Abstract
Aim
The naturally occurring interlukin-1 receptor antagonist (IL-1RA) markedly protects rodents against ischaemic, excitotoxic and traumatic brain injury, suggesting it may be of therapeutic value. The aim was to determine the pharmacokinetics of IL-1RA in cerebrospinal fluid (CSF) of patients, to allow modelling that would aid development of therapeutic regimens.
Methods
When administered intravenously to patients soon after stroke, IL-1RA is safe and reduces the peripheral inflammatory response. However, IL-1RA is a large protein (17 kDa), which may limit brain penetration, thereby limiting its potential utility in brain injury. In seven patients with subarchnoid haemorrhage (SAH), IL-1RA was administered by intravenous bolus, then infusion for 24 h, and both blood and CSF, via external ventricular drains, were sampled during and after stopping the infusion.
Results
Plasma steady-state concentrations were rapidly attained and maintained throughout the infusion, whereas CSF concentrations rose slowly towards a plateau during the 24-h infusion, reaching at best only 4% of that in plasma. Plasma kinetic parameters were within the literature range. Modelling of the combined data yielded rate constants entering and leaving the CSF of 0.0019 h−1[relative standard error (RSE) = 19%] and 0.1 h−1 (RSE = 19%), respectively.
Conclusions
Peripherally administered IL-1RA crosses slowly into and out of the CSF of patients with SAH. However, there is a large concentration gradient of IL-1RA between plasma and CSF. These CSF:plasma data are consistent with very low permeation of IL-1RA into the CSF and elimination kinetics from it controlled by the volumetric turnover of CSF.
What is already known about this subject?
The naturally occurring interlukin-1 receptor antagonist (IL-1RA) markedly protects rodents against ischaemic, excitotoxic and traumatic brain injury, suggesting it may be of therapeutic value.
When administered intravenously to patients soon after stroke, IL-1RA is safe and reduces the peripheral inflammatory response.
However, IL-1RA is a large protein (17 kDa), which may limit brain penetration, thereby limiting its potential utility in brain injury.
What this study adds
The purpose of these experiments was to determine the pharmacokinetics of IL-1RA in cerebrospinal fluid (CSF) of patients, to allow modelling that would aid development of therapeutic regimens.
Peripherally administered IL-1RA crosses slowly into and out of the CSF of patients with subarachnoid haemorrhage and, at steady state, CSF IL-1RA concentration (range 115–886 ng ml−1) was similar to that found to be neuroprotective in rats (range 91–232 ng ml−1), although there was considerable variability among patients.
However, there is a large concentration gradient of IL-1RA between plasma and CSF.
These CSF:plasma data are consistent with very low permeation of IL-1RA into the CSF and elimination kinetics from it controlled by the volumetric turnover of CSF.
Keywords: interleukin-1 receptor antagonist, pharmacokinetics, CSF, patients, modelling, subarachnoid haemmorage, ischemia
Introduction
There is considerable evidence implicating inflammatory molecules such as interleukin (IL)-1 in disease processes, not least in the central nervous system (CNS) [1–3]. Rapid upregulation of IL-1 occurs in response to experimental brain ischaemia and, when administered to rodents centrally or peripherally, worsens brain injury [4–7]. In rodents, interleukin-1 receptor antagonist (IL-1RA) markedly inhibits neuronal injury induced by focal or global, reversible or permanent cerebral ischaemia, excitotoxicity, traumatic brain injury and seizures [7, 8]. IL-1RA has proved safe in patients with sepsis [9] and is licensed for clinical use in the treatment of rheumatoid arthritis [10]. We have tested its safety in a small, randomized, double-blinded study, where IL-1RA, or placebo, was administered as an intravenous (i.v.) bolus, followed by infusion, to patients with stroke [11]. No serious adverse events were observed and IL-1RA markedly attenuated the levels of circulating inflammatory molecules and cells, which have been correlated with poor clinical outcome. These data suggest that IL-1RA may be beneficial in brain disorders associated with ischaemic or excitotoxic processes, including stroke, subarachnoid haemorrhage (SAH) and brain trauma. Although brain penetration of IL-1RA may be expected to be limited, due to its size (17 kDa), a few studies have shown that very high doses of IL-1RA, given subcutaneously, are neuroprotective in rodents [12, 13], and an active uptake transport mechanism into brain has been reported in rats [14]. The extent to which a drug is distributed into the cerebrospinal fluid (CSF) depends on the relationship between rates of transport into and out of the CSF (i.e. the influx and efflux clearances) relative to the concentrations driving those rates. Furthermore, the description of drug transport at the plasma–CSF barrier requires a parametric (usually compartmental) model [15].
The aim of this study was to develop a multicompartment model to describe adequately and interpret the pharmacokinetic profiles of IL-1RA in plasma and CSF, after i.v. administration to patients suffering SAH, as a step in the development of a possible therapeutic regime.
Methods
SAH patients
Eight patients (six female, two male) were recruited from the Intensive Care or Neuro High Dependency Units (Hope Hospital, Salford, UK) between September 2002 and January 2005. Of these, seven were available for analysis. All patients had confirmed aneurysmal SAH and required external ventricular drainage for treatment of hydrocephalus. Patients were White, with a mean age of 51 years (range 39–69 years) and a mean weight of 70 kg (range 64–150 kg). The Salford and Trafford NHS Trust Local Research Ethics Committee and the University of Manchester Research Ethics Committee approved the study, which was performed in accordance with the Declaration of Helsinki. Informed written consent was obtained from patients or, where this was not possible, written assent was obtained from their representative. Patients were excluded if they had a clinically significant medical condition, if they were pregnant or breastfeeding or if they had a history of sensitivity to products derived from Escherichia. coli, or any component of IL-1RA. Initial data collected included full physical examination and clinical history with additional demographic. The presenting Glasgow Coma Score [16] ranged from 8 to 15, 6/8 patients being grade ≥13. The distribution of subarachnoid blood on computed tomography varied greatly, but the majority of patients had thin or thick vertical patterns of haemorrhage (revised Fisher grades 2 and 3 [17]). All patients had normal functional ability as measured by the Barthel index prior to their SAH [18]. In addition, all patients had good functional status as measured by the modified Rankin score (mRS 0 or 1 [19]). Participants were sequentially coded A–H.
Pharmacokinetics in patients with SAH
All patients received the IL-1RA (as Kineret®) within 5 days of their haemorrhage. Baseline blood samples for measurement of IL-1RA, haematological and biochemical profiles were obtained within 6 h before IL-1RA infusion. Participants received an i.v. bolus injection (100 mg) of IL-1RA, immediately followed by a continuous i.v. infusion at 2 mg kg−1 h−1 (in 0.9% sterile NaCl) for 24 h, via a volumetric infusion pump (Graseby, Watford, UK). One patient with impaired renal function (plasma creatinine concentration >177 µmol l−1; creatinine clearance of 47 ml min−1) received 1 mg kg−1 h−1 infusion as per the manufacturer's guidelines. The parameters used to determine the dosage regimens were based on prior pharmacokinetic information [20]. Three millilitres of CSF [from the extravascular drain (EVD)] and 5 ml blood were obtained for measurement of IL-1RA at each time point. Samples were collected (pyrogen-free heparinized tubes −10 IU ml−1) and centrifuged (15 min at 2000 g). The resulting supernatant was stored at −70°C until analysis. Assays for IL-1RA were performed using an enzyme-linked immunosorbent assay, as described previously [11]. An assessment of blood–brain barrier (BBB) function was made using a ratio of CSF albumin (mg l−1) to plasma albumin (g l−1), with values >9 taken to signified BBB breakdown [21].
Safety in patients
All adverse events were reported to an independent data monitoring committee. A full physical examination was performed on days 0, 1, 2, 3, 4, 10 and 30. Haematology and blood biochemistry were monitored at these time points and CSF was obtained for microscopy and bacteriological culture, where available via EVD.
Pharmacokinetic analysis
Two- and one-compartment linear models were used to describe the time course of plasma and CSF drug concentrations, respectively. Both the peripheral and CSF compartments were linked to the central (plasma) compartment (Figure 1). However, unlike the peripheral compartment, as discussed below, drug is eliminated from the CSF compartment and does not return from it to plasma. The relevant differential equations defining the structural pharmacokinetic model are given in the Appendix. Equation 1 describes the two-compartment i.v. infusion pharmacokinetic model, which was fit to the plasma data, whereas Equation 5 represents a one-compartment pharmacokinetic model used to fit the CSF data. Equations 2–4 list the interrelationships between the estimated parameters (rate constants) and the physiological parameters.
Figure 1.
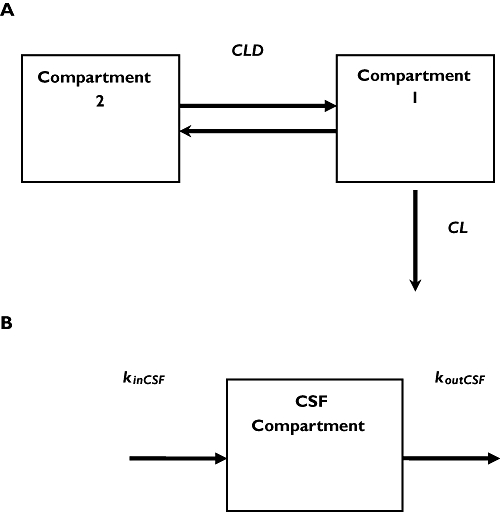
(A) Two-compartment model, resulting from analysis of the plasma data. Compartment 1 is the central compartment (plasma); compartment 2 is the peripheral compartment; CLD, intercompartmental clearance between the central and peripheral compartments; and CL, total clearance. (B) One-compartment model for CSF. kinCSF is the rate constant for drug transfer from the central to the CSF compartment; koutCSF is rate constant out from CSF
With the exception of data from the patient with impaired renal function, which were analysed separately, the concentration data for all patients were analysed simultaneously using nonlinear mixed effects modelling in NONMEM, ADVAN 9 [22, 23]. The plasma data were first modelled, the resulting estimated parameters then fixed, before modelling the CSF data. Pharmacokinetic analyses of the plasma were parameterized using clearances and distribution volumes, whereas the equation for CSF was parameterized in terms of rate constants. Initially, the pharmacokinetic model was developed to fit only the plasma data. Finally, the plasma and CSF data were combined to yield the full model. Between-subject variability in the parameters was modelled with an exponential random effects term, and the residual error was modelled as additive, multiplicative or both, independently for plasma and CSF. Group mean parameter estimates and between-subject variability together with their associated uncertainties [expressed as relative standard error (RSE), defined as the percentage ratio between standard error and mean estimates] were calculated.
Results
A total of eight patients, of whom seven were available for pharmacokinetic analysis, met the inclusion criteria and were recruited to the study. Patient demographics are described in detail in Methods.
Safety in patients and BBB function assessment
Six patients completed the full 24-h infusion of IL-1RA. Patient B developed diabetes insipidus and hypernatraemia, and the infusion was stopped at 8 h. Patient F developed severe cardiac instability, requiring large concentrations of positive inotropic agents, and the infusion was stopped at 11 h. Pharmacokinetic data for F were excluded from analysis. In the 30 days of the study, the single serious adverse event (death) was not attributable to test treatment, since the patient was extremely unwell due to haemorrhage prior to study entry. In the same 30-day time scale there were 12 adverse events in six patients. Of these, eight were infections (two CSF, four respiratory, one urinary and one gastrointestinal). The three remaining adverse events were haematemesis, diabetes insipidus and bleeding from a surgical wound. All adverse events were referred to an independent data monitoring committee, who deemed that none of these was attributable to test treatment. For the duration of the study, IL-1RA had no significant effect on haemoglobin concentration or circulating leucocyte or platelet numbers. In addition, there was no significant alteration in biochemical, renal or hepatic parameters related to infusion of IL-1RA and it had no apparent effect on heart rate, blood pressure or aural temperature during the course of the study.
The proportion of IL-1RA that crosses the BBB was higher in our study than previously reported [14], where only a single i.v. bolus dose was administered. This difference could be attributed to the continuous-rate i.v. infusion regime we used, or possibly enhanced permeability of the BBB, caused by the stroke or SAH, may have contributed to the increase transfer. Since patients with SAH have leakage of blood (and consequently albumin) into the subarachnoid space at the time of haemorrhage, estimating BBB integrity by monitoring entry of plasma proteins is problematic. However, in our series the albumin concentrations did not rise during the infusion period, suggesting that there was no further significant compromise of the BBB after the initial bleed. Although this would represent a limitation in extrapolating these results to those with a normal BBB, it presents less compromise in terms of considering therapeutic potential in patients with CNS pathology.
Plasma and CSF kinetics
As seen in Figure 2, in all patients the combination of the bolus followed by the constant-rate infusion immediately attained and thereafter maintained a relatively constant plasma concentration of IL-1RA, from which the estimated clearance ranged from 6.18 to 12.5 l h−1, except for renally impaired patient B, in whom it was 1.4 l h−1. On stopping the infusion the plasma concentration of IL-1RA fell initially rapidly and then more slowly, with a terminal half-life ranging from 5.3 to 6 h.
Figure 2.
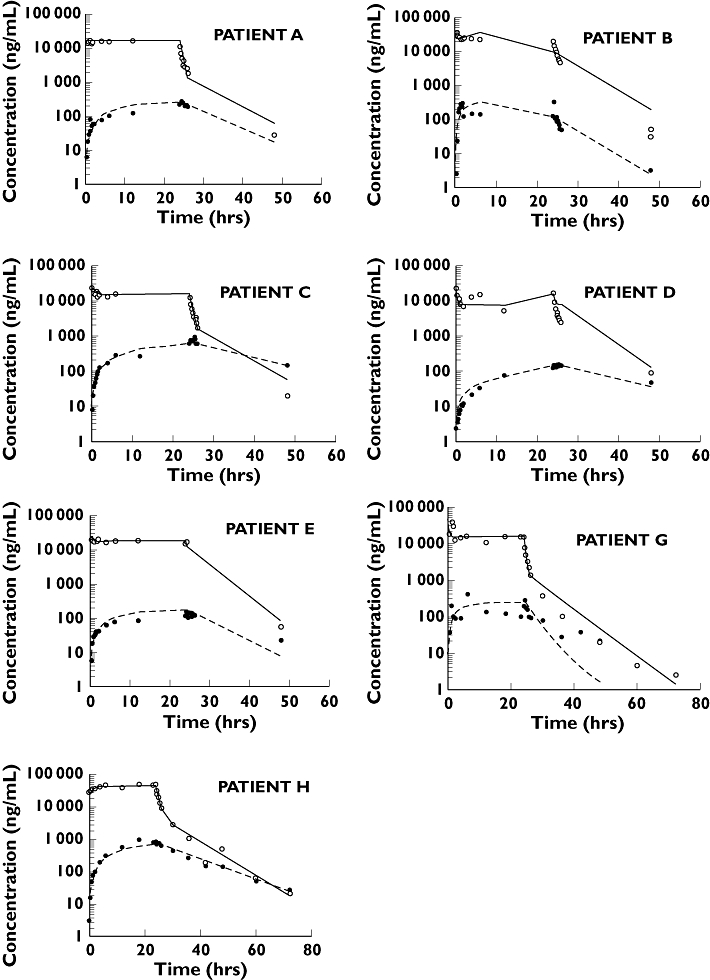
Predicted plasma and CSF concentrations, assuming the model given in Figure 1. Open and closed circles are plasma and CSF data, respectively; solid and dotted black lines are the individual predictions for plasma and CSF concentrations
During the infusion, the CSF concentration rose slowly, tending to reach steady state by about 24 h, mean concentration (range) 358 ng/ml (115–886), although in some patients it clearly had not reached a plateau even by then (Figure 2). At 24 h, the end of the infusion, the ratio of CSF to plasma concentration was very low, ranging from 0.01 to 0.04. After stopping the infusion, unlike events in plasma, the concentration in CSF fell very slowly at all times, so that by 24 h post infusion the concentration was either comparable to, or exceeded, that in plasma. In all cases the kinetics of decline in CSF was either parallel to or slower than the terminal half-life in plasma.
Pharmacokinetic parameter estimates
A linear two-compartment model, with elimination from the central compartment (Figure 1a), provided the best fit to the plasma data. The corresponding mean parameter estimates are listed in Table 1. The mean clearance of IL-1RA was 6.96 l h−1 for the patients with normal renal function with standard error, expressed as a percent coefficient of variation (RSE), of 23%, and only 1.41 l h−1 (with 14% RSE) in the renal impaired patient. The initial volume of distribution of IL-1RA (Vs) was 5.1 l, and the steady-state volume (Vss = V1 + V2) 10.1 l. Similar values were found for the renally impaired patient: specifically, V1 was 3.94 l and steady-state volume 8.25 l.
Table 1.
Population kinetic parameters for IL-1RA in plasma and CSF, assuming the model given in Figure 1. (Patients with normal renal function)
| Parameter | Parameter estimates Value | Between-subject variability CV (%) | RSE* | RSE* |
|---|---|---|---|---|
| CL (l h−1) | 6.96 | 23 | 28 | 57 |
| V1 (l) | 5.14 | 7 | 22 | 116 |
| CLD (l h−1) | 0.63 | 21 | NA | NA |
| V2 (l) | 4.96 | 26 | 10 | 476 |
| kinCSF (h−1) | 0.0019 | 19 | 66 | 43 |
| koutCSF (h−1) | 0.1 | 19 | 41 | 41 |
| Plasma within-subject variability % (proportional error model) | 25 | 59 | ||
| CSF within-subject variability % (proportional error model) | 38 | 52 |
Standard error expressed as percent coefficient of variation; NA not applicable.
The CSF data were best fit globally by a one-compartment model (Figure 1b). Figure 2 shows the individual fits of the total model to both plasma and CSF data for each patient. The goodness of fit of the model to the data for IL-1RA is illustrated in Figure 3. The pharmacokinetic model was assessed in terms of group-weighted residuals (Figure 3, lower panel). The residuals appeared to be distributed evenly around the group mean predicted concentrations and the majority of the weighed residuals lay between ±2 SD from the mean. The CSF data rate constants, kin,CSF and kout,CSF, characterizing input and output from the CSF compartment, were calculated by fitting the model. Mean and RSE estimates for patients with normal renal function were 0.0019 h−1 (19%) and 0.1 h−1 (19%) for kin,CSF and kout,CSF, respectively. Data for the one patient with impaired renal function, analysed separately, yielded estimates for kin,CSF and kout,CSF of 0.0008 h−1 and 0.1 h−1, respectively. The residual variability for this patient with proportional error models in plasma and CSF was 40% and 63%, respectively. Had more patients with renal impairment been available, renal function could have been included as a covariate in the population model, and all patients analysed simultaneously.
Figure 3.
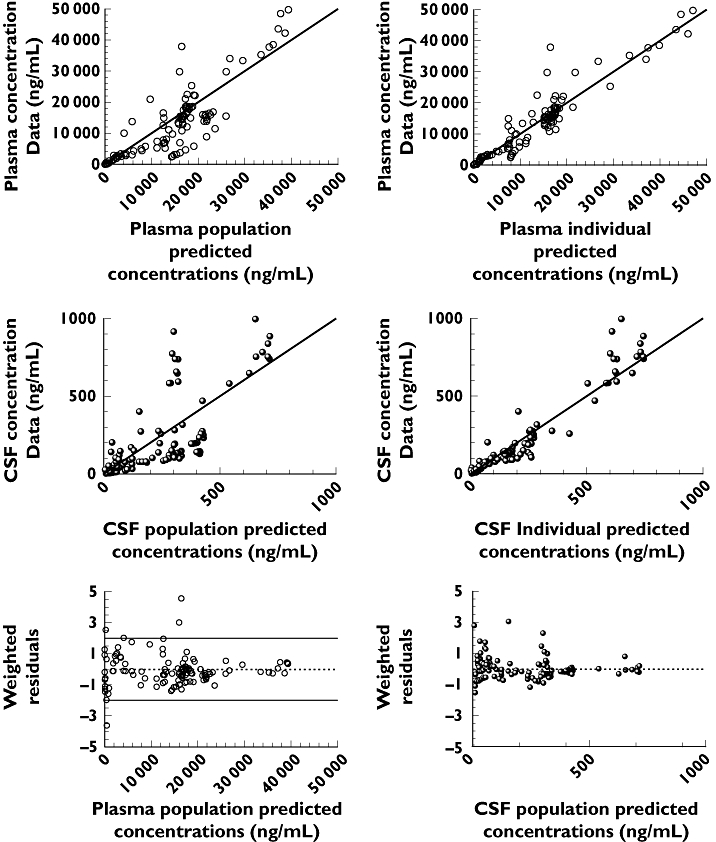
Goodness-of-fit plots of the two-compartment pharmacokinetic model for plasma and one-compartment model for CSF for IL-1RA. Upper panels: measured concentrations in plasma vs. predictions for the population (left) and for the individuals (right). Middle panels: measured concentrations in CSF vs. predictions for the population and for the individuals. Lower panel: weighted residuals vs. population predictions for plasma (left) and CSF (right)
Discussion
The study design of an i.v. bolus of 100 mg, followed by an i.v. infusion of 2 mg kg−1 h−1, was chosen to immediately attain and maintain a steady-state plasma concentration, based on a regimen used in patients with sepsis and stroke, and was the maximum daily dose of IL-1RA that had been given previously to any patient [11, 24]. Within the realms of safety, this regimen aimed to ensure the most rapid rise to, and highest CSF concentrations at, steady state. In most of the patients the 24-h (approximately steady state) CSF IL-1RA concentration (range 115–886 ng ml−1) was similar to those found to be neuroprotective in rats (range 91–232 ng ml−1), although there was considerable variability among patients [25, 26]. Furthermore, it generally took at least 6 h and sometimes longer to reach this range, and it would have taken even longer had not bolus doses supplemented the infusion. Although these data suggest that, at the employed regimen, IL-1RA is a promising potential treatment of SAH and cerebral ischaemia, if higher CSF concentrations are required earlier, even larger bolus doses or perhaps higher initial infusion rates would be needed, which are beyond those that have so far been clinically investigated. Nevertheless, the information gained from the present study should allow prediction of likely plasma and CSF concentration with time associated with various dosing strategies.
IL-1RA reached mean plasma concentrations in humans similar to those reported previously for the same bolus dose and infusion rate in sepsis in human [11], and the observed plasma kinetics were identical to those observed in healthy volunteers [20]. It is relatively uncommon to have the opportunity for rich longitudinal sampling of CSF. This specific study design allowed a detailed characterization of exchange of drug between plasma and CSF, which can be used in the design of larger studies in the targeted population where sparse data are usually collected.
A compartment model for IL-1RA was used to quantify and help interpret processes occurring at the plasma–CSF barrier and within CSF in patients with SAH. Although it is likely that reversible exchange of IL-1RA occurs between CSF and plasma, the modelling assumes that no IL-1RA in CSF returns to plasma. This assumption was made for practical reasons. Theory dictates that each peripheral compartment reversibly connected to the central (plasma) compartment adds an additional exponential term to the plasma concentration–time data. Yet, even if it occurs, the mass transfer between CSF and plasma is too small to affect the events in plasma, so that, had a reversible process been included, the model for plasma would be overparameterized, yielding poor parameter estimates. The lack of impact of events in CSF on those in plasma is also evident from the failure of the slower fractional decline in CSF in some patients to be noticeably reflected in the terminal decline in plasma. This procedure of having no return of drug from CSF to plasma is analogous to the same procedure frequently employed when modelling pharmacodynamics, based on the knowledge that in most cases the amount of drug located at the site of action is only a small fraction of total drug in the body [27].
We found that IL-1RA had a mean clearance of 6.96 l h−1 in patients with normal renal function, which was reduced to 1.41 l h−1 in the patient with renal impairment. This finding is consistent with a previous study [20], which compared plasma IL-1RA pharmacokinetics in healthy volunteers with that in patients with renal function impairment. This yielded clearances of 8.2 and 1.2 l h−1 in the two groups, respectively, and emphasizes the importance of the kidney as the major organ for elimination of IL-1RA, which primarily involves metabolism, as little unchanged drug is found in urine [20].
The small initial volume of distribution of IL-1RA (5.1 l) is consistent with the difficulty that this large polypeptide has in distributing out into tissues. Renal disease does not appear to influence the extent of distribution, because Vss values were very similar between the patient with renal disease (8.25 l) and the other patients (mean 10.1 l), again in agreement with those published [20], namely Vss of 9.8 l and 8.7 l for the two groups, respectively.
The mean parameters (Table 1) were estimated with small RSEs, not exceeding 30%, indicative of high precision in the estimates. Between-subject variability was <30% for the plasma parameters and <70% for rate constants to and from the CSF. However, these random effect parameters tended to have high standard errors, suggesting lower precision in their estimation than some other variance terms. This can be explained by the small group sample size, which does not allow precise estimation of between-subject variance. The residual variance terms were 25% and 38% for plasma and CSF, respectively, with RSEs of 59% and 52%.
The mean withdrawal rate of CSF (0.073 ml min−1) during the duration of the study was considerably lower than the average CSF production rate of 0.28 ml min−1[28] and so should have minimal effect on events in CSF. The slow rise in CSF concentrations during the infusion and subsequent slow fall on stopping administration, relative to events in plasma, indicate that the temporal events in CSF are controlled primarily by processes occurring there rather than by those in plasma. Certainly, during the infusion period, when the plasma concentration, and hence rate of entry of drug into CSF, is relatively constant, as with any such input–output system, the rise in CSF with time to plateau is controlled only by the output rate constant koutCSF. But with the plasma concentration, and hence input rate, falling so precipitously on stopping the infusion, the decline in CSF IL-1RA is also controlled by kinCSF.
There are two possible routes of loss of IL-1RA from CSF. One is return of drug from CSF to plasma by passive diffusion across the plasma–CSF barrier, and the other is loss by other mechanisms. Both were ignored with respect to events in plasma, as described above, but obviously cannot be when considering events in CSF. Of these two mechanisms, the 25–100-fold steady-state plasma : CSF concentration ratio of IL-1RA indicates that loss by other process(es) by far predominates. One possible explanation is slow passive permeability across the plasma–CSF barrier, expected for such a large molecule (17 kDa), coupled with effective active efflux from CSF into plasma. Although this mechanism cannot be discounted, there is no evidence that IL-1RA is a substrate for any CSF efflux transporters. Nor can the present data allow the characterization of one important feature of a transport process, namely concentration dependence, as only one infusion rate was explored. Another possible, and more likely, mechanism is slow passive penetration followed by volumetric removal of compound with CSF as it drains down the spinal canal into the systemic vasculature, as postulated to explain the very low CSF : plasma concentration gradients of proteins, such as albumin [29]. One piece of evidence supporting this latter hypothesis comes from turnover concepts. We note that CSF turns over three to four times daily, which gives a CSF turnover time of between 6 and 8 h [30]. Using the inversely proportional relationship between output rate constant and turnover time, the value for koutCSF associated with the volumetric turnover of CSF lies between 0.17 h−1 and 0.13 h−1, which is close to our estimated value for koutCSF of 0.1 h−1 in the SAH patients. This would suggest that the CSF dynamics in SAH patients do not differ substantially from those in healthy adults.
One would like to know the physiologically relevant parameters P, the permeability–surface product of the plasma–CSF barrier, and CLCSF, the CSF clearance of IL-1RA. However, only kin,CSF (= P/VCSF) and kout,CSF (= CLCSF/VCSF) can be estimated (see Appendix), so that without knowing VCSF, the volume of distribution of IL-1RA, no further interpretation of the CSF data can be made. Estimation of VCSF requires introduction of IL-1RA directly in the CSF, but given the large relatively polar nature, one reasonable approximation is to set VCSF equal to the physical volume of CSF, 150 ml [31]. In doing that, the corresponding mean values of P and CLCSF are 0.29 ml h−1 and 0.3 ml min−1, respectively. The latter value is close to the reported flow rate of CSF of 8.5 ml min−1 (range 0.4–18.5 ml min−1) [32], further supporting the view that the kinetic events of IL-1RA in CSF are controlled by, and reflect, CSF physiology.
In conclusion, intravenously administered IL-1RA crosses into the CSF of patients with SAH, but very slowly. Furthermore, even at steady state there is a large concentration gradient of IL-1RA in favour of plasma. The most likely explanation for these observations is low permeability of the CSF barrier to IL-1RA coupled with its removal kinetics from CSF being controlled by the turnover of this fluid.
Acknowledgments
Kineret was generously provided by the Amgen Corporation, but the company was not involved in the design, conduct or analysis of the study. The University of Manchester holds the use patent for treatment of brain disease. This study was supported by the Sir Jules Thorn Charitable Trust, a Research into Ageing research fellowship funded by the Oglesby Charitable Trust (S.R.C.) and MRC Professorship (N.J.R.). We are grateful to P. Kirkpatrick and E. Warburton (University of Cambridge) for acting as an independent data monitoring committee. Our thanks are also due to the patients who kindly participated in this study, and the neurosurgical team at Hope Hospital for their support.
Appendix
Pharmacokinetic compartmental models For plasma
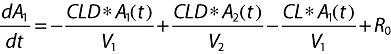 |
(1) |
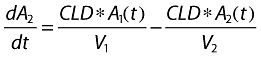 |
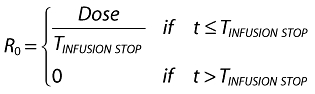 |
For CSF
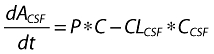 |
(2) |
Expressing in terms of concentration by dividing by VCSF
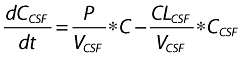 |
(3) |
Now
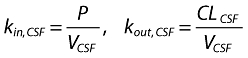 |
(4) |
Hence
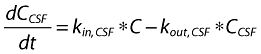 |
(5) |
where A1 (t), A2 (t) and ACSF (t) are the amounts of drug in the central, peripheral and CSF compartments, respectively, at any time t, V1, V2 and VCSF are the volumes of distribution of the central, peripheral and CSF compartments, C and CCSF are the drug concentrations in the central and CSF compartments, CL is the total body clearance, CLD is the intercompartmental clearance between the central and peripheral compartments, CLCSF is the clearance of drug from CSF, P is the permeability–surface area product of drug at the plasma–brain barrier, kinCSF is the rate constant for drug transfer from the central to CSF compartment, koutCSF is the rate constant out from CSF compartment, and R0 is the infusion rate.
References
- 1.Lipton P. Ischemic cell death in brain neurons. Physiol Rev. 1999;79:1432–516. doi: 10.1152/physrev.1999.79.4.1431. [DOI] [PubMed] [Google Scholar]
- 2.Ginsberg MD. Adventures in the pathophysiology of brain ischemia: penumbra, gene expression, neuroprotection. The 2002 Thomas Willis lecture. Stroke. 2002;34:214–33. doi: 10.1161/01.str.0000048846.09677.62. [DOI] [PubMed] [Google Scholar]
- 3.Allan SM, Rothwell NJ. Inflammation in central nervous system injury. Philos Trans R Soc Lond B Biol Sci. 2003;358:1669–77. doi: 10.1098/rstb.2003.1358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rothwell NJ, Loddick SA, Stroemer P. Interleukins and cerebral ischaemia. Int Rev Neurobiol. 1997;40:281–98. doi: 10.1016/s0074-7742(08)60724-2. [DOI] [PubMed] [Google Scholar]
- 5.Rothwell NJ, Allan SM, Toulmond S. The role of interleukin 1 in acute neurodegeneration and stroke: pathophysiological and therapeutic implications. J Clin Invest. 1997;100:2648–52. doi: 10.1172/JCI119808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Allan SM, Tyrrell PJ, Rothwell NJ. Interleukin-1 and neuronal injury. Nat Rev Immunol. 2005;5:629–40. doi: 10.1038/nri1664. [DOI] [PubMed] [Google Scholar]
- 7.Relton JK, Rothwell NJ. Interleukin-1 receptor antagonist inhibits ischaemic and excitotoxic neuronal damage in the rat. Brain Res Bull. 1992;29:243–6. doi: 10.1016/0361-9230(92)90033-t. [DOI] [PubMed] [Google Scholar]
- 8.Touzani O, Boutin H, Chuquet J, Rothwell NJ. Potential mechanisms of interleukin-1 involvement in cerebral ischaemia. J Neuroimmunol. 1999;100:203–15. doi: 10.1016/s0165-5728(99)00202-7. [DOI] [PubMed] [Google Scholar]
- 9.Fisher CJ, Dhainaut JA, Opal SM, Pribble JP, Balk RA, Slotman GJ, Iberti TJ, Rackow EC, Greenman RL. Recombinant human interleukin-1 receptor antagonist in the treatment of patients with sepsis syndrome: results from a randomised, double blind, placebo controlled trial. JAMA. 1994;271:1836–43. [PubMed] [Google Scholar]
- 10.Nuki G, Bresnihan B, Bear MB, McCabe D. Long term safety and maintenance of clinical improvement following treatment with Anakinra (recombinant human interleukin-1 receptor antagonist) in patients with rheumatoid arthritis. Arthritis Rheum. 2002;46:2838–46. doi: 10.1002/art.10578. [DOI] [PubMed] [Google Scholar]
- 11.Emsley HCA, Smith CJ, Georgiou RF, Vail A, Hopkins SJ, Rothwell NJ, Tyrell PJ. A randomised phase II study of interleukin-1 receptor antagonist in acute stroke patients. J Neurol, Neurosurg, Psychiatry. 2005;76:1366–72. doi: 10.1136/jnnp.2004.054882. for the IL-1ra in acute stroke investigators. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Martin D, Chinookoswong N, Miller G. The interleukin-1 receptor antagonist (rhIL-1ra) protects against cerebral infarction in a rat model of hypoxia-ischemia. Exp Neurol. 1994;130:362–7. doi: 10.1006/exnr.1994.1215. [DOI] [PubMed] [Google Scholar]
- 13.Relton JK, Martin D, Thompson RC, Russell DA. Peripheral administration of interleukin-1 receptor antagonist inhibits brain damage after focal cerebral ischemia in the rat. Exp Neurol. 1996;138:206–13. doi: 10.1006/exnr.1996.0059. [DOI] [PubMed] [Google Scholar]
- 14.Gutierrez EG, Banks WA, Kastin AJ. Blood-borne interleukin-1 receptor antagonist crosses the blood–brain barrier. J Neuroimmunol. 1994;55:153–60. doi: 10.1016/0165-5728(94)90005-1. [DOI] [PubMed] [Google Scholar]
- 15.Hammarlund-Udenaes M. The use of microdialysis in CNS drug delivery studies. Pharmacokinetic perspectives and results with analgesics and antiepileptics. Adv Drug Deliv Rev. 2000;45:283–94. doi: 10.1016/s0169-409x(00)00109-5. [DOI] [PubMed] [Google Scholar]
- 16.Teasdale GM, Jennett B. Assessment of coma and impaired consciousness. A practical scale. Lancet. 1974;2:81–4. doi: 10.1016/s0140-6736(74)91639-0. [DOI] [PubMed] [Google Scholar]
- 17.Fisher CM, Kistler JP, Davis JM. Relation of cerebral vasospasm to subarachnoid haemorrhage visualised by computerized tomographic scanning. Neurosurgery. 1981;6:1–9. doi: 10.1227/00006123-198001000-00001. [DOI] [PubMed] [Google Scholar]
- 18.Mahoney FI, Barthel DW. Functional evaluation: the Barthel index. Maryland State Med J. 1965:61–5. [PubMed] [Google Scholar]
- 19.van Swieten JC, Koudstaal PJ, Visser MC, Schouten HJ, van Gijn J. Interobserver agreement for the assessment of handicap in stroke patients. Stroke. 1988;19:604–7. doi: 10.1161/01.str.19.5.604. [DOI] [PubMed] [Google Scholar]
- 20.Yang BB, Baughman S, Sullivan JT. Pharmacokinetics of anakinra in subjects with different levels of renal function. Clin Pharmacol Ther. 2003;74:85–94. doi: 10.1016/S0009-9236(03)00094-8. [DOI] [PubMed] [Google Scholar]
- 21.Al Kassab S, Skyhoj OT, Skriver EB. Blood–brain barrier integrity in patients with cerebral infarction investigated by computed tomography and serum-CSF-albumin. Acta Neurol Scand. 1981;64:438–45. doi: 10.1111/j.1600-0404.1981.tb04421.x. [DOI] [PubMed] [Google Scholar]
- 22.Beal S, Sheiner LB. The NONMEM system. Am Stat. 1980;34:118–9. [Google Scholar]
- 23.Beal S, Sheiner LB. NONMEM Users Guides. University of California at San Fransisco: NONMEM Project Group; 1998. [Google Scholar]
- 24.Granowitz EV, Porat R, Mier JW, Pribble JP, Syiles DM, Bloedow DC, Catalano MA, Wolff SM, Dinarello CA. Pharmacokinetics, safety and immunomodulatory effects of human recombinant interleukin-1 receptor antagonist in healthy humans. Cytokine. 1992;4:353–60. doi: 10.1016/1043-4666(92)90078-6. [DOI] [PubMed] [Google Scholar]
- 25.Clark SR, Stuart A, Rothwell JN. Peripheral administartion of interlukin-1 receptor antagonist (IL-1RA) reduces ischaemic brain damage in the rat. J Cerebral Flow Metab. 2005;25:S45. [Google Scholar]
- 26.Clark SR, McMahon CJ, Gueorguieva I, Rowland M, Scarth S, Georgiou R, Tyrell PJ, Hopkins SJ, Rothwell NJ. Interleukin-1 receptor antagonist penetrates human brain at experimentally therapeutic concentrations. J. Cerebral Flow Metab. 2007 doi: 10.1038/sj.jcbfm.9600537. Epub ahead of print. doi: 10.1038/sj.jcbfm.9600537. [DOI] [PubMed] [Google Scholar]
- 27.Verotta D, Sheiner LB. A general conceptual model for non steady-state pharmacokinetic/pharmacodynamic data (plus response and rejoinder) J Pharmacokin Biopharm. 1995;23:1–10. doi: 10.1007/BF02353780. [DOI] [PubMed] [Google Scholar]
- 28.Kosteljanetz M. Cerebrospinal production in subarchnoid haemorrhage. Br J Neurosurg. 1988;2:161–7. doi: 10.3109/02688698808992665. [DOI] [PubMed] [Google Scholar]
- 29.Rapoport SI. Blood–Brain Barrier in Physiology and Medicine. New York: Raven Press; 1976. [Google Scholar]
- 30.Kandel ER, Schwartz JH, Jessell TM. Principles of Neural Science. New York: McGraw-Hill; 2000. pp. 1296–7. [Google Scholar]
- 31.Berne RM, Levy MN. Principles of Physiology. Boston: Mosby; 1990. pp. 66–7. [Google Scholar]
- 32.Luetmer P, Huston J, Friedman JA, Dixon GR, Petersen RC, Jack CR, MaccLelland RL, Ebersold MJ, Milhorat TH, Hodge CJ. Measuremnt of cerebrospinal fluid flow at the cerebral aqueduct by use of phase-contrast magnetic resonance imaging: technique validation and utility in diagnosing idiopathic normal pressure hydrocephalus. Neurosurgery. 2002;50:534–43. doi: 10.1097/00006123-200203000-00020. [DOI] [PubMed] [Google Scholar]


