Abstract
Mother-infant attachment is facilitated in altricial rodents through unique neural mechanisms that include impaired neonatal fear conditioning until the time that pups first begin to leave the nest (sensitive period). Here, we confirmed the developmental emergence of odor fear conditioning in neonatal rat pups, and examined synaptic plasticity of inputs to the basolateral amygdala in vitro. Coronal slices through the amygdala were obtained from sensitive (< 10 days) and post-sensitive (> 10, < 19 days) period pups. Field potentials were recorded in the basolateral amygdala in response to stimulation of either the external capsule (neocortical inputs) or fibers from the cortical nucleus of the amygdala (olfactory inputs). The effects of tetanic stimulation were examined in each pathway. In both pathways, tetanic stimulation induce significant long-term synaptic plasticity in post-sensitive period pups, but no significant plasticity in sensitive period pups incapable of learning odor aversions. GABAA receptor blockade in post-sensitive period slices reverts synaptic plasticity to sensitive period characteristics. The results suggest that sensitive period deficits in fear conditioning may be related to impaired amygdala synaptic plasticity and the immature state of GABAergic inhibition and/or it modulation in the neonatal amygdala.
Keywords: emotion, olfaction, fear memory, infant attachment, GABA, long-term potentiation
1. INTRODUCTION
Forming social attachments is fundamentally important for survival in many altricial species. This is highlighted by the presence of specialized learning circuits during ‘sensitive periods’ of social attachment formation where some forms of learning are facilitated, while others are attenuated. For example, altricial rat pups are dependent on maternal care for survival and exhibit facilitated sensitive period odor preference learning to the maternal odor, which is then used for approach to the caregiver and nipple attachment. Sensitive period pups also show attenuated aversion learning, presumably to prevent pups from learning to avoid the maternal odor. For rat pups, the temporal association of maternal odor with a variety of other maternally generated stimuli, such as grooming, warmth, or milk results in learned approach, nipple attachment and behavioral activation responses by the neonate on subsequent presentation of that odor (Galef and Sherry, 1973, Johanson and Hall, 1979, Johanson and Teicher, 1980, Brake, 1981, Pedersen et al., 1982, Alberts and May, 1984, Sullivan et al., 1986a, Sullivan et al., 1986b, Wilson and Sullivan, 1994). Importantly, the range of interactions with the mother includes painful stimuli, such as biting and being stepped upon, yet neonates fail to learn an aversion to odors paired with such painful stimulation and instead learn to prefer the odor (Haroutunian and Campbell, 1979, Sullivan et al., 1986a, Sullivan et al., 1986b, Camp and Rudy, 1988, Sullivan et al., 2000, Moriceau and Sullivan, 2004a, Roth and Sullivan, 2005). As pups mature and begin to explore the extra-nest environment around postnatal day (PN) 10 (Bolles and Woods, 1964), more ‘adult-like’ fear and inhibitory learning emerges (Haroutunian and Campbell, 1979, Blozovski and Dumery, 1987, Camp and Rudy, 1988, Sullivan et al., 2000, Moriceau and Sullivan, 2004a, Roth and Sullivan, 2005).
Here we explore the neural correlates of attenuated aversion learning and the emergence of fear conditioning in sensitive period and post-sensitive period pups. In adult rats, the amygdala plays a critical role in fear conditioning (Sananes and Campbell, 1989, Rosenkranz and Grace, 2002, Davis et al., 2003, Fanselow and Gale, 2003, LeDoux, 2003, Debiec and Ledoux, 2004, Sevelinges et al., 2004, Schroeder and Shinnick-Gallagher, 2005). Association of a conditioned stimulus and, for example, footshock in juvenile or adult rats causes activation of the amygdala, and induces a modification of conditioned stimulus-evoked responses of amygdala neurons (Rosenkranz and Grace, 2002). Lesions of the amygdala prevent or retard fear learning and memory (LaBar and LeDoux, 1996, Setlow et al., 2000, Gale et al., 2004). Furthermore, synaptic plasticity of cortical and thalamic inputs to the basolateral nucleus of the amygdala appears necessary for normal fear conditioning (Blair et al., 2001, Maren, 2005), such that manipulations that impair or enhance such plasticity also impair or enhance acquisition of behaviorally expressed learned fear (e.g., (Campeau et al., 1992, Davis et al., 1994, Szinyei et al., 2007).
The failure of odor-pain association to induce learned fear in neonates may in part be due, therefore, to the immature state of amygdala circuitry during the early postnatal period. In the adult, neocortical and thalamic inputs to basolateral nucleus neurons demonstrate long-term synaptic plasticity following tetanic stimulation, and this plasticity may either be expressed as potentiation or depression depending on the conditions and presence or absence of GABAA receptor antagonists (Rogan et al., 1997, Heinbockel and Pape, 2000, Rammes et al., 2001, Kaschel et al., 2004). Furthermore, plasticity is expressed at both excitatory and inhibitory synapses (Rogan et al., 1997, Bauer and LeDoux, 2004, Szinyei et al., 2007). Both amygdala synaptic plasticity and learned fear are modulated by a number of factors, including neuromodulators (Rosenkranz and Grace, 2002, Azad et al., 2004), steroid hormones (Setlow et al., 2000) and level of GABAergic inhibition (Watanabe et al., 1995, Rammes et al., 2000). Importantly however, while GABA synthetic enzymes (e.g., GAD (Stork et al., 2000)) and receptor subunits (Zhang et al., 1991) are present at birth in the amygdala, they do not attain adult levels there until several weeks later, suggesting a potential late emergence for the mature expression of amygdala synaptic plasticity (Gilbert and Cain, 1981). In fact, odor-foot shock association that induces amygdala activation (e.g., c-fos labeling) and learned fear in PN12 rat pups, induces neither amygdala activation nor fear in PN10 pups (Sullivan et al., 2000, Moriceau and Sullivan, 2004b, Roth and Sullivan, 2005). It should be noted that pain threshold to footshock is very similar across this age range of pups (Emerich et al., 1985, Barr, 1995, Sullivan et al., 2000, Fitzgerald and Beggs, 2001).
The present report was an examination of synaptic plasticity in two afferent pathways to the basolateral nucleus of the amygdala in vitro, before and after the age at which fear conditioning emerges in the rat. Given that neonatal maternal recognition is primarily olfactory mediated, we examined the putative input from the cortical nucleus of the amygdala to the basolateral nucleus. The cortical nucleus of the amygdala receives direct input from the olfactory bulb (Shipley and Ennis, 1996), and olfactory evoked responses within the amygdala are known to be modified by fear conditioning (Rosenkranz and Grace, 2002). To allow our results to be compared to the extant literature on amygdala synaptic plasticity, we also examined the neocortical input to basolateral nucleus. We hypothesized that the during the sensitive period for learned odor-guided attachment to the mother, plasticity within circuits mediating fear conditioning would be impaired or abnormal. The results suggest a GABAergic-dependent change in synaptic plasticity of both pathways coinciding with developmental emergence of learned fear.
2. RESULTS
Behavior
As shown in Figure 1, behavioural Y maze testing revealed that sensitive period (PN8) pups that received the paired odor-shock conditioning made a larger proportion of choices towards the CS odor, indicating a learned odor preference. Older, post-sensitive period (PN12) pups that received the same conditioning made a smaller proportion of choices towards the CS odor demonstrating a learned odor aversion (ANOVA, condition X age interaction, F (2, 28) = 41.47, p < 0.001; post hoc Fisher test revealed that the paired pups were significantly different from each of the age-matched control groups). This replicates previous results (Sullivan et al., 2000).
Figure 1.
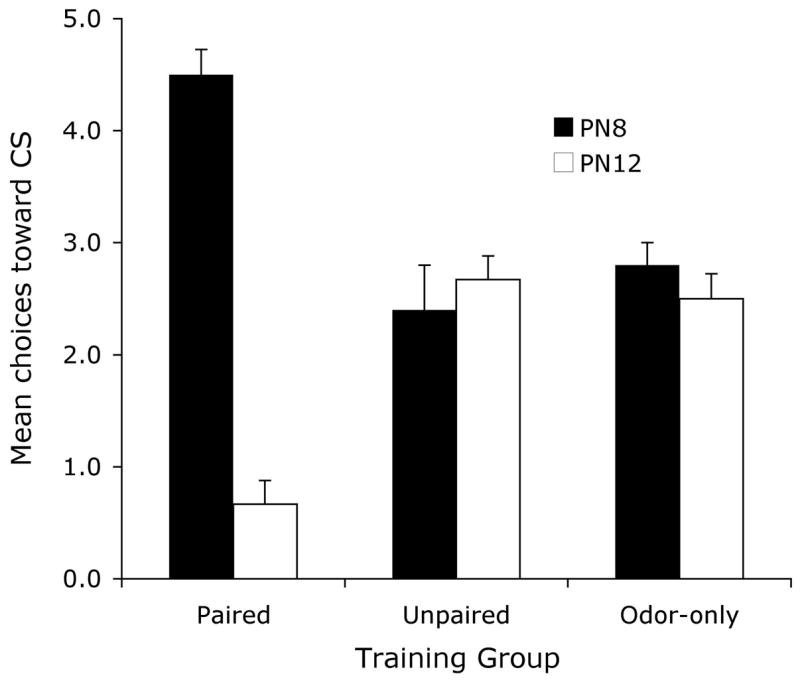
Mean (± sem) number of choices toward the conditioned stimulus (CS) odor during the Y-maze test (total of 5 trials) for PN8 and PN12 pups.
Electrophysiology
As shown in Fig. 2, both stimulation of the dorsal edge of lateral nucleus near the external capsule and stimulation of putative fibers from the cortical nucleus of the amygdala evoked short-latency, negative evoked responses recorded in the basolateral nucleus. The lateral nucleus input pathway has been well described by multiple groups, and is a glutamatergic excitatory response. Similarly, responses to the cortical nucleus input could be reversibly blocked by the glutamatergic receptor antagonist kynurenic acid (10 mM; n =3, data not shown).
Figure 2.
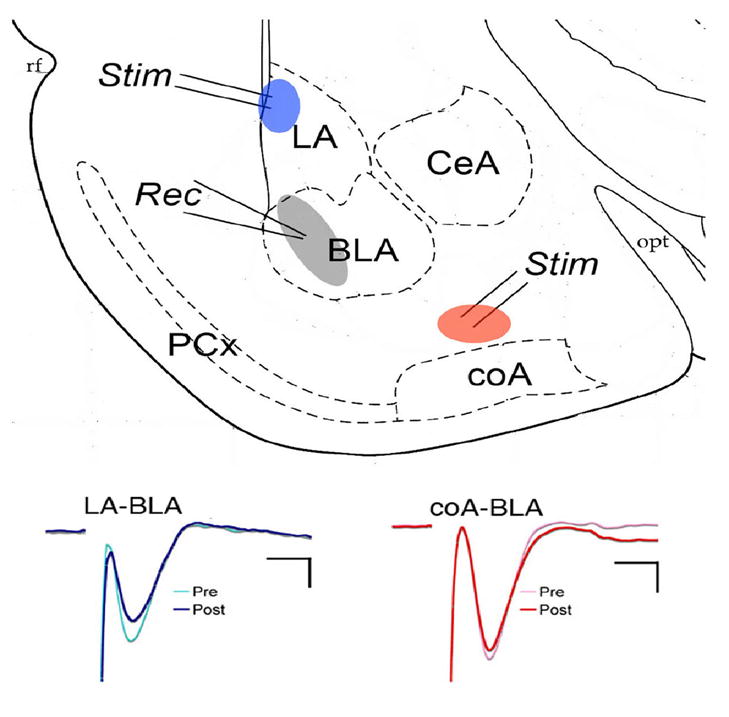
(TOP) Schematic representation of coronal amygdala slice showing approximate stimulation (Stim) and recording (Rec) electrode placements. Only one pathway was tested in each slice. (BOTTOM) Examples of evoked potentials before and after tetanic stimulation of the two pathways. LA = lateral nucleus, BLA = basolateral nucleus, ceA = central nucleus, coA = cortical nucleus, PCx = piriform cortex. Calibration is 2 ms and 2 mV for LA-BLA pathway and 2 ms and 5 mV for coA-BLA pathway.
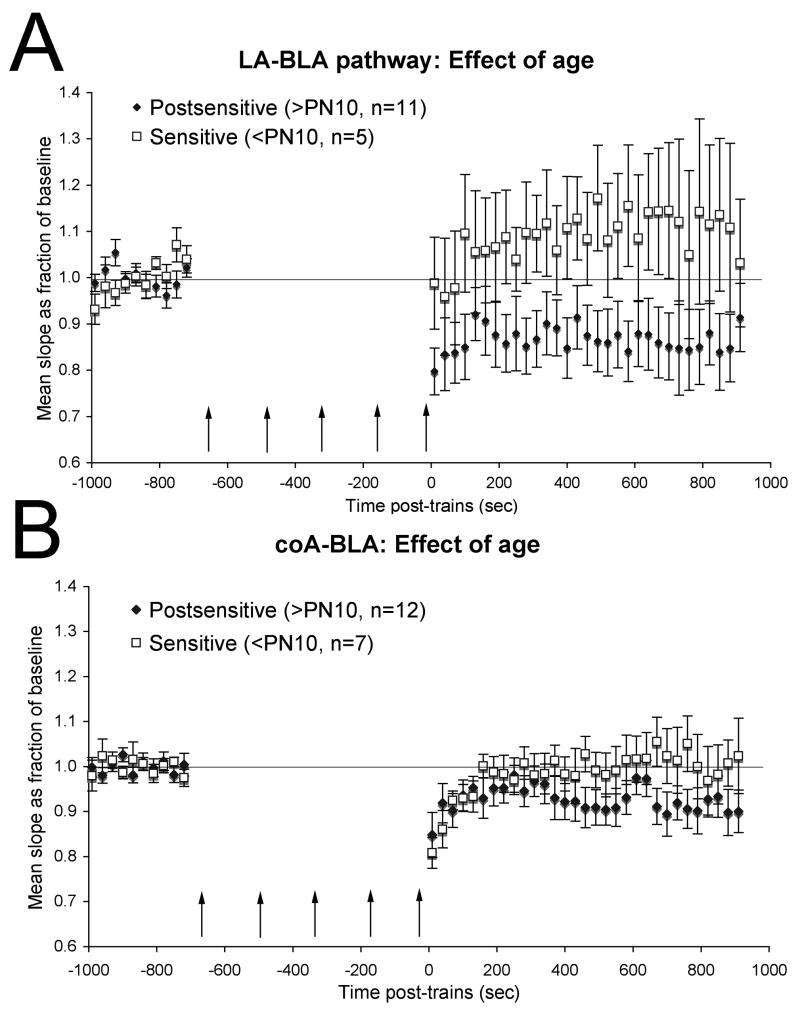
Tetanic stimulation of the lateral nucleus/external capsule pathway in post-sensitive period pups (> PN11; Fig. 3A) induced a long-term depression of responses (repeated measures ANOVA main effect of time, F(30,300) = 3.49, p < 0.001), as has been reported previously (Heinbockel and Pape, 2000, Rammes et al., 2001, Kaschel et al., 2004). In contrast, in slices from sensitive period pups, the same stimulation protocol induced no significant change (repeated measures F(30,120) = 1.12, N.S.), though there was a clear trend toward potentiation. There was a significant difference between sensitive period and post-sensitive period responses post-trains (ANOVA, F(1,46) = 11.77, p < 0.01).
Figure 3.
Effect of age on synaptic plasticity in the basolateral amygdala. (A). Mean slope of evoked field potential as a proportion of baseline recorded in the BLA before and after tetanic stimulation of LA inputs for slices from postsensitive and sensitive period pups. Tetanic stimulation of the lateral amygdala/external capsule induced a long-term depression of synaptic potentials recorded in the basolateral amygdala of post-sensitive period pups. Similar stimulation in slices from sensitive period pups produced no statistically significant change, thought there was a mild potentiation. (B). Same as A, for stimulation of coA inputs. Tetanic stimulation of the putative cortical nucleus input to the basolateral amygdala also produced a significant long-term depression in slices from post-sensitive period pups, but no long-term change in slices from sensitive period pups.
In the cortical nucleus – basolateral nucleus pathway (Fig. 3B), tetanic stimulation in post-sensitive period pups induced a long-term depression, similar to that seen in the lateral nucleus pathway (repeated measures ANOVA, F(30,330) = 2.66, p < 0.001). The magnitude of this change was not as great as that in the external capsule pathway, reflecting either a real difference in plasticity in these two pathways, or perhaps more likely a difference in density of fibers being stimulated at the two sites. Nonetheless, no significant long-term change in synaptic strength was observed after tetanic stimulation of the cortical nucleus pathway in sensitive period pups (repeated measures F(30,180) = 0.87, N.S.). There was a significant difference between sensitive period and post-sensitive period responses post-trains (ANOVA, F(1,55) = 4.74, p < 0.05).
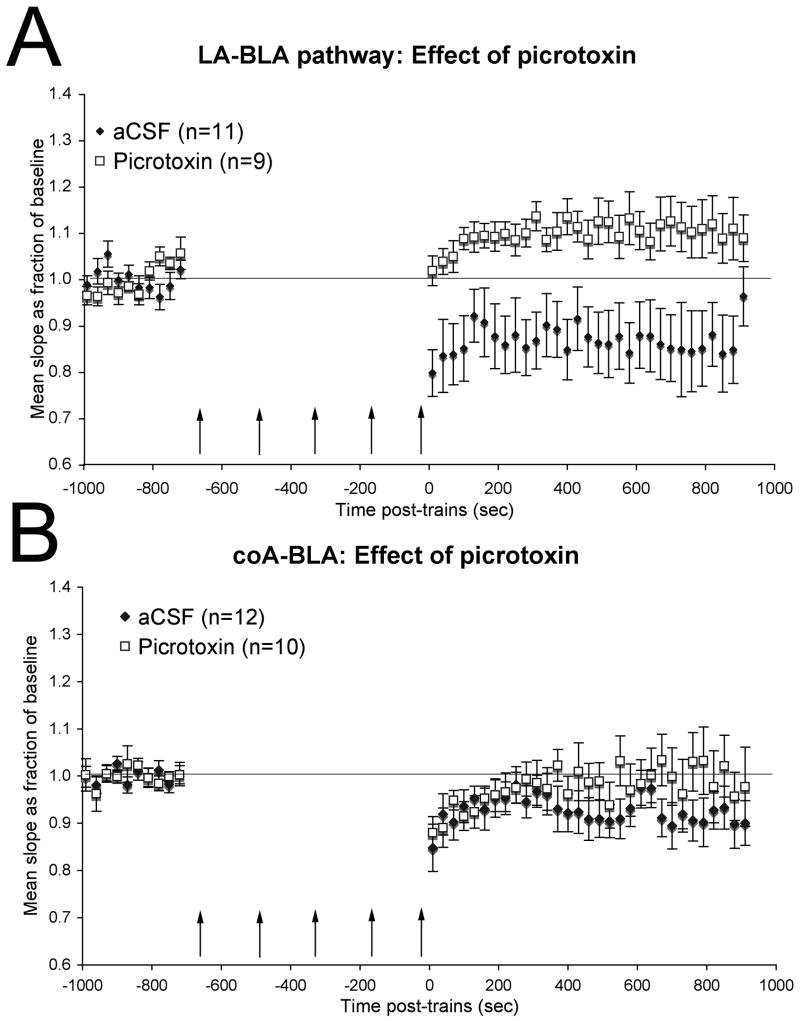
To determine whether the late maturation of GABAergic circuits could contribute to the changes in synaptic plasticity observed here around the time of sensitive period termination, we examined the effect of the GABAA receptor antagonist picrotoxin (100 μM) on plasticity in these two pathways in slices from post-sensitive period pups. Blockade of GABAA receptors in post-sensitive period slices induced changes in slice physiology comparable to that observed in sensitive period pups. In the lateral nucleus to basolateral nucleus pathway of slices from post-sensitive period pups (Fig. 4A), tetanic stimulation in the presence of picrotoxin induced a significant long-term potentiation (repeated measures ANOVA F(30,240) = 4.32, p < 0.01), in contrast to the long-term depression observed under the same conditions in the presence of aCSF above. There was a significant difference between aCSF and picrotoxin treated slices post-trains (ANOVA, F(1, 58) = 23.03, p < 0.01).
Figure 4.
GABAA receptor blockade in post-sensitive period slices reverts synaptic plasticity to sensitive period characteristics. Data for aCSF recordings is the same as shown in Fig. 3. (A) Mean slope of evoked field potential as a proportion of baseline recorded in the BLA before and after tetanic stimulation of LA inputs for slices from postsensitive period pups with and without added picrotoxin. Tetanic stimulation of the lateral amygdala input to basolateral amygdala induced long-term depression in post-sensitive period control slices but long-term potentiation in the presence of picrotoxin. (B) Same as A, for stimulation of coA inputs. Picrotoxin blocked the long-term depression of cortical nucleus input to basolateral amygdala seen in control post-sensitive period slices.
Similarly, in the cortical nucleus to basolateral pathway of slices from post-sensitive period pups (Fig. 4B), picrotoxin reduced the magnitude of long-term depression, though a short-term depression remained. Analysis of train effects greater than 8 min post trains revealed a significant depression in aCSF-treated slices (repeated measures ANOVA, F(9,99) = 3.05, p < 0.01), but no lasting depression in picrotoxin treated slices (repeated measures ANOVA, F(9,81) = 0.68, N.S.). The difference between aCSF and picrotoxin treated post-train effects in this pathway did not quite reach statistical significance (ANOVA, F(1,64) = 3.58, p = 0.062).
3. DISCUSSION
An association of odor and 0.5mA footshock in sensitive period rat pups (< PN10) induces a relative preference for that odor, while similar conditioning in post-sensitive period pups induces a relative aversion, replicating previous results (Camp and Rudy, 1988, Sullivan et al., 2000). While it may seem paradoxical for infant rats to learn to prefer an odor paired with pain, this attenuated learning can prevent avoidance of the mother and increase caregiver proximity-seeking behaviors, regardless of the quality of caretaker treatment. Similar paradoxical attachment occurs in other species, including chicks, infant dogs, nonhuman primates and children (e.g., (Harlow and Harlow, 1965, Salzen, 1967)).
In adults, conditioned fear of stimuli in a variety of sensory modalities is associated with synaptic plasticity of inputs to the basolateral amygdala (Rogan et al., 1997, Rosenkranz and Grace, 2002). While adults display long-term potentiation of afferent input to the amygdala associated with learning in vivo (Rogan et al., 1997), in adult in vitro slices this plasticity is expressed as either long-term potentiation or long-term depression, depending on the stimulation characteristics and presence or absence of GABAA antagonists in the bath, similar to that shown in the post-sensitive period slices here. The present results suggest that at an age where pups do not acquire learned odor aversions, neither neocortical nor cortical nucleus of the amygdala inputs to the basolateral nucleus display the mature form of long-term synaptic plasticity. In fact, in the cortical to the basolateral nucleus pathway, which conveys odor input from the olfactory bulb, no stable synaptic plasticity could be evoked in sensitive period pups that fail to learn aversions. Several days later, when normal learned odor fear can be acquired, normal long-term synaptic plasticity is expressed. Age-dependent changes were observed in both afferent pathways, though the magnitude of plasticity was less in the cortical nucleus of the amygdala pathway compared to the external capsule pathway. These results suggest that modified or impaired synaptic plasticity of inputs to the basolateral amygdala during early development may contribute to the reduced ability of animals at this age to learn odor aversions, and in turn contribute to infant-maternal attachment.
Furthermore, the abnormal characteristics of sensitive period synaptic plasticity can be mimicked in post-sensitive period pups by blockade of GABAA receptors with picrotoxin in both pathways. This finding, combined with the known slow ontogeny of GABA receptors in the amygdala (Zhang et al., 1991, Stork et al., 2000) and limbic system, leads to the hypothesis that developmental emergence of amygdala plasticity and amygdala-dependent behaviors may reflect, in part, maturation of GABAergic function. In support of this hypothesis, manipulations known to modulate GABAergic function also modulate the age of sensitive period termination. For example, corticosterone (CORT) modulates GABAergic inhibition and principal cell excitability in the adult amygdala (Duvarci and Pare, 2007) and other limbic regions (Verkuyl et al., 2005). Elevating CORT levels within the amygdala of sensitive period pups allows precocial emergence of fear conditioning (Moriceau and Sullivan, 2004a), while reducing CORT levels in post-sensitive period pups either by adrenalectomy or maternal presence (Wiedenmayer et al., 2003) reinstates the sensitive period blockade on fear conditioning (Moriceau and Sullivan, 2004b, Moriceau and Sullivan, 2006).
The potential link between the developmental emergence of fear learning with GABAergic function and modulation of amygdala synaptic plasticity also has ramifications for the known long-term effects of early stress and fear learning. For example, early neonatal handling and maternal separation are correlated with changes in limbic system GABA receptor sub-unit expression and receptor function in adults (Hsu et al., 2003), as well as several behavioral effects such as reduced fear responses (Ladd et al., 2000). Similarly, the postnatal sensitive period odor-shock conditioning paradigm used in the present study reduces odor fear conditioning when those animals are later trained as adults (Sevelinges et al., in press). Integrating the effects of early experience on GABAergic maturation, endocrine function and amygdala synaptic plasticity may provide important clues toward understanding the behavioral consequences of infant stress and trauma.
4. EXPERIMENTAL PROCEDURES
The subjects were male and female preweanling Long Evans hooded rat pups bred and born in the University of Oklahoma animal care facilities. Rats were housed in rectangular polypropylene cages (34×29×17 cm) lined with wood chips and kept in a temperature (20o C) and light (0800–2000 hr) controlled room with ad lib food and water. The birth date was considered PN0, litters were culled to 12 pups on PN1 and only 1 male and/or female from each litter was used in any training/test condition. Animal care and experimental procedures conformed to US Public Health Service Policy on Humane Care and Use of Laboratory Animals and were approved by the Institutional Animal Care and Use Committee.
Behavior
Pups were randomly assigned to one of three groups for odor-shock conditioning. The paired group (n=6 PN8, n = 6 PN12)received 10 pairings of the unconditioned stimulus (US -moderate tail shock; 0.5 mA, 1 sec.) paired with the last second of a 30 sec conditioned stimulus (CS, 2L/min, 1:10 peppermint vapor:air) delivered through an olfactometer controlled by a Chrontrol at a 4 min inter-trial interval. The unpaired group (n=5 PN8, n = 6 PN12)received the shock 1.5–2 min after the CS odor presentation, while the odor-only group (n=5 PN8, n = 6 PN12) received only the odor CS. Pups were removed from the nest immediately prior to conditioning and given 10 min to habituate to the training apparatus (600 ml Pyrex jars) prior to the training session, and returned immediately to the nest following training. Pups’ behaviors were monitored during training and all pups in the paired odor-shock group showed learning curves (documented by a behavioral activity scale related to the number of limbs moving), while control pups showed no indication of an acquisition curve. All pups also showed strong responsiveness to shock regardless of the conditioning groups.
The next day, pups were tested in a Y-maze to assess their expression of an odor preference or aversion using a video-tracking system (Columbus Instruments). The Y-maze required pups to choose between the CS odor and a familiar odor (the same wood shavings used as nest bedding but clean) placed at the end of the two arms of a Plexiglas Y-maze (choice arms: 8.5x24x8 cm). Pups were placed in the start box for 5 sec, the alley doors opened, and the pups were given 60 sec to choose an arm (pup’s entire body was past the alley entrance). Each pup completed 5 trials. Between trials, the pup was placed in a holding cage for 5 sec and he floor wiped clean with water and dried.
Electrophysiology
Amygdala slices were obtained from pups in one of two age groups, PN7-PN10 (<20g, sensitive period pups) and PN11-PN19 (>20g post-sensitive period pups). Brains were rapidly dissected from isoflurane anesthetized pups and placed in ice-cold oxygenated artificial cerebrospinal fluid (aCSF) composed of 124mM NaCl, 5 mM KCl, 1.24 mM KH2PO4, 2.4 mM CaCl, 1.3 mM MgSO4, 26 mM NaHCO3 and 10 mM glucose. Coronal, 400μ thick slices including the amygdala were cut with a vibratome. Slices were incubated at room temperature for 1 hour in aCSF. The slices were then moved to a superfusion chamber at room temperature for recording. In all procedures described below, electrical stimulation was provided with a concentric bipolar stainless steel stimulating electrode and field potentials were recorded using tungsten microelectrodes (A-M Systems). Amplified and bandpass (5Hz-1kHz) filtered signals were acquired at 5 kHz and analyzed using Spike2 software (CED, Inc).
Evoked responses and plasticity were examined in two different pathways afferent to the basolateral amygdala in different slices. Amygdalar nuclei were located using neuroanatomical landmarks. Activation of neocortical inputs was mimicked by electrical stimulation of the dorsal lateral amygdala near the external capsule. Activation of inputs from the olfactory system was mimicked by stimulation near the dorsal edge of the cortical nucleus of the amygdala, which receives direct input from mitral/tufted cells of the ipsilateral olfactory bulb (Shipley and Ennis, 1996). Following recording of baseline responses to 0.1 ms duration, 10–100 μA test pulses as 0.1 Hz, high frequency tetanic stimulation was delivered (100 Hz, 1 sec trains with 3 min between trains, 5 trains total, same pulse duration and intensity as the test stimuli), followed by a return to 0.1 Hz test pulse stimulation for up to 60 min post-trains. Only one pathway was tested in each slice.
In a subset of slices, the effect of the GABAA receptor antagonist picrotoxin (100 μM) on response to tetanic stimulation was tested. Following recording of baseline responses in aCSF, slices were superfused with picrotoxin for at least 10 min, followed by the stimulation protocol described above.
Evoked responses were quantified by measuring the slope of the initial phase of evoked waveforms averaged from three stimuli. Effects of the tetanic stimulation were statistically examined with repeated measures ANOVA over data from 3 min pre-trains and the first 10 min post-trains. Mean effect of tetanic stimulation was compared across age groups or drug condition with ANOVA of the data at least 8 min post-trains.
Acknowledgments
This work was funded by grants NIDCD-DC03906 to DAW and NICHD-HD33402, NSF IOB-0544406 & the Oklahoma Center for the Advancement of Science and Technology to RMS.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alberts JR, May B. Nonnutritive, thermotactile induction of filial huddling in rat pups. Dev Psychobiol. 1984;17:161–181. doi: 10.1002/dev.420170207. [DOI] [PubMed] [Google Scholar]
- Azad SC, Monory K, Marsicano G, Cravatt BF, Lutz B, Zieglgansberger W, Rammes G. Circuitry for associative plasticity in the amygdala involves endocannabinoid signaling. J Neurosci. 2004;24:9953–9961. doi: 10.1523/JNEUROSCI.2134-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barr GA. Ontogeny of nociception and antinociception. NIDA Res Monogr. 1995;158:172–201. [PubMed] [Google Scholar]
- Bauer EP, LeDoux JE. Heterosynaptic long-term potentiation of inhibitory interneurons in the lateral amygdala. J Neurosci. 2004;24:9507–9512. doi: 10.1523/JNEUROSCI.3567-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blair HT, Schafe GE, Bauer EP, Rodrigues SM, LeDoux JE. Synaptic plasticity in the lateral amygdala: a cellular hypothesis of fear conditioning. Learn Mem. 2001;8:229–242. doi: 10.1101/lm.30901. [DOI] [PubMed] [Google Scholar]
- Blozovski D, Dumery V. Development of amygdaloid cholinergic mediation of passive avoidance learning in the rat. II. Nicotinic mechanisms. Exp Brain Res. 1987;67:70–76. doi: 10.1007/BF00269454. [DOI] [PubMed] [Google Scholar]
- Bolles RC, Woods PJ. The ontogeny of behaviour in the albino rat. Animal Behaviour. 1964;12:427–441. [Google Scholar]
- Brake SC. Suckling infant rats learn a preference for a novel olfactory stimulus paired with milk delivery. Science. 1981;211:506–508. doi: 10.1126/science.7192882. [DOI] [PubMed] [Google Scholar]
- Camp LL, Rudy JW. Changes in the categorization of appetitive and aversive events during postnatal development of the rat. Dev Psychobiol. 1988;21:25–42. doi: 10.1002/dev.420210103. [DOI] [PubMed] [Google Scholar]
- Campeau S, Miserendino MJ, Davis M. Intra-amygdala infusion of the N-methyl-D-aspartate receptor antagonist AP5 blocks acquisition but not expression of fear-potentiated startle to an auditory conditioned stimulus. Behav Neurosci. 1992;106:569–574. doi: 10.1037//0735-7044.106.3.569. [DOI] [PubMed] [Google Scholar]
- Davis M, Rainnie D, Cassell M. Neurotransmission in the rat amygdala related to fear and anxiety. Trends Neurosci. 1994;17:208–214. doi: 10.1016/0166-2236(94)90106-6. [DOI] [PubMed] [Google Scholar]
- Davis M, Walker DL, Myers KM. Role of the amygdala in fear extinction measured with potentiated startle. Ann N Y Acad Sci. 2003;985:218–232. doi: 10.1111/j.1749-6632.2003.tb07084.x. [DOI] [PubMed] [Google Scholar]
- Debiec J, Ledoux JE. Disruption of reconsolidation but not consolidation of auditory fear conditioning by noradrenergic blockade in the amygdala. Neuroscience. 2004;129:267–272. doi: 10.1016/j.neuroscience.2004.08.018. [DOI] [PubMed] [Google Scholar]
- Duvarci S, Pare D. Glucocorticoids enhance the excitability of principal basolateral amygdala neurons. J Neurosci. 2007;27:4482–4491. doi: 10.1523/JNEUROSCI.0680-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emerich DF, Scalzo FM, Enters EK, Spear NE, Spear LP. Effects of 6-hydroxydopamine-induced catecholamine depletion on shock-precipitated wall climbing of infant rat pups. Dev Psychobiol. 1985;18:215–227. doi: 10.1002/dev.420180303. [DOI] [PubMed] [Google Scholar]
- Fanselow MS, Gale GD. The amygdala, fear, and memory. Ann N Y Acad Sci. 2003;985:125–134. doi: 10.1111/j.1749-6632.2003.tb07077.x. [DOI] [PubMed] [Google Scholar]
- Fitzgerald M, Beggs S. The neurobiology of pain: developmental aspects. Neuroscientist. 2001;7:246–257. doi: 10.1177/107385840100700309. [DOI] [PubMed] [Google Scholar]
- Gale GD, Anagnostaras SG, Godsil BP, Mitchell S, Nozawa T, Sage JR, Wiltgen B, Fanselow MS. Role of the basolateral amygdala in the storage of fear memories across the adult lifetime of rats. J Neurosci. 2004;24:3810–3815. doi: 10.1523/JNEUROSCI.4100-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galef BG, Jr, Sherry DF. Mother’s milk: a medium for transmission of cues reflecting the flavor of mother’s diet. J Comp Physiol Psychol. 1973;83:374–378. doi: 10.1037/h0034665. [DOI] [PubMed] [Google Scholar]
- Gilbert ME, Cain DP. A developmental study of kindling in the rat. Brain Res. 1981;254:321–328. doi: 10.1016/0165-3806(81)90041-9. [DOI] [PubMed] [Google Scholar]
- Harlow HF, Harlow MK. The Effect of Rearing Conditions on Behavior. Int J Psychiatry. 1965;1:43–51. [PubMed] [Google Scholar]
- Haroutunian V, Campbell BA. Emergence of interoceptive and exteroceptive control of behavior in rats. Science. 1979;205:927–929. doi: 10.1126/science.472715. [DOI] [PubMed] [Google Scholar]
- Heinbockel T, Pape HC. Input-specific long-term depression in the lateral amygdala evoked by theta frequency stimulation. J Neurosci. 2000;20:RC68. doi: 10.1523/JNEUROSCI.20-07-j0002.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu FC, Zhang GJ, Raol YS, Valentino RJ, Coulter DA, Brooks-Kayal AR. Repeated neonatal handling with maternal separation permanently alters hippocampal GABAA receptors and behavioral stress responses. Proc Natl Acad Sci U S A. 2003;100:12213–12218. doi: 10.1073/pnas.2131679100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johanson IB, Hall WG. Appetitive learning in 1-day-old rat pups. Science. 1979;205:419–421. doi: 10.1126/science.451612. [DOI] [PubMed] [Google Scholar]
- Johanson IB, Teicher MH. Classical conditioning of an odor preference in 3-day-old rats. Behav Neural Biol. 1980;29:132–136. doi: 10.1016/s0163-1047(80)92596-0. [DOI] [PubMed] [Google Scholar]
- Kaschel T, Schubert M, Albrecht D. Long-term depression in horizontal slices of the rat lateral amygdala. Synapse. 2004;53:141–150. doi: 10.1002/syn.20045. [DOI] [PubMed] [Google Scholar]
- LaBar KS, LeDoux JE. Partial disruption of fear conditioning in rats with unilateral amygdala damage: correspondence with unilateral temporal lobectomy in humans. Behav Neurosci. 1996;110:991–997. doi: 10.1037//0735-7044.110.5.991. [DOI] [PubMed] [Google Scholar]
- Ladd CO, Huot RL, Thrivikraman KV, Nemeroff CB, Meaney MJ, Plotsky PM. Long-term behavioral and neuroendocrine adaptations to adverse early experience. Prog Brain Res. 2000;122:81–103. doi: 10.1016/s0079-6123(08)62132-9. [DOI] [PubMed] [Google Scholar]
- LeDoux J. The emotional brain, fear, and the amygdala. Cell Mol Neurobiol. 2003;23:727–738. doi: 10.1023/A:1025048802629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maren S. Building and burying fear memories in the brain. Neuroscientist. 2005;11:89–99. doi: 10.1177/1073858404269232. [DOI] [PubMed] [Google Scholar]
- Moriceau S, Sullivan RM. Corticosterone influences on Mammalian neonatal sensitive-period learning. Behav Neurosci. 2004a;118:274–281. doi: 10.1037/0735-7044.118.2.274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moriceau S, Sullivan RM. Unique neural circuitry for neonatal olfactory learning. J Neurosci. 2004b;24:1182–1189. doi: 10.1523/JNEUROSCI.4578-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moriceau S, Sullivan RM. Maternal presence serves as a switch between learning fear and attraction in infancy. Nat Neurosci. 2006;9:1004–1006. doi: 10.1038/nn1733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedersen PE, Williams CL, Blass EM. Activation and odor conditioning of suckling behavior in 3-day-old albino rats. J Exp Psychol Anim Behav Process. 1982;8:329–341. [PubMed] [Google Scholar]
- Rammes G, Eder M, Dodt HU, Kochs E, Zieglgansberger W. Long-term depression in the basolateral amygdala of the mouse involves the activation of interneurons. Neuroscience. 2001;107:85–97. doi: 10.1016/s0306-4522(01)00336-0. [DOI] [PubMed] [Google Scholar]
- Rammes G, Steckler T, Kresse A, Schutz G, Zieglgansberger W, Lutz B. Synaptic plasticity in the basolateral amygdala in transgenic mice expressing dominant-negative cAMP response element-binding protein (CREB) in forebrain. Eur J Neurosci. 2000;12:2534–2546. doi: 10.1046/j.1460-9568.2000.00108.x. [DOI] [PubMed] [Google Scholar]
- Rogan MT, Staubli UV, LeDoux JE. Fear conditioning induces associative long-term potentiation in the amygdala. Nature. 1997;390:604–607. doi: 10.1038/37601. [DOI] [PubMed] [Google Scholar]
- Rosenkranz JA, Grace AA. Dopamine-mediated modulation of odour-evoked amygdala potentials during pavlovian conditioning. Nature. 2002;417:282–287. doi: 10.1038/417282a. [DOI] [PubMed] [Google Scholar]
- Roth TL, Sullivan RM. Memory of early maltreatment: neonatal behavioral and neural correlates of maternal maltreatment within the context of classical conditioning. Biol Psychiatry. 2005;57:823–831. doi: 10.1016/j.biopsych.2005.01.032. [DOI] [PubMed] [Google Scholar]
- Salzen EA. Imprinting in birds and primates. Behaviour. 1967;28:232–254. doi: 10.1163/156853967x00028. [DOI] [PubMed] [Google Scholar]
- Sananes C, Campbell B. Role of the central nucleus of the amygdala in olfactory heart rate conditioning. Behavioral Neuroscience. 1989;103:519–525. [PubMed] [Google Scholar]
- Schroeder BW, Shinnick-Gallagher P. Fear learning induces persistent facilitation of amygdala synaptic transmission. Eur J Neurosci. 2005;22:1775–1783. doi: 10.1111/j.1460-9568.2005.04343.x. [DOI] [PubMed] [Google Scholar]
- Setlow B, Roozendaal B, McGaugh JL. Involvement of a basolateral amygdala complex-nucleus accumbens pathway in glucocorticoid-induced modulation of memory consolidation. Eur J Neurosci. 2000;12:367–375. doi: 10.1046/j.1460-9568.2000.00911.x. [DOI] [PubMed] [Google Scholar]
- Sevelinges Y, Gervais R, Messaoudi B, Granjon L, Mouly AM. Olfactory fear conditioning induces field potential potentiation in rat olfactory cortex and amygdala. Learn Mem. 2004;11:761–769. doi: 10.1101/lm.83604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sevelinges Y, Moriceau S, Holman P, Miner C, Muzny K, Gervais R, Mouly AM, Sullivan RM. Enduring effects of infant memories: Infant odor-shock conditioning attenuates amygdala activity and adult fear conditioning. Biol Psychiatry. doi: 10.1016/j.biopsych.2007.04.025. in press. [DOI] [PubMed] [Google Scholar]
- Shipley MT, Ennis M. Functional organization of olfactory system. J Neurobiol. 1996;30:123–176. doi: 10.1002/(SICI)1097-4695(199605)30:1<123::AID-NEU11>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- Stork O, Ji FY, Kaneko K, Stork S, Yoshinobu Y, Moriya T, Shibata S, Obata K. Postnatal development of a GABA deficit and disturbance of neural functions in mice lacking GAD65. Brain Res. 2000;865:45–58. doi: 10.1016/s0006-8993(00)02206-x. [DOI] [PubMed] [Google Scholar]
- Sullivan RM, Brake SC, Hofer MA, Williams CL. Huddling and independent feeding of neonatal rats can be facilitated by a conditioned change in behavioral state. Dev Psychobiol. 1986a;19:625–635. doi: 10.1002/dev.420190613. [DOI] [PubMed] [Google Scholar]
- Sullivan RM, Hofer MA, Brake SC. Olfactory-guided orientation in neonatal rats is enhanced by a conditioned change in behavioral state. Dev Psychobiol. 1986b;19:615–623. doi: 10.1002/dev.420190612. [DOI] [PubMed] [Google Scholar]
- Sullivan RM, Landers M, Yeaman B, Wilson DA. Good memories of bad events in infancy. Nature. 2000;407:38–39. doi: 10.1038/35024156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szinyei C, Narayanan RT, Pape HC. Plasticity of inhibitory synaptic network interactions in the lateral amygdala upon fear conditioning in mice. Eur J Neurosci. 2007;25:1205–1211. doi: 10.1111/j.1460-9568.2007.05349.x. [DOI] [PubMed] [Google Scholar]
- Verkuyl JM, Karst H, Joels M. GABAergic transmission in the rat paraventricular nucleus of the hypothalamus is suppressed by corticosterone and stress. Eur J Neurosci. 2005;21:113–121. doi: 10.1111/j.1460-9568.2004.03846.x. [DOI] [PubMed] [Google Scholar]
- Watanabe Y, Ikegaya Y, Saito H, Abe K. Roles of GABAA, NMDA and muscarinic receptors in induction of long-term potentiation in the medial and lateral amygdala in vitro. Neurosci Res. 1995;21:317–322. doi: 10.1016/0168-0102(94)00867-f. [DOI] [PubMed] [Google Scholar]
- Wiedenmayer CP, Magarinos AM, McEwen BS, Barr GA. Mother lowers glucocorticoid levels of preweaning rats after acute threat. Ann N Y Acad Sci. 2003;1008:304–307. doi: 10.1196/annals.1301.038. [DOI] [PubMed] [Google Scholar]
- Wilson DA, Sullivan RM. Neurobiology of associative learning in the neonate: early olfactory learning. Behav Neural Biol. 1994;61:1–18. doi: 10.1016/s0163-1047(05)80039-1. [DOI] [PubMed] [Google Scholar]
- Zhang JH, Sato M, Tohyama M. Region-specific expression of the mRNAs encoding beta subunits (beta 1, beta 2, and beta 3) of GABAA receptor in the rat brain. J Comp Neurol. 1991;303:637–657. doi: 10.1002/cne.903030409. [DOI] [PubMed] [Google Scholar]