Abstract
Many groups of organisms show greater species richness in the tropics than in the temperate zone, particularly in tropical montane regions. Forty years ago, Janzen suggested that more limited temperature seasonality in the tropics leads to greater climatic zonation and more climatic barriers to organismal dispersal along elevational gradients in the tropics relative to temperate regions. These factors could lead to differences in how species arise in tropical versus temperate regions and possibly contribute to greater tropical diversity. However, no studies have compared the relationships among climate, elevational distribution and speciation in a group inhabiting both tropical and temperate regions. Here, we compare elevational and climatic divergence among 30 sister-species pairs (14 tropical, 16 temperate) within a single family of salamanders (Plethodontidae) that reaches its greatest species richness in montane Mesoamerica. In support of Janzen's hypothesis, we find that sister species are more elevationally and climatically divergent in the tropics than in the temperate zone. This pattern seemingly reflects regional variation in the role of climate in speciation, with niche conservatism predominating in the temperate zone and niche divergence in the tropics. Our study demonstrates how latitudinal differences in elevational climatic zonation may increase opportunities for geographical isolation, speciation and the associated build-up of species diversity in the tropics relative to the temperate zone.
Keywords: elevation, climate, latitude, niche evolution, speciation, species richness
1. Introduction
Forty years ago, Janzen published a paper on the patterns of climatic variation across latitudes, with far-reaching implications for studies of evolution, ecology and conservation. Janzen (1967) showed that limited seasonal temperature variation in the tropics creates greater climatic stratification across elevational gradients (figure 1). He suggested that this stratification could lead to natural selection for organisms with narrower climatic and physiological tolerances in the tropics than in the temperate zone. A tendency for greater climatic zonation on tropical mountain slopes might lead to latitudinal variation in speciation mechanisms and contribute to high species richness in tropical regions. For example, if the limited temperature seasonality at sites in the tropics selects for narrow climatic and physiological tolerances, then there should be less overlap in the climatic tolerances of organisms at different elevations in the tropics, more climatic barriers to dispersal along tropical mountain slopes and thus more opportunities for climate-driven geographical isolation, divergence and speciation (Ghalambor et al. 2006).
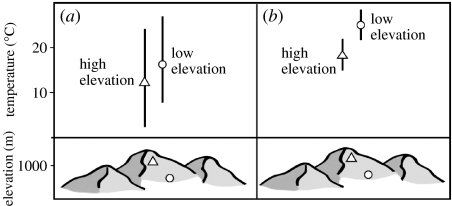
Figure 1.
Latitudinal and elevational patterns of temperature variation and overlap as predicted by Janzen's (1967) hypothesis. The annual range of ambient temperatures experienced by low and high elevation sites is depicted for (a) temperate and (b) tropical montane regions. Greater temperature seasonality in temperate regions results in high overlap in the temperatures experienced by organisms at low and high elevation sites. In contrast, sites separated by the same elevations in the tropics show much less overlap owing to the lack of temperature seasonality. Janzen hypothesized that these climatic differences lead to the evolution of organisms with broadly overlapping thermal and physiological tolerances at low and high elevations in temperate mountains, resulting in greater opportunity for dispersal between elevationally separated sites in the temperate zone compared with the tropics. Conversely, in the tropics, it is more difficult for organisms to disperse between different elevations, leading to greater opportunities for climatically driven speciation along tropical mountain slopes.
Since Janzen's climatic zonation hypothesis was first proposed, numerous studies have documented latitudinal variation in species' geographical distributions and climatic tolerance ranges that are consistent with the idea that climate might serve as a more effective barrier to dispersal in tropical montane regions. For example, the faunal overlap of elevationally separated sites is greater in temperate than tropical mountains (Heyer 1967; Wake & Lynch 1976; Huey 1978). Furthermore, a variety of studies have documented that elevational ranges of species (Heyer 1967; Wake & Lynch 1976; Terborgh 1977; Lieberman et al. 1996; Rahbek & Graves 2001; Navas 2003) and the thermal tolerances of species (Snyder & Weathers 1975; van Berkum 1988; Addo-Bediako et al. 2000) become greater with increasing latitude. However, these studies did not address whether latitudinal variation in the climatic tolerances of species might actually be associated with processes that drive the formation of new evolutionary lineages (i.e. geographical isolation and speciation). Understanding this potential link between climate and speciation requires comparisons of the climatic regimes occupied by sister species in both tropical and temperate montane regions.
Climatic zonation in montane regions may drive speciation through at least two mechanisms. First, montane habitats separated by intervening lowland areas (or lowland habitats separated by mountains) may serve as refugia where populations become geographically isolated from each other as they track suitable habitats to higher or lower elevations in response to climate change (for example, two mesic-adapted species separated by warmer, drier lowland environments, or two xeric-adapted species separated by cool, moist mountain ranges; Wiens 2004; Kozak & Wiens 2006). Alternatively, new species may originate as populations adapt to different climates in distinct elevational zones (Patton & Smith 1992; Bates & Zink 1994; Hall 2005). Referred to as the refuge and gradient models of speciation (Moritz et al. 2000), these models predict contrasting roles for natural selection in speciation. During refuge speciation, niche conservatism and the inability of populations to adapt to new environmental conditions play the primary role in geographical isolation (Wiens 2004; Kozak & Wiens 2006). In contrast, during gradient speciation, climatic niche divergence and adaptation to the different environmental conditions in distinct elevational zones drive population isolation (Moritz et al. 2000). Under the gradient model, gene flow between incipient species at different elevations will be limited by the combination of their different climatic distributions and narrow climatic tolerances, regardless of whether these species are allopatric or parapatric (Moritz et al. 2000).
These alternative models of montane speciation may potentially be distinguished by integrating data on the phylogenetic history, elevational ranges and climatic regimes of species. Under the refuge/niche conservatism model, sister species are expected to occupy similar elevational distributions and climatic regimes that correspond to current and/or historical refugia (Patton & Smith 1992; Moritz et al. 2000; Wiens 2004; Kozak & Wiens 2006). In contrast, the gradient model predicts that sister species should occupy divergent climatic regimes and have elevationally non-overlapping geographical distributions (Patton & Smith 1992; Moritz et al. 2000).
The relative frequencies by which species originate through niche conservatism and gradient speciation in tropical and temperate mountains might be important for understanding the disparity in species diversity between these regions. For example, populations of a tropical species occurring at different elevations should experience less overlap in climate and may have a greater propensity for gradient speciation than populations of a temperate species occurring over the same range of elevations. These latitudinal differences in elevational climatic zonation might lead to greater opportunities for speciation along tropical elevational gradients compared with temperate ones. Many studies in tropical systems have found evidence suggesting that speciation involved divergence between populations inhabiting distinct elevational and climatic zones (Moritz et al. 2000; Ogden & Thorpe 2002; Graham et al. 2004; Hall 2005; but see Peterson et al. 1999). In contrast, recent studies from the temperate zone suggest that niche conservatism, the tendency for species to retain similar ecological characteristic over evolutionary time scales (see review in Wiens & Graham 2005), plays an important role in geographical isolation and speciation in temperate montane regions (Knowles 2001; Carstens et al. 2005; Kozak & Wiens 2006). However, understanding whether there are latitudinal differences in the relationship between climatic variation and speciation has been hindered by a lack of comparative analyses across a single clade that inhabits both tropical and temperate regions.
Here, we investigate the relationships among elevation, climate and speciation in plethodontid salamanders from tropical Mesoamerica (subfamily Bolitoglossinae) and temperate eastern North America (subfamily Plethodontinae, genera Desmognathus and Plethodon). Plethodontid salamanders are well suited for comparative analyses of the relationship between speciation and climate in tropical and temperate montane regions. Plethodontid species diversity peaks in the temperate Appalachian Mountains of eastern North America (Duellman & Sweet 1999) and the tropical Mesoamerican Highlands, with regional species richness being greatest in the Mesoamerican Highlands (Wake & Lynch 1976; Amphibia Web 2007; Wiens et al. 2007). Phylogenetic relationships among species are sufficiently well known so that extant sister species can be reasonably hypothesized (Kozak et al. 2005, 2006; Wiens et al. 2006a, 2007). Finally, the combination of georeferenced specimen localities and geographical information system (GIS)-based climatic data (Hijmans et al. 2005) permits quantification of climatic variation throughout the geographical ranges of these species.
In this study, we first revisit Janzen's hypothesis that tropical species have narrower climatic regimes than temperate species. Specifically, we use GIS climate maps and georeferenced museum specimens to estimate the range of temperatures experienced by select tropical and temperate plethodontid species throughout their known geographical ranges. We use published phylogenetic estimates to identify 14 tropical and 16 temperate sister-species pairs. We then calculate the degree of elevational and climatic overlap between each sister-species pair. Next, we examine whether tropical sister-species pairs show lesser elevational and climatic overlap than temperate sister-species pairs, as predicted by the climatic zonation hypothesis. Finally, we examine whether there are differences between tropical and temperate regions in the relative frequency of speciation through niche conservatism versus niche divergence.
2. Material and methods
(a) Identification of sister species and their divergence times
We used published molecular phylogenetic analyses and divergence time estimates to identify 30 sister-species pairs (14 tropical and 16 temperate) and their ages. Phylogenies for Desmognathus and Plethodon are nearly complete in terms of described species within these genera (Kozak et al. 2005, 2006), suggesting that we have identified actual, extant sister species in these clades. Although the phylogenies used were based only on mitochondrial DNA (mtDNA) data, analyses of these genera incorporating nuclear data give similar relationships and divergence times (Wiens et al. 2006a; K.H. Kozak & J.J. Wiens 2007, unpublished data). Three of the pairs that we used represent lineages that are distinct and divergent but are still awaiting formal description as separate species (Crespi et al. 2003; Weisrock & Larson 2006).
To select bolitoglossine sister species, we used the most complete phylogeny assembled to date (Wiens et al. 2007), which is based on mtDNA data for 133 of the 228 known species and is generally concordant with morphology-based taxonomy. Thus, due to incomplete taxon sampling, it is not possible to identify extant sister-species pairs from all described genera or species groups of bolitoglossines. Accordingly, we chose our sister-species pairs from genera and species groups for which all described species (or almost all) were included in the phylogeny. When possible, we verified the status of putative sister species by considering additional studies that examined geographical patterns of intra- and interspecific variation in mtDNA and/or nuclear-encoded allozymes (see electronic supplementary material). The sister species we selected are spread across the bolitoglossine tree (i.e. Bolitoglossa, Ixalotriton, Oedipina, Pseudoeurycea) and are collectively distributed from sea level to higher than 3500 m. Furthermore, they cover nearly the entire latitudinal extent of Mesoamerica. Therefore, it seems unlikely that we have introduced any systematic biases into our analyses which could arise from focusing on a geographically, ecologically or phylogenetically non-random subset of bolitoglossine taxa. A list of all sister species included in our analyses is provided in the electronic supplementary material. For both bolitoglossines and plethodontines, divergence times were estimated in the original studies using penalized likelihood analysis (Sanderson 2002), a molecular clock method that incorporates fossil calibration points and does not require constant rates of molecular evolution.
(b) Elevational and climatic data
To quantify the elevational ranges and climatic distributions of species, we mapped 3602 georeferenced specimen localities (see electronic supplementary material for sample sizes per species) from museum collections and the literature using Diva-GIS v. 5.2 (http://diva-gis.org). Desmognathus locality data were gathered from published systematic studies in which specimen collection localities were georeferenced by the original collectors. Georeferenced localities for Plethodon were obtained from the US National Museum of Natural History; the collecting sites and taxonomic identities of these specimens were verified by the original collector, R. Highton. Bolitoglossine locality data were obtained from the Museum of Vertebrate Zoology's online database, which have been georeferenced based on geographical descriptions of locations provided by the original collectors. In theory, some collection sites with unique coordinates could represent different verbal descriptions of the same general area. However, this redundancy should not bias our results because we are interested in the elevational and climatic overlap of sister species and because geographically proximate sites tend to have identical or very similar climates.
For each locality, we extracted elevation and temperature data (see below for specific variables) from the WORLDCLIM database with 1 km2 spatial resolution (Hijmans et al. 2005). Each species' elevational range was calculated as the difference between its observed minimum and maximum elevations. The degree of overlap between sister species' elevational ranges was then calculated by dividing the amount of elevational overlap for those species by the elevational range of the species with the smaller elevational range. This index ranges from 0.0 to 1.0. An index of 0.0 describes allopatric or parapatric species with completely non-overlapping elevational ranges. In contrast, because none of the sister species we included are sympatric, an index of 1.0 describes allopatric sister species distributed over identical elevational ranges (i.e. they are distributed over the same elevations, but found on different mountaintops).
Field body temperature ranges of plethodontid salamanders closely parallel ambient variation in temperature (Feder & Lynch 1982; Feder et al. 1982; see also electronic supplementary material). We therefore used GIS maps of ambient temperature variation as a proxy for the thermal environment individuals experience across the species' geographical range. To estimate the width of a species ambient temperature regime, we first extracted the maximum temperature of the warmest month (Bio5) and the minimum temperature of the coldest month (Bio6) for each of its collection sites. We then calculated the width of a species' temperature regime as the difference between its maximum observed value of Bio5 and minimum observed value of Bio6. We note that Worldclim does not account for temperature variations associated with aspect, which may be important determinants of an organism's elevational limits on north- versus south-facing slopes (Porter et al. 2002). However, not accounting for climatic variation associated with aspect should have negligible impact on our results since we are interested in large-scale patterns of temperature variation across species' entire geographical ranges.
To calculate the temperature overlap of sister species, we first estimated the ambient temperature range each species experiences each month by calculating the mean minimum (mean min.) and maximum (mean max.) across all of its collection sites. We then the calculated temperature overlap for each sister-species pair using the following formula:
where RAi and RBi are the ambient temperature ranges experienced by species A and B, for month i, respectively, and oi is the overlap of RA and RB (in degree Celsius) for month i. Thus, for each month, overlap ranges between 0 and 1, with the total annual temperature overlap ranging between 0 and 12.
The hypothesis that niche conservatism causes populations to become isolated as they track suitable climatic conditions into separate refugia predicts that regions occupied by allopatric sister taxa will be climatically very similar to each other, but not to regions separating their geographical distributions (Kozak & Wiens 2006). To test this hypothesis for allopatric sister-species pairs (seven tropical; eight temperate), we calculated separately (i) the overlap between the temperature regimes of sister species and (ii) the overlap of the temperature regimes of each species and their corresponding absence locations between their geographical ranges (locations where closely related species have been collected, but not the species in question). Thus, there was a total of 15 temperature overlaps measured between sister taxa, and a total of 30 between sister taxa and their absence locations (see electronic supplementary material for a figure illustrating the procedure used to calculate the temperate overlap of sister species and their corresponding absence locations). Owing to a scarcity of absence locations separating the ranges of allopatric species pairs in the tropics, we compared the climatic overlap between each species to pseudoabsence locations between their geographical ranges. To generate pseudoabsence locations, we enclosed the known locations of each species in a minimum convex polygon in ArcGIS v. 8.3 (ESRI, Redland, CA). We then randomly sampled points in the geographical space in between their polygons. The number of pseudoabsence locations generated for each sister-species comparison was set to be equal to the total number of unique sampling locations for that species pair (e.g. if species A and B are known from 10 and 15 locations, respectively, 25 pseudoabsence locations would be sampled in between their geographical ranges).
We also quantified regional variation in the width and overlap of sister species' precipitation regimes. However, we found no evidence for differences in the degree of precipitation overlap for tropical and temperate sister species (results not shown). Given these non-significant results, and that Janzen's climatic hypothesis specifically emphasized temperature zonation along elevation gradients, we focus exclusively on the regional patterns of temperature overlap experienced by sister species.
(c) Statistical analyses
Janzen's climatic zonation hypothesis predicts that tropical species should experience a narrower range of temperatures across their geographical ranges than temperate species. As a corollary, it also suggests that tropical species might have narrower elevational distributions than temperate species (Ghalambor et al. 2006). However, less extensive sampling in the tropics relative to the temperate zone might lead to the underestimation of the widths of temperature regimes and elevational ranges for tropical species. To account for this potential bias, we used linear regressions to estimate the relationship between the number of geographical locations sampled per species and temperature-regime width and elevational range. To test for regional differences in species' temperature-regime widths and elevational ranges, we conducted two separate ANOVAs (one for temperature-regime width and one for elevational range) in which we designated the least-squares residuals from each regression as the dependent variable and region (tropical versus temperate) as the factor.
The hypothesis that greater climatic zonation at lower latitudes leads to greater opportunities for speciation in distinct elevational zones predicts that tropical sister species should exhibit lesser elevational and climatic overlap than temperate sister species. However, a variety of factors could potentially bias the results of tests for regional differences (i.e. tropical versus temperate) in the relationships among elevation, climate and speciation. For example, the observation of lower climatic overlap between sister species within one region may be an artefact of their greater age (and thus greater time for climatic divergence) or failure to discover localities in the geographical area separating their known geographical ranges. Climatic overlap might also be underestimated if species that we hypothesize as sister taxa are not so (i.e. owing to undiscovered taxa or recent extinction). Finally, more intensive sampling in one region could lead to larger estimates of the elevational ranges for species and more overlap between sister species.
To test for regional differences in the overlap of sister species' elevational distributions and temperature regimes, we conducted analyses of covariance where we treated region (i.e. tropical versus temperate sister species) as a factor and treated as covariates the divergence times of sister species, the geographical distances separating sister-species distributions (calculated as the distance in kilometres between the two closest sampling locations) and the number of localities sampled per sister-species pair. Consideration of divergence times accounts both for potential latitudinal differences in species ages (Weir & Schluter 2007) and for species pairs that are not actually sister taxa (i.e. assuming climatic divergence between sister species generally increases with time, and that non-sister-species pairs are older than sister-species pairs). Similarly, considering the geographical distances between species and their elevational distributions permits a detailed dissection of whether inadequate sampling might bias regional patterns of overlap between sister species. Lastly, considering the number of locations sampled per sister-species pair permits an assessment of whether greater sampling effort in the temperate zone leads to larger estimates of the geographical and elevational ranges for species and more overlap between sister species. We tested the assumption of equal slopes among groups by testing for significant interaction effects between the factor (tropics versus temperate zone) and covariate (i.e. divergence time, geographical distance, elevational range) for each model. We found no significant interaction between the factor and covariates for any of the models. We therefore excluded the interaction terms from all subsequent ANCOVAs (Engvist 2005). All ANCOVAs were conducted using JMP v. 3.2.1.
Finally, to test whether allopatric sister species show greater climatic overlap with each other than they do with absence locations separating their geographical ranges, we conducted t-tests using the overlap type (i.e. between sister species and between species and absence locations) as the treatment effect and the degree of temperature overlap as the dependent variable. Since the absence localities for each sister-species pair were used in multiple comparisons of temperature overlap, the assumption of independence among data points was violated. We therefore evaluated the significance of the t-statistic by randomizing the temperature overlaps among treatments using PopTools v. 2.6 (http://cse.csiro.au/poptools). If fewer than 5% of the 999 randomizations had a t-statistic greater than the observed one, we considered the result significant.
3. Results
As predicted by Janzen's hypothesis, we found that the sample-size corrected estimates of tropical species thermal regimes are narrower than those of temperate species (ANOVA: F1,58=82.64, p=0.0001). In contrast, we found no evidence that tropical species have narrower elevational ranges than temperate species (ANOVA: F1,58=0.52, p=0.47). We acknowledge that regional differences in temperature-regime widths alone are only weak evidence for latitudinal variation in thermal tolerance ranges. Nonetheless, in tropical and temperate plethodontids, a strong relationship exists between species' field body temperature ranges and estimates of their thermal-regime widths calculated from GIS-based data on temperature variation (see electronic supplementary material). Furthermore, these data are consistent with ecophysiological studies of ectotherms that demonstrate that species' thermal-tolerance ranges increase with latitude (Snyder & Weathers 1975; Feder 1976; van Berkum 1988).
Tropical sister species exhibit lesser overlap in their elevational ranges than temperate sister species, a pattern that is not driven by the greater ages of tropical sister species (ANCOVA: region effect, F1,26=5.36, p=0.03; divergence-time effect, F1,26=0.34, p=0.56), sampling gaps between the geographical ranges of tropical sister species (ANCOVA: region effect, F1,26=4.57, p=0.04; distance effect, F1,26=3.97, p=0.06) or differences in sampling intensity between tropical and temperate regions (ANCOVA: region effect, F1,26=4.24, p=0.04; sample-size effect, F1,26=0.02, p=0.89).
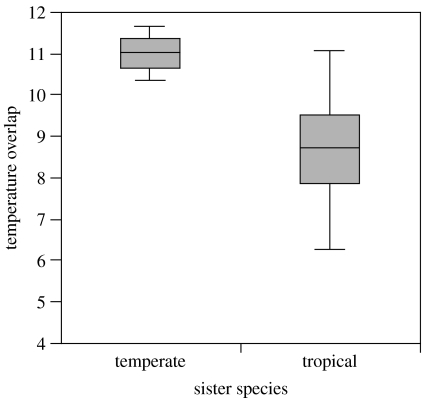
Likewise, tropical sister species also show lesser overlap in the temperature regimes they inhabit than do temperate sister species (figure 2). Our analyses indicate that this pattern is not explained by the greater ages of tropical sister species (ANCOVA: region effect, F1,26=16.47, p=0.0004; divergence-time effect, F1,26=1.73, p=0.19), sampling gaps between the geographical ranges of tropical sister species (ANCOVA: region effect, F1,26=13.61, p=0.001; distance effect, F1,26=0.00, p=0.98) or differences in sampling between the tropics and temperate zone (where sample size is the number of localities per species pair; ANCOVA: region effect, F1,26=11.96, p=0.002; sample-size effect, F1,26=0.03, p=0.87). Furthermore, the lower temperature overlap of tropical sister-species pairs is not an artefact of the greater topographic relief of Mesoamerica compared with eastern North America, given that sister species in the tropics show lesser temperature overlap per unit of elevational overlap in the tropics than in the temperate zone (ANOVA of residual temperature overlap by region, F1,28=7.75, p=0.01).
Figure 2.
Overlap in temperature regimes of tropical and temperate sister species. Boxes enclose the mean and its 95% confidence limits; vertical bars indicate the standard deviation. Tropical sister species show lesser overlap in their temperature regimes than temperate sister species. See text for results of statistical analyses.
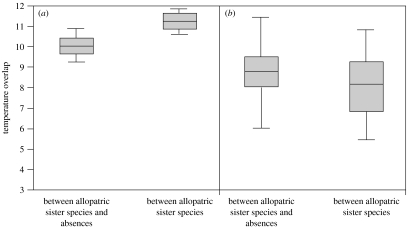
Comparisons of allopatric sister-species pairs (six tropical and eight temperate) and their absence localities reveal regional differences in the relationship between climatic zonation, geographic isolation, and speciation. Consistent with the niche conservatism hypothesis, we found that most allopatric sister species in the temperate zone (six out of eight) show much greater temperature overlap with each other than they do with their intervening absence locations (F1,22=5.21, p=0.03; figure 3a; see also Kozak & Wiens 2006). This pattern of temperature overlap is also found for some (two out of six) tropical sister-species pairs, suggesting that niche conservatism has also contributed to allopatric speciation in tropical montane regions in some cases. However, on average, allopatric sister species in the tropics show no more temperature overlap with each other than they do with the locations separating their ranges (F1,16=0.13, p=0.71; figure 3b). Instead, we found that speciation in the tropics has more frequently been associated with isolation in climatically distinct elevational zones. The temperature regimes occupied by allopatric sister species in the tropics tend to be more strongly differentiated from each other and from their absence locations in the tropics relative to the temperate zone (figure 3a,b). Similarly, we found that sister species with geographically abutting ranges (parapatric; seven tropical pairs and eight temperate pairs) show significantly lesser temperature overlap in the tropics than in the temperate zone (F1,13=6.23, p=0.03).
Figure 3.
Regional comparisons of the temperature overlap of geographically isolated sister species and the absence locations separating their geographical ranges. Boxes enclose the mean and its 95% confidence limits; vertical bars indicate the standard deviation. (a) Temperate sister species show significantly greater overlap in their temperature regimes with each other than they do with their intervening absence locations (suggesting speciation through niche conservatism). (b) Tropical sister species show no greater overlap with each other than they do with absence locations separating their geographical ranges (suggesting speciation through niche divergence). See text for results of statistical analyses.
4. Discussion
For over two centuries, biologists have documented that most groups of organisms show greater species diversity in the tropics (Willig et al. 2003). Recent studies have explored how geographical variation in the timing and rate of diversification may explain high tropical biodiversity (Cardillo 1999; Cardillo et al. 2005; Ricklefs 2006; Wiens et al. 2006b; Weir & Schluter 2007; Wiens 2007). In this study, we address how latitudinal variation in climate over space (elevation) and time (seasonality) may influence the way in which new species arise in the tropics relative to the temperate zone.
Our study uncovered strong regional differences in the elevational and climatic overlap of sister species. First, as predicted by Janzen's hypothesis, we found that the temperature regimes inhabited by tropical species are significantly narrower than those of temperate species (see also van Berkum 1988). Second, tropical sister species exhibit lesser overlap in their elevational ranges and in the temperature regimes they inhabit than do temperate sister species. Finally, we found that tropical sister species exhibit lesser temperature overlap per unit of elevational overlap than do temperate sister species. Together, these results suggest that tropical species exhibit a greater propensity for climate-associated isolation, divergence and speciation in distinct elevational zones relative to temperate sister species.
Our regional comparisons of the temperature overlap between allopatric sister-species pairs further suggest contrasting roles of elevational climatic zonation in speciation between tropical and temperate regions. Despite their geographical separation, we found that allopatric sister species in the temperate zone show much greater temperature overlap with each other than they do with their intervening absence locations, a pattern that supports the hypothesis that niche conservatism underlies their past and present isolation (Kozak & Wiens 2006). In contrast, patterns of climatic overlap among sister species in the tropics support a role for climatic niche divergence during speciation; we found that tropical sister species are no more similar to each other than they are to absence locations separating their geographical ranges.
Similarly, parapatric sister species show lesser climatic overlap in the tropics compared with the temperate zone. It is unclear whether these species pairs originated in situ (as in parapatric speciation) or whether they diverged in allopatry and came into secondary contact along climatic gradients. Nevertheless, the lower climatic overlap of tropical sister species, coupled with their narrower thermal tolerances and reduced ability to undergo thermal acclimation relative to temperate species (Feder 1976; Feder & Lynch 1982), may play an important role in speciation by reducing gene flow between incipient species in primary or secondary zones of contact. Supporting this idea, García-París et al. (2000) found deep phylogeographic structuring among parapatric populations of tropical bolitoglossine species, and found that this structuring was strongly associated with elevational zonation in climate. This combination of conditions is expected to promote speciation along environmental gradients (Gavrilets 2000).
Janzen's climatic zonation hypothesis rests on the assumption that temperature restricts the distribution of species through direct effects on the performance and fitness of organisms. However, interactions between biotic and climatic factors might also be important for setting range limits and driving divergence and speciation in tropical montane regions. For example, range limits may also be set by vegetation (e.g. creating necessary microhabitats), which is influenced by climatic gradients (Heyer 1967). Similarly, parapatric sister species whose range margins are influenced by competitive interactions might have their range limits further reinforced by specialization to different climatic regimes at different elevations in the tropics. However, biotic interactions alone do not seem to explain the regional differences in elevational and thermal overlap of sister species. Allopatric sister species (whose ranges by definition cannot be explained by competitive interactions between them) also show lesser elevational and thermal overlap in the tropics than they do in the temperate zone.
We caution that some tropical species may contain morphologically cryptic species (García-París et al. 2000; Parra-Olea et al. 2002), as few intraspecific phylogeographic studies have been conducted on tropical bolitoglossines compared with temperate plethodontids. Nevertheless, it seems unlikely that the regional differences in elevational and climatic divergence that we found can be explained entirely by incomplete knowledge of species limits in the tropics. Although incomplete taxon sampling is expected to lead to overestimating the actual divergence times of species pairs, analyses of covariance demonstrate that our results cannot be attributed to the greater ages of tropical sister species. Moreover, given the greater topographic relief and climatic heterogeneity of Mesoamerica compared with eastern North America (habitats along Mesoamerican elevational gradients range from wet and dry forests in the lowlands to cloud forest and paramo in the highlands; Holdridge 1964), we predict that many new tropical species will occupy unique climatic regimes compared with their sister taxa.
The strong regional differences in the relationships among elevation, climate and isolation of sister species that we report for plethodontid salamanders may be taxonomically and geographically widespread. For example, studies in tropical montane regions have found evidence suggesting that speciation is associated with divergence in distinct elevational and climatic zones in a variety of taxa (Bates & Zink 1994; Ogden & Thorpe 2002; Graham et al. 2004; Hall 2005; but for possible examples of niche conservatism in the tropics see Patton & Smith 1992 and Peterson et al. 1999). In contrast, recent studies from the temperate zone suggest that niche conservatism is important in promoting speciation in temperate montane regions (Knowles 2001; Carstens et al. 2005; Kozak & Wiens 2006). By focusing on speciation patterns in closely related tropical and temperate clades, our study suggests that these differences between studies are neither idiosyncratic nor taxon specific. Rather, our study suggests that these regional differences in speciation patterns may be linked to the greater spatial and temporal stability of different climatic zones in tropical montane regions (Janzen 1967; Jansson & Dynesius 2002).
Our findings are particularly relevant to understanding the origin and maintenance of high tropical biodiversity. Many tropical montane regions around the world are centres of species diversity and endemism for a variety of groups (Fjeldså & Lovett 1997; Roy 1997; Rahbek & Graves 2001; Fjeldså & Rahbek 2006; Smith et al. 2007), including plethodontid salamanders (Wake & Lynch 1976; Wiens et al. 2007), which show accelerated rates of diversification in the tropics relative to the temperate zone (Wiens 2007). Recent studies have identified important ecological correlates of high species richness (see Willig et al. 2003), but how these factors interact with the processes that directly increase or decrease species diversity within a region (i.e. speciation, extinction and dispersal) remains poorly understood (Ricklefs 2004; Wiens & Donoghue 2004; Mittelbach et al. 2007). The latitudinal differences in elevational and climatic overlap of sister species that we report here suggest that there are more opportunities for climatic specialization, climate-driven geographical isolation and speciation in tropical montane regions. Paradoxically, the same processes that seem to have contributed to the remarkable build-up of species diversity in tropical mountains (i.e. selection for narrow climatic tolerances) may also promote high rates of extinction in the face of global climate change (Parra-Olea et al. 2005).
Here, we have focused on the differences in speciation mechanisms between tropical Mesoamerica and temperate eastern North America within a single group of organisms. Further comparative analyses of the relationship between climatic zonation and speciation are needed for other taxa and different tropical and temperate regions. Latitudinal variation in climatic zonation and thermal tolerances may play a general role in contributing to the origin and maintenance of high tropical species richness. The latitudinal variation in seasonality noted by Janzen (1967) is likely to hold for many tropical and temperate regions across the world (with some exceptions; see Ghalambor et al. 2006). In addition, recent studies that have examined the degree of physiological specialization along latitudinal gradients have found that the thermal-tolerance ranges of species generally increase with latitude (Snyder & Weathers 1975; van Berkum 1988; Addo-Bediako et al. 2000; Ghalambor et al. 2006). Future comparative studies of tropical and temperate speciation should also include comparisons of physiological tolerances, dispersal levels along mountain slopes and analyses of the mechanisms that lead to intrinsic reproductive isolation. Nevertheless, our study shows how Janzen's climatic zonation hypothesis might lead to latitudinal variation in the processes of speciation and contribute to the high biodiversity of many tropical montane regions.
Acknowledgments
We thank R. Highton, S. G. Tilley, D. Wake, M. García-París, G. Parra-Olea and the museum staff members at the Museum of Vertebrate Zoology at the University of California, Berkeley and the US National Museum of Natural History, all of whom helped compile sampling records that made this study possible. K. Tighe, C. Wolfe, and especially A. Wynn provided invaluable assistance with the acquisition and verification of species records. We thank J. Flowers, M. Gifford, C. Graham, D. Moen, C. Ulloa, and especially D. Wake for discussions that improved the manuscript. This research was supported by NSF Bioinformatics postdoctoral fellowship DBI-0434728 to K.H.K. and NSF grants DEB-0129142 and DEB-0331747 to J.J.W.
Supplementary Material
The 60 species included in all analyses along with the number of localities (N), their temperature regime widths and their elevational ranges
The 30 sister-species pairs included along with their distribution types, estimated divergence times, and temperature and elevational overlaps
Relationship between annual field-body temperature range and thermal-regime widths
General illustration depicting the procedure used to calculate the temperature overlap for allopatric sister species and their corresponding absence locations
References
- Addo-Bediako A, Chown S.L, Gaston K.J. Thermal tolerance, climatic variability and latitude. Proc. R. Soc. B. 2000;267:739–745. doi: 10.1098/rspb.2000.1065. doi:10.1098/rspb.2000.1065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amphibia Web 2007 Information on amphibian biology and conservation (web application), Amphibia Web, Berkeley, CA. See http://amphibiaweb.org/ (Last accessed: 24 April 2007.)
- Bates J.M, Zink R.M. Evolution into the Andes: molecular evidence for species relationships in the genus Leptopogon. Auk. 1994;111:507–515. [Google Scholar]
- Cardillo M. Latitude and diversification rates in birds and butterflies. Proc. R. Soc. B. 1999;266:1221–1225. doi:10.1098/rspb.1999.0766 [Google Scholar]
- Cardillo M, Orme C.D.L, Owens I.P.F. Testing for latitudinal bias in rates of species diversification: an example using New World birds. Ecology. 2005;86:2278–2287. doi:10.1890/05-0112 [Google Scholar]
- Carstens B.C, Brunsfeld S.J, Demboski J.R, Good J.M, Sullivan J. Investigating the evolutionary history of the Pacific northwest mesic forest ecosystem: hypothesis testing within a comparative phylogeographic framework. Evolution. 2005;59:1639–1652. doi:10.1554/04-661.1 [PubMed] [Google Scholar]
- Crespi E.J, Rissler L.J, Browne R.A. Testing Pleistocene refugia theory: phylogeographical analysis of Desmognathus wrighti, a high-elevation salamander in the southern Appalachians. Mol. Ecol. 2003;12:969–984. doi: 10.1046/j.1365-294x.2003.01797.x. doi:10.1046/j.1365-294X.2003.01797.X [DOI] [PubMed] [Google Scholar]
- Duellman W.E, Sweet S.S. Distribution patterns of amphibians in the Nearctic region of North America. In: Duellman W.E, editor. Patterns of distribution of amphibians. Johns Hopkins University Press; Baltimore, MD: 1999. pp. 31–106. [Google Scholar]
- Engvist L. Mistreatment of covariate interaction terms in linear model analyses of behavioural and evolutionary ecology studies. Anim. Behav. 2005;70:967–971. doi:10.1016/j.anbehav.2005.01.016 [Google Scholar]
- Feder M.E. Environmental variability and thermal acclimation of metabolism in neotropical and temperate zone salamanders. Phys. Zool. 1976;51:7–16. [Google Scholar]
- Feder M.E, Lynch J.F. Effects of latitude, season, elevation, and microhabitat on field body temperatures of neotropical and temperate zone salamanders. Ecology. 1982;63:1657–1664. doi:10.2307/1940107 [Google Scholar]
- Feder M.E, Lynch J.F, Shaffer H.B, Wake D.B. Field body temperatures of tropical and temperate zone salamanders. Smithsonian Herpetol. Inf. Serv. Publ. 1982;52:1–23. [Google Scholar]
- Fjeldså J, Lovett J.C. Geographical patterns of young and old species in African forest biota: the significance of specific montane areas as evolutionary centres. Biodivers. Conserv. 1997;6:325–346. doi:10.1023/A:1018356506390 [Google Scholar]
- Fjeldså J, Rahbek C. Diversification of tanagers, a species-rich bird group, from the lowlands to montane regions of South America. Integr. Comp. Biol. 2006;46:72–81. doi: 10.1093/icb/icj009. doi:10.1093/icb/icj009 [DOI] [PubMed] [Google Scholar]
- García-París M, Good D.A, Parra-Olea G, Wake D.B. Biodiversity of Costa Rican salamanders: implications of high levels of genetic differentiation and phylogeographic structure for species formation. Proc. Natl Acad. Sci. USA. 2000;97:1640–1647. doi: 10.1073/pnas.97.4.1640. doi:10.1073/pnas.97.4.1640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gavrilets S. Waiting time to parapatric speciation. Proc. R. Soc. B. 2000;267:2483–2492. doi: 10.1098/rspb.2000.1309. doi:10.1098/rspb.2000.1309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghalambor C.K, Huey R.B, Martin P.R, Tewksbury J.J, Wang G. Are mountain passes higher in the tropics? Janzen's hypothesis revisted. Integr. Comp. Biol. 2006;46:5–17. doi: 10.1093/icb/icj003. doi:10.1093/icb/icj003 [DOI] [PubMed] [Google Scholar]
- Graham C.H, Ron S.R, Santos J.C, Schneider C.J, Moritz C. Integrating phylogenetics and environmental niche models to explore speciation mechanisms in dendrobatid frogs. Evolution. 2004;58:1781–1793. doi: 10.1111/j.0014-3820.2004.tb00461.x. [DOI] [PubMed] [Google Scholar]
- Hall J.P. Montane speciation patterns in Ithomiola butterflies (Lepidoptera: Rhiodinidae): are they consistently moving up in the world? Proc. R. Soc. B. 2005;272:2457–2466. doi: 10.1098/rspb.2005.3254. doi:10.1098/rspb.2005.3254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heyer R.W. A herpetofaunal study of an ecological transect through the Cordillera de Tilarán, Costa Rica. Copeia. 1967;1967:259–271. doi:10.2307/1442113 [Google Scholar]
- Hijmans R.J, Cameron S.E, Parra J.L, Jones P.G, Jarvis A. Very high resolution interpolated climate surfaces for global land areas. Int. J. Climatol. 2005;25:1965–1978. doi:10.1002/joc.1276 [Google Scholar]
- Holdridge L.R.Life zone ecology1964Tropical Science Center; San Jose, Costa Rica [Google Scholar]
- Huey R.B. Latitudinal pattern of between-altitude faunal similarity: mountains might be higher in the tropics. Am. Nat. 1978;112:225–229. doi:10.1086/283262 [Google Scholar]
- Jansson R, Dynesius M. The fate of clades in a world of recurrent climate change: Milankovitch oscillations and evolution. Annu. Rev. Ecol. Syst. 2002;33:741–777. doi:10.1146/annurev.ecolsys.33.010802.150520 [Google Scholar]
- Janzen D.H. Why mountain passes are higher in the tropics. Am. Nat. 1967;101:233–249. doi:10.1086/282487 [Google Scholar]
- Knowles L.L. Did the Pleistocene glaciations promote divergence? Tests of explicit refugial models in montane grasshoppers. Mol. Eol. 2001;10:691–701. doi: 10.1046/j.1365-294x.2001.01206.x. doi:10.1046/j.1365-294x.2001.01206.x [DOI] [PubMed] [Google Scholar]
- Kozak K.H, Wiens J.J. Does niche conservatism promote speciation? A case study in North American salamanders. Evolution. 2006;60:2604–2621. [PubMed] [Google Scholar]
- Kozak K.H, Larson A, Bonett R.M, Harmon L.J. Phylogenetic analysis of ecomorphological divergence, community structure, and diversification rates in dusky salamanders (Plethodontidae: Desmognathus) Evolution. 2005;59:2000–2016. [PubMed] [Google Scholar]
- Kozak K.H, Weisrock D.W, Larson A. Rapid lineage accumulation in a non-adaptive radiation: phylogenetic analysis of diversification rates in eastern North American woodland salamanders (Plethodontidae: Plethodon) Proc. R. Soc. B. 2006;273:539–546. doi: 10.1098/rspb.2005.3326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieberman D, Lieberman M, Peralta R, Hartshorn G.S. Tropical forest structure and composition on a large-scale altitudinal gradient in Costa Rica. J. Ecol. 1996;84:137–152. doi:10.2307/2261350 [Google Scholar]
- Mittelbach G.G, et al. Evolution and the latitudinal diversity gradient: speciation, extinction, and biogeography. Ecol. Lett. 2007;10:315–331. doi: 10.1111/j.1461-0248.2007.01020.x. doi:10.1111/j.1461-0248.2007.01020.x [DOI] [PubMed] [Google Scholar]
- Moritz C, Patton J.L, Schneider C.J, Smith T.B. Diversification of rainforest faunas: an integrated molecular approach. Annu. Rev. Ecol. Syst. 2000;31:533–563. doi:10.1146/annurev.ecolsys.31.1.533 [Google Scholar]
- Navas C.A. Herpetological diversity along Andean elevational gradients: links with physiological ecology and evolutionary physiology. Comp. Biochem. Physiol. 2003;133:469–485. doi: 10.1016/s1095-6433(02)00207-6. doi:10.1016/S1095-6433(02)00207-6 [DOI] [PubMed] [Google Scholar]
- Ogden R, Thorpe R.S. Molecular evidence for ecological speciation in tropical habitats. Proc. Natl Acad. Sci. USA. 2002;99:13 612–13 615. doi: 10.1073/pnas.212248499. doi:10.1073/pnas.212248499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parra-Olea G, García-París M, Papenfuss T.J, Wake D.B. Systematics of the Pseudoeurycea belli (Caudata: Plethodontidae) species complex. Herpetologica. 2002;61:145–158. doi:10.1655/03-02 [Google Scholar]
- Parra-Olea G, Martinez Meyer E, de Leon G.F.P. Forecasting climate change effects on salamander distributions in the highlands of central Mexico. Biotropica. 2005;37:202–208. doi:10.1111/j.1744-7429.2005.00027.x [Google Scholar]
- Patton J.L, Smith M.F. mtDNA phylogeny of Andean mice: a test of diversification across ecological gradients. Evolution. 1992;46:174–183. doi: 10.1111/j.1558-5646.1992.tb01992.x. doi:10.2307/2409812 [DOI] [PubMed] [Google Scholar]
- Peterson A.T, Soberón J, Sánchez-Cordero V. Conservatism of ecological niches in evolutionary time. Science. 1999;285:1265–1267. doi: 10.1126/science.285.5431.1265. doi:10.1126/science.285.5431.1265 [DOI] [PubMed] [Google Scholar]
- Porter W.P, Sabo J.L, Tracy C.R, Reichman O.J, Ramankutty N. Physiology on a landscape scale: plant–animal interactions. Integr. Comp. Biol. 2002;42:431–453. doi: 10.1093/icb/42.3.431. doi:10.1093/icb/42.3.431 [DOI] [PubMed] [Google Scholar]
- Rahbek C, Graves G.R. Multiscale assessment of patterns of avian species richness. Proc. Natl Acad. Sci. USA. 2001;98:4534–4539. doi: 10.1073/pnas.071034898. doi:10.1073/pnas.071034898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ricklefs R.E. A comprehensive framework for global patterns in biodiversity. Ecol. Lett. 2004;7:1–15. doi:10.1046/j.1461-0248.2003.00554.x [Google Scholar]
- Ricklefs R.E. Global variation in the diversification rate of passerine birds. Ecology. 2006;87:2468–2478. doi: 10.1890/0012-9658(2006)87[2468:gvitdr]2.0.co;2. doi:10.1890/0012-9658(2006)87[2468:GVITDR]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Roy M.S. Recent diversification of African greenbuls (Pycnonotidae: Andropadus) supports a montane speciation model. Proc. R. Soc. B. 1997;264:1337–1344. doi:10.1098/rspb.1997.0185 [Google Scholar]
- Sanderson M.J. Estimating absolute rates of molecular evolution and divergence times: a penalized likelihood approach. Mol. Biol. Evol. 2002;19:101–109. doi: 10.1093/oxfordjournals.molbev.a003974. [DOI] [PubMed] [Google Scholar]
- Smith S.A, Nieto Montes de Oca A, Reeder T.W, Wiens J.J. A phylogenetic perspective on elevational species richness patterns in Middle American treefrogs: why so few species in lowland tropical rainforests? Evolution. 2007;61:1188–1207. doi: 10.1111/j.1558-5646.2007.00085.x. doi:10.11111/j.1558-5646.2007.00085.x [DOI] [PubMed] [Google Scholar]
- Snyder G.K, Weathers W.W. Temperature adaptations in amphibians. Am. Nat. 1975;109:93–101. doi:10.1086/282976 [Google Scholar]
- Terborgh J. Bird species diversity on an Andean elevational gradient. Ecology. 1977;58:1007–1019. doi:10.2307/1936921 [Google Scholar]
- van Berkum F.H. Latitudinal patterns of thermal sensitivity of sprint speed in lizards. Am. Nat. 1988;132:327–343. doi:10.1086/284856 [Google Scholar]
- Wake D.B, Lynch J.F. The distribution, ecology, and evoloutionary history of plethodontid salamanders in tropical America. Sci. Bull. Nat. Hist. Mus. Los Angeles Co. 1976;25:1–65. [Google Scholar]
- Weir J.T, Schluter D. The latitudinal gradient in recent speciation and extinction rates of birds and mammals. Science. 2007;315:1928–1933. doi: 10.1126/science.1135590. doi:10.1126/science.1135590 [DOI] [PubMed] [Google Scholar]
- Weisrock D.W, Larson A. Testing hypotheses of speciation in the Plethodon jordani species complex with allozymes and Mitochondrial DNA sequences. Biol. J. Linn. Soc. 2006;89:25–51. doi:10.1111/j.1095-8312.2006.00655.x [Google Scholar]
- Wiens J.J. Speciation and ecology revisited: phylogenetic niche conservatism and the origin of species. Evolution. 2004;58:193–197. doi: 10.1111/j.0014-3820.2004.tb01586.x. [DOI] [PubMed] [Google Scholar]
- Wiens J.J. Global patterns of species richness and diversification in amphibians. Am. Nat. 2007;170:S86–S106. doi: 10.1086/519396. doi:10.1086/519396 [DOI] [PubMed] [Google Scholar]
- Wiens J.J, Donoghue M.J. Historical biogeography, ecology, and species richness. Trends Ecol. Evol. 2004;19:639–644. doi: 10.1016/j.tree.2004.09.011. doi:10.1016/j.tree.2004.09.011 [DOI] [PubMed] [Google Scholar]
- Wiens J.J, Graham C.H. Niche conservatism: integrating evolution, ecology, and conservation biology. Ann. Rev. Ecol. Evol. Syst. 2005;36:519–539. doi:10.1146/annurev.ecolsys.36.102803.095431 [Google Scholar]
- Wiens J.J, Engstrom T.N, Chippindale P.T. Rapid diversification, incomplete isolation, and the “speciation clock” in North American salamanders (genus: Plethodon): testing the hybrid swarm hypothesis of rapid radiation. Evolution. 2006a;60:2585–2603. [PubMed] [Google Scholar]
- Wiens J.J, Graham C.H, Moen D.S, Smith S.A, Reeder T.W. Evolutionary and ecological causes of the latitudinal diversity gradient in hylid frogs: treefrog trees unearth the roots of high tropical diversity. Am. Nat. 2006b;168:579–596. doi: 10.1086/507882. doi:10.1086/507882 [DOI] [PubMed] [Google Scholar]
- Wiens J.J, Parra-Olea G, García-París M, Wake D.B. Phylogenetic history underlies elevational biodiversity patterns in tropical salamanders. Proc. R. Soc. B. 2007;274:919–928. doi: 10.1098/rspb.2006.0301. doi:10.1098/rspb.2006.0301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willig M.R, Kaufman D.M, Stevens R.D. Latitudinal gradients of biodiversity: pattern, process, scale, and synthesis. Annu. Rev. Ecol. Evol. Syst. 2003;34:273–309. doi:10.1146/annurev.ecolsys.34.012103.144032 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The 60 species included in all analyses along with the number of localities (N), their temperature regime widths and their elevational ranges
The 30 sister-species pairs included along with their distribution types, estimated divergence times, and temperature and elevational overlaps
Relationship between annual field-body temperature range and thermal-regime widths
General illustration depicting the procedure used to calculate the temperature overlap for allopatric sister species and their corresponding absence locations