Cyclophosphamide (CPA) is widely used as an anticancer and immunosuppressive agent in various indications, and the dosage given may vary considerably depending on the disease treated. A recent review [1] has described the pharmacokinetics of CPA, a prodrug of which 70–80% of the administered dose is activated by cytochrome P450 (CYP) to the active alkylating species 4-hydroxycyclophosphamide (4OH-CPA). Several CYPs are responsible for CPA activation, mainly CYP2B6 and, secondarily, CYP2C9, CYP2C19 and CYP3A4/5 (Figure 1) [2]. Systemic exposure to cyclophosphamide metabolites after fixed doses of cyclophosphamide may vary by up to 10-fold between patients [1]. Genetic variants of CYPs involved in CPA metabolism may contribute to its pharmacokinetic variability and genetic polymorphisms of glutathione S-transferases may influence its toxicity.
Figure 1.
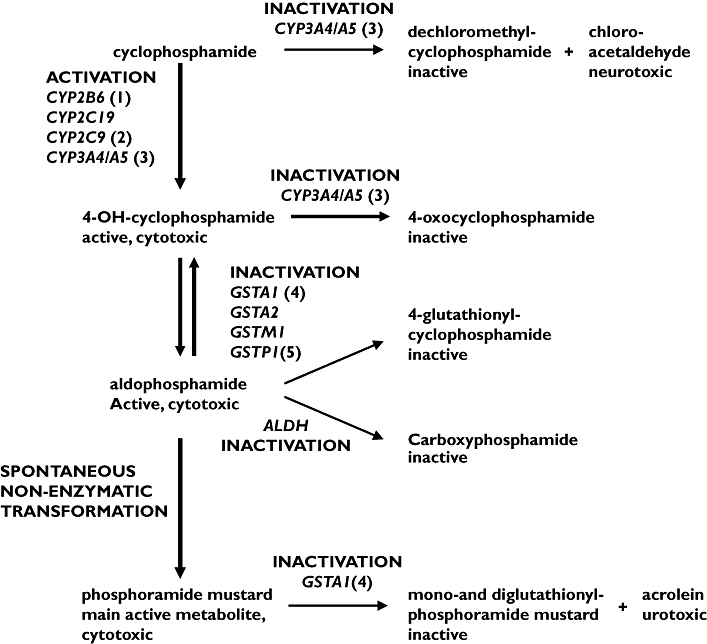
Cyclophosphamide metabolic pathways (adapted from Timm et al.[2]). The mutations (1) CYP2B6516TT, (2) CYP2C9*1/*3, (3) CYP3A5*3/*3, (4) GSTA1*A/*B, and (5) GSTP1*A/*B were identified in the presented case
We report a case of a patient who experienced severe toxicity (pancytopenia) with small dosages of CPA (1.60 mg kg−1 day−1). A 25-year-old female patient presenting with extramembranous glomerulonephritis (with tubulointerstitial lesions) began treatment with cyclophosphamide in association with prednisone (40 mg day−1). She also received the following treatments: an angiotensin-converting enzyme inhibitor, an oral anticoagulant, a statin and recombinant erythropoietin. When cyclophosphamide treatment was initiated, creatinine clearance was 32 ml min−1, leucocytes 6500 μl−1, absolute neutrophil count (ANC) 4550 μl−1, platelet count 345 000 μl−1 and haemoglobin 11.6 g dl−1. After 14 days of treatment (day 14), the patient was admitted to hospital because of an intense asthenia accompanied by fever (38.8–39.5°C) which had appeared 78 h previously. The patient had ulcerative pharyngitis with dysphagia, about 20 pyodermitis lesions infected by Staphylococcus epidermidis, total body hair loss and painful diarrhoea. Severe neutropenia (ANC < 100 μl−1) and anaemia (haemoglobin 8.8 g dl−1) were observed, leucocyte and platelet absolute counts were 6000 μl−1 and 178 000 μl−1, respectively. Hepatic enzymes were within normal range. All the treatments were stopped and antibiotherapy was initiated in association with an erythropoiesis-stimulating agent and recombinant granulocyte colony-stimulating factor. At day 20 the platelet count was 34 000 μl−1 and haemoglobin 5.8 g dl−1. At day 21, the patient was apyretic. Aplasia ceased on day 22. Pharyngitis and pyodermitis were cured on day 23 and hairiness was normal 3 months after cyclophosphamide discontinuation. As all the drugs previously used were recommenced without any side-effect and as the clinical symptoms observed caused a CPA overdosage, we therefore suspected an alteration of CPA metabolism or detoxification pathways. Genetic variants of enzymes involved in these pathways were explored. Results were the following: CYP2B6516TT, CYP2C9*1/*3, CYP2C19*1/*1, CYP3A4*1 A/*1 A, CYP3A5*3/*3, GSTA1*A/*B, GSTM1+ (presence of gene), GSTT1+, GSTM3*B/*B and GSTP1*A/*B.
The major side-effects observed were aplasia and total body hair loss. They are more likely to be observed when cyclophosphamide is used as a cancer treatment (500–4000 mg m−2 day−1) than for nonmalignant kidney disorders (glomerulonephritis) (100–200 mg m−2 day−1). As leukopenia, thrombocytopenia and anaemia are dose-related, we hypothesized that the aplasia observed in the present patient, despite the low dose of CPA, could be explained by enhanced 4OH-CPA exposure because of increased activation pathway of CPA into 4OH-CPA and less active detoxification pathways of 4OH-CPA and phosphoramide mustard. This was specially as the CLCr was 32 ml min−1 and hepatic function was normal. Indeed, Xie et al.[3] have demonstrated in vivo (patients with haematological malignancies) that the CYP2B6516T variant allele contribution to CPA elimination clearance was about twice that from wild-type gene. Moreover GSTA1*B/*B polymorphism, corresponding to lower detoxification, was correlated with greater survival than GSTA1*A/*B or GSTA1*A/*A in breast cancer therapy with CPA [4]. The detoxification pathway of anticancer drugs was decreased in carriers of GSTP1 variant alleles [5]. Nakajima et al.[6] have demonstrated in vitro that inhibition of GSTP1 enhances the antitumour activity of CPA.
The genetic polymorphisms observed in our patient might contribute to decreased detoxification pathways and to increased exposure to 4OH-CPA and could explain the reported adverse effects. Further studies are needed to confirm these results.
Acknowledgments
The authors are indebted to M. Cammas for her excellent technical assistance.
References
- 1.de Jonge ME, Huitema AD, Rodenhuis S, Beijnen JH. Clinical pharmacokinetics of cyclophosphamide. Clin Pharmacokinet. 2005;44:1135–64. doi: 10.2165/00003088-200544110-00003. [DOI] [PubMed] [Google Scholar]
- 2.Timm R, Kaiser R, Lotsch J, Heider U, Sezer O, Weisz K, Montemurro M, Roots I, Cascorbi I. Association of cyclophosphamide pharmacokinetics to polymorphic cytochrome P450 2C19. Pharmacogenomics J. 2005;5:365–73. doi: 10.1038/sj.tpj.6500330. [DOI] [PubMed] [Google Scholar]
- 3.Xie H, Griskevicius L, Stahle L, Hassan Z, Yasar U, Rane A, Broberg U, Kimby E, Hassan M. Related pharmacogenetics of cyclophosphamide in patients with hematological malignancies. Eur J Pharm Sci. 2006;27:54–61. doi: 10.1016/j.ejps.2005.08.008. [DOI] [PubMed] [Google Scholar]
- 4.Sweeney C, Ambrosone CB, Joseph L, Stone A, Hutchins LF, Kadlubar FF, Coles BF. Association between a glutathione S-transferase A1 promoter polymorphism and survival after breast cancer treatment. Int J Cancer. 2003;103:810–4. doi: 10.1002/ijc.10896. [DOI] [PubMed] [Google Scholar]
- 5.Ishimoto TM, Ali-Osman F. Allelic variants of the human glutathione S-transferase P1 gene confer differential cytoprotection against anticancer agents in Escherichia coli. Pharmacogenetics. 2002;12:543–53. doi: 10.1097/00008571-200210000-00006. [DOI] [PubMed] [Google Scholar]
- 6.Nakajima T, Takayama T, Miyanishi K, Nobuoka A, Hayashi T, Abe T, Kato J, Sakon K, Naniwa Y, Tanabe H, Niitsu Y. Reversal of multiple drug resistance in cholangiocarcinoma by the glutathione S-transferase-pi-specific inhibitor O1-hexadecyl-gamma-glutamyl-S-benzylcysteinyl-D-phenylglycine ethylester. J Pharmacol Exp Ther. 2003;306:861–9. doi: 10.1124/jpet.103.052696. [DOI] [PubMed] [Google Scholar]


