Abstract
Background
The percentage of asthma cases attributable to atopy is the subject of debate.
Objectives
The objectives were to estimate the percentage of asthma cases in the U.S. population attributable to atopy and to examine associations between allergen-specific skin tests and asthma.
Methods
Data were obtained from NHANES III in which subjects aged 6–59 years were skin tested with 10 allergens. Atopy was defined as at least one positive allergen-specific test. Doctor-diagnosed current asthma was assessed by questionnaire.
Results
In the U.S., 56.3% of the asthma cases were attributable to atopy, and that percentage was greater among males than females, among persons in the highest education category than in lower education categories, and among persons living in highly populated metropolitan areas than in all other areas. Each allergen-specific test was strongly associated with asthma before adjustment (odds ratios varied from 2.1 to 4.5); however, after adjustment by all the allergens, only tests to cat, Alternaria, white oak, and perennial rye were independently associated with asthma. Perennial rye was inversely associated with asthma. Of the 10 allergens, a positive response to cat accounted for the highest percentage of asthma cases (29.3%).
Conclusions
About half of the current asthma cases in the U.S. population represented by NHANES III were attributable to atopy. Some allergen-specific skin tests were not independently associated with asthma.
Clinical Implications
If atopy could be prevented or reversed, or its effect on asthma blocked, then a large percentage of asthma cases in the U.S. population could be prevented.
Keywords: allergens, allergic sensitization, allergy skin test, asthma, atopy, epidemiology, NHANES III, skin prick test, survey
INTRODUCTION
Atopy, defined as “the genetic propensity to develop immunoglobulin E (IgE) antibodies in response to exposure to allergen”,1 is an established risk factor for asthma.1–6 However, the percentage of asthma cases attributable to atopy is the subject of debate. In a meta-analysis of population-based studies, mostly conducted in Western countries, Pearce et al. reported that 38% of asthma cases in children and 37% of asthma cases in adults were attributable to atopy, as defined by allergy skin test positivity.7 With atopy defined as total serum IgE > 100 IU/ml, the average population attributable risk across studies of children and adults was 33%.7 Those authors concluded that the importance of atopy as a cause of asthma may have been previously overestimated, perhaps leading to “an under-recognition of, and insufficient research into, other possible etiological mechanisms for the development of asthma.”7 Similarly, from a study of 4-year-old children on the Isle of Wight, Arshad et al. reported that atopy (positive skin prick test) attributed to 35% of asthma cases, which led them to conclude that 60–70% of cases were attributable to other factors.1 In contrast, other researchers have argued that most all asthma cases are IgE mediated, challenging the concept that there are allergic and nonallergic forms of asthma.8
In the third National Health and Nutrition Examination Survey (NHANES III), conducted from 1988–1994, prick-puncture allergy skin tests to 10 allergens were administered to subjects aged 6 to 59 years. This nationally representative survey provided the opportunity to estimate the percentage of asthma cases in the U.S. population attributable to skin test positivity, an indirect measurement of atopy (serum IgE was not measured in NHANES III). In addition, the large sample size, over 10,000 individuals, allowed for the comprehensive assessment of the associations between allergen-specific skin test responses and asthma.
METHODS
Data
Data were obtained from NHANES III, a complex survey designed to represent the civilian noninstitutionalized population of the U.S. Questionnaires were administered to and medical examinations and laboratory tests conducted on 31,311 individuals aged 2 months to 90 years. A subsample of 12,106 subjects consisting of all subjects aged 6–19 years and a random hal/ample of subjects aged 20–59 years were selected for allergy skin testing.
Allergy Skin Testing and Atopy
A panel of 10 allergens and 2 controls (positive and negative) were administered by the prick-puncture method. For this analysis, a skin test panel was considered valid if the difference in mean wheal diameters between the positive and negative controls was at least 1 mm. An allergen-specific skin test was considered positive if the skin test panel was valid and the difference in mean wheal diameters between the allergen-specific test and negative control was at least 3 mm. Of the 12,106 subjects age-eligible for skin testing, 1069 refused or were unavailable for testing and 174 were excluded for medical reasons.9 The number of subjects with a valid test and results for all 10 allergens was 10,508. Details of the allergy skin testing may be found elsewhere.10
In the main analysis, atopy was defined as a positive response to at least one allergen. In secondary analyses, atopy was defined as at least 2 and at least 3 positive responses.
Assessment of Asthma
The disease outcome for this analysis was doctor-diagnosed current asthma assessed by questionnaire. Cases were individuals who answered in the affirmative to the questions, “Has a doctor ever told you that you had asthma?” and “Do you still have asthma?” Non-cases were subjects who responded in the negative to either question.
Statistical Analyses
Differences in prevalences across subject characteristics were assessed with Chi-square statistics. Unadjusted and adjusted odds ratios for associations between atopy and asthma and between allergen-specific skin test responses and asthma were estimated with logistic regression. Odds ratios were adjusted by potential risk factors for allergy or asthma, which are listed in Table 1. The sample size used in the estimation of adjusted odds ratios was 10,375 (of the 10,508 subjects with valid skin tests and results for all 10 allergens, 10,479 had information on asthma and 10,375 had information on asthma and all of the subject characteristics in Table 1). Odds ratios stratified by the subject characteristics were estimated from models containing two-way interaction terms. Statistical differences across stratified odds ratios were determined from the P-value of the interaction term.
Table 1.
Percentage of asthma cases in the U.S. population aged 6–59 years attributable to atopy (at least one positive skin test response to any of 10 allergens) stratified by subject characteristics.
| Subject Characteristics | N | Percent (SE) asthmatic | Percent (SE) atopic | Percent (SE) atopic among asthmatics | Adjusted Odds ratio (95% CI)* | Percent of cases attributable to atopy (95% CI) |
|---|---|---|---|---|---|---|
| Overall | 10479 | 5.2 (0.38) | 54.2 (0.99) | 78.9 (3.21) | 3.5 (2.3–5.3) | 56.3 (42.1–69.5) |
| Age | ||||||
| 06–19 | 5640 | 6.4 (0.57) | 52.7 (1.18) | 78.0 (4.74) | 3.4 (1.9–6.2) | 55.2 (35.1–73.8) |
| 20–39 | 2975 | 4.6 (0.54) | 58.1 (1.27) | 82.4 (6.18) | 3.8 (1.6–9.0) | 60.6 (32.0–83.5) |
| 40–59 | 1864 | 4.9 (0.60) | 49.9 (1.93)† | 75.3 (4.55) | 3.2 (1.8–5.8) | 52.1 (33.6–69.9) |
| Sex | ||||||
| Male | 5046 | 4.8 (0.56) | 59.3 (1.21) | 88.9 (3.22) | 6.0 (3.0–12.0) | 74.1 (55.8–86.7) |
| Female | 5433 | 5.5 (0.61) | 49.1 (1.23)† | 70.4 (4.43)† | 2.6 (1.6–4.2)† | 43.2 (26.6–61.6)† |
| Race-ethnicity | ||||||
| Non-Hispanic white | 3030 | 5.3 (0.50) | 51.2 (1.15) | 76.8 (4.24) | 3.5 (2.1–5.9) | 54.8 (37.6–71.0) |
| Non-Hispanic black | 3461 | 6.0 (0.51) | 62.0 (1.27) | 80.4 (3.72) | 2.6 (1.6–4.2) | 49.3 (31.2–67.6) |
| Mexican American | 3511 | 3.3 (0.39) | 57.1 (1.31) | 81.1 (5.27) | 3.2 (1.7–6.1) | 55.8 (33.6–75.9) |
| Other | 477 | 4.6 (1.03)† | 63.9 (2.83)† | 93.1 (4.64) | 8.1 (2.0–32.3) | 81.6 (45.7–95.9) |
| Education (of family referent‡) | ||||||
| < 12th grade | 4109 | 5.4 (0.75) | 54.3 (1.73) | 69.5 (5.83) | 2.0 (1.1–3.8) | 35.5 (14.8–63.5) |
| 12th grade | 3290 | 5.0 (0.58) | 51.8 (1.71) | 71.6 (6.59) | 2.5 (1.2–4.9) | 42.5 (18.3–70.9) |
| > 12th grade | 3007 | 5.2 (0.74) | 55.9 (1.74) | 88.7 (2.95)† | 7.2 (3.9–13.4)† | 76.5 (61.8–86.7)† |
| Census region | ||||||
| Northeast | 1245 | 6.5 (0.69) | 57.7 (2.70) | 86.9 (3.48) | 5.6 (2.6–11.9) | 71.3 (51.7–85.1) |
| Midwest | 1903 | 5.2 (0.81) | 52.7 (2.57) | 76.8 (9.06) | 3.2 (1.0–9.8) | 52.8 (18.9–84.3) |
| South | 4539 | 4.6 (0.57) | 50.8 (1.40) | 77.9 (5.57) | 3.8 (1.9–7.6) | 57.6 (35.8–76.8) |
| West | 2792 | 4.7 (0.88) | 57.8 (1.52)† | 72.8 (7.40) | 2.1 (0.9–4.5) | 37.4 (10.8–74.7) |
| Urbanization | ||||||
| Metropolitan ≥ 1 million pop. | 5377 | 5.5 (0.55) | 55.8 (1.61) | 85.4 (2.91) | 5.4 (3.1–9.2) | 69.5 (54.8–81.1) |
| All other areas | 5102 | 4.8 (0.48) | 52.4 (1.43) | 71.0 (5.39)† | 2.3 (1.3–4.1)† | 40.7 (20.7–64.3)† |
| Serum cotinine (ng/mL) | ||||||
| 0.035–0.100 | 2251 | 4.7 (0.82) | 56.8 (2.18) | 84.5 (7.62) | 4.6 (1.4–15.1) | 66.3 (30.1–90.0) |
| 0.100–10.00 | 5460 | 5.7 (0.60) | 55.3 (1.61) | 79.9 (3.97) | 3.5 (2.0–6.3) | 57.2 (39.2–73.6) |
| 10.00–1080.0 | 1900 | 5.0 (0.81) | 51.0 (1.48) | 75.2 (7.57) | 3.2 (1.4–7.6) | 51.9 (22.8–79.7) |
| Missing/Unknown§ | 868 | 3.8 (1.38) | 51.1 (2.41) | 65.3 (15.50) | 2.0 (0.5–7.9) | 32.2 (1.08–95.4) |
| Anyone smoke inside the home? | ||||||
| Yes | 4254 | 4.8 (0.61) | 52.0 (1.45) | 78.5 (5.25) | 3.7 (1.9–7.0) | 57.3 (36.7–75.6) |
| No | 6214 | 5.4 (0.50) | 55.6 (1.38) | 79.1 (3.50) | 3.4 (2.1–5.3) | 55.6 (39.6–70.5) |
| Body mass index | ||||||
| 11.2–18.4 (underweight) | 2409 | 5.0 (0.62) | 48.8 (1.79) | 79.2 (5.99) | 4.3 (2.0–9.3) | 61.0 (37.3–80.4) |
| 18.5–24.9 (normal weight) | 4337 | 5.1 (0.64) | 54.3 (1.77) | 84.9 (4.77) | 5.2 (2.4–11.4) | 68.6 (46.1–84.8) |
| 25.0–29.9 (overweight) | 2166 | 3.7 (0.55) | 56.5 (2.03) | 75.4 (8.18) | 2.5 (1.0–6.2) | 45.4 (14.7–80.0) |
| 30.0–79.6 (obese) | 1541 | 7.5 (1.14)† | 54.3 (2.02)† | 70.0 (5.83) | 2.2 (1.2–3.9) | 37.7 (17.8–62.9) |
Odds ratio for atopy-asthma association adjusted by all of the subject characteristics in this table
A difference between categories exists at P ≤ 0.05
Knowledgeable household member 17 years or older who owned or rented the dwelling unit.
Missing/unknown category was included in regression models in order not to lose 868 observations
The percentages of asthma cases in the population attributable to atopy and to allergen-specific skin test responses were calculated from the population attributable risk formula, PAR = P·(RR−1)/(RR), where RR is the relative risk (RR must be ≥ 1) and P is the percentage of disease cases with the risk factor.11 The relative risk was estimated by the adjusted odds ratio. To calculate the confidence intervals for the PARs, a logit transformation was first applied to the PARs to bound the resulting intervals between 0% and 100%. The variance of the logit(PARs) were calculated by Fay’s method, as described by Judkins,12 with perturbation factor of 50% and 500 replicates. Confidence intervals were calculated using this variance in a normal approximation, then transformed back with an inverse logit. Tests of significance were performed using variance and covariance derived from Fay’s method on the logit(PARs) in a Wald-type test.
All reported statistics other than numbers of subjects were weighted to represent the civilian, noninstitutionalized population of the United States aged 6–59 years. Standard errors (SE) were adjusted for the complex survey design using SUDAAN statistical software (Release 9.0.1, Research Triangle Institute, Research Triangle Park, NC). Statistical significance was set at P ≤ 0.05.
RESULTS
Prevalence of Asthma
The prevalence of asthma in the U.S. population aged 6–59 years was 5.2%. Of the subject characteristics evaluated, the prevalence of asthma differed significantly by race-ethnicity (P < 0.01) and body mass index (P = 0.03) (Table 1). For race-ethnicity, asthma was most prevalent among non-Hispanic blacks, and for body mass index (BMI), asthma was most prevalent among persons categorized as obese. As categorized, there was no statistical difference by age (P = 0.06); however, the prevalence of asthma was highest among persons in the youngest category.
Prevalence of Atopy
The prevalence of atopy in the U.S. population aged 6–59 years, defined as at least one positive skin test response, was 54.2%. Atopy differed significantly by age (P < 0.01), sex (P < 0.01), race-ethnicity (P < 0.01), census region (P = 0.01), and body mass index (P = 0.02) (Table 1). In contrast to asthma, the prevalence of atopy was higher in males than females and in the middle age category than the younger or older category. A detailed analysis of predictors of skin test positivity in NHANES III was previously published.9
The prevalence of atopy among asthmatics, one of the two statistics used in the calculation of PAR, was 78.9%. Among asthmatics, atopy was more prevalent among males than females (P < 0.01), among persons in the highest education category than in the lower education categories (P = 0.01), and among persons in highly populated metropolitan areas than in all other areas (P = 0.04) (Table 1).
Association Between Atopy and Asthma
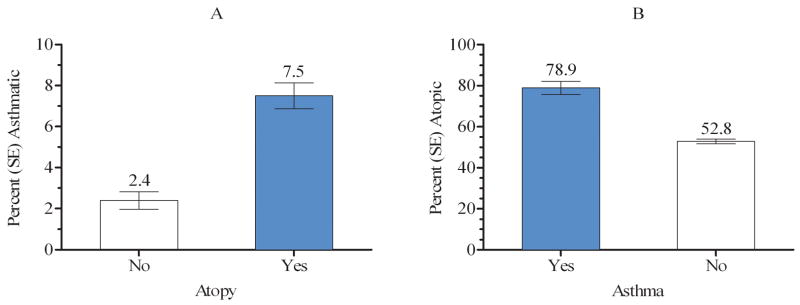
The prevalence of asthma was significantly higher among atopic than non-atopic individuals (Figure 1A), and conversely, the prevalence of atopy was significantly higher among asthmatic than non-asthmatic individuals (Figure 1B). The unadjusted odds of being atopic was 3.3 (95% CI: 2.2–5.1) times greater for asthmatic than non-asthmatic individuals.
Figure 1.
Prevalence of asthma by atopy (defined by at least one positive skin test response) (A) and prevalence of atopy by asthma (B)
The adjusted odds ratio for the atopy-asthma association, the second statistic used in the calculation of PAR, was 3.5 (2.3–5.3). The adjusted odds ratios were significantly higher for males than females (interaction P = 0.03), for persons in the highest education category than in the lower education categories (P = 0.01), and persons in highly populated metropolitan areas than in all other areas (P = 0.04).
Population Attributable Risk (PAR)
Among the U.S. population aged 6–59 years, 56.3% of the asthma cases were attributable to atopy (PAR). Across the subject characteristics in Table 1, the PARs varied from a low of 35.5% to a high of 81.6%. The PAR was greater among males than females (P < 0.01), among persons in the highest education category than in lower education categories (P = 0.02), and among persons living in highly populated metropolitan areas than in all other areas (P = 0.04) (Table 1).
The effect on the PAR by varying the definition of atopy is shown in Table 2. Atopy defined as at least 1, 2, and 3 positive skin test responses, respectively, resulted in decreasing PARs: 56.3%, 50.4%, and 43.7%. Those reductions were due to the decreasing prevalences of atopy among asthmatics (Table 2).
Table 2.
Percentage of asthma cases in the U.S. population aged 6–59 years attributable to atopy according to varying definitions of atopy
| Definition of Atopy | Percent (SE) atopic among asthmatics | Adjusted Odds ratio (95% CI)* | Percent of cases attributable to atopy (95% CI) |
|---|---|---|---|
| At least 1 positive skin test response | 78.9 (3.21) | 3.5 (2.3–5.3) | 56.3 (42.1–69.5) |
| At least 2 positive skin test responses | 68.0 (3.93) | 3.9 (2.7–5.6) | 50.4 (38.3–62.4) |
| At least 3 positive skin test responses | 58.4 (4.08) | 4.0 (2.8–5.6) | 43.7 (33.0–55.1) |
Odds ratio for the atopy-asthma association adjusted by all of the subject characteristics in Table 1
Allergen-Specific Skin Tests and Asthma
Table 3 shows the odds ratios for the associations between the allergen-specific skin tests and asthma. All unadjusted and partially adjusted odds ratios (adjusted by the subject characteristics in Table 1) were significantly greater than 1.0. However, full adjustment by the subject characteristics and all of the allergen-specific tests resulted in significant odds ratios only for cat, Alternaria, white oak, and perennial rye. The fully adjusted odds ratio for perennial rye was 0.6 (0.4–0.9), indicating an inverse association with asthma.
Table 3.
Percentage of asthma cases in the U.S. population aged 6–59 years attributable to allergen-specific skin test responses
| Allergen-specific skin test | Percent (SE) skin test positive among asthmatics | Odds ratio (95% CI) for the association between the allergen-specific skin test and asthma | Percent of cases attributable to allergen-specific response (95% CI) | ||
|---|---|---|---|---|---|
| Unadjusted | Partially adjusted* | Fully adjusted† | |||
| Cat | 45.2 (4.01) | 4.5 (3.3–6.1) | 4.7 (3.5–6.4) | 2.9 (1.8–4.5) | 29.3 (18.8–42.6) |
| Alternaria | 34.5 (3.69) | 4.0 (2.8–5.8) | 4.3 (2.9–6.3) | 2.6 (1.6–4.0) | 21.1 (13.0–32.4) |
| White oak | 34.6 (3.55) | 3.9 (2.9–5.4) | 4.4 (3.3–5.8) | 2.5 (1.5–4.4) | 20.9 (12.1–33.8) |
| Short ragweed | 49.1 (3.62) | 2.9 (2.1–3.9) | 3.1 (2.2–4.2) | 1.3 (0.8–2.0) | 10.5 (0.0–29.6) |
| Dust mite | 46.7 (3.39) | 2.5 (1.9–3.2) | 2.4 (1.8–3.2) | 1.3 (0.9–1.8) | 10.1 (1.8–40.7) |
| Russian thistle | 29.6 (3.84) | 2.5 (1.6–3.8) | 2.7 (1.8–4.0) | 1.2 (0.7–2.0) | 4.3 (0.0–17.4) |
| Bermuda grass | 33.9 (3.33) | 2.5 (1.8–3.4) | 2.6 (1.9–3.6) | 0.8 (0.4–1.5) | ‡ |
| Peanut | 17.9 (2.12) | 2.5 (1.8–3.4) | 2.7 (2.0–3.7) | 0.8 (0.5–1.2) | ‡ |
| Perennial rye | 43.8 (3.35) | 2.2 (1.7–2.9) | 2.3 (1.8–3.1) | 0.6 (0.4–0.9) | ‡ |
| G. cockroach | 40.8 (2.83) | 2.1 (1.6–2.7) | 2.2 (1.7–2.8) | 1.2 (0.9–1.6) | 7.6 (1.2–36.2) |
A positive response to cat allergen had the highest PAR (29.3%), followed by positive responses to Alternaria (21.1%), white oak (20.9%), dust mite (10.1%), and cockroach (7.6%) (Table 3). The PARs for short ragweed and Russian thistle were 10.5% and 4.3%, respectively; however, their confidence intervals included 0. The PARs for Bermuda grass, peanut, and perennial rye were not calculable because their odds ratios were less than 1.0 (the PAR formula requires an odds ratio ≥ 1.0). The PARS for dust mite and German cockroach allergens had confidence intervals that excluded 0 even though the confidence intervals for their odds ratios included 1. This inconsistency was possible because the confidence interval of the PAR is influenced by the variances of both the odds ratio and percent atopic among asthmatics as well as their covariance, which is not shown. Further, the PAR confidence interval was based on Fay’s method; as such there is an additional inherent variability associated with the jackknife estimation process.
A secondary analysis was performed to determine how similar odds ratios for solitary responses to specific allergens would be to the fully adjusted, allergen-specific odds ratios shown in Table 3. In a separate model for each allergen (adjusted for the subject characteristics), the allergen-specific skin test response was categorized as 1) negative to any allergen (reference category), 2) positive to that specific allergen but negative to all others, i.e., a solitary response, 3) positive to that specific allergen and at least one other, and 4) negative to that specific allergen but positive to at least one other. Those results are shown in Table E1 in the Online Supplement, but summarized here. With a few exceptions, odds ratios for solitary responses were similar to the fully-adjusted allergen-specific odds ratios in Table 3, although the 95% confidence intervals for the solitary responses were very wide due to the low frequencies: cat = 3.9 (0.9–16.4), Alternaria = 2.7 (0.9–8.0), white oak = 2.4 (0.2–24.2), short ragweed = 1.8 (0.5–6.4), dust mite = 1.3 (0.5–3.3), perennial rye = 0.5 (0.1–2.5), and German cockroach = 1.4 (0.6–3.3). The odds ratio for a solitary response to Bermuda grass was 2.3 (0.2–22.4), unlike the odds ratio of 0.8 in Table 3, and odds ratios for Russian thistle and peanut could not be estimated because there were no asthma cases among persons with solitary responses to those allergens. If the allergen-specific response occurred with a response to at least one other allergen, the allergen-specific odds ratio was at least 4.4 and the 95% confidence interval excluded 1.0 (Table E1).
Table E1.
Associations between allergen-specific skin tests and asthma with each allergen-specific test categorized into four categories as opposed to positive versus negative.
| Allergen-specific skin test | Mean (min., max.) number of positive tests | Asthmatic subjects/total subjects | Adjusted OR (95% CI)* |
|---|---|---|---|
| Cat | |||
| No positive skin tests | 0.0 (0, 0) | 104/4629 | 1.0 (reference) |
| Positive to cat only | 1.0 (1, 1) | 9/99 | 3.9 (0.9–16.4) |
| Positive to cat and at least one other | 5.8 (2, 10) | 185/1450 | 7.4 (4.8–11.2) |
| Negative to cat, positive to at least one other | 2.7 (1, 9) | 222/4197 | 2.1 (1.3–3.4) |
| Alternaria | |||
| No positive skin tests | 0.0 (0, 0) | 104/4629 | 1.0 (reference) |
| Positive to Alternaria only | 1.0 (1, 1) | 7/137 | 2.7 (0.9–8.0) |
| Positive to Alternaria and at least one other | 5.9 (2, 10) | 183/1300 | 7.9 (4.6–13.4) |
| Negative to Alternaria, positive to at least one other | 2.9 (1, 9) | 226/4309 | 2.5 (1.6–3.9) |
| White oak | |||
| No positive skin tests | 0.0 (0, 0) | 104/4629 | 1.0 (reference) |
| Positive to oak only | 1.0 (1, 1) | 1/13 | 2.4 (0.2–24.2) |
| Positive to oak and at least one other | 6.5 (2, 10) | 140/1157 | 7.5 (4.9–11.6) |
| Negative to oak, positive to at least one other | 2.6 (1, 9) | 275/4576 | 2.5 (1.6–4.0) |
| Short ragweed | |||
| No positive skin tests | 0.0 (0, 0) | 104/4629 | 1.0 (reference) |
| Positive to ragweed only | 1.0 (1, 1) | 8/173 | 1.8 (0.5–6.4) |
| Positive to ragweed and at least one other | 5.5 (2, 10) | 250/2550 | 5.0 (3.3–7.7) |
| Negative to ragweed, positive to at least one other | 2.0 (1, 9) | 158/3023 | 2.5 (1.5–4.1) |
| Dust mite | |||
| No positive skin tests | 0.0 (0, 0) | 104/4629 | 1.0 (reference) |
| Positive to dust mite only | 1.0 (1, 1) | 14/441 | 1.3 (0.5–3.3) |
| Positive to dust mite and at least one other | 4.9 (2, 10) | 238/2340 | 4.7 (3.1–7.3) |
| Negative to dust mite, positive to at least one other | 2.7 (1, 9) | 164/2965 | 2.9 (1.8–4.8) |
| Russian thistle | |||
| No positive skin tests | 0.0 (0, 0) | 104/4629 | 1.0 (reference) |
| Positive to thistle only | 1.0 (1, 1) | 0/34 | undefined |
| Positive to thistle and at least one other | 6.2 (2, 10) | 157/1493 | 5.2 (3.1–8.9) |
| Negative to thistle, positive to at least one other | 2.5 (1, 9) | 259/4219 | 3.0 (1.9–4.6) |
| Bermuda grass | |||
| No positive skin tests | 0.0 (0, 0) | 104/4629 | 1.0 (reference) |
| Positive to Bermuda only | 1.0 (1, 1) | 1/51 | 2.3 (0.2–22.4) |
| Positive to Bermuda and at least one other | 6.1 (2, 10) | 189/1869 | 4.9 (3.3–7.3) |
| Negative to Bermuda, positive to at least one other | 2.3 (1, 8) | 226/3826 | 2.9 (1.8–4.9) |
| Peanut | |||
| No positive skin tests | 0.0 (0, 0) | 104/4629 | 1.0 (reference) |
| Positive to peanut only | 1.0 (1, 1) | 0/22 | undefined |
| Positive to peanut and at least one other | 7.1 (2, 10) | 99/849 | 5.9 (3.6–9.4) |
| Negative to peanut, positive to at least one other | 2.9 (1, 9) | 317/4875 | 3.2 (2.0–4.9) |
| Perennial rye | |||
| No positive skin tests | 0.0 (0, 0) | 104/4629 | 1.0 (reference) |
| Positive to rye only | 1.0 (1, 1) | 4/192 | 0.5 (0.1–2.5) |
| Positive to rye and at least one other | 5.5 (2, 10) | 246/2593 | 4.4 (2.9–6.5) |
| Negative to rye, positive to at least one other | 1.9 (1, 9) | 166/2961 | 3.0 (1.8–5.1) |
| German cockroach | |||
| No positive skin tests | 0.0 (0, 0) | 104/4629 | 1.0 (reference) |
| Positive to cockroach only | 1.0 (1, 1) | 19/558 | 1.4 (0.6–3.3) |
| Positive to cockroach and at least one other | 4.8 (2, 10) | 216/2484 | 4.4 (2.8–6.9) |
| Negative to cockroach, positive to at least one other | 2.9 (1, 9) | 181/2704 | 3.1 (2.0–4.9) |
Adjusted by all subject characteristics in Table 1
DISCUSSION
In the U.S. population aged 6–59 years, 56.3% of current asthma cases were attributable to atopy, as measured by a positive skin test response to any of 10 allergens. The PAR was significantly greater among males than females, among persons in the highest education category than in lower education categories, and among persons living in highly populated metropolitan areas than in all other areas.
The PAR estimate for the U.S. population is higher than the PAR reported in several studies. In the meta-analysis by Pearce et al., the mean PAR was 38% across studies of children and 37% across studies of adults.7 Among 4-year-old children on the Isle of Wight, Arshad et al. found that atopy (skin prick test positivity to any of 12 allergens) attributed to 35% of asthma cases.1 In a study of adults in the Pirkanmaa District of Southern Finland, Jaakkola et al. reported a PAR (defined by total and specific IgE) of 30%.13 Among Australian children aged 8–10 years, Ponsonby et al. found that atopy (defined as skin test positivity to any of 10 aeroallergens) attributed to 33% of asthma cases, although the percentage was 54% for past hospital attendance for asthma.14 And, in the European Community Respiratory Heath Study, a 36-center study of adults in 16 countries, the mean PAR across centers (atopy was defined as specific serum IgE > 0.35 kU/L to any of 5 allergens) was 30%.15 However, the U.S. estimate fell within the range of center-specific PARs (4% to 61%). Centers with a PAR similar to the U.S. estimate were Huelva, Spain (61%); Groningen, the Netherlands (58%); Antwerp, Belgium (55%); Bordeaux, France (55%); Wellington, New Zealand (52%); and Umea, Sweden (50%). The variation in PARs across the studies could reflect differences in environmental exposures and genetics between the populations as well as differences in study methodologies, such as the assessments of atopy and asthma and subject selection.
In the U.S. population, the PAR differed significantly by sex, education, and urbanization categories. Across categories of those characteristics, the atopy-asthma odds ratios differed significantly. Undoubtedly, there are cofactors associated with these characteristics that strengthen or weaken the effects of atopy on asthma. One possible cofactor—and there are likely to be many—is allergen exposure, which was not measured in NHANES III. Data from the National Survey of Lead and Allergens in Housing have shown that people in the above high school education category have a higher mean level of cat allergen in their homes than people in the lower education categories whereas people living in highly populated metropolitan areas are more likely to have elevated levels of cockroach allergen in their homes than people living in other areas.16, 17 In addition, studies from rural areas in Europe have consistently shown that children growing up on farms are less likely to develop atopy and allergic disease,18 the presumption being that certain microbial exposures in early childhood may be beneficial. However, in NHANES III, the urbanization category “all other areas” consists only partially of rural or farm families, so it is not known whether any differences in the PAR by urbanization can be attributed to a protective effect of rural or farm exposures. Whereas females are more likely than males to have asthma, this study found that males are more likely to have atopic asthma. Why the etiology of asthma would differ between males and females is not known, but the difference suggests that reproductive hormones may play a role. If reproductive hormones suppressed atopic asthma or promoted non-atopic asthma in females, or if testosterone suppressed non-atopic asthma in males, a difference in the asthma-atopy association by sex would occur. Leptin, a hormone associated with obesity, might also play a role in the observed difference. A recent study indicated that serum concentrations of leptin was a stronger risk factor for asthma in females than males, and the same study found that BMI was associated with asthma in females but not males.19 However, the authors of that study did not indicate whether leptin or BMI influenced asthma through atopic or non-atopic pathways. Besides hormones, differences in environmental exposures, such as tobacco smoke, alcohol, diet, and occupations, are potential explanations for the differences in PAR by sex.
Of the ten allergens included in the skin test panel, only cat, Alternaria, and white oak showed significant, positive associations with asthma after adjustment by the subject characteristics and all other allergens. Cat allergen had the largest fully-adjusted odds ratio, and a positive test to cat allergen accounted for the highest percentage of asthma cases (29.3%). Whereas some studies have shown that exposure to cats may be protective for development of allergic sensitization and disease,20 sensitization to cat appears to be a strong risk factor for asthma. In the European Community Respiratory Heath Survey, sensitization to cat allergen had the highest odds ratio for asthma, although sensitization to house dust mite and Timothy grass accounted for more cases of asthma (PARs were 18.2% for dust mite, 17.1% for Timothy grass, and 14.1% for cat).15 In a study of Swedish adults, Plaschke et al. reported that skin prick test positivity to cats and dogs had the strongest associations with asthma, while associations with dust mites and grass were less pronounced.21
Why did most of the allergen-specific skin tests lose statistical significance with asthma after adjustment by all the allergen-specific tests? One potential explanation is that some allergens were associated with asthma before adjustment not because they were independently associated with asthma but because they often occurred with one or more allergens that were. The mean number of positive skin test responses among persons with at least one positive response was 3.5. Thus, for any given allergen, a positive test to that allergen usually occurred with a positive response to other allergens, perhaps other allergens that were more strongly associated with asthma. For example, on average, people who tested positive to dust mite allergen tested positive to 4.9 allergens (Table E1). Unadjusted, the odds ratio for dust mite was 2.5 (Table 3); however, as a solitary response (Table E1) or as a response fully adjusted by all the allergens (Table 3), the odds ratio was 1.3.
Because the allergen-specific results represent averages across the U.S. population, some caution must be used in interpreting them when considering an individual. For a given individual, or among individuals within specific regions or subpopulations of the U.S., some of the allergen-specific sensitizations may play a more or less predominant role in asthma.
The most important limitation to the study is that the data is cross-sectional, a limitation common to many of the published papers that have examined the percentage of asthma cases attributable to atopy. The estimation of population attributable risk assumes that the exposure was present before the disease—a criterion for causality. However, because asthma and skin test responses were assessed at the same point in time, the temporal relationship between the two variables cannot be established. If the onset of asthma occurred prior to the onset of allergic sensitization in a significant percentage of asthma cases, then this study has overestimated the contribution of atopy to asthma.
The estimation of PAR also assumes that the relative risk estimate is unconfounded.11 Because adjustment by the 9 subject characteristics had little effect on the atopy-asthma association (unadjusted OR = 3.3 and adjusted OR = 3.5), confounding was likely well controlled in the analyses of atopy. However, for the allergen-specific skin tests, the odds ratios changed dramatically with adjustment by other allergens. Because many allergens were not skin tested, it is possible that the fully-adjusted allergen-specific odds ratios reported in Table 3 were confounded.
Other important limitations were that the assessment of atopy was limited to the skin testing of only 10 allergens, neither total nor allergen-specific serum IgE was measured, and neither subjects younger than 6 years nor older than 59 years were skin tested in NHANES III. Without a larger panel of allergens or the availability of serum IgE measurements, this study may have underestimated the prevalence of atopy, which would cause the PARs to be underestimated. In an attempt to address this limitation, atopy was redefined in a secondary analysis as either hay fever or a positive skin test response. The inclusion of hay fever into the definition only increased the prevalence of atopy in the total population from 54.2% to 56.0%, which suggests that the 10-allergen skin test panel included allergens to which most hay fever cases would respond. With the PAR peaking in the middle category (Table 1), it is possible that there would have been an age effect if all ages had been included. In NHANES 2005–2006, total IgE and specific IgE to 19 allergens was measured on all subjects aged 1 year and older. Those data, when made available, will allow for a more precise estimate of atopy and a broader assessment of age effects.
The results of this study have two implications. First, the population attributable risk conveys a sense of how much disease can be prevented by eliminating the exposure or blocking its effects.22 Therefore, if atopy could be prevented, reversed, or blocked, then a large percentage of current asthma cases could be prevented. Atopy, by definition, is the result of gene-environment interactions; therefore, at least in theory, intervention at either the genetic or environmental level could prevent atopy. However, intervention at the genetic level is not yet possible and intervention at the environmental level by altering allergen and other exposures has had mixed results, perhaps because of our limited understanding of the environmental exposures that influence atopy and how to modify those exposures. Blocking the pathway between atopy and asthma through immunotherapy, medication, or reduction in allergen and other environmental exposures should also lead to a large reduction in asthma cases. Second, this study’s results highlight the need for research into the non-atopic causes of asthma since one-third to one-half of the asthma cases apparently have a non-atopic etiology. Pearce et al. have suggested that atopic asthma research has been at the expense of non-atopic asthma research;7 however, for the burden of asthma in the population to be significantly reduced, asthma research needs to address the causes of and interventions for both atopic and non-atopic asthma.
Acknowledgments
This research was funded by the Intramural Research Programs of the National Institute of Environmental Health Sciences and the National Institute of Allergy and Infectious Diseases, the National Institutes of Health
ABBREVIATIONS AND ACRONYMS
- IgE
immunoglobulin E
- IU/ml
international units per milliliter
- NHANES III
Third National Health and Nutrition Examination Survey
- PAR
population attributable risk
References
- 1.Arshad SH, Tariq SM, Matthews S, Hakim E. Sensitization to common allergens and its association with allergic disorders at age 4 years: a whole population birth cohort study. Pediatrics. 2001;108:E33. doi: 10.1542/peds.108.2.e33. [DOI] [PubMed] [Google Scholar]
- 2.Gergen PJ, Turkeltaub PC. The association of individual allergen reactivity with respiratory disease in a national sample: data from the second National Health and Nutrition Examination Survey, 1976–80 (NHANES II) J Allergy Clin Immunol. 1992;90:579–88. doi: 10.1016/0091-6749(92)90130-t. [DOI] [PubMed] [Google Scholar]
- 3.Squillace SP, Sporik RB, Rakes G, Couture N, Lawrence A, Merriam S, et al. Sensitization to dust mites as a dominant risk factor for asthma among adolescents living in central Virginia. Multiple regression analysis of a population-based study. Am J Respir Crit Care Med. 1997;156:1760–4. doi: 10.1164/ajrccm.156.6.9704026. [DOI] [PubMed] [Google Scholar]
- 4.Lanphear BP, Kahn RS, Berger O, Auinger P, Bortnick SM, Nahhas RW. Contribution of residential exposures to asthma in US children and adolescents. Pediatrics. 2001;107:E98. doi: 10.1542/peds.107.6.e98. [DOI] [PubMed] [Google Scholar]
- 5.Sporik R, Holgate ST, Platts-Mills TA, Cogswell JJ. Exposure to house-dust mite allergen (Der p I) and the development of asthma in childhood. A prospective study. N Engl J Med. 1990;323:502–7. doi: 10.1056/NEJM199008233230802. [DOI] [PubMed] [Google Scholar]
- 6.Lau S, Illi S, Sommerfeld C, Niggemann B, Bergmann R, von Mutius E, et al. Early exposure to house-dust mite and cat allergens and development of childhood asthma: a cohort study. Multicentre Allergy Study Group. Lancet. 2000;356:1392–7. doi: 10.1016/s0140-6736(00)02842-7. [DOI] [PubMed] [Google Scholar]
- 7.Pearce N, Pekkanen J, Beasley R. How much asthma is really attributable to atopy? Thorax. 1999;54:268–72. doi: 10.1136/thx.54.3.268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Burrows B, Martinez FD, Halonen M, Barbee RA, Cline MG. Association of asthma with serum IgE levels and skin-test reactivity to allergens. N Engl J Med. 1989;320:271–7. doi: 10.1056/NEJM198902023200502. [DOI] [PubMed] [Google Scholar]
- 9.Arbes SJ, Jr, Gergen PJ, Elliott L, Zeldin DC. Prevalences of positive skin test responses to 10 common allergens in the US population: Results from the Third National Health and Nutrition Examination Survey. J Allergy Clin Immunol. 2005;116:377–83. doi: 10.1016/j.jaci.2005.05.017. [DOI] [PubMed] [Google Scholar]
- 10.National Center for Health Statistics (U.S.) Public use data tape documentation: allergy skin testing: tape number 5309: National Health and Nutrition Examination Survey, 1976–80. Hyattsville, Md: U.S. Dept. of Health and Human Services, Public Health Service, National Center for Health Statistics; 1986. [Google Scholar]
- 11.Miettinen OS. Proportion of disease caused or prevented by a given exposure, trait or intervention. Am J Epidemiol. 1974;99:325–32. doi: 10.1093/oxfordjournals.aje.a121617. [DOI] [PubMed] [Google Scholar]
- 12.Judkins DR. Fay’s method for variance estimation. Journal of Official Statistics. 1990;6:223–39. [Google Scholar]
- 13.Jaakkola MS, Ieromnimon A, Jaakkola JJ. Are atopy and specific IgE to mites and molds important for adult asthma? J Allergy Clin Immunol. 2006;117:642–8. doi: 10.1016/j.jaci.2005.11.003. [DOI] [PubMed] [Google Scholar]
- 14.Ponsonby AL, Gatenby P, Glasgow N, Mullins R, McDonald T, Hurwitz M. Which clinical subgroups within the spectrum of child asthma are attributable to atopy? Chest. 2002;121:135–42. doi: 10.1378/chest.121.1.135. [DOI] [PubMed] [Google Scholar]
- 15.Sunyer J, Jarvis D, Pekkanen J, Chinn S, Janson C, Leynaert B, et al. Geographic variations in the effect of atopy on asthma in the European Community Respiratory Health Study. J Allergy Clin Immunol. 2004;114:1033–9. doi: 10.1016/j.jaci.2004.05.072. [DOI] [PubMed] [Google Scholar]
- 16.Arbes SJ, Jr, Cohn RD, Yin M, Muilenberg ML, Friedman W, Zeldin DC. Dog allergen (Can f 1) and cat allergen (Fel d 1) in US homes: results from the National Survey of Lead and Allergens in Housing. J Allergy Clin Immunol. 2004;114:111–7. doi: 10.1016/j.jaci.2004.04.036. [DOI] [PubMed] [Google Scholar]
- 17.Cohn RD, Arbes SJ, Jr, Jaramillo R, Reid LH, Zeldin DC. National prevalence and exposure risk for cockroach allergen in U.S. households. Environ Health Perspect. 2006;114:522–6. doi: 10.1289/ehp.8561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.von Mutius E. Asthma and allergies in rural areas of europe. Proc Am Thorac Soc. 2007;4:212–6. doi: 10.1513/pats.200701-028AW. [DOI] [PubMed] [Google Scholar]
- 19.Sood A, Ford ES, Camargo CA., Jr Association between leptin and asthma in adults. Thorax. 2006;61:300–5. doi: 10.1136/thx.2004.031468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Platts-Mills T, Vaughan J, Squillace S, Woodfolk J, Sporik R. Sensitisation, asthma, and a modified Th2 response in children exposed to cat allergen: a population-based cross-sectional study. Lancet. 2001;357:752–6. doi: 10.1016/S0140-6736(00)04168-4. [DOI] [PubMed] [Google Scholar]
- 21.Plaschke P, Janson C, Norrman E, Bjornsson E, Ellbjar S, Jarvholm B. Association between atopic sensitization and asthma and bronchial hyperresponsiveness in swedish adults: pets, and not mites, are the most important allergens. J Allergy Clin Immunol. 1999;104:58–65. doi: 10.1016/s0091-6749(99)70114-4. [DOI] [PubMed] [Google Scholar]
- 22.Rothman KJ. Modern epidemiology. 1. Boston: Little Brown; 1986. [Google Scholar]