Abstract
Aims
To investigate the effect of moderate exercise on the absorption of inhaled insulin.
Methods
A single-centre, randomized, open-label, three-period cross-over trial was carried out in 12 nonsmoking healthy subjects. A dose of 3.5 mg inhaled human insulin was administered via a nebulizer and followed in random order by either 1) no exercise (NOEX), 2) 30 min exercise starting immediately after dosing (EX0), or 3) 30 min exercise starting 30 min after dosing (EX30). The study was carried out as a 10 h euglycaemic glucose clamp (90 mg dl−1 (5.0 mmol l−1)).
Results
The absorption of insulin over the first 2 h after start of exercise was 16% increased for EX0 (ratio (95%CI) 1.16 (1.04, 1.30), P = 0.01) and 20% increased for EX30 (1.20 (1.05, 1.36), P < 0.01), both compared with NOEX; the overall insulin absorption during 6 h and 10 h after dosing was not influenced by exercise. The maximum insulin concentration (Cmax) increased by 32% for EX0 and 35% for EX30 (both P < 0.01) compared with NOEX, while the time to Cmax was 31 min faster for EX0 (P < 0.01), but not significantly different after EX30, compared with NOEX.
Conclusions
A significant and clinically relevant increase of insulin absorption over the first 2 h after the beginning of exercise was observed. Until data from studies using the specific insulin inhalers exists, patients using inhaled insulin should be made aware of a potential increased absorption and higher concentration of insulin in connection with exercise.
What is already known about this subject
Exercise is known to affect absorption of other inhaled substances, but so far there are no reports on the effect of exercise on the absorption of inhaled insulin in humans.
What this paper adds
This report is the first to investigate the effect of exercise on the absorption of inhaled insulin.
In this study in healthy volunteers we found that exercise early after dosing increased absorption (15–20%) of inhaled insulin over the first 2 h after start of exercise, with an approximately 30% increase in maximal insulin concentration, and unchanged overall absorption.
Keywords: administration, aerosol, drug safety, inhalation, lung, pharmacodynamics, pharmacokinetics
Introduction
Insulin is the cornerstone for the treatment of patients with type 1 diabetes, and a commonly used treatment for patients with type 2 diabetes as well. While subcutaneous (s.c) injection of insulin has been the standard for over 80 years, pulmonary administration is now an alternative route for insulin delivery with the first product on the market and several systems in development [1]. Generally, the pharmacokinetic profile of inhaled human insulin shows a more rapid absorption and a shorter time to peak concentration than fast-acting human insulin administered s.c. [2–5] and with an onset of action like a rapid-acting insulin analogue and a duration of action comparable with s.c. fast-acting human insulin [6, 7].
Regular physical exercise is recommended to all groups of people with diabetes [8]; information is however, lacking about the effects of exercise on insulin absorption when administered via the pulmonary route.
Exercise leads to increased ventilation, as well as to changes of regional ventilation and perfusion of the lung [9, 10], and is therefore likely to induce a change in the absorption pattern. In humans, exercise has been shown to increase the pulmonary absorption rate of inhaled 99 m-technetium labelled diethylene triamine penta-acetic acid (99mTc-DTPA) [11, 12]. In line with this, exercise has been shown to give a faster absorption as well as an increased maximum plasma concentration of terbutaline [13], and increased pulmonary absorption of nedocromil sodium [14].
In a study in rabbits we have shown that large tidal volume ventilation (LTVV) for 120 min after inhalation of human insulin leads to an approximately 150% increase in insulin absorption [15]. Furthermore, pulmonary function test manoeuvres (forced expiratory manoeuvres), which include deep breathing, have been shown to lead to increased serum concentrations of inhaled human insulin [16] as well as increased pulmonary absorption of nedocromil sodium [14]. Thus, exercise and deep ventilation in itself appear to influence absorption of inhaled substances.
The purpose of this study was to investigate the effect of moderate exercise on the absorption of inhaled human insulin in healthy subjects.
Methods
Twelve nonsmoking, healthy male and female subjects were included in the trial. Inclusion criteria were age between 18 and 65 years (inclusive), body mass index (BMI) ≤ 29 kg m−2 and normal pulmonary function, defined as forced vital capacity (FVC) and forced expiratory volume in 1 s (FEV1) > 80% and FEV1/FVC ≥ 75% of predicted values. Subjects with past or present pulmonary disease were excluded as were subjects with any clinically significant findings from a cardiopulmonary exercise test performed at screening.
The study was approved by the Ethics Committee at Medical University Graz, Graz, Austria where the study was carried out according to the principles of the Declaration of Helsinki [17] and Good Clinical Practice [18]. All subjects gave written informed consent before any trial related activities.
Procedures
This investigator sponsored, single-centre, open-label, randomized, three-period cross-over, euglycaemic clamp trial, consisted of five visits: a screening visit (visit 1) to evaluate the subject's eligibility for participation in the trial, three dosing days (visits 2–4), and a follow-up visit (visit 5).
There were three treatments: 1) inhaled insulin and no exercise, 2) inhaled insulin and 30 min moderate exercise starting immediately after dosing, and 3) inhaled insulin and 30 min moderate exercise starting 30 min after dosing. The time point immediately after dosing was chosen to match previous studies with exercise [13], while 30 min after dosing was chosen as the earliest clinically relevant time of exercise after insulin inhalation. The three treatments were given to all subjects on separate dosing days in randomized order in accordance with a randomization list prepared based on the six possible treatment combinations as stated in the protocol. Each treatment sequence was sealed until randomization.
The subjects attended each dosing day in the morning in a fasting state, and received a dose of 3.5 mg (emitted dose) inhaled human insulin (1500 U ml−1 insulin human inhalation solution, Novo Nordisk A/S, Bagsvaerd, Denmark) administered by a commercial nebulizer (MedicAidpro-Jaeger Aerosol Provocation System (APS), Viasys Healthcare GmbH, Wuerzburg, Germany). As there was no access to a dedicated insulin inhaler, this nebulizer system was chosen as the best available alternative. The dose was delivered during 23 slow, deep breaths from t = 0, and administered as calculated by the software (Jaeger APS-Bronchial test V4.65b, Jaeger, Viasys Healthcare GmbH, Wuerzburg, Germany). Nebulization time per breath was 1 s breath−1, and trigger volume for nebulization was 0.1 l breath−1. The same settings were used for all subjects. Each subject was trained by administration of isotonic saline solution. The volume median diameter (VMD) of the aerosol was 4.4 µm with a span value of 1.6,measured using a Malvern Insitec laser diffraction instrument (Malvern/Insitec, California, USA).
Physical activity level was characterized at screening using the International Physical Activity Questionnaire (IPAQ) [19]. A cardiopulmonary exercise test was done at screening to establish each subject's maximal oxygen uptake (VO2max). The initial workload was 20 W with 15 W increments every minute until exhaustion. A constant workload corresponding to 50% of maximal oxygen uptake (VO2max), i.e. moderate exercise [8], was used at the 2 dosing days with exercise, where ergometer exercise with a pedal intensity of 60–80 min−1 was carried out for 30 min, followed by a 2 min cool down period at 40 W. Just before start of exercise and every 10 min thereafter, heart rate, ventilation rate, tidal volume, minute ventilation and Borg rating of relative perceived exertion (RPE) [20] were recorded.
A hand or antecubital vein was cannulated and kept in a thermo regulated box (approximately 50°C) for the sampling of arterialized venous blood. An antecubital vein on the contralateral arm was cannulated for the infusion of glucose.
Arterialized venous blood samples for plasma glucose measurements were obtained on average every 5–10 min during the experiment, and plasma glucose was measured in duplicate using the Beckman Glucose Analyser II (Beckman Instruments, Fullerton, California, USA). Euglycaemia at 90 ± 20 mg dl−1 (5.0 ± 1.1 mmol l−1) was maintained by variable glucose infusion (Glucose 10%; Braun Infusomat FM, Melsungen, Germany). Except for dosing and exercise period, the subjects were in a supine position during the dosing day.
Blood samples for serum insulin were drawn in intervals of 5–60 min, while samples for serum C-peptide for control of suppression of endogenous insulin were drawn less frequently (at time points −10, 0, 30, 90, 120, 180, 240, 300, 360 and 600 min). Serum insulin was measured using Ultrasensitive Insulin Enzyme-Linked Immunosorbent Assay (ELISA) (Mercodia, Uppsala, Sweden; interassay CV 1.3–7.2%), while C-peptide was measured using Ultrasensitive C-peptide ELISA (Mercodia, Uppsala, Sweden; interassay CV 3.7–8.4%).
Safety assessments included adverse events, pulmonary function tests, physical examination, electrocardiogram, vital signs and standard laboratory safety parameters.
Statistical analysis
The sample size of 12 was based on ability to detect a 30% treatment difference in area under the curve (AUC) for serum insulin from 0 to 120 min (AUCins(0,120 min)) with a two sided significance level of 0.05 and a power of 0.80. The period of 2 h from start of exercise was chosen as a period including 30 min exercise as well as a period after exercise, where an effect of exercise could still be present.
AUCins(0,120 min) was calculated by means of the trapezoidal rule, and was analyzed by an anova, including treatment as fixed effect, the subject as random effect and log-transformed baseline insulin concentration as covariate. Baseline insulin concentration was calculated as the mean of the −10 and the 0 insulin concentrations. AUCins(0,120 min) was transformed with the natural logarithm prior to analysis.
The maximal insulin concentration, Cmax, was estimated together with tmax, the corresponding time point. Initial rate of increase was calculated as the slope of the best fitting line from 0 to tmax. The terminal rate constant (λz) was estimated by a log-linear regression on the terminal log-linear part of insulin concentration time curve. Mean residence time (MRT) was calculated as AUMC/AUC (AUC being the area under the insulin curve from zero to infinity, and AUMC being the corresponding area under the moment curve). AUC and AUMC were calculated with standard pharmacokinetic formulae. In order to allow a valid interpolation of the insulin profiles to infinity, insulin data used for calculation of MRT and λz were corrected by subtracting the lowest measured insulin value from all values.
The other endpoints, AUCins(30,150 min), AUCins(0,360 min), AUCins(0,600 min), Cmax, tmax, initial rate of increase, the terminal rate constant and MRT were analyzed similar to AUCins(0,120 min), but without baseline insulin as covariate. All endpoints were calculated from measured serum insulin, and except tmax and initial rate of increase, all were transformed with the natural logarithm prior to analysis.
As control for no systematic difference in endogenous insulin contribution, mean concentration of C-peptide from 30 to 200 min (C-peptide(30,200 min)) and C-peptide(0,600 min) were calculated and analyzed (natural logarithm transformed) in a anova model with treatment as fixed effect and subject as random. The period of 30–200 min for mean C-peptide was chosen as the period best representing insulin concentrations from 0 to 150 min, as the half-life of C-peptide is longer than that of insulin [21, 22].
The pharmacodynamic endpoints, AUC for the glucose infusion rate (GIR) from 0 to 120 min (AUCGIR(0,120 min)), AUCGIR(30,150 min), AUCGIR(0,360 min) AUCGIR(0,600 min) were calculated and analyzed similar to the corresponding pharmacokinetic endpoints.
Individual GIR profiles were smoothed using a locally weighted regression technique as implemented in SAS proc LOESS. GIRmax and time to maximal glucose infusion rate (tGIRmax) were calculated from smoothed GIR profiles as the maximal GIR of the smoothed GIR profile and the corresponding time point, and analyzed similar to Cmax and tmax.
Data from the IPAQ questionnaire were summarized [23] in categories, and in MET-minutes (MET-min), where MET-min is approximately equivalent to kilocalories for a 60 kg person.
A significance level of 0.05 was used throughout, and all tests were made against no exercise treatment.
Results
Fifteen subjects were screened, and 12 subjects (five women and seven men; all Caucasians), age 28.6 (range 22.7–36.4) years, and BMI 23.2 (range 20.0–28.0) kg m−2 were randomized in the trial. All subjects completed the trial and were included in the analyses.
The subjects were generally active with seven subjects being in the IPAQ ‘Moderate’ activity category, and five being IPAQ ‘High’ activity category, and with a relative VO2max of 50 ± 9 ml min−1 kg−1 and a total activity score of 2698 (659–11226) MET-min/week (median (minimum − maximum)). The physiological response to exercise was reproducible between the 2 exercise days (Table 1).
Table 1.
Exercise summary
| EX0 Before exercise | During exercise | EX30 Before exercise | During exercise | |
|---|---|---|---|---|
| Work load (W) | – | 111 ± 35 | – | 111 ± 35 |
| Heart rate (beats min−1) | 87 ± 20 | 129 ± 8 | 86 ± 15 | 127 ± 8 |
| Ventilation rate (min−1) | 16 ± 4 | 18 ± 5 | 16 ± 3 | 18 ± 6 |
| Tidal volume (l) | 1.2 ± 0.8 | 2.2 ± 0.7 | 1.0 ± 0.5 | 2.3 ± 0.8 |
| Minute ventilation (l min−1) | 18 ± 9 | 38 ± 11 | 16 ± 7 | 40 ± 12 |
| RPE (Borg scale) | 6 (6–6) | 11 (9–12) | 6 (6–8) | 11 (9–12) |
Mean ± SD for all except for RPE (median (min–max)). n = 12 for all. Before exercise is just before start of exercise. During exercise represents mean (median) values for the three time points during the last 20 min of exercise. EX0, exercise starting immediately after dosing; EX30, exercise starting 30 min after dosing; RPE, relative perceived exertion.
Pharmacokinetics
The mean insulin concentration profiles are shown in Figure 1. The absorption of insulin for 2 h after the start of exercise was increased compared with no exercise. Exercise starting immediately after dosing resulted in a 16% increase in AUCins(0,120 min) (P = 0.01), and exercise starting 30 min after dosing resulted in a 20% increased in AUCins(30,150 min) (P < 0.01) (Table 2, Figure 1). The overall absorption from 0 to 360 or from 0 to 600 min was, however, not significantly different after exercise (all P > 0.30).
Figure 1.
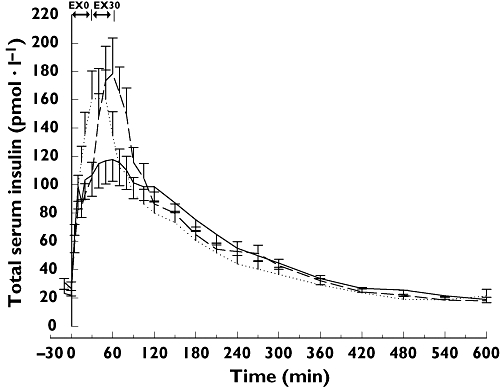
Mean serum insulin concentration curves (mean ± SEM). Straight line, no exercise (NOEX); Dotted line, exercise starting immediately after dosing (EX0); Dashed line, exercise starting 30 min after dosing (EX30)
Table 2.
Pharmacokinetic endpoints
| NOEX | EX0 | EX30 | |||||
|---|---|---|---|---|---|---|---|
| Mean (95% CI) | Mean (95% CI) | Mean ratio (95% CI) | P | Mean (95% CI) | Mean ratio (95% CI) | P | |
| AUCins(0,120 min) (pmol l−1 min) | 11 222 (9 116, 13 814) | 13 046 (10 600, 16 055) | 1.16 (1.04, 1.30) | 0.0121 | – | – | – |
| AUCins(30,150 min) (pmol l−1 min) | 11 757 (9 505, 14 544) | – | – | – | 14 069 (11 373, 17 401) | 1.20 (1.05, 1.36) | 0.0096 |
| AUCins(0,360 min) (pmol l−1 min) | 25 594 (21 239, 30 841) | 24 416 (20 265, 29 419) | 0.95 (0.83, 1.09) | 0.4756 | 27 076 (22 469, 32 627) | 1.06 (0.92, 1.21) | 0.3953 |
| AUCins(0,600 min) (pmol l−1 min) | 31 473 (26 142, 37 892) | 29 305 (24 341, 35 277) | 0.93 (0.81, 1.07) | 0.2983 | 32 177 (26 726, 38 739) | 1.02 (0.89, 1.17) | 0.7443 |
| Cmax (pmol l−1) | 128 (100, 164) | 169 (131, 216) | 1.32 (1.10, 1.58) | 0.0043 | 173 (135, 222) | 1.35 (1.13, 1.62) | 0.0022 |
| tmax (min) | 67.1 (51.5, 82.7) | 35.8 (20.2, 51.4) | 31.3 (10.9, 51.6)a | 0.0042 | 59.2 (43.6, 74.8) | 7.9 (−12.4, 28.2)a | 0.4279 |
| Initial rate of increase (pmol l−1 min−1) | 1.30 (0.85, 1.99) | 3.26 (2.13, 5.00) | 2.50 (1.62, 3.85) | 0.0002 | 2.24 (1.46, 3.43) | 1.72 (1.11, 2.64) | 0.0166 |
All means are geometric means (exp(Least Squares Means)) based on anova model, except for tmax, which is Least Squares Means. n = 12 for all. All tests are against no exercise. NOEX, no exercise; EX0, exercise starting immediately after dosing; EX30, exercise starting 30 min after dosing.
For tmax based on mean difference.
Exercise also led to an increased Cmax with a 32% increase for exercise immediately after dosing (P < 0.01) and a 35% increase for exercise 30 min after dosing (P < 0.01), both compared with no exercise. For exercise starting immediately after dosing tmax came 31 min earlier than with no exercise (P < 0.01), whereas tmax was not significantly different after exercise starting 30 min after dosing (Table 2).
The initial appearance of insulin in serum was significantly faster on the exercise days with the initial rate of increase of serum concentration being significantly higher for both exercise days compared with the day with no exercise (P < 0.001 and P = 0.02) (Table 2).
There was no statistical significant effect of exercise on λz (overall P = 0.68), nor on MRT (overall P = 0.15).
Inspection of the C-peptide profiles confirmed a suppression of endogenous insulin after 30 min, and no statistical significant differences in the mean C-peptide concentrations between treatments were found (C-peptide(30,200 min): overall P = 0.79; C-peptide (0,600 min): overall P = 0.11).
Pharmacodynamics
Exercise increased the need for glucose during 2 h after start of exercise; with 95% increased AUCGIR(0, 120 min) for exercise starting immediately after dosing (ratio (95% CI) 1.95 (1.32, 2.87), P < 0.01), and 123% increased AUCGIR(30,150 min) for exercise starting 30 min after dosing (2.23 (1.44, 3.44), P < 0.001); both compared with no exercise (Figure 2). The effect of exercise on the overall need for glucose over 6 and 10 h did not reach statistical significance for exercise starting immediately after dosing (AUCGIR(0,360 min) 1.22 (0.73, 2.05), P = 0.43; AUCGIR(0,600 min) 1.21 (0.71, 2.07), P = 0.47) or for exercise starting 30 min after dosing (AUCGIR(0,360 min) (mean (95% CI) 1.54 (0.92, 2.58), P = 0.10; AUCGIR(0,600 min): 1.53 (0.89, 2.61), P = 0.11); both compared with no exercise. However, as can be seen from the 95% CIs, a great variability was observed for test estimates.
Figure 2.
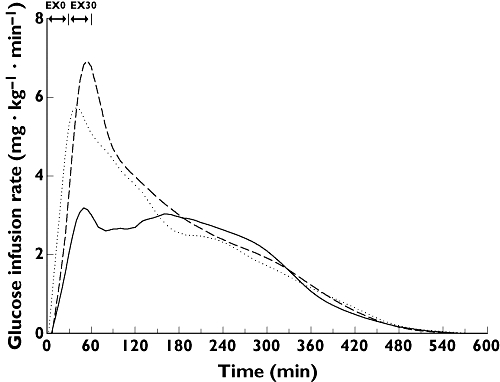
Mean glucose infusion rate curves (smoothed means). Straight line, no exercise (NOEX); Dotted line, exercise starting immediately after dosing (EX0); Dashed line, exercise starting 30 min after dosing (EX30)
GIRmax was increased by 59% by exercise starting immediately after dosing (1.59 (1.10, 2.31), P = 0.02) and by 93% by exercise starting 30 min after dosing (1.93 (1.33, 2.80), P < 0.01). The tGIRmax was lower with exercise; 66 min lower for exercise starting immediately after (difference (95% CI), −66, (−98, −35) min, P < 0.001), and 38 min lower for exercise starting 30 min after dosing (−38 (−70, −7) min, P = 0.02).
Safety
A total of 11 adverse events were observed in nine subjects. All were mild, except for one headache that was severe. No adverse events were judged related to inhaled insulin, and there were no clinically relevant changes in any safety parameter.
Discussion
This study investigated for the first time the effect of exercise on the absorption of inhaled insulin in humans. A significant and clinically relevant increase of insulin absorption over the first 2 h after begin of exercise was observed. If confirmed in people with diabetes this finding may have important impact on the safety and treatment guidelines of the use of inhaled insulin.
Exercise influenced the insulin profile primarily around the period of exercise. Based on the insulin profiles, the primary contribution to the absorption over the first 2 h after start of exercise appears to come from the increased plasma concentration reached during the 30 min exercise period, indicating that an increased absorption is taking place during exercise, and afterwards the effect decreases. This would be in line with studies with DTPA showing an immediate effect on clearance rate of DTPA upon change of ventilation [24]. There is no indication that the overall bioavailability was changed due to exercise.
Similar effects of exercise have been shown for other inhaled drugs, such as terbutaline [13], nedocromil sodium [14] and 99mTc-DTPA [11], indicating that the effect of exercise on absorption is general for inhaled hydrophilic drugs. The exercise induced deep ventilation with stretching of the lung is considered the primary mechanism for the increased absorption. The mechanism of this is not fully understood, but stretching of the lung has been shown to lead to more permeable transcellular pores [25], and expansion of the surface by stretching of caveolae of the capillary endothelium and type I cells might also contribute [26]. Although the lungs have a high blood flow in resting state, and solute absorption is not flow-dependent to an appreciable extent [27–29], a minimal influence of exercise induced increase in perfusion cannot be ruled out.
The response to exercise is very individual, which is also reflected in the variability of the pharmacodynamic results. Generally, people with diabetes are advised to reduce the meal related insulin dose and/or increase carbohydrate intake in connection with exercise, but each person has to find his/her individual response to exercise [30]. Increased absorption rates of insulin due to exercise is also seen in connection with s.c. insulin administered in the thigh [31–33] but not in the abdomen [32]. Even without exercise, the intrasubject variability of s.c. insulin is around 20–25% [34]. Thus any factor that will further increase this variability should be eliminated where possible. Thus, in order to remove one source of variation in connection with exercise, people with diabetes are advised to use the abdomen as injection site [32]. Change of administration site is however, not possible for inhaled insulin, and if confirmed, the results from this study indicate that patients using inhaled human insulin and exercising early after dosing can expect an increased absorption and higher concentration of insulin during the first 2 h after start of exercise, but not over 6 or 10 h. Further, it should be noted that time of exercise, especially very soon after inhaling insulin, might influence the time of insulin peak concentration, but if the patient is aware of this, adjustment of insulin dose and/or carbohydrate intake, to account for this effect, would be possible.
After this first trial investigating the effect of exercise on inhaled insulin, there are more clinical questions that need to be answered. Firstly, this study only investigated the effect of moderate exercise, as this was evaluated to be the most relevant for the general population. However, it can be assumed that more intensive or longer exercise might further augment the effect of exercise. Secondly, it cannot be judged from this study what happens if patients exercise later after dosing. Thirdly, a direct comparison to s.c. insulin would be advantageous to compare dosing recommendations. Fourthly, the effects of exercise on absorption of inhaled insulin should preferably be investigated in people with diabetes, knowing that this population has a different exercise response than healthy subjects [35], and possibly a different lung diffusion response to exercise [36]. Finally, although the effect of exercise on absorption of inhaled hydrophilic substances seems to be general, and the nebulizer system was chosen as the best representative system, the functionality is different from a handheld inhaler, and it cannot be ruled out that the response will be different with different inhalers. Thus, the effect of exercise should be investigated with the insulin inhaler in question.
In summary, the present study has shown that moderate exercise starting immediately or 30 min after dosing leads to increased absorption over the first 2 h after start of exercise, with increased maximal insulin concentration, but unchanged overall absorption, thereby indicating that people with diabetes using inhaled insulin, and exercising shortly after inhaling, might expect an enhanced effect of the insulin. Further studies in patients with diabetes with the different insulin inhalers, and possibly also at other time points after dosing are required. Until results from such studies exist, health care professionals and patients using inhaled insulin should be aware of a potential increased absorption and higher blood concentration of insulin in connection to exercise.
Acknowledgments
The authors are grateful to Device Chemistry & Biology, Novo Nordisk A/S for aerosol characterization. The study was part of a PhD program supported by Ministry of Science Technology and Innovation, Denmark, as well as Novo Nordisk A/S, Denmark.
References
- 1.Fineberg SE. Diabetes therapy trials with inhaled insulin. Expert Opin Inv Drug. 2006;15:743–62. doi: 10.1517/13543784.15.7.743. [DOI] [PubMed] [Google Scholar]
- 2.Brunner GA, Balent B, Ellmerer M, Schaupp L, Siebenhofer A, Jendle JH, Okikawa J, Pieber TR. Dose–response relation of liquid aerosol inhaled insulin in type I diabetic patients. Diabetologia. 2001;44:305–8. doi: 10.1007/s001250051618. [DOI] [PubMed] [Google Scholar]
- 3.Kapitza C, Heise T, Fishman RS, Shapiro DA, Gopalakrishnan V, Rave K, Bott S, Perera AD, Heinemann L. Impact of particle size and aerosolization time on the metabolic effect of an inhaled insulin aerosol. Diabetes Technol Ther. 2004;6:119–27. doi: 10.1089/152091504773731302. [DOI] [PubMed] [Google Scholar]
- 4.Steiner S, Pfutzner A, Wilson BR, Harzer O, Heinemann L, Rave K. Technosphere/Insulin – proof of concept study with a new insulin formulation for pulmonary delivery. Exp Clin Endocrinol Diabetes. 2002;110:17–21. doi: 10.1055/s-2002-19989. [DOI] [PubMed] [Google Scholar]
- 5.Heinemann L, Traut T, Heise T. Time-action profile of inhaled insulin. Diabet Med. 1997;14:63–72. doi: 10.1002/(SICI)1096-9136(199701)14:1<63::AID-DIA298>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 6.Rave K, Bott S, Heinemann L, Sha S, Becker RHA, Willavize SA, Heise T. Time-action profile of inhaled insulin in comparison with subcutaneously injected insulin Lispro and regular human insulin. Diabetes Care. 2005;28:1077–82. doi: 10.2337/diacare.28.5.1077. [DOI] [PubMed] [Google Scholar]
- 7.Plank J, Petersen AH, Bock G, Wutte A, Haahr H, Ronn B, Pieber TR. Onset of action of inhaled human insulin via the AERx (R) iDMS compared to subcutaneous human insulinand subcutaneous insulin aspart (Abstract) Diabetologia. 2005;48:A31. [Google Scholar]
- 8.Zinman B, Ruderman N, Campaigne BN, Devlin JT, Schneider SH. American Diabetes Association. Physical activity/exercise and diabetes. Diabetes Care. 2004;27(Suppl 1):S58–S62. doi: 10.2337/diacare.26.2007.s73. [DOI] [PubMed] [Google Scholar]
- 9.Bryan AC, Bentivoglio LG, Beerel F, Macleish H, Zidulka A, Bates DV. Factors affecting regional distribution of ventilation and perfusion in the lung. J Appl Physiol. 1964;19:395–402. doi: 10.1152/jappl.1964.19.3.395. [DOI] [PubMed] [Google Scholar]
- 10.Harf A, Pratt T, Hughes JM. Regional distribution of VA/Q in man at rest and with exercise measured with krypton-81m. J Appl Physiol: Respiratory, Environ Exercise Physiol. 1978;44:115–23. doi: 10.1152/jappl.1978.44.1.115. [DOI] [PubMed] [Google Scholar]
- 11.Meignan M, Rosso J, Leveau J, Katz A, Cinotti L, Madelaine G, Galle P. Exercise increases the lung clearance of inhaled technetium-99m DTPA. J Nucl Med. 1986;27:274–80. [PubMed] [Google Scholar]
- 12.Lorino AM, Meignan M, Bouissou P, Atlan G. Effects of sustained exercise on pulmonary clearance of aerosolized 99mTc-DTPA. J Appl Physiol. 1989;67:2055–9. doi: 10.1152/jappl.1989.67.5.2055. [DOI] [PubMed] [Google Scholar]
- 13.Schmekel B, Borgström L, Wollmer P. Exercise increases the rate of pulmonary absorption of inhaled terbutaline. Chest. 1992;101:742–5. doi: 10.1378/chest.101.3.742. [DOI] [PubMed] [Google Scholar]
- 14.Ghosh SK, Neale MG, Patel KR. The effect of physiological manoeuvres on the absorption of inhaled nedocromil sodium. Br J Clin Pharmacol. 1994;37:305–8. doi: 10.1111/j.1365-2125.1994.tb04281.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Petersen AH, Laursen T, Ahrén B, Pieber TR, Clauson P, Wollmer P. The impact of large tidal Volume ventilation on the absorption of inhaled insulin in rabbits. Eur J Pharm Sci. 2007;30:351–7. doi: 10.1016/j.ejps.2006.12.002. [DOI] [PubMed] [Google Scholar]
- 16.Farr SJ, McElduff A, Mather LE, Okikawa J, Ward ME, Gonda I, Licko V, Rubsamen RM. Pulmonary insulin administration using the AERx system: physiological and physicochemical factors influencing insulin effectiveness in healthy fasting subjects. Diabetes Technol Ther. 2000;2:185–97. doi: 10.1089/15209150050025131. [DOI] [PubMed] [Google Scholar]
- 17.World Medical Association World Medical Association Declaration of Helsinki. Ethical Principles for Medical Research Involving Human Subjects. 52nd WMA General Assembly, Edinburgh, Scotland. 2004. Last amended with Note of Clarification on Paragraph 30 added by the WMA General Assembly, Tokyo.
- 18.International Conference on Harmonisation; ICH Harmonised Tripartite GuidelineGuideline for Good Clinical Practice E6InclPost Step 4 Corrections. [Google Scholar]
- 19.IPAQ Research Committee. [30 May 2006]. International physical activity questionnaire – short last 7 days self-administered format. Available from http://www.ipaq.ki.se.
- 20.Borg GA. Psychophysical bases of perceived exertion. Med Sci Sports Exerc. 1982;14:377–81. [PubMed] [Google Scholar]
- 21.Faber OK, Hagen C, Binder C, Markussen J, Naithani VK, Blix PM, Kuzuya H, Horwitz DL, Rubenstein AH, Rossing N. Kinetics of human connecting peptide in normal and diabetic subjects. J Clin Invest. 1978;62:197–203. doi: 10.1172/JCI109106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kuzuya T, Matsuda A. Disappearance rate of endogenous human C-peptide from blood. Diabetologia. 1976;12:519–21. doi: 10.1007/BF01219517. [DOI] [PubMed] [Google Scholar]
- 23.IPAQ Research Committee. Guidelines for Data Processing and Analysis of the International Physical Activity Questionnaire (IPAQ) – Short and Long Forms (November 2005) [30 May 2006]. Available from http://www.Ipaq.Ki.Se.
- 24.Woolman PS, Jones DK, Barber RW, Higenbottam TW. The rapid reversibility of effects of changing lung Volume on the clearance rate of inhaled 99Tcm-DTPA in man. Nucl Med Commun. 1987;8:881–7. doi: 10.1097/00006231-198711000-00005. [DOI] [PubMed] [Google Scholar]
- 25.Egan EA, Nelson RM, Olver RE. Lung inflation and alveolar permeability to non-electrolytes in the adult sheep in vivo. J Physiol. 1976;260:409–24. doi: 10.1113/jphysiol.1976.sp011522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Patton JS. Mechanisms of macromolecule absorption by the lungs. Adv Drug Deliv Rev. 1996;19:3–36. [Google Scholar]
- 27.Rizk NW, Luce JM, Hoeffel JM, Price DC, Murray JF. Site of deposition and factors affecting clearance of aerosolized solute from canine lungs. J Appl Physiol: Respiratory, Environ Exercise Physiol. 1984;56:723–9. doi: 10.1152/jappl.1984.56.3.723. [DOI] [PubMed] [Google Scholar]
- 28.Rinderknecht J, Shapiro L, Krauthammer M, Taplin G, Wasserman K, Uszler JM, Effros RM. Accelerated clearance of small solutes from the lungs in interstitial lung disease. Am Rev Respir Dis. 1980;121:105–17. doi: 10.1164/arrd.1980.121.1.105. [DOI] [PubMed] [Google Scholar]
- 29.Oberdorster G, Utell MJ. Weber DA, Ivanovich M, Hyde RW, Morrow PE. Lung clearance of inhaled 99mTc-DTPA in the dog. J Appl Physiol: Respiratory, Environ Exercise Physiol. 1984;57:589–95. doi: 10.1152/jappl.1984.57.2.589. [DOI] [PubMed] [Google Scholar]
- 30.Galbo H, Richter EA. Exercise. In: DeFronzo RA, Ferrannini E, Keen H, Zimmet P, editors. International textbook of diabetes mellitus. 3. West Sussex: John Wiley & Sons Ltd; 2004. pp. 771–94. [Google Scholar]
- 31.Fernqvist E, Linde B, Ostman J, Gunnarsson R. Effects of physical exercise on insulin absorption in insulin-dependent diabetics. A comparison between human and porcine insulin. Clin Physiol. 1986;6:489–97. doi: 10.1111/j.1475-097x.1986.tb00782.x. [DOI] [PubMed] [Google Scholar]
- 32.Koivisto VA, Felig P. Effects of leg exercise on insulin absorption in diabetic patients. N Engl J Med. 1978;298:79–83. doi: 10.1056/NEJM197801122980205. [DOI] [PubMed] [Google Scholar]
- 33.Kemmer FW, Berchtold P, Berger M, Starke A, Cüppers HJ, Gries FA, Zimmermann H. Exercise-induced fall of blood glucose in insulin-treated diabetics unrelated to alteration of insulin mobilization. Diabetes. 1979;28:1131–7. doi: 10.2337/diab.28.12.1131. [DOI] [PubMed] [Google Scholar]
- 34.Galloway JA, Spradlin CT, Howey DC, Dupre J. Intrasubject differences in pharmacokinetic and pharmacodynamic responses: The immutable problem of present-day treatment? In. In: Serrano-Rios M, Lefebvre PJ, editors. Diabetes. New York: Excerpta Medica; 1985. pp. 877–86. [Google Scholar]
- 35.Tuominen JA, Ebeling P, Koivisto VA. Exercise increases insulin clearance in healthy man and insulin-dependent diabetes mellitus patients. Clin Physiol. 1997;17:19–30. doi: 10.1046/j.1365-2281.1997.01717.x. [DOI] [PubMed] [Google Scholar]
- 36.Niranjan V, McBrayer DG, Ramirez LC, Raskin P, Hsia CC. Glycemic control and cardiopulmonary function in patients with insulin-dependent diabetes mellitus. Am J Med. 1997;103:504–13. doi: 10.1016/s0002-9343(97)00251-9. [DOI] [PubMed] [Google Scholar]


