMed] [Google Scholar]
- McIntosh D.N, Reichmann-Decker A, Winkielman P, Wilbarger J.L. When the social mirror breaks: deficits in automatic, but not voluntary, mimicry of emotional facial expressions in autism. Dev. Sci. 2006;9:295–302. doi: 10.1111/j.1467-7687.2006.00492.x. doi:10.1111/j.1467-7687.2006.00492.x [DOI] [PubMed] [Google Scholar]
- Press C, Bird G, Flach R, Heyes C. Robotic movement elicits automatic imitation. Brain Res. Cogn. Brain Res. 2005;25:632–640. doi: 10.1016/j.cogbrainres.2005.08.020. doi:10.1016/j.cogbrainres.2005.08.020 [DOI] [PubMed] [Google Scholar]
- Rogers S.J, Hepburn S.L, Stackhouse T, Wehner E. Imitation performance in toddlers with autism and those with other developmental disorders. J. Child Psychol. Psychiatry. 2003;44:763–781. doi: 10.1111/1469-7610.00162. doi:10.1111/1469-7610.00162 [DOI] [PubMed] [Google Scholar]
- Russell J. Oxford University Press; New York, NY: 1997. Autism as an executive disorder. [Google Scholar]
- Sebanz N, Knoblich G, Stumpf L, Prinz W. Far from action-blind: representation of others' actions in individuals with autism. Cogn. Neuropsychol. 2005;22:433–454. doi: 10.1080/02643290442000121. doi:10.1080/02643290442000121 [DOI] [PubMed] [Google Scholar]
- Spezio M.L, Adolphs R, Hurley R.S, Piven J. Abnormal use of facial information in high-functioning autism. J. Autism Dev. Disord. 2007;37:929–939. doi: 10.1007/s10803-006-0232-9. doi:10.1007/s10803-006-0232-9 [DOI] [PubMed] [Google Scholar]
- Tai Y.F, Scherfler C, Brooks D.J, Sawamoto N, Castiello U. The human premotor cortex is ‘mirror’ only for biological actions. Curr. Biol. 2004;14:117–120. doi: 10.1016/j.cub.2004.01.005. doi:10.1016/j.cub.2004.01.005 [DOI] [PubMed] [Google Scholar]
- Wechsler D. 3rd edn. Harcourt Assessment; London, UK: 1999. Wechsler adult intelligence scale. [Google Scholar]
- Williams J.H, Whiten A, Singh T. A systematic review of action imitation in autistic spectrum disorder. J. Autism Dev. Disord. 2004;34:285–299. doi: 10.1023/b:jadd.0000029551.56735.3a. doi:10.1023/B:JADD.0000029551.56735.3a [DOI] [PubMed] [Google Scholar]
- Williams J.H, Waiter G.D, Gilchrist A, Perrett D.I, Murray A.D, Whiten A. Neural mechanisms of imitation and ‘mirror neuron’ functioning in autistic spectrum disorder. Neuropsychologia. 2006;44:610–621. doi: 10.1016/j.neuropsychologia.2005.06.010. doi:10.1016/j.neuropsychologia.2005.06.010 [DOI] [PubMed] [Google Scholar]
- Yang M.S, Gill M. A review of gene linkage, association and expression studies in autism and an assessment of convergent evidence. Int. J. Dev. Neurosci. 2007;25:69–85. doi: 10.1016/j.ijdevneu.2006.12.002. doi:10.1016/j.idevneu.2006.12.002 [DOI] [PubMed] [Google Scholar]