Abstract
Aims
To examine the potential effect of danshen extract on the pharmacokinetics of theophylline.
Methods
In a sequential cross-over study with two phases, 12 volunteers took 100 mg theophylline on day 1 and day 15. From day 2 to day 15, volunteers received danshen extract tablets three times daily, four tablets each time for 14 days. On day 15, they received four danshen extract tablets with 100 mg theophylline. Plasma concentrations of theophylline were measured on days 1 and 15 periodically for 24 h.
Results
The 90% confidence interval of Cmax, t1/2 and CL/F of theophylline with 14-day danshen extract tablets vs. without comedication were (101.42, 121.36) (84.57, 106.72) and (88.82, 105.72), respectively. The time to peak plasma theophylline concentration was unchanged by danshen (P > 0.05). The pharmacokinetics parameter of theophylline was unaffected by danshen extract.
Conclusions
Danshen extract does not influence the metabolism of theophylline in healthy volunteers. Dose adjustment of theophylline thus may not be necessary in patients receiving concurrent therapy with danshen extract tablets.
What is already known about this subject
Danshen extract is widely used for the treatment and prevention of coronary heart disease and other diseases of senility in Asia.
Danshen extract and theophylline may be prescribed together to treat patients with asthma.
In human, theophylline with low therapeutic index is mainly metabolized by CYP1A2.
In vitro findings have shown that human CYP1A2 is inhibited by the ethyl acetate extract of danshen and danshen pharmaceutical product.
There may be drug interactions between danshen extract and theophylline (CYP1A2 substrate).
What this study adds
This study concerned drug interactions between danshen extract and theophylline in Chinese volunteers.
Long-term oral intake of danshen extract does not change the basic pharmacokinetic parameters of theophylline.
Dose adjustment of theophylline thus may not be necessary in patients receiving concomitant therapy with danshen extract.
Keywords: CYP1A2, danshen extract, drug interaction, theophylline
Introduction
Danshen, the dried root of Salvia miltiorrhiza, is used as a traditional Chinese medicine for promoting circulation and improving blood stasis. It is also widely used for the treatment and prevention of coronary heart disease, hyperlipidaemia and cerebrovascular disease [1–3]. Numerous danshen extract products are commercially available, especially in Asia. These consist of tablets, capsules, granules, injection preparations, oral liquids, dripping pills and sprays of either Danshen or Fufang Danshen, which is the composite of S. miltiorrhiza, Panax notoginseng and Cinnamomum camphora.
In recent years, some studies have reported the effect of danshen extract composed of lipophilic and hydrophilic components on cytochrome P450 (CYP). Chan et al. have reported that treatment of rats with the aqueous extract of danshen (hydrophilic components) elevated the AUC of warfarin, suggesting an inhibitory effect of danshen on CYP. Warfarin is mainly metabolized by CYP2C9 and, to a lesser extent, by CYP1A2 and CYP3A4 [4]. A study has shown that there are mouse CYP1A-, CYP2C- and CYP3A-inducing agents present in the ethyl acetate extract of danshen (lipophilic components) [5–7]. The in vitro study showed that human CYP1A2 is inhibited by the ethyl acetate extract of danshen and danshen pharmaceutical product. These findings suggest that drug interactions between danshen and CYP1A2 substrates require further study in humans.
Theophylline has been used for many years to treat acute asthma and chronic obstructive pulmonary disease [8, 9]. Oral absorption of theophylline is almost complete, with peak plasma concentrations generally achieved 2 h after administration, although this can be influenced by coadministered medications [10]. The therapeutic index of theophylline is low with the therapeutic concentration ranges of 5–20 µg ml−1, and signs of toxicity or therapeutic failure may occur with relatively small changes in plasma concentrations of the drug [11].
In humans, theophylline is eliminated almost exclusively by CYP-mediated hepatic oxidation, predominantly to 1,3-dimethyluric acid, 1-methyluric acid, and 3-methylxanthine by CYP1A2, and, to a lesser extent, to 1,3-dimethyluric acid by CYP2E1 [12]. Inhibition of CYPlA2 activity may increase plasma theophylline by inhibiting hepatic clearance and may contribute to the emergence of adverse effects. In contrast, induction of cytochrome isozymes may reduce plasma theophylline to subtherapeutic concentrations.
Since danshen extract and theophylline may be prescribed together to treat patients with asthmatic disease, herb–drug interaction may crucially affect the therapeutics of theophylline with a narrow therapeutic index. Although some in vitro findings have suggested that there are drug interactions between danshen extract and CYP1A2 substrates, no in vivo studies have investigated the influence of danshen extract on theophylline metabolism. The purpose of this study was to investigate whether danshen extract can influence CYP1A2 activity and consequently alter the pharmacokinetics of theophylline in healthy volunteers.
Methods
The quality and reliability of Danshen [13]
The extract was obtained from the dried root of danshen. Danshen extract tablet used in this study was produced according to the methods of the Chinese Pharmacopoeia (2005) [14], which contained an extract of 1 g danshen (lot no. 051003) manufactured by Shanghai Leiyong Shong Pharmaceutical Limited Company (China). This product had been registered for clinical use for decades in China.
The hydrophilic and lipophilic components of Danshen extract tablet were separately determined by high-performance liquid chromatography (HPLC) [15–18]. The Waters HPLC system (Waters Corporation, Milford, MA, USA), used for determination of the components of danshen, consisted of a 515 binary HPLC pump, a 717 plus autosampler, a column incubator, a 2487 ultraviolet detector, and Breeze Software. A Lichrospher C18 (150 × 4.6 mm, 5-µm) column was used for analysis.
For determination of hydrophilic components, the mobile phase was 0.5% acetic acid:methanol (80 : 20, v/v). Elution was carried out at a flow rate of 1 ml min−1 and at a column temperature of 35°C. The detection wavelength was set to 282 nm.
For determination of the lipophilic components, the mobile phase was 0.5% acetic acid:methanol (17 : 83, v/v). The flow rate was 1.0 ml min−1. The detection wavelength was set to 254 nm.
The contents of the lipophilic components in each table found were: cryptotanshinone (0.25 ± 0.06 mg, n = 5), tanshinone I (0.5 ± 0.1 mg, n = 5) and tanshinone IIA (0.35 ± 0.04 mg, n = 5); the contents of the major hydrophilic components were: danshensu (1.6 ± 0.3 mg, n = 5), protocatechuic acid (0.2 ± 0.04 mg, n = 5) and salvianolic acid B (13 ± 2 mg, n = 6). All analyses were performed in triplicate. The following reference standards were used: cryptotanshinone, tanshinone I, tanshinone IIA, danshensu, protocatechuic acid and salvianolic acid B purchased from the National Institute for the Control of Pharmaceutical and Biological Products (Beijing, China).
Subjects
All subjects were nonsmokers and were healthy on the basis of medical history, physical examination, electrocardiogram and routine tests of urine, biochemistry and haematology. Furthermore, all volunteers were required to have no laboratory evidence of hepatitis B, hepatitis C or human immunodeficiency virus infection.
Participants were excluded if they had any relevant medical history 4 weeks before admission, use of any prescription or over-the-counter drugs (including herbal medicines or supplement) within 4 weeks before enrolment or during the study. Twelve healthy subjects were randomly selected from a pool of healthy volunteers. The ethics committee of Yijishan Hospital, affiliated to Wannan Medical College, approved the clinical protocol and informed consent form. All subjects signed an informed consent form before the study.
Study design
The study design was a sequential, open-label, two-period, cross-over trial (single dose P450 probe followed by multiple dose investigated drug + single dose probe [19]) conducted at the Drug Clinical Research Organization of Yijishan Hospital. On the morning of day 1, after oral administration of a single dose of 100 mg theophylline (0.1 g per tablet, immediate release dose form, lot no. 0508150; Shangdong Xinhua Pharmaceutical Co. Ltd, Zhongdian, China), 4-ml blood samples were taken at 0, 0.5, 1, 2, 3, 4, 5, 6, 8, 10, 12 and 24 h. On day 2, subjects received danshen extract tablets three times daily, four tablets each time for 14 days. On day 15, they received four danshen extract tablets together with 100 mg theophylline. Blood samples were obtained from forearm veins; blood samples were taken at the same as on day 1. The plasma was centrifuged immediately and stored at −70°C until analysis. Before morning dosing of day 1 and day 15, the subjects had fasted overnight. A light standard meal was served 4 h after medication intake on 2 days. Smoking and consumption of alcohol, coffee, tea and any drugs were prohibited during the test days.
Plasma sample preparation and analysis [20, 21]
Plasma samples were analysed for theophylline concentration using a validated HPLC method. The Waters HPLC system consisted of a 515 binary HPLC pump, a 717 plus autosampler, a column incubator, a 2487 ultraviolet detector and Breeze Software. A Lichrospher C18 (250 × 4.6 mm, 5 µm) column was used for analysis. The mobile phase was methanol:water (20 : 80, v/v) (pH 6.7 by triethylamine and acetic acid adjustment. The flow rate was 1.0 ml min−1. Recovery of theophylline was >80%. This assay has a lower limit of quantification (LOQ) of 50.0 ng ml−1, with a calibration curve ranging from 68.0 to 8712.0 ng ml−1. Intra- and interday precision was 10.9%, 5.7%, 11.8% and 7.3%, 4.0%, 6.0%, respectively, based on QC samples of 136.0, 1089.0, 4356.0 ng ml−1. All mean accuracy values were within 95.5–99.0% for both the standards and QC samples. The detection wavelength was set to 270 nm. The retention time of theophylline was 10.0 min under the described conditions.
To 250 µl of plasma sample, in 1.5 ml MaxyClear microtubes, 100 µl perchloric acid was added. The samples were extracted by vortex mixing for 30 s and centrifuged at 9652 g for 10 min. Only 10 µl of supernatant was injected into the HPLC column.
Safety evaluation
Safety and tolerability were evaluated through adverse events (AEs) reported by the doctors and subjects. AEs were assessed by the doctors with regard to severity (mild, moderate, severe, and life threatening) and relationship (reasonably or possibly related, not reasonably or possibly related) to study treatment.
Pharmacokinetic analysis
The plasma concentration–time data of theophylline obtained on days 1 and 15 were analysed by model-independent approaches. The maximum plasma drug concentration (Cmax) and time to Cmax (Tmax) were directly obtained from the plasma concentration–time data. The elimination half-life (t1/2) was calculated as 0.693/Ke, where Ke, the elimination rate constant, was calculated from semilog regression on the terminal phase of the plasma concentration–time curve. The AUC from time 0 to infinity (AUC0–∞) was estimated as AUC0–t + Ct/Ke, where Ct is the plasma concentration of the last measurable sample and AUC0–t was calculated according to the linear trapezoidal rule. Total plasma clearance (CL/F) was calculated as dose/AUC0–∞.
Statistical analysis
The principal pharmacokinetic parameters (Cmax, AUC0–∞, T1/2 and CL/F) were ln-transformed. Results for Cmax, AUC0–∞, T1/2 and CL/F were reported as 90% confidence intervals (CIs) about the ratio of the geometric least-squares (LS) means of the pharmacokinetic measures between without comedication and with 14-day danshen treatment. The resulting confidence limits were transformed by exponentiation and reported on the original measurement scale. Tmax was analysed using Wilcoxon's signed rank test. The DAS statistical analysis system (version 1.0) was used.
Results
Mean plasma theophylline concentration–time profiles before and after 14 days of Danshen extract tablets are presented in the Figure 1. It was shown that long-term oral intake of Danshen extract tablets had little effect on the plasma concentrations of theophylline.
Figure 1.
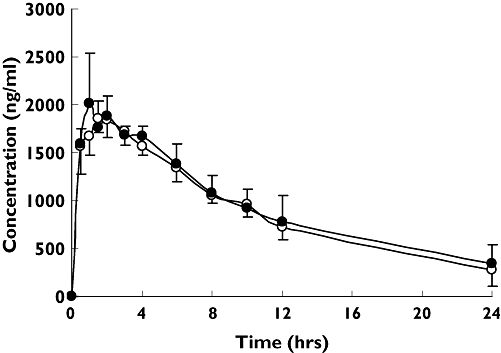
Plasma theophylline concentration (mean ± SD) vs. time profiles in 12 healthy volunteers before (○) and after (•) coadministration of 14-day Danshen extract tablets
Table 1 summarizes the pharmacokinetic parameters of theophylline before and after 14-days' treatment with Danshen extract tablets. Values of Cmax were 1882.11 and 2134.21 ng ml−1, CL/F was 4.37 and 4.47 l h−1 and tmax was 1.6 h and 1.3 h, respectively, for 14-day Danshen extract tablet treatment and before comedication with Danshen extract tablets.
Table 1.
Effects of Danshen extract on pharmacokinetic parameters of theophylline in healthy volunteers
| PK parameter | Baseline (n = 12) | Danshen (n = 12) | Geometric LS mean ratio, % | 90% CI | P-value |
|---|---|---|---|---|---|
| t1/2 (h) | 6.66 ± 0.81 | 6.69 ± 1.02 | 94.78 | (84.57, 106.22) | – |
| Tmax (h) | 1.60 ± 0.22 | 1.30 ± 0.45 | – | – | 0.285* |
| Cmax (mg l−1) | 1 882.11 ± 154.2 | 2 134.21 ± 404.6 | 110.94 | (101.42, 121.36) | – |
| CL/F (l h−1) | 4.37 ± 0.28 | 4.47 ± 0.85 | 96.90 | (88.82, 105.72) | – |
| AUC0–∞ (mg l−1) | 22 969.6 ± 1470.05 | 23 031.11 ± 4 457.24 | 103.42 | (94.82, 112.80) | – |
Wilcoxon signed rank test.
Ratios of geometric LS means of Cmax, AUC0–∞, t1/2 and CL/F (with 14-day danshen vs. without comedication) were 110.94%, 103.42%, 94.78% and 96.90%, respectively.
The 90% CIs of the Cmax, AUC0–∞, t1/2 and CL/F were within the range of bioequivalence (80–125%). A Wilcoxon signed rank test indicated that Tmax was not significantly different (P > 0.05).
Twelve subjects completed the study ‘per protocol’ and all tolerated well the Danshen extract tablets and theophylline.
Discussion
Because many composite preparations containing danshen are available on market, Danshen extract tablets were selected as a test preparation in order to avoid the interference of other plant components.
In this study, 14 days of treatment with Danshen extract tablets had no effect on the Cmax of theophylline. Moreover, none of the other pharmacokinetic parameters for theophylline (Tmax, AUC, CL/F, t1/2) were significantly altered by concomitant administration of Danshen extract tablets. The bioequivalence of theophylline in the absence and presence of danshen was shown by the 90% CIs, and there was no difference in plasma concentration–time curves of theophylline with 14-day Danshen extract tablets and without comedication.
Previous in vitro findings have suggested that lipophilic constituents play a role in the induction or inhibition of CYP1A2 [5–7]. All chemical constituents and the concentration of danshen absorbed into the blood stream were unidentified, but we did not explore plasma concentrations of tanshinone IIA, tanshinone I and cryptotanshinone, after following the Danshen extract tablet by the LC/MS/MS method, as described previously [22–24]. Our findings are consistent with previous results [25, 26]. Tanshinone IIA absorption was poor, with an absolute bioavailability of <3.5%. The poor absorption of Tanshinone IIA may have been caused by its low aqueous solubility and limited membrane permeability [26].
The lipophilic components of Danshen extract have low bioavailability, therefore they have little effect on CYP1A2 which mainly locates on the hepatocyte after oral administration. Since theophylline is mainly metabolized by CYP1A2, the metabolism of theophylline is not likely to be influenced by long-term oral administration of Danshen extract.
In conclusion, long-term oral administration of Danshen extract tablets did not change the basic pharmacokinetic parameters of theophylline. Thus, dose adjustment of theophylline may not be necessary in patients receiving concomitant therapy with Danshen extract tablets.
Acknowledgments
This study was supported by a grant from the Health Department of Anhui province, China (2002A039), and by a grant from National ‘863’ Project (2003AA2Z347A). The assistance of J. J. Yang, P. Wu, L. X. Zhou and X. L. Hu is gratefully acknowledged.
References
- 1.Danshen. A popular Chinese cardiac herbal drug. J Am Coll Cardiol. 2006;47:1498. doi: 10.1016/j.jacc.2006.01.001. [DOI] [PubMed] [Google Scholar]
- 2.Ji XY, Tan BK, Zhu YZ. Salvia miltiorrhiza and ischemic diseases. Acta Pharmacol Sin. 2000;21:1089–94. [PubMed] [Google Scholar]
- 3.Zhou W, Ruigrok TJ. Protective effect of danshen during myocardial ischemia and reperfusion: an isolated rat heart study. Am J Chin Med. 1990;18:19–24. doi: 10.1142/S0192415X90000046. [DOI] [PubMed] [Google Scholar]
- 4.Chan TY. Interaction between warfarin and danshen (Salvia miltiorrhiza) Ann Pharmacother. 2001;35:501–4. doi: 10.1345/aph.19029. [DOI] [PubMed] [Google Scholar]
- 5.Kuo YH, Lin YL, Don MJ, Chen RM, Ueng YF. Induction of cytochrome P450 dependent monooxygenase by extracts of the medicinal herb Salvia miltiorrhiza. J Pharm Pharmacol. 2006;58:521–6. doi: 10.1211/jpp.58.4.0012. [DOI] [PubMed] [Google Scholar]
- 6.Ueng YF, Kuo YH, Wang SY, Lin YL, Chen CF. Induction of CYP1A by a diterpene quinone tanshinone IIA isolated from a medicinal herb Salvia miltiorrhiza in C57BL/6J but not in DBA/2J mice. Life Sci. 2004;74:885–96. doi: 10.1016/j.lfs.2003.07.035. [DOI] [PubMed] [Google Scholar]
- 7.Ueng YF, Kuo YH, Peng HC, Chen TL, Jan WC, Peter GF, Lin YL. Diterpene quinone tanshinone IIA selectively inhibits mouse and human cytochrome p4501A2. Xenobiotica. 2003;33:603–13. doi: 10.1080/0049825031000105769. [DOI] [PubMed] [Google Scholar]
- 8.Sullivan P, Bekir S, Jaffear Z, Page CP, Jeffery P, Costello JF. Anti-inflammatory effects of low-dose oral theophylline in atopic asthma. Lancet. 1994;343:1006–8. doi: 10.1016/s0140-6736(94)90127-9. [DOI] [PubMed] [Google Scholar]
- 9.Pauwels R. The effect of theophylline and enprophylline on allergen-induced bronchoconstriction. J Allergy Clin Immunol. 1985;76:583–90. doi: 10.1016/0091-6749(85)90779-1. [DOI] [PubMed] [Google Scholar]
- 10.Page CP, Cotter T, Kilfeather S, Sullivan P, Spina D, Costello JF. Effect of chronic theophylline treatment on the methacholine dose–response curve in allergic asthmaticsubjects. Eur Respir J. 1998;12:24–9. doi: 10.1183/09031936.98.12010024. [DOI] [PubMed] [Google Scholar]
- 11.Desoky E, Meinshausen J, Buhl K, Engel G, Harings-Kaim A, Drewelow B, Klotz U, Rolan PE. Generation of pharmacokinetic data during routine therapeutic drug monitoring: Bayesian approach vs pharmacokinetic studies. Ther Drug Monit. 1993;15:281–8. doi: 10.1097/00007691-199308000-00004. [DOI] [PubMed] [Google Scholar]
- 12.Sarkar MA, Hunt C, Guzelian PS, Karnes HT. Characterization of human liver cytochromes P-450 involved in theophylline metabolism. Drug Metab Dispos. 1992;20:3l–7. [PubMed] [Google Scholar]
- 13.Gagnier JJ, Boon H, Rochon P, Moher D, Barnes J, Bombardier C. Reporting randomized, controlled trials of herbal interventions: an elaborated CONSORT statement. Ann Intern Med. 2006;144:364–7. doi: 10.7326/0003-4819-144-5-200603070-00013. [DOI] [PubMed] [Google Scholar]
- 14.State Pharmacopoeia Commission of the People's Republic of China. Pharmacopoeia of the People's Republic of China. 2005. Bei jing: Chemical Industry Press; 2005. p. 394. [Google Scholar]
- 15.Sun D, Liu WY, Liang C. Simultaneous quantitative determination of four active components in Salvia miltiorrhiza tablets by HPLC. J China Pharmaceut University. 2007;38:51–4. [Google Scholar]
- 16.Guo YX, Zhang DJ, Wang H, Xiu ZL, Wang LX, Xiao HB. Hydrolytic kinetics of lithospermic acid B extracted from roots of Salvia miltiorrhiza. J Pharmaceut Biomed. 2007;43:435–9. doi: 10.1016/j.jpba.2006.07.046. [DOI] [PubMed] [Google Scholar]
- 17.Shi ZH, He JT, Yao TT, Chang WB, Zhao MP. Simultaneous determination of cryptotanshinone, tanshinone I and tanshinone IIA in traditional Chinese medicinal preparations containing Radix salvia miltiorrhiza by HPLC. J Pharmaceut Biomed. 2005;37:481–6. doi: 10.1016/j.jpba.2004.11.015. [DOI] [PubMed] [Google Scholar]
- 18.Zhou LM, Chow M, Zuo Z. Improved quality control method for Danshen products – consideration of both hydrophilic and lipophilic active components. J Pharmaceut Biomed. 2006;41:744–50. doi: 10.1016/j.jpba.2005.12.032. [DOI] [PubMed] [Google Scholar]
- 19.Bjornsson TD, Callaghan JT, Einolf HJ, Fischer V, Gan L, Grimm S, Kao J, King SP, Miwa G, Ni L, Kumar G, Mcleod J, Obach RS, Roberts S, Shah ARA, Snikeris F, Sullivan J, Tweedie D, Vega JM, Walsh J, Wrighton SA. The conduct of in vitro and in vivo drug–drug interaction studies: a phRMA perspective. J Clin Pharmacol. 2003;43:443–69. [PubMed] [Google Scholar]
- 20.Holland DT, Godfredsen KA, Page T, Connor JD. Simple high performance liquid chromatography method for the simultaneous determination of serum caffeine and paraxanthine following rapid sample preparation. J Chromatogra B. 1998;707:105–10. doi: 10.1016/s0378-4347(97)00590-2. [DOI] [PubMed] [Google Scholar]
- 21.Rasmussen BB, Brosen K. Determination of theophylline and its metabolites in human urine and plasma by high performance liquid chromatography. J Chromatogra B. 1996;676:169–74. doi: 10.1016/0378-4347(95)00374-6. [DOI] [PubMed] [Google Scholar]
- 22.Pei WJ, Zhao XF, Zhu ZM, Lin CZ, Zha WM, Zheng XH. Study of the determination and pharmacokinetics of compound danshen dripping pills in human serum by column switching liquid chromatography electrospray ion trap mass spectrometry. J Chromatogr B. 2004;809:237–42. doi: 10.1016/j.jchromb.2004.06.028. [DOI] [PubMed] [Google Scholar]
- 23.Li XC, Yu C, Cai YB, Liu GY, Jia JY, Wang YP. Simultaneous determination of six phenolic constituents of danshen in human serum using liquid chromatography/tandem mass spectrometry. J Chromatogr B. 2005;820:41–7. doi: 10.1016/j.jchromb.2005.03.005. [DOI] [PubMed] [Google Scholar]
- 24.Hao HP, Wang GJ, Li P, Li J, Ding ZQ. Simultaneous quantification of cryptotanshinone and its active metabolite tanshinone IIA in plasma by liquid chromatography/tandem mass spectrometry (LC–MS/MS) J Pharmaceut Biomed. 2006;40:382–8. doi: 10.1016/j.jpba.2005.07.029. [DOI] [PubMed] [Google Scholar]
- 25.Kim HH, Kim J, Ji HY, Kim YC, Sohn DH, Lee BM, Lee HS. Pharmacokinetics of lithospermic acid B isolated from Salvia miltiorrhiza in rats. J Toxicol Environ Health A. 2005;68:2239–47. doi: 10.1080/15287390500182222. [DOI] [PubMed] [Google Scholar]
- 26.Hao HP, Wang GJ, Cui N, Li J, Xie L, Ding ZQ. Pharmacokinetics, absorption and tissue distribution of tanshinone IIA solid dispersion. Planta Med. 2006;72:1311–7. doi: 10.1055/s-2006-951698. [DOI] [PubMed] [Google Scholar]


