Abstract
Aim
To investigate the effect of intensive muscular exercise (weightlifting) on clinical chemistry parameters reflecting liver function in healthy men.
Methods
Fifteen healthy men, used to moderate physical activity not including weightlifting, performed an 1 h long weightlifting programme. Blood was sampled for clinical chemistry parameters [aspartate aminotransferase (AST), alanine aminotransferase (ALT), lactate dehydrogenase (LD), gamma-glutamyl transferase (γGT), alkaline phosphatase (ALP), bilirubin, creatine kinase (CK) and myoglobin] at repeated intervals during 7 days postexercise and at a follow-up examination 10–12 days postexercise.
Results
Five out of eight studied clinical chemistry parameters (AST, ALT, LD, CK and myoglobin) increased significantly after exercise (P < 0.01) and remained increased for at least 7 days postexercise. Bilirubin, γGT and ALP remained within the normal range.
Conclusion
The liver function parameters, AST and ALT, were significantly increased for at least 7 days after the exercise. In addition, LD and, in particular, CK and myoglobin showed highly elevated levels. These findings highlight the importance of imposing restrictions on weightlifting prior to and during clinical studies. Intensive muscular exercise, e.g. weightlifting, should also be considered as a cause of asymptomatic elevations of liver function tests in daily clinical practice.
What is already known about this subject
The occurrence of idiosyncratic drug hepatotoxicity is a major problem in all phases of clinical drug development and the leading cause of postmarketing warnings and withdrawals.
Physical exercise can result in transient elevations of liver function tests.
There is no consensus in the literature on which forms of exercise may cause changes in liver function tests and to what extent.
What this study adds
Weightlifting results in profound increases in liver function tests in healthy men used to moderate physical activity, not including weightlifting.
Liver function tests are significantly increased for at least 7 days after weightlifting.
It is important to impose relevant restrictions on heavy muscular exercise prior to and during clinical studies.
Keywords: clinical trials, liver function tests, physical exercise
Introduction
The liver is the main organ for conversion of one chemical species to another and this interconversion is the main route for preparing drugs for excretion from the body. The metabolism of drugs can lead to the formation of chemically reactive intermediates that may play a significant role in the induction of hepatic injury. It is important that potentially hepatotoxic effects of new drugs are recognized early during drug development. Therefore, in Phase I clinical trials, monitoring of liver function parameters is mandatory. The occurrence of asymptomatic elevations in liver function tests is a problem during all phases of drug development. An asymptomatic elevation of, for example, liver transaminases during clinical trials could be drug related, but other factors, such as exercise [1] and diet [2], may also have had this effect.
It has long been known that physical exercise results in transient elevations of liver function tests [3, 4]. Subjects studied in Phase I clinical trials are often young healthy volunteers who in their normal life perform some kind of recreational exercise, and during outpatient trials the volunteers usually continue with their normal life, including exercise. We have observed that healthy subjects performing intensive weightlifting during clinical trials may exhibit altered liver function tests [elevations of aspartate aminotransferase (AST) and alanine aminotransferase (ALT)], but the influence of weightlifting on clinical chemistry parameters is poorly described.
There is no consensus on what forms of exercise can cause changes in clinical chemistry parameters, which parameters may be affected, or to what extent. Several studies have described enzyme elevations in response to running [5, 6], whereas only a few have dealt with the effects of weightlifting [7, 8]. The effects of muscular exercise on clinical chemistry parameters may also vary depending on gender and on the fitness level of the individual [9]. However, no study to our knowledge has examined the possible effect of weightlifting on clinical chemistry parameters, commonly used to evaluate liver function, and the duration of such an effect.
The primary objective of the present study was to investigate the effect of intensive muscular exercise (weightlifting) on a single occasion on clinical chemistry parameters, reflecting liver function in healthy men not used to performing weightlifting on a regular basis. A secondary objective was to investigate the effect of a single occasion of intensive muscular exercise (weightlifting) on clinical chemistry parameters reflecting muscle damage, i.e creatine kinase (CK) and myoglobin.
Methods
Study design
This was an open study consisting of five separate visits to one centre (AstraZeneca Clinical Pharmacology Unit, Lund, Sweden); screening and weightlifting ‘test’ (visits 1 and 2), baseline blood sampling before weightlifting (visit 3), weightlifting with blood sampling at repeated intervals up to 1 week post exercise (visit 4), and follow-up (visit 5). Visit 1 was to be performed within 21 days of visit 3, and visit 2 was to take place at least 10 days before visit 3. Visit 5 was to be performed within 10–12 days after the weightlifting exercise at visit 4.
The study involved 15 healthy men aged 18–45 years with a body mass index (BMI) between 18 and 30 and a minimum weight of 60 kg. They were all used to moderate physical exercise, but not used to performing weightlifting on a regular basis. The subjects were not allowed to perform strenuous physical exercise within 2 weeks of visit 1 and they had to abstain from physical exercise during the study, other than study-specific exercise. They were not allowed to have any clinically relevant abnormalities in physical examination, clinical chemistry parameters, human immunodeficiency virus (HIV) and/or hepatitis B/C serology, haematology, urinalysis, ECG or vital signs. All subjects were required to have AST, ALT, alkaline phosphatase (ALP), CK, gamma glutamyl transferase (γGT), lactate dehydrogenase (LD), myoglobin and bilirubin within the appropriate reference ranges at visit 3. They were not allowed to use any medication (except for occasional intake of paracetamol) within 2 weeks of visit 1 and they had to maintain a normal diet and constant weight during the study. In addition, they had to abstain from alcohol consumption for 48 h before each visit, between visits 2 and 3, and during visits, for as long as blood sampling for clinical chemistry parameters were being performed.
Weightlifting programme
At visit 2, subjects tested the weightlifting equipment and the maximum weight possible for each exercise and subject was estimated. At visit 4, subjects used 70% of the obtained maximum weight in each exercise.
Before starting the programme at visit 4, subjects warmed up by cycling at moderate speed for 5 min. They conducted an approximately 1 h long weightlifting programme going through the major muscle groups in the body (Table 1). Every part of the programme was performed in three sets, with approximately a 1-min pause between sets, to a maximum number of repetitions per set with the aim of reaching 12 repetitions. If 12 repetitions were not reached during a set, the weight had to be adjusted before the start of the next (not applicable for push-ups, sit-ups and back-raises). If 12 repetitions were easily reached, the weight was increased.
Table 1.
Weightlifting programme
| Weightlifting exercise | Muscle groups |
|---|---|
| Lat pull down behind neck | Trapezius, teres major, latissimus dorsi, rhomboideus, brachioradialis, brachialis, biceps brachii |
| Cable machine seated row | Trapezius, rhomboideus major, latissimus dorsi, teres major, deltoideus, erector spinae, brachioradialis |
| Back raise* | Gluteus maximus, semitendinosus |
| Sit-ups | Rectus abdominus |
| Push-ups | Pectoralis major and minor, triceps brachii, deltoideus |
| Reversed curls with barbell | Biceps brachii, brachialis |
| Machine side shoulder raise | Deltoideus |
| Seated leg extension | Quadriceps femoris |
| Hamstring curl | Biceps femoris, semitendinosus, semimembranosus |
| Leg press | Gluteus maximus, quadriceps femoris, biceps femoris |
With or without a weight.
After the programme was completed, the subjects had to stretch the major muscles.
Laboratory analysis
At visit 1, blood samples for haematology (haemoglobin, leucocyte count, leucocyte differential count, platelets), clinical chemistry (AST, ALT, ALP, γGT, creatinine, albumin, glucose, C-reactive protein, potassium, calcium, sodium) and HIV and hepatitis B and C serology were taken. A mid-stream urine sample for urinalysis and a drugs of abuse screen (RapidTest d.a.u.® 10 kit; Syva Co., Marburg, Germany) was also performed.
At visit 2, after at least 10 h fasting, a blood sample for plasma bilirubin was taken to exclude subjects with Gilbert's syndrome.
Blood samples were taken for clinical chemistry parameters in plasma (AST, ALT, ALP, CK, γGT, LD, bilirubin) and in serum (myoglobin) at visits 3–5.
At visit 3 baseline values were obtained. At visit 4, blood sampling for these clinical chemistry parameters was performed immediately before, immediately after and at 1, 3, 6, 24, 48, 72, 96, 120, 144 and 168 h after the weightlifting programme. If the enzyme levels were normalized before 168 h, blood sampling was interrupted. Follow-up blood sampling was performed at visit 5, 10–12 days after the weightlifting programme.
Alcohol breath tests were performed at visits 2, 3 and 4.
Ethics
Before the study, approval was obtained by the Ethics Committee of Lund University, Sweden. The study was performed in accordance with the ethical principles of the Declaration of Helsinki and all subjects gave their written informed consent before participation.
Statistics
The study was explorative in nature and not powered with respect to any prespecified difference of interest. Primary data were analysed using descriptive statistics and graphical illustrations. Changes from baseline to different time points after exercise were assessed using Wilcoxon's signed rank sum test.
All hypothesis testing was done using two-sided alternative hypotheses. P-values < 5% were considered to be statistically significant.
The statistical analysis was done using Gauss from Aptech Systems Inc. (Black Diamond, WA, USA).
Results
A total of 35 subjects were enrolled at one centre. Twenty subjects were excluded because eligibility criteria were not met (n = 7) or for other reasons (n = 13). The 15 subjects included were all men with a mean age of 24.5 years and a mean BMI of 22.8 kg m−2. The mean exercise duration was 73.1 min and the mean total workload was 10.5 tonnes. One subject (no. 14) withdrew 1 day after performing the weightlifting programme due to personal circumstances. In total, 14 subjects were used for the statistical analysis (all except subject 14).
Changes in AST and ALT
The individual value curves for AST and ALT are illustrated in Figures 1 and 2. On day 1 postexercise, six subjects showed AST above the upper reference limit, whereas none of the subjects showed increased ALT. On day 2 post exercise, five subjects showed an increased ALT. All 14 subjects had AST above the upper reference limit 3, 4 and 5 days post exercise. The increase was higher for AST; the highest value obtained was 16.0 µkat l−1 in two subjects (nos. 1 and 12) as shown in Table 2. The maximum increase in ALT was 4.1 µkat l−1 (subjects 11 and 15). Seven days postexercise, AST and ALT were still significantly increased compared with the pre-exercise levels (P ≤ 0.01).
Figure 1.
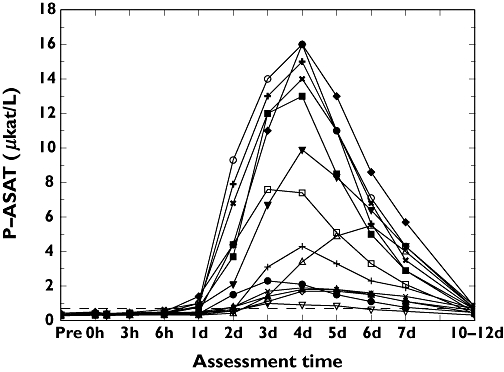
Individual changes over time for aspartate aminotransferase (µkat l−1). 1, (○); 2, (□); 3, (▵); 4, (+); 5, (◊); 6, (▿); 7, (×); 8, (•); 9, (▪); 10, (▴); 11, (+); 12, (♦); 13, (▾); 15 (×)
Figure 2.

Individual changes over time for alanine aminotransferase (µkat l−1). 1, (○); 2, (□); 3, (▵); 4, (+); 5, (◊); 6, (▿); 7, (×); 8, (•); 9, (▪); 10, (▴); 11, (+); 12, (♦); 13, (▾); 15 (×)
Table 2.
Individual responders (above upper reference limit) by parameter
| Maximum value (if responder)* | ||||||||
|---|---|---|---|---|---|---|---|---|
| Subject | ALT (µkat l−1) | AST (µkat l−1) | γGT (µkat l−1) | ALP (µkat l−1) | LD (µkat l−1) | CK (µkat l−1) | Bilirubin (µmol l−1) | Myoglobin (µg l−1) |
| 1 | 3.3 | 16.0 | 26.0 | 857.0 | 24 | >2999† | ||
| 2 | 2.9 | 7.6 | 12.0 | 377.0 | 2874 | |||
| 3 | 1.3 | 5.5 | 2.1 | 7.7 | 233.0 | 1385 | ||
| 4 | 1.3 | 4.3 | 5.9 | 152.0 | 742 | |||
| 5 | 0.7 | 1.7 | 3.8 | 50.4 | 415 | |||
| 6 | 1.0 | 3.7 | 37.3 | 193 | ||||
| 7 | 0.7 | 1.9 | 3.7 | 57.4 | 607 | |||
| 8 | 0.8 | 2.3 | 4.5 | 94.0 | 585 | |||
| 9 | 2.6 | 13.0 | 31.0 | 822.0 | >2999† | |||
| 10 | 0.7 | 1.8 | 3.9 | 62.2 | 532 | |||
| 11 | 4.1 | 15.0 | 14.0 | 891.0 | >2999† | |||
| 12 | 3.7 | 16.0 | 23.0 | 812.0 | >2999† | |||
| 13 | 2.3 | 9.9 | 12.0 | 392.0 | 1954 | |||
| 15 | 4.1 | 14.0 | 10.0 | 507.0 | >2999† | |||
Only results from subjects with values above local upper reference limit are shown; alanine aminotransferase (ALT) (>0.7 µkat l−1), aspartate aminotransferase (AST) (>0.7 µkat l−1), gamma-glutamyl transferase (γGT) (>0.6 µkat l−1), alkaline phosphatase (ALP) (>1.8 µkat l−1), lactate dehydrogenase (LD) (>3.5 µkat l−1), creatine kinase (CK) (>3.3 µkat l−1), bilirubin (>20 µmol l−1), and myoglobin (>72 µg l−1).
The upper detection limit for myoglobin with method used was 2999 µg l−1.
The AST/ALT ratio was >1.0 in all subjects from 6 h to 7 days post exercise. At the follow-up visit, the mean value curve for ALT showed higher values than for AST and 12 of the subjects had a ratio < 1.0. The highest AST/ALT ratio was 6.2, observed in subject 1 on day 2 post exercise. The lowest ratio was 0.36 in subject 2 at follow-up (Figure 3).
Figure 3.

The aspartate aminotransferase/alanine aminotransferase ratio during the study period. Each box shows the median and the interquartile range values; lines show the total range
Changes in LD
The individual changes in LD are illustrated in Figure 4. All subjects showed an increased LD at some time point during the assessment period and the highest value obtained was 31.0 µkat l−1 (subject 9; Table 2). On day 7, LD was still significantly increased compared with the pre-exercise levels (P ≤ 0.01).
Figure 4.
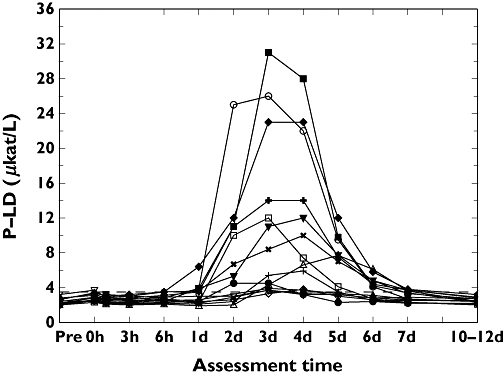
Individual changes over time for lactate dehydrogenase (µkat l−1). 1, (○); 2, (□); 3, (▵); 4, (+); 5, (◊); 6, (▿); 7, (×); 8, (•); 9, (▪); 10, (▴); 11, (+); 12, (♦); 13, (▾); 15 (×)
Changes in ALP, γGT and bilirubin
Bilirubin, γGT and ALP were almost unaltered during the 7-day measurement period. The maximum values for individual responders are given in Table 2. There was one responder for ALP (subject 3; 2.1 µkat l−1) and one for bilirubin (subject 1; 24 µmol l−1). There were no responders for γGT.
Changes in CK and myoglobin
Creatine kinase and myoglobin showed significantly increased values during the full measurement period (Figures 5 and 6). On day 2, both CK and myoglobin were above the reference limit in all 14 subjects. Four subjects had CK levels >800 µkat l−1 and five subjects had myoglobin levels above the maximum detectable level of 2999 µg l−1.
Figure 5.

Individual changes over time for creatine kinase (µkat l−1). 1, (○); 2, (□); 3, (▵); 4, (+); 5, (◊); 6, (▿); 7, (×); 8, (•); 9, (▪); 10, (▴); 11, (+); 12, (♦); 13, (▾); 15 (×)
Figure 6.
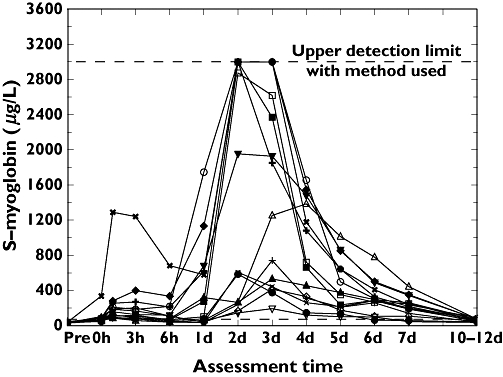
Individual changes over time for myoglobin (µg l−1). 1, (○); 2, (□); 3, (▵); 4, (+); 5, (◊); 6, (▿); 7, (×); 8, (•); 9, (▪); 10, (▴); 11, (+); 12, (♦); 13, (▾); 15 (×)
On day 7, these two parameters were still significantly increased compared with pre-exercise levels (P < 0.01). The maximum values for individual responders are given in Table 2.
Time pattern of changes in clinical chemistry parameters
The time to reach tmax, time to first passage above upper local reference limit and time of renormalization [last passage below upper local reference limit or defined as day 8 (192 h) if still increased on day 7] was calculated for five of the studied clinical chemistry parameters (Table 3). Myoglobin had the shortest tmax (0.08 h) and ALT the longest tmax and time to first passage above upper local reference limit, whereas LD had the shortest time to first passage below upper local reference limit. Except for LD, >50% of the subjects had values above the upper local reference limit on day 7.
Table 3.
Median time to response
| Median times (decimal hours) | |||||
|---|---|---|---|---|---|
| Parameter | ALT | AST | LD | CK | Myoglobin |
| tmax | 120 | 96 | 78 | 96 | 60 |
| Above ref. limit | 59 | 28 | 26 | 2.0 | 0.08 |
| Below ref. limit* | 192 | 192 | 142 | 192 | 192 |
If above reference limit on day 7, day 8 (192 h) was used as estimate. ALT, Alanine aminotransferase; AST, aspartate aminotransferase; LD, lactate dehydrogenase; CK, creatine kinase.
Discussion
The occurrence of idiosyncratic drug hepatotoxicity is a major problem in all phases of clinical drug development and the leading cause of postmarketing warnings and withdrawals [10]. Asymptomatic elevations of liver function tests during clinical trials could be drug-related, but other factors, such as strenuous exercise, have resulted in increased serum transaminase levels [11]. As we had observed that healthy subjects performing intensive weightlifting during clinical trials exhibited altered liver function tests (elevations of AST, ALT; unpublished observations), we conducted a study to clarify the effects of weightlifting on liver function tests.
The effects of weightlifting on CK and myoglobin levels have been thoroughly described [8, 12], but there is no information regarding the effect on clinicalchemistry parameters commonly used to evaluate liver function. However, other types of strenuous physical exercise, such as marathon running, are known to affect liver function tests [13].
In this study, it has been shown that weightlifting resulted in profound increases in the liver function parameters, AST and ALT, as well as in LD, CK and myoglobin levels. Furthermore, we have been able to show that this effect was prolonged and that most subjects still had increased enzyme concentrations 1 week after performing the weightlifting programme. The duration of increased markers for muscle damage (myoglobin, CK, AST, ALT and LD) was in line with a recent study using strenuous one-arm exercise, in which all markers of muscle damage were significantly increased for up to 10 days after exercise [14]. There was, however, considerable variability in the extent of response to the heavy muscular exercise. One possible explanation for this could be that the subjects were used to varying amounts of physical activity in daily life. Other factors, e.g. ethnicity and diet, could also have contributed to this variability.
These findings highlight the importance of imposing relevant restrictions on weightlifting prior to and during clinical studies, and illustrate the need to consider weightlifting and probably other forms of intense muscular activity as possible causes of asymptomatic elevations of liver function tests in daily clinical practice.
The weightlifting programme used in this study resulted in CK values consistent with exercise-induced rhabdomyolysis in most of the subjects. Eight subjects (57%) had maximum CK levels >167 µkat l−1 (10 000 U l−1), a threshold commonly used to diagnose severe rhabdomyolysis [15]. Furthermore, seven out of these eight subjects had CK levels >250 µkat l−1 (15 000 U l−1), which have been associated with an increased risk of renal failure due to rhabdomyolysis [16]. Although renal function was not monitored during the study, none of the subjects had an affected renal function at follow-up. Our findings are in accordance with previous studies [14, 17], and clinicians shouldbe aware of these observations when evaluating patients who have performed a heavy work-out such as weightlifting.
Bilirubin, γGT and ALP were almost unaltered during the 7-day measurement period. This finding was expected, as these enzymes are not present in muscle tissue, and is also in accordance with a previous study [18].
The time pattern of changes in clinical chemistry parameters after the weightlifting programme were also investigated. Myoglobin has the shortest tmax and time to first passage above upper reference limit, followed by LD, CK and AST, whereas ALT has the longest tmax and time to first passage above upper reference limit. The myoglobin peaked 36 h before the CK, a finding consistent with results from a cohort of critically ill patients with rhabdomyolysis treated at an intensive care unit [19].
The AST/ALT ratio was >1 in almost all subjects during 7 days post exercise. However, at the follow-up (10–12 days post exercise) the majority of subjects had an AST/ALT ratio < 1 and ALT concentrations above the upper reference range. This could be explained by the longer half-life of ALT (47 h) compared with AST (17 h) [20, 21]. If liver fuction tests are performed at that time point, a misleading picture may result, suggesting mild liver disease.
The time pattern of enzyme activity following exercise compared with following acute myocardial infarction (AMI) has been reported previously [22]. It is considered that a distinguishing characteristic of LD activity post exercise is that this enzyme peaks at least 40 h sooner than maximal LD activity following AMI. This was not true for our results, where tmax for LD was 78 h postexercise compared with tmax for LD following an AMI, which is about 48 h. One reason for the discrepancy between our results and those of the previous study [22] may be that the type of exercise was different in the respective studies – a weightlifting programme and a 6–10-mile run, respectively. One similarity, however, between these studies was that LD peaked about 16 h earlier than CK in both studies.
Conclusion
Liver function tests are significantly increased for at least 7 days after weightlifting among men used to moderate physical activity, but not used to performing weightlifting on a regular basis. In accordance with these results, and in order to exclude potential exercise-related effects on liver function tests, it is important to impose training restrictions on weightlifting for at least 1 week before the start of clinical trials. Furthermore, the study also illustrates the importance of considering weightlifting and probably other types of intense muscular training as causes of asymptomatic elevations of liver function tests in daily clinical practice. This will reduce the risk of erroneously attributing changes in liver function tests to a drug effect.
The underlying mechanisms of asymptomatic elevations of clinical chemistry parameters caused by muscular exercise are to a large extent unknown and need to be explored further.
References
- 1.Giboney PT. Mildly elevated liver transaminase levels in the asymptomatic patient. Am Fam Physician. 2005;71:1105–10. [PubMed] [Google Scholar]
- 2.Purkins L, Love ER, Eve MD, Wooldridge CL, Cowan C, Smart TS, Johnson PJ, Rapeport WG. The influence of diet upon liver function tests and serum lipids in healthy male volunteers resident in a Phase I unit. Br J Clin Pharmacol. 2004;57:199–208. doi: 10.1046/j.1365-2125.2003.01969.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Loll H, Hilscher A. Change of substrate concentration and enzyme activity in serum by physical activity [in German] Artzl Forsch. 1958;12:II/85–6. [PubMed] [Google Scholar]
- 4.Halonen P, Konttinen A. Effect of physical exercise on some enzymes in the serum. Nature. 1962;193:942–4. doi: 10.1038/193942a0. [DOI] [PubMed] [Google Scholar]
- 5.Hong CZ, Lien IN. Metabolic effects of exhaustive training of athletes. Arch Phys Med Rehabil. 1984;65:362–5. [PubMed] [Google Scholar]
- 6.Apple FS, McGue MK. Serum enzyme changes during marathon training. Am J Clin Pathol. 1983;79:716–9. doi: 10.1093/ajcp/79.6.716. [DOI] [PubMed] [Google Scholar]
- 7.Manore MM, Thompson J, Russo M. Diet and exercise strategies of a world-class bodybuilder. Int J Sport Nutr. 1993;3:76–86. doi: 10.1123/ijsn.3.1.76. [DOI] [PubMed] [Google Scholar]
- 8.Vincent HK, Vincent KR. The effect of training status on the serum creatine kinase response, soreness and muscle function following resistance exercise. Int J Sports Med. 1997;18:431–7. doi: 10.1055/s-2007-972660. [DOI] [PubMed] [Google Scholar]
- 9.Koutedakis Y, Raafat A, Sharp NC, Rosmarin MN, Beard MJ, Robbins SW. Serum enzyme activities in individuals with different levels of physical fitness. J Sports Med Phys Fitness. 1993;33:252–7. [PubMed] [Google Scholar]
- 10.Kaplowitz N. Idiosyncratic drug hepatotoxicity. Nat Rev Drug Dis. 2005;4:489–99. doi: 10.1038/nrd1750. [DOI] [PubMed] [Google Scholar]
- 11.Malinoski FJ. Strenuous exercise simulating hepatic injury during vaccine trials. Vaccine. 1992;10:39–42. doi: 10.1016/0264-410x(92)90417-i. [DOI] [PubMed] [Google Scholar]
- 12.Paul GL, DeLany JP, Snook JT, Seifert JG, Kirby TE. Serum and urinary markers of skeletal muscle tissue damage after weight lifting exercise. Eur J Appl Physiol Occup Physiol. 1989;58:786–90. doi: 10.1007/BF00637392. [DOI] [PubMed] [Google Scholar]
- 13.Smith JE, Garbutt G, Lopes P, Tunstall Pedoe D. Effects of prolonged strenuous exercise (marathon running) on biochemical and haematological markers used in the investigation of patients in the emergency department. Br J Sports Med. 2004;38:292–4. doi: 10.1136/bjsm.2002.002873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Clarkson PM, Kearns AK, Rouzier P, Rubin R, Thompson PD. Serum creatine kinase and renal function measures in exertional muscle damage. Med Sci Sports Exerc. 2006;38:623–7. doi: 10.1249/01.mss.0000210192.49210.fc. [DOI] [PubMed] [Google Scholar]
- 15.De Meijer AR, Fikkers BG, De Keijzer MH, Van Engelen BGM, Drenth JPH. Serum creatine kinase as a predictor of clinical course in rhabdomyolysis: a 5-year intensive care survey. Intens Care Med. 2003;29:1121–5. doi: 10.1007/s00134-003-1800-5. [DOI] [PubMed] [Google Scholar]
- 16.Veenstra J, Smit VM, Krediet RT, Arisz L. Relationship between elevated creatine phosphokinase and the clinical spectrum of rhabdomyolysis. Nephrol Dial Transplant. 1994;9:637–41. doi: 10.1093/ndt/9.6.637. [DOI] [PubMed] [Google Scholar]
- 17.Sinert R, Kohl L, Rainone T, Scalea T. Exercise-induced rhabdomyolysis. Ann Emerg Med. 1994;23:1301–6. doi: 10.1016/s0196-0644(94)70356-6. [DOI] [PubMed] [Google Scholar]
- 18.Statland BE, Winkel P, Bokelund H. Factors contributing to intra-individual variation of serum constituents: 2. Effects of exercise and diet on variation of serum constituents in healthy subjects. Clin Chem. 1973;19:1380–3. [PubMed] [Google Scholar]
- 19.Mikkelsen TS, Toft P. Prognostic value, kinetics and effect of CVVHDF on serum of the myoglobin and creatine kinase in critically ill patients with rhabdomyolysis. Acta Anaesthesiol Scand. 2005;49:859–64. doi: 10.1111/j.1399-6576.2005.00577.x. [DOI] [PubMed] [Google Scholar]
- 20.Dajani LK, Paus E, Warren DJ. Development of a rapid and sensitive immunofluorometric assay for gluthatione S-transferase A. Clin Chem. 2001;47:867–73. [PubMed] [Google Scholar]
- 21.Knapen M, Mulder T, Bisseling J, Penders R, Peters W, Steegers E. Plasma gluthatione S-transferase alpha 1-1: a more sensitive marker for hepatocellular damage than serum alanine aminotransferase in hypertensive disorders of pregnancy. Am J Obstet Gynecol. 1998;178:161–5. doi: 10.1016/s0002-9378(98)70645-3. [DOI] [PubMed] [Google Scholar]
- 22.Kaman RL, Goheen B, Patton R, Raven P. The effects of near maximum exercise on serum enzymes: the exercise profile versus the cardiac profile. Clin Chim Acta. 1977;81:145–52. doi: 10.1016/0009-8981(77)90006-7. [DOI] [PubMed] [Google Scholar]


