Abstract
Aim
To evaluate the effect of an electronic prompt in dispensing software on the frequency of clinical interventions recorded by community pharmacists.
Method
An electronic decision-support prompt identifying patients for a targeted proactive clinical intervention was developed and implemented. Each time an oral antidiabetic agent was dispensed, a prompt was displayed reminding pharmacists to discuss the suitability of aspirin therapy in eligible patients with diabetes. The prompt was randomly assigned to 31 of 52 metropolitan pharmacies in Melbourne (Australia) for 6 weeks, with the remaining pharmacies as controls.
Results
One hundred and fifty pharmacists in 52 pharmacies recorded a total of 2396 clinical interventions at an intervention rate of 0.92 interventions per 100 patients [95% confidence interval (CI) 0.58, 1.23]. Pharmacists recorded a total of 201 target interventions related to aspirin therapy in diabetes at an intervention rate of 2.55 interventions per 100 diabetic patients (95% CI 0.85, 4.24). All of the targeted clinical interventions were recorded in the prompt arm; no targeted interventions were recorded in the control group. The effect of the prompt decreased over the study period and was not maintained after prompt deactivation.
Conclusion
An electronic prompt significantly increased pharmacists' recording of the targeted clinical intervention in diabetic patients. An electronic decision-support prompt has significant potential to promote community pharmacists' contribution to the quality use of medicines.
What is already known about this subject
Computerized prompts and reminders have been shown to be effective in changing the behaviour of health professionals in a variety of settings.
There is little literature describing or evaluating electronic decision-support for pharmacists.
What this study adds
An electronic prompt in dispensing software for a targeted clinical intervention has a significant effect on pharmacists' behaviour. A markedly increased rate of recording and performing the targeted clinical intervention was found.
The effect of the prompt reduces markedly once the prompt is deactivated.
Keywords: clinical interventions, decision support, pharmacist
Introduction
The practice of community pharmacy has changed dramatically. The primary activities of pharmacists have traditionally been procuring, preparing and dispensing medicines. In recent years, the emphasis has shifted to patient care, and pharmacists now undertake a variety of clinical roles, including patient counselling and education, individual medication reviews, drug use evaluation, medication compliance monitoring, prescribing error detection and intervention and monitoring of therapeutic outcomes (e.g. blood pressure, blood glucose level). The emerging patient-focused roles for community pharmacists will require improved access to clinical knowledge and appropriate decision-support tools [1].
Computerized reminders and alerts are an increasingly common means of delivering support to doctors and other health professionals, and their use is likely to increase as electronic medical records become more prevalent [2]. Computerized prompts and reminders have been shown to be effective in changing the behaviour of health professionals in a variety of settings. In a systematic review of electronic decision-support, Garg et al.[3] have shown improved practitioner performance in 62 of the 97 studies assessed. Reminders and alerts can improve the quality of care by reducing reliance on memory and presenting accepted, evidence-based clinical guidelines at the point-of-care [4].
There is little literature regarding clinicians' views and preferences to guide the design of effective alerts. Bates et al.[5] have studied the impact of decision-support in a number of fields over the past decade and found a number of elements important for success, including speed of access, ‘fit’ with user workflow, usability, simplicity, minimal manual entry of information, early monitoring of impact with feedback and adjustment if necessary, and maintenance and tailoring of the knowledge base.
As community pharmacists generally use a computer for dispensing prescriptions, the potential for using decision-support as a means of promoting clinical interventions that improve patient care and enhance pharmacists' professional role is enormous. Electronic decision-support in community pharmacy dispensing systems in Australia is limited and currently includes alerts for drug–drug interactions, drug allergy warnings, drug–food interactions, therapeutic duplication and/or drug dose checking. There are no reports of critical evaluations of decision-support features of these systems or of their impact or outcome [1].
We implemented and evaluated a computerized prompt system which identified community pharmacy patients potentially eligible for a specific clinical intervention – a recommendation for the addition of low-dose aspirin therapy to the medication regimen of high-risk patients with diabetes. This recommendation is supported by current guidelines [6]. The prompt was incorporated into the workflow of the dispensing process, and a partially automated electronic documentation system was used by pharmacists to record details of the intervention, allowing measurement of the effect of the prompt on pharmacists' behaviour. The aims of the study were to evaluate the effectiveness of the prompt on the rate and recording of clinical interventions by pharmacists and assess pharmacists' opinions of the prompt.
Methods
This project was part of a larger project – Pharmacy Recording of Medication Incidents and Services (PROMISe) [7]– which developed and evaluated an electronic documentation system to record pharmacists' clinical interventions. The PROMISe study was approved by the Human Research Ethics Committee (University of Tasmania).
Prompt
A single, specific, proactive clinical intervention was chosen for the prompt. The clinical intervention was chosen on the basis that there was clear supporting evidence for the intervention.
Functional specifications were developed specifying the content and appearance of the prompt; the drugs for which the prompt should appear; associated material for patients and pharmacists, and dates the prompt should be activated and de-activated. A pharmacy software vendor (PCA NU Systems, Melbourne, Australia) was contracted to implement the prompt system in their dispensing software (WiniFRED) according to the functional specifications. This dispensing software is the market leader in the community pharmacy sector in Australia, with a presence in almost half of all community pharmacies.
Pharmacists were presented with the prompt (Figure 1) each time they dispensed an oral hypoglycaemic agent (Table 1) for a patient, indicating a potential clinical intervention [i.e. the computer identified a patient with diabetes who was potentially eligible for low-dose aspirin for the prevention of cardiovascular disease (CVD)].
Figure 1.
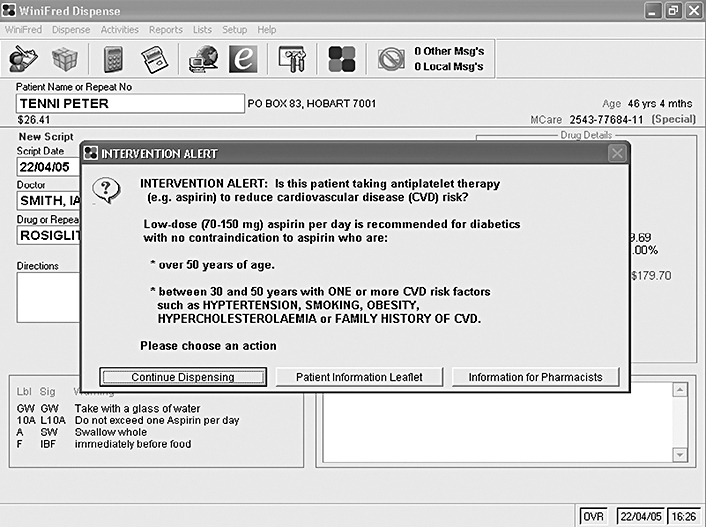
The ‘prompt’ dialogue in dispensing software indicating a potential clinical intervention. Buttons on the prompt allowed pharmacists to: (a) dismiss the prompt; (b) print a patient leaflet; (c) view background material and a protocol for performing the intervention
Table 1.
Oral hypoglycaemic drugs linked to prompt
| Acarbose | Glimepiride | Pioglitazone | Rosiglitazone |
| Glibenclamide | Glipizide | Repaglinide | Metformin/glibenclamide combination |
| Gliclazide | Metformin |
Sample size
The sample size (number of pharmacies) was determined by calculating opportunities for performing the targeted intervention and considering estimates of pharmacists' clinical intervention rates from other studies of approximately 0.5 interventions per 100 prescriptions [8, 9]. An approximation of the opportunities for performing the targeted intervention was based on: the expected numbers of prescriptions dispensed over the study period for each of the oral hypoglycaemic agents available in Australia [10]; an estimation of number of patients eligible for the targeted intervention based on underutilization of aspirin prophylaxis in diabetics of around 50% [11]; and an estimation of the mean number of prescriptions dispensed per week in each pharmacy.
Participants
On behalf of the project team, the collaborating software vendor distributed a letter to 260 pharmacies in metropolitan Melbourne (Australia) with the WiniFRED dispensing software, inviting participation in the study. After exclusion due to software or hardware issues, 52 (20%) of the 260 pharmacies were recruited to participate in the study. Pharmacies were evenly matched for ownership status (chain vs. individual), annual turnover, number of prescriptions dispensed, opening hours and staffing levels. The proprietor for each of the 52 community pharmacies recruited for the project signed agreements relating to nondisclosure, privacy, ethics and use of the data.
Proprietors identified 150 pharmacists who anticipated working at least one full day during the trial period in any one of the 52 recruited pharmacies. These pharmacists were required to attend an orientation and training session detailing the PROMISe documentation module as it appeared in the WiniFRED dispensing system. No information about the prompt was provided or shown at these sessions. They were then given 6 weeks to complete online training for classifying interventions using the DOCUMENT classification scheme [12] (Appendix).
The 52 pharmacies were randomized with a computer-generated list of random numbers to a ‘prompt’ or a ‘no-prompt’ arm at a 3 : 2 ratio, i.e. 31 of the 52 pharmacies had the prompt activated. The prompt was activated in the dispensing software of the intervention pharmacies for 6 weeks, with data collected for approximately 8 weeks in order to capture a potential decline in intervention rate following deactivation. Half of the pharmacies in the prompt arm (15) were allocated an observer (Figure 2). Differences in clinical intervention rates between the two study arms were used to assess the effectiveness of the prompt. Within the prompt arm, the effect of observers on clinical intervention rate was also assessed.
Figure 2.
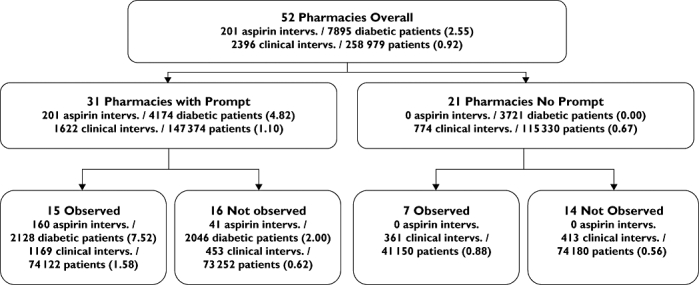
Interventions (Aspirin and Total) per 100 diabetic or total patients during trial period showing prompt and observer arms
The seven observers recruited for the study were experienced pharmacists familiar with the dispensing system. They were trained in the classification of clinical interventions using the DOCUMENT classification scheme and in use of the documentation module developed for the interface. Their role was to assist in documentation and to remind the pharmacists to document, but not to prompt them to perform an intervention. On average, observers were present in 21 of the 52 pharmacies for 3 h on nine separate occasions during the initial 3 weeks of the study, alternating between morning and afternoon.
Pharmacist questionnaire
At the completion of the study, a questionnaire was sent to the 150 pharmacists involved. Pharmacists were asked to mark a 10-point Likert scale to indicate their agreement or disagreement with each of nine questions (0 = strongly disagree; 10 = strongly agree) with the aim of determining their opinion of the usefulness of the prompt. There was an option to tick a box if unsure.
Clinical intervention
A clinical intervention was defined as ‘any professional activity (outside of the basic dispensing and counselling procedures) directed towards improving health outcomes or the quality use of medicines, or the provision of health-related information’. An opportunity for an aspirin intervention occurred when a patient with diabetes presented to the pharmacist, i.e. when a patient presented a prescription for an oral hypoglycaemic agent. As a single patient might take more than one oral hypoglycaemic agent, clinical interventions were analysed per unique patient with diabetes rather than by oral hypoglycaemic prescription items.
Documentation
Pharmacists in all 52 pharmacies used an electronic intervention documentation module (PROMISe) [7] to document clinical interventions performed each day, including the intervention relating to the prompt (for those who had the prompt activated). The DOCUMENT classification scheme (Appendix) used in the PROMISe module has codes, categories and definitions for:
•Type of activity (seven categories, 30 subcategories)
•Action(s) taken to clarify the extent of the problem (seven subcategories)
•Recommendation(s) made to resolve the problem (18 subcategories)
•Outcome (whether the recommendation was accepted) (four subcategories); and
•Clinical significance of the activity (five subcategories).
Transmission of data to a secure repository
Clinical interventions documented by the pharmacist were recorded in the patient history file and automatically uploaded (with pharmacy daily dispense history) once per day to an external secure repository server. Information sent to the repository was formatted with Health Level 7 messaging. Messages were encrypted with Public Key Infrastructure, then encapsulated in a http message as a Secure Multi-Purpose Internet Mail Extensions attachment. An SQL database on the external repository was used to store data received from pharmacies.
Data analysis
The overall number of clinical interventions recorded by pharmacists, the number of targetted interventions relating to the prompt and the opportunities for interventions (i.e. prescription numbers and numbers of ‘diabetics presenting to pharmacy’) were measured. The primary end-point was the rate of recorded interventions. In this study, prescription numbers means individual items (medicines) on a prescription. Data were analysed using the Mann–Whitney U-test, given the data was nonparametric. Statistical analysis was performed using SPSS version 13.0 (SPSS Inc., Chicago, IL, USA).
Results
Clinical interventions resulting from use of prompt
Figure 2 shows the rate of targeted interventions documented as a result of the aspirin prompt (per 100 patients with diabetes). Pharmacists recorded a total of 201 target interventions related to aspirin therapy in diabetes – an intervention rate of 2.55 interventions per 100 diabetic patients [95% confidence interval (CI) 0.85, 4.24]. All 201 of the targeted clinical interventions recommending aspirin therapy for patients with diabetes were recorded in the prompt arm (at a rate of 4.82 interventions per 100 diabetic patients); no targeted interventions were recorded in the control group. Nearly all the targeted interventions occurred during the 6 weeks in which the prompt was active. In the prompt arm, 21 of 31 pharmacies with the prompt activated documented at least one intervention as a direct result of the prompt; 10 pharmacies did not document any aspirin-prompt interventions. The range of ‘aspirin-prompt’ interventions per pharmacy for the trial period was 0–33. Aspirin-prompt interventions accounted for 77% of all interventions associated with oral antidiabetic drugs during the trial period in all arms.
The mean rate of aspirin-prompt interventions documented per 100 oral hypoglycaemic agents dispensed was 6.52. If only the 21 pharmacies that documented the aspirin-prompt interventions are considered, the mean rate was approximately 10 interventions per 100 oral hypoglycaemic agents dispensed. In the prompt arm of the trial, 104 pharmacists dispensed one or more prescriptions for oral hypoglycaemic agents. Forty of these pharmacists documented one or more aspirin-prompt interventions (mean 5.05 interventions per pharmacist; range 1–33).
Clinical interventions overall
Figure 2 also shows the overall rate of clinical interventions (all types) documented per 100 patients. The 150 pharmacists in the 52 pharmacies recruited for the study recorded a total of 2396 clinical interventions over an 8-week period. During this period, 435 520 prescriptions were dispensed for 258 979 patients, i.e. an intervention rate of 0.92 per 100 patients. The majority of clinical interventions were classified by pharmacists into one of three categories: drug selection problems (22.7%), dosage problems (19.4%) or the provision of education or information (17.4%).
The overall clinical intervention documentation rate was analysed for prompt and no-prompt arms to ascertain if there were any differences in rate of documentation between the two groups. Analysis of prescription data showed a total of 7895 patients with diabetes presented to all pharmacies during the 6-week study period; 4174 in the aspirin-prompt arm and 3721 in the no-prompt arm. The overall documented clinical intervention rate while the aspirin prompt was active was found to be 1.74 per 100 patients (95% CI 1.55, 1.93), nearly double the clinical intervention rate for the no-prompt arm, which was 0.91 (0.77, 1.05) (Mann–Whitney U-test, P < 0.001).
Effect of observers on recording of interventions
The presence of observers increased the rate of recorded clinical interventions in the prompt arm of the trial. For the ‘observer arm’ of the trial, the rate was approximately four times that of the ‘non-observed arm’ (7.52 vs. 2.00 interventions per 100 diabetic patients).
Figure 3 shows cumulative aspirin interventions documented over the trial period. The top line (observer arm) indicates interventions documented in pharmacies that had been assigned an observer at the beginning of the trial period. The bottom line indicates clinical interventions documented in pharmacies in the non-observed arm. The rate of documented clinical interventions tended towards zero after approximately 4 weeks.
Figure 3.
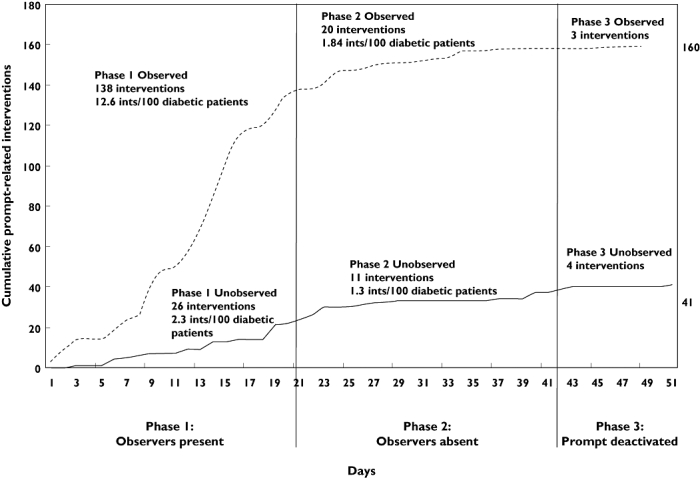
Cumulative aspirin interventions recorded during the 6-week study period and 10-days poststudy with phases indicated
The results were analysed by constructing phases (based on whether the observers were visiting pharmacies and whether the aspirin prompt was active or inactive; Figure 3) and comparing different phases of the trial to illustrate more clearly the effect of observers and prompt on clinical intervention rate. The 8-week data collection period was split into three phases: prompt activated and observer arm received visits from observers; prompt activated but observers no longer visited; and prompt deactivated and no observer visits. The rate of recording of aspirin interventions in phase 1 was 12.6 per 100 diabetic patients in the observer arm and only 2.3 in the non-observed arm. When the observers were no longer present in the observed pharmacies, the rate declined significantly to 1.84, only marginally higher than the rate in the non-observed arm (1.3). In phase 3, when no observers were present, seven aspirin interventions were documented; data analysis revealed that most had been undertaken in phase 2 but were documented in phase 3. Therefore, the clinical intervention rate for aspirin interventions once the prompt was ‘turned off’ was effectively zero.
Pharmacist questionnaire
A total of 63 of 150 pharmacists answered the questionnaire (response rate = 31%); three questionnaires were considered invalid and were excluded from analysis. The results are shown in Figure 4.
Figure 4.
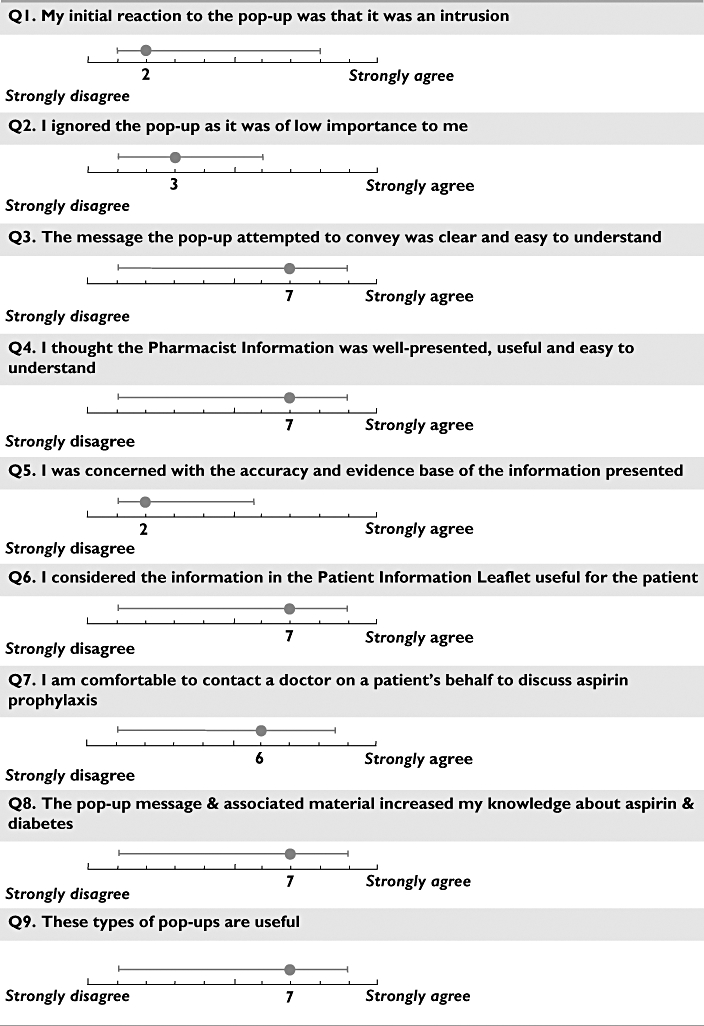
Pharmacist responses to questionnaire about aspirin prompt (medians, with range lines plotted at the 10th and 90th percentile)
Discussion
The effect of the prompt on pharmacist behaviour was marked – 201 clinical interventions recommending aspirin therapy for patients with diabetes were recorded in direct response to the prompt; no targeted interventions were recorded in the control group. Pharmacists in the intervention group also recorded a higher overall clinical intervention rate compared with the control group. This was an unexpected and additional positive effect of the prompt. One possibility is that the prompt reminded the dispensing pharmacist to consider the possibility of a clinical intervention, and this flowed on to other prescriptions being dispensed. Another reason may be that it provided an ‘example’ of a clinical intervention, and increased the capability of pharmacists to identify (and record) other clinical interventions.
Effective electronic decision-support provides actionable recommendations, in a simple format [5]. The prompt provided a short evidence-based message to pharmacists with an immediately actionable item. Sequist et al.[13] have suggested that reminders and prompts are likely to be most successful if they focus on aspects of care for which there is very little disagreement on appropriate management. This strategy avoids the pitfalls of generating distrust with the reminder system while also capturing those patients in most need of improved disease management [13]. The action in the prompt – to recommend aspirin to eligible patients with diabetes – was not considered controversial, and clear rationale for the recommendation was provided to pharmacists. Aspirin is underutilized in people with diabetes – it has been estimated that more than half of diabetic patients with risk factors for CVD are not using aspirin prophylaxis [11]. The action in the prompt was likely to be relevant to a high proportion of people with diabetes.
At least two studies have demonstrated that requiring clinician acknowledgement of reminders may be a critical step in achieving success [13, 14]. Requiring pharmacists to respond to the prompt, by presenting the message in a modal dialogue box (i.e. the pharmacist must click to dismiss the modal dialogue box) may have been an important feature contributing to the marked effect of the prompt.
The rate of clinical interventions arising from the prompt was higher in the pharmacies that had regular observation visits. There are two possible reasons for this: pharmacists may have increased the frequency of performing clinical interventions while the observer was present, or they might have increased the frequency of documentation of clinical interventions while the observer was present.
The decline in clinical interventions documented throughout the study may have been due to ‘fatigue’ with the prompt, i.e. pharmacists became desensitized to the prompt. This desensitization effect has been well-described in the literature and is often greatest when prompts are perceived as repetitious, time consuming, inappropriate or not relevant to the decision at hand [15]. Users often override alerts without reading them [16–22]; even when prompt systems are seen as helpful, users are often frustrated by being delayed by the alert, particularly if they have difficulty interpreting the alert or receive the same prompt repeatedly [12, 15, 23–25]. Prior qualitative work looking at the content and urgency of the message, mode of presentation, workflow fit, and usability may be critical to the effectiveness of the prompt [23, 26]. At least one other study has shown that the effect of reminders deteriorates over time, although the reason for this is unclear [27]. One study has shown that a user feedback mechanism might reduce this effect [4]. Another study found significant variation in clinician adherence to prompts by clinic, individual clinician and type of prompt [28].
It is also possible that the opportunities for a clinical intervention for aspirin therapy decreased as the trial progressed, as a number of patients with diabetes re-presented to the pharmacy for a second or third time. Therefore, the relevance of the prompt might decrease over time. Several pharmacists commented that the prompt should appear only once per patient and/or there should be an option to turn the prompt off. Further research on acceptable mechanisms for delivering this type of information to users is needed.
This study did not attempt to measure the effect of the clinical intervention performed by the pharmacist in terms of patient outcomes, i.e. the percentage of patients recommended aspirin who actually commenced aspirin. Nor did it assess longer term outcomes of the intervention. The aim of the study was to measure the effect on pharmacists' behaviour, rather than clinical outcome. However, this should be the subject of further research.
Pharmacists' opinions of the aspirin prompt were positive. Pharmacists did not find the alert an intrusion into their work flow; thought the alert was important; and indicated these types of alerts were useful. Pharmacists indicated that the prompt and linked supporting material helped to increase their confidence to perform an intervention and had increased their knowledge about aspirin therapy and diabetes.
In conclusion, this study has shown that an electronic prompt implemented in dispensing software had a significant effect on pharmacists' behaviour – specifically increasing pharmacists' recording of the targeted clinical intervention. The effect of the prompt reduced markedly once the prompt was deactivated. Pharmacists found the prompt to be beneficial and provided positive feedback. This type of decision-support has potential to promote community pharmacists' contribution to the quality use of medicines.
Appendix
Appendix: DOCUMENT classification scheme for classifying pharmacists' interventions
| Category | Subcategory | |
|---|---|---|
| Type of drug-related problem | ||
| D | Drug choice | Duplication (D1); Drug interaction (D2); Wrong drug (D3); Wrong dosage form (D4); Other drug selection problem (D0) |
| O | Over/underdose prescribed | Dose too high (O1); Dose too low (O2); Other dose problem (O0) |
| C | Compliance | Taking too little (C1); Taking too much (C2); Intentional drug misuse (C3); Difficulty using dosage form (C4); Other compliance problem (C0) |
| U | Untreated indications | Condition not adequately treated (U1); Preventive therapy required (U2); Other untreated indication problem (U0) |
| M | Monitoring required | Laboratory monitoring (M1); Nonlaboratory monitoring (M3); Other monitoring problem (M0) |
| E | Education or counselling advice | Patient drug information request (E1); Confusion about therapy (E2); Demonstration of device (E3); Disease management or advice (E4); Other education or information problem (E0) |
| N | Non-clinical | Non-clinical (N0) |
| T | Toxicity/adverse effect | Toxicity caused by dose (T1); Toxicity caused by drug interaction (T2); Toxicity evident (T3);Other toxicity/adverse effect problem (T0) |
| Actions to investigate the problem | ||
| Action | Investigation: written material (A1); Investigation: software (A2); Investigation: patient history (A3); Investigation: other (A4); Contacted prescriber (A5); Discussion with patient or carer (A6); Corrected without discussion (A7) | |
| Recommendations to resolve the problem | ||
| Recommendation | Dose change (R1); Drug change (R2); Drug formulation change (R3); Drug brand change (R4); Dose frequency/schedule change (R5); Prescription not dispensed (R6); Other changes to therapy (R7); Refer to prescriber (R8); Refer to hospital (R9); Refer for medication review (R10); Other referral required (R11); Education/counselling session (R12); Written summary of medications (R13); Commence dose administration aid (R14); Other written information (R15); Monitoring: nonlaboratory (R16); Monitoring: laboratory test (R17); No recommendation necessary (R18) | |
| Outcome – acceptance of pharmacist's resolution of the problem | ||
| Outcome | Unknown (O0); Accepted (O1); Partially accepted (O2); Not accepted (O3) | |
| Clinical significance of the problem | ||
| Significance | Nil (S0); Low (S1); Mild (S2); Moderate (S3); High (S4) | |
References
- 1.Calabretto J-P, Warren J, Bird L. Pharmacy decision support: where is it? A systematic literature review. Int J Pharm Pract. 2005;13:157–64. [Google Scholar]
- 2.Sacchetti A, Sacchetti C, Carraccio C, Gerardi M. The potential for errors in children with special health care needs. Acad Emerg Med. 2000;7:1330–3. doi: 10.1111/j.1553-2712.2000.tb00484.x. [DOI] [PubMed] [Google Scholar]
- 3.Garg AX, Adhikari NK, McDonald H, Rosas-Arellano MP, Devereaux PJ, Beyene J, Sam J, Haynes RB. Effects of computerized clinical decision support systems on practitioner performance and patient outcomes: a systematic review. JAMA. 2005;293:1223–38. doi: 10.1001/jama.293.10.1223. [DOI] [PubMed] [Google Scholar]
- 4.Saleem JJ, Patterson ES, Militello L, Render ML, Orshansky G, Asch SM. Exploring barriers and facilitators to the use of computerized clinical reminders. J Am Med Inform Assoc. 2005;12:438–47. doi: 10.1197/jamia.M1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bates DW, Kuperman GJ, Wang S, Gandhi T, Kittler A, Volk L, Spurr C, Khorasani R, Tanasijevic M, Middleton B. Ten commandments for effective clinical decision support: making the practice of evidence-based medicine a reality. J Am Med Inform Assoc. 2003;10:523–30. doi: 10.1197/jamia.M1370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.American Diabetes Association. Clinical practice recommendations. Aspirin therapy in diabetes. Diabetes Care. 1997;20:1772–3. doi: 10.2337/diacare.20.11.1772. [DOI] [PubMed] [Google Scholar]
- 7.Peterson GM, Tenni PT, Kruup H, Hasan O, Reeve JF. Evaluation of Clinical Interventions within Community Pharmacy (PROMISe II) Canberra: Pharmacy Guild of Australia; 2003. [22 April 2007]. Available at http://beta.guild.org.au/research/project_display.asp?id=270. [Google Scholar]
- 8.Caleo S, Benrimoj SI, Collins D, Lauchlan R, Stewart K. Clinical evaluation of community pharmacists' interventions. Int J Pharmacy Prac. 1996;4:221–7. [Google Scholar]
- 9.Benrimoj SI, Langford JH, Berry G, Collins D, Lauchlan R, Stewart K. Clinical intervention rates in community pharmacy: a randomised trial of the effect of education and a professional allowance. Int J Pharm Pract. 2003;11:71–80. doi: 10.2165/00019053-200018050-00005. [DOI] [PubMed] [Google Scholar]
- 10.Medicare Australia. Expenditure and Prescriptions Twelve Months to 30 June. Canberra: Medicare Australia; 2004. [13 May 2007]. Available at http://www.health.gov.au/internet/wcms/publishing.nsf/Content/Pharmaceutical+Benefits+Scheme+%28PBS%29-3. [Google Scholar]
- 11.Persell SD, Baker DW. Aspirin use among adults with diabetes: recent trends and emerging sex disparities. Arch Intern Med. 2004;164:2492–9. doi: 10.1001/archinte.164.22.2492. [DOI] [PubMed] [Google Scholar]
- 12.Peterson G, Tenni P. Identifying, prioritising and documenting drug-related problems. Aust Pharmacist. 2004;23:706–9. [Google Scholar]
- 13.Sequist TD, Gandhi TK, Karson AS, Fiskio JM, Bugbee D, Sperling M, Cook EF, Orav EJ, Fairchild DG, Bates DW. A randomized trial of electronic clinical reminders to improve quality of care for diabetes and coronary artery disease. J Am Med Inform Assoc. 2005;12:431–7. doi: 10.1197/jamia.M1788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Litzelman DK, Dittus RS, Miller ME, Tierney WM. Requiring physicians to respond to computerized reminders improves their compliance with preventive care protocols. J Gen Intern Med. 1993;8:311–7. doi: 10.1007/BF02600144. [DOI] [PubMed] [Google Scholar]
- 15.Ahearn MD, Kerr SJ. General practitioners' perceptions of the pharmaceutical decision-support tools in their prescribing software. Med J Aust. 2003;179:34–7. doi: 10.5694/j.1326-5377.2003.tb05415.x. [DOI] [PubMed] [Google Scholar]
- 16.Bates DW, Teich JM, Lee J, Seger D, Kuperman GJ, Ma'Luf N, Boyle D, Leape L. The impact of computerized physician order entry on medication error prevention. J Am Med Inform Assoc. 1999;6:313–21. doi: 10.1136/jamia.1999.00660313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Major K, Shabot MM, Cunneen S. Wireless clinical alerts and patient outcomes in the surgical intensive care unit. Am Surg. 2002;68:1057–60. [PubMed] [Google Scholar]
- 18.Kuperman GJ, Teich JM, Gandhi TK, Bates DW. Patient safety and computerized medication ordering at Brigham and Women's Hospital. Jt Comm J Qual Improv. 2001;27:509–21. doi: 10.1016/s1070-3241(01)27045-x. [DOI] [PubMed] [Google Scholar]
- 19.Lesar TS, Briceland L, Stein DS. Factors related to errors in medication prescribing. JAMA. 1997;277:312–7. [PubMed] [Google Scholar]
- 20.Leape LL, Kabcenell AI, Gandhi TK, Carver P, Nolan TW, Berwick DM. Reducing adverse drug events: lessons from a breakthrough series collaborative. Jt Comm J Qual Improv. 2000;26:321–31. doi: 10.1016/s1070-3241(00)26026-4. [DOI] [PubMed] [Google Scholar]
- 21.Weingart SN, Toth M, Sands DZ, Aronson MD, Davis RB, Phillips RS. Physicians' decisions to override computerized drug alerts in primary care. Arch Intern Med. 2003;163:2625–31. doi: 10.1001/archinte.163.21.2625. [DOI] [PubMed] [Google Scholar]
- 22.Abookire SA, Teich JM, Sandige H, Paterno MD, Martin MT, Kuperman GJ, Bates DW. Improving allergy alerting in a computerized physician order entry system. Proc AMIA Symp. 2000:2–6. [PMC free article] [PubMed] [Google Scholar]
- 23.Feldstein A, Simon SR, Schneider J, Krall M, Laferriere D, Smith DH, Sittig DF, Soumerai SB. How to design computerized alerts to safe prescribing practices. Jt Comm J Qual Saf. 2004;30:602–13. doi: 10.1016/s1549-3741(04)30071-7. [DOI] [PubMed] [Google Scholar]
- 24.Magnus D, Rodgers S, Avery AJ. GPs' views on computerized drug interaction alerts: questionnaire survey. J Clin Pharm Ther. 2002;27:377–82. doi: 10.1046/j.1365-2710.2002.00434.x. [DOI] [PubMed] [Google Scholar]
- 25.Glassman PA, Simon B, Belperio P, Lanto A. Improving recognition of drug interactions: benefits and barriers to using automated drug alerts. Med Care. 2002;40:1161–71. doi: 10.1097/00005650-200212000-00004. [DOI] [PubMed] [Google Scholar]
- 26.Krall MA, Sittig DF. Clinician's assessments of outpatient electronic medical record alert and reminder usability and usefulness requirements. Proc AMIA Symp. 2002:400–4. [PMC free article] [PubMed] [Google Scholar]
- 27.Demakis JG, Beauchamp C, Cull WL, Denwood R, Eisen SA, Lofgren R, Nichol K, Woolliscroft J, Henderson WG. Improving residents' compliance with standards of ambulatory care: results from the VA cooperative study on computerized reminders. JAMA. 2000;284:1411–6. doi: 10.1001/jama.284.11.1411. [DOI] [PubMed] [Google Scholar]
- 28.Agrawal A, Mayo-Smith MF. Adherence to computerized clinical reminders in a large healthcare delivery network. Medinfo. 2004;11:111–4. [PubMed] [Google Scholar]


