Abstract
AIM
To determine the effectiveness of theophyllines in real clinical practice on moderate to severe exacerbations.
METHODS
A cohort of 36 492 chronic obstructive pulmonary disease (COPD) patients aged ≥50 years was reconstructed from the health administrative databases of the province of Quebec, Canada, between 1 January 1995 and 31 December 2002 to compare users of theophyllines with users of inhaled corticosteroids (ICS) and users of long-acting β2-agonists (LABA) on their rate of moderate to severe COPD exacerbations.
RESULTS
Users of theophyllines were found to be less likely than users of LABA [crude rates 84 vs. 91 per 100 patient-years, adjusted rate ratio (RR) 0.89, 95% confidence interval (CI) 0.84, 0.95] and users of theophyllines plus ICS were found to be less likely than users of LABA plus ICS (crude rates 114 vs. 112 per 100 patient-years, adjusted RR 0.89, 95% CI 0.87, 0.92) to have moderate to severe COPD exacerbations. Users of theophyllines were found to be more likely than users of ICS to have a COPD exacerbation (crude rates 84 vs. 77 per 100 patient-years, adjusted RR 1.07, 95% CI 1.04, 1.10), and this association was even stronger among patients who had at least three exacerbations in the year prior to cohort entry (crude rates 273 vs. 213 per 100 patient-years, adjusted RR 1.28, 95% CI 1.19, 1.38).
CONCLUSION
The use of theophyllines was found to be associated with a reduction in the rate of COPD exacerbations among all COPD patients, but to be less effective than ICS among patients with frequent exacerbations.
WHAT IS ALREADY KNOWN ABOUT THIS SUBJECT
Despite active research, none of the existing medications used to treat chronic obstructive pulmonary disease (COPD) has been shown to modify the long-term decline in lung function.
Theophyllines have been recognized for their bronchodilating effects and anti-inflammatory properties, but at the same time they are associated with the risk of adverse events due to their narrow therapeutic range and potential for drug interactions.
To our knowledge, no study has investigated the effects of theophylline on outcomes that can reflect the overall morbidity of COPD patients.
WHAT THIS STUDY ADDS
The use of theophyllines is associated with a reduction in the rate of COPD exacerbations compared with long-acting β2-agonists among COPD patients.
Theophyllines could be seen as an interesting alternative in the treatment of COPD, because they are much less expensive than long-acting β2-agonists, and, from the patient's perspective, an oral formulation might be easier to take than an inhaled formulation.
Keywords: administrative database, cohort study, COPD, exacerbations, theophylline
Introduction
In Canada, chronic obstructive pulmonary disease (COPD) affects 4.3% of adults aged ≥35 years, making it the fourth most common cause of illness and death [1]. Worldwide, COPD is also the fourth leading cause of death [2] and the 12th leading cause of disability [3]. Acute exacerbations are the most frequent cause of hospital admissions and death among COPD patients [4].
Unfortunately, recent guidelines for the diagnosis, management and prevention of COPD report that none of the existing medications for COPD has been shown to modify the long-term decline in lung function [5]. Although the goals of COPD management include the prevention of disease progression and the reduction of mortality, they also include symptom relief, treatment and prevention of exacerbations and the improvement of health status [5]. The step care approach proposed by the Global Initiative for Chronic Obstructive Disease (GOLD) treatment guidelines recommends theophyllines as the third line of treatment when symptoms are still persistent despite treatment with short- and long-acting inhaled bronchodilators [5]. The recommendation for inhaled corticosteroids (ICS) use is more restricted, being recommended only for patients with frequent exacerbations, as their efficacy to improve lung function has been shown to be limited, whereas they have been shown to reduce the risk of exacerbations [6–8]. In the ISOLDE trial, the annual rate of exacerbations among moderate to severe COPD patients was 1.90 (SD 2.63) for placebo group and 1.43 (SD 1.93) for patients on ICS after 3 years of treatment [7]. In this randomized controlled trial (RCT), an exacerbation was defined as worsening of respiratory symptoms that required treatment with oral corticosteroids or antibiotics, or both [7].
Theophyllines have been recognized for their bronchodilating effects since the early 1950s, but have recently been shown also to have anti-inflammatory properties in patients with asthma [9]. Several clinical trials have shown that theophyllines improve lung function and dyspnoea [10, 11] in patients with COPD. Theophyllines may also improve mucociliary clearance [12, 13], cardiovascular function [14], gas exchange [10] and exercise capacity [14]. Despite these proven clinical benefits, the use of theophyllines in the treatment of COPD has decreased over the past years [15], mainly due to their narrow therapeutic range and potential for drug interactions [5]. At the same time, the use of ICS in the treatment of COPD has increased dramatically [16], despite the guidelines' recommendations and the lack of scientific evidence to demonstrate their efficacy to reduce disease progression [6–8, 17–19].
As in many chronic diseases, in the treatment of COPD there is an important gap between the guidelines' recommendations and the use of prescribed medications in clinical practice [16, 20]. This situation commends the evaluation of drug effectiveness in real clinical practice, since guidelines' recommendations are mainly based on the results of RCTs that might not be easily generalized into practice. To our knowledge, there is no study comparing the effectiveness of theophyllines with other available treatment options to reduce the risk of COPD exacerbations in clinical practice. We therefore conducted a large population-based cohort study to evaluate and compare the rate of moderate to severe COPD exacerbations between users of oral theophyllines, ICS and long-acting inhaled β2-agonists (LABA) among patients aged ≥50 years.
Methods
Source of data
This study used claims data from the health administrative database of the Régie de l'Assurance Maladie du Quebec (RAMQ) and data from the MED-ECHO database from the Canadian province of Quebec. During the study period, close to 97% of elderly (>874 000 persons aged ≥65 years) and 508 000 persons aged between 50 and 64 years [21] were covered by the RAMQ medical services plan and the drug plan. The RAMQ Prescriptions Drugs Database contains information on prescriptions filled at community pharmacies, i.e. name, dose, form, quantity of medication dispensed, date and duration of prescription, as well as the identification and specialty of the prescribing physician. The RAMQ Medical Services Database contains claims data on medical services dispensed either at hospitals, emergency departments or medical clinics, i.e. date of service, where the service was dispensed, diagnosis coded with International Classification of Diseases (ICD)-9 codes, as well as specialty and identification of the treating physician. The RAMQ database also contains patients' socio-demographic data, such as age, gender and date of death, as well as a variable indicating whether a patient is receiving social assistance or a supplement added to the Old Age Security pension. The MED-ECHO database contains information on all admissions to acute care hospitals in Quebec, such as date of admission, length of stay, diagnosis coded with ICD-9 codes (admission, principal and secondary), identification of hospital and treating physician. The RAMQ and MED-ECHO databases were linked using an encrypted unique patient's identifier included in all databases. The RAMQ and MED-ECHO databases have been used extensively for epidemiological studies, and the information related to medications has been proven valid and comprehensive [22–24]. Moreover, medical diagnoses related to COPD recorded in the RAMQ Medical Services database have been found to be valid for research purposes [25].
Study population and design
From the RAMQ databases a large cohort of COPD patients aged ≥50 years between 1 January 1995 and 31 December 1999 was selected. To be included in the cohort, patients should have: (i) filled at least six prescriptions of a short-acting inhaled β2-agonists (SABA) (epinephrine, orciprenaline, salbutamol, terbutaline, fenoterol or pirbuterol) or of an ipratropium bromide in the year preceding cohort entry (six prescriptions per year of inhaled bronchodilator correspond to 3.6 inhalations per day, on average, which is based on the recommended minimum dose of two inhalations qid when needed [5]); (ii) received at least one medical service for COPD (service billed for the following ICD-9 diagnostic codes: chronic bronchitis codes 491.0, 491.1, 491.2, 491.8 and 491.9; emphysema codes 492.0, 492.8; and chronic airway obstruction code 496) in the year preceding cohort entry; and (iii) been covered by the RAMQ drug plan for at least 1 year prior to cohort entry. Patients were excluded if they received oral corticosteroids as a continuous therapy (at least six filled prescriptions of ≥28 days, or at least 21 filled prescriptions regardless of duration), were hospitalized for >30 days in one hospitalization (because no information was available on medication in RAMQ database when patients were hospitalized) or received any medical service for asthma (ICD-9 codes 493) in the year preceding cohort entry. Corticosteroid-dependent patients were excluded, because this treatment might have been prescribed for a disease other than COPD and continuous use of oral corticosteroids would preclude the evaluation of our main outcome, which is based on markers of COPD exacerbations, such as a filled prescription of oral corticosteroids. Cohort entry was defined as 1 January 1996, 1997, 1998, 1999 or 2000. Patients were followed for a maximum of 7 years. Follow-up was stopped either when patients reached the end of the study period, i.e. 31 December 2002, or died. Patients were also censored if they left the RAMQ drug plan insurance, received one asthma diagnosis, were hospitalized for >30 days or started a continuous therapy of oral corticosteroids, because these events would preclude the evaluation of our main outcome. Data on medical and pharmacy services were obtained 1 year before cohort entry and during study follow-up.
Assessment of exposure
Follow-up time was divided into treatment episodes. A treatment episode was defined by the number of consecutive days a patient remained under the same treatment regimen. No minimum was required for the duration of a treatment period for inclusion in the analysis. The duration of a treatment period was based on the duration of the filled prescriptions and a delay was allowed of two times the duration of the prescription between renewals before considering that a patient had stopped his treatment. Each treatment episode was classified into one of seven treatment regimens for COPD based on filled prescriptions. The first regimen was formed of SABA and/or ipratropium bromide only. For all the other regimens, there was at least one medication added to the SABA and/or ipratropium bromide and one to three adjuvant therapies, i.e. theophyllines (aminophylline, theophylline or oxtriphylline in oral formulation); ICS (beclomethasone, triamcinolone, flunisolide, budesonide or fluticasone); LABA (salmeterol or formoterol); theophyllines and ICS concurrently; ICS and LABA concurrently; and theophyllines, ICS and LABA concurrently. Patients remained in a treatment episode until they added another medication to their treatment regimen, switched, stopped a medication or reached the end of the study follow-up. Patients could thus contribute to more than one episode of treatment during the study follow-up. An example of the assessment of exposure for a patient during the entire follow-up is presented in Figure 1.
Figure 1.
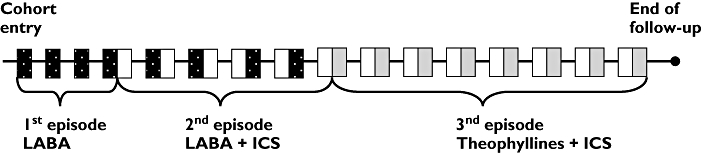
Example of assessement of exposure to chronic obstructive pulmonary disease treatment for one patient. Rx, Prescription; ICS, inhaled corticosteroids; LABA, long-acting β2-agonists. Rx LABA, ( ); Rx ICS, (□); Rx theophyllines, (
); Rx ICS, (□); Rx theophyllines, ( )
)
Outcome
The main outcome was the rate of moderate to severe COPD exacerbations, i.e. the number of exacerbations divided by the person-days during episodes of a specific COPD treatment regimen. An episode was defined by the number of consecutive days a patient remained under the same treatment regimen. An exacerbation was defined either as a filled prescription of oral corticosteroids, a visit to an emergency department for COPD or a hospitalization for COPD (admission or principal ICD-9 codes equal to 491.x, 492.x or 496). Only a single exacerbation was considered if more than one marker of exacerbation [prescription of oral corticosteroids, Emergency Department (ED) visits for COPD or hospitalizations for COPD] occurred within a period of 15 days.
Potential confounders
Potential confounders included patient's age at cohort entry, gender, socio-economic status [poor (receiving social assistance or receiving Guaranteed Income Supplement added to the Old Age Security Pension) vs. others] and the calendar year of cohort entry (to adjust for prescribing habits that may vary over time). Potential confounders measured in the year prior to cohort entry were also included, such as a medication-based comorbidity score [26] and a medical visit-based continuity of care score [27]. Markers of COPD severity included the number of prescriptions of oral corticosteroids, ED visits for COPD and hospitalizations for COPD in the year prior to cohort entry, as well as a medical visit with a respiratory physician, the average daily dose of SABA and ipratropium bromide (one dose equals two inhalations) and the number of prescriptions of antibiotics for COPD filled in the 3 months prior to each specific treatment episode.
Statistical analysis
The crude rate of moderate to severe COPD exacerbations was estimated for all treatment regimens. Poisson regression models were also performed to estimate the adjusted rate ratios of moderate to severe COPD exacerbations comparing patients who had regimens with same number of adjuvant therapies: (i) theophyllines vs. ICS; (ii) theophyllines vs. LABA; and (iii) theophyllines plus ICS vs. LABA plus ICS. Treatment episodes with theophyllines plus LABA were excluded from the comparison because of the small number of patients under this treatment regimen. All comparisons were performed twice, once among all COPD patients and secondly among patients who had three exacerbations of COPD or more in the year prior to cohort entry. This stratification was done to be coherent with the Canadian guidelines, which recommend regular use of ICS only in patients with moderate to severe COPD who have three or more acute exacerbations per year [28]. Other Poisson regression models were used to obtain adjusted rate ratios for COPD exacerbations in association with the average daily doses of theophyllines and ICS. All potential confounder variables were included in the models and analyses were carried out using the SAS system version 8.2 (SAS Institute Inc., Cary, NC, USA).
Results
The cohort was formed of 36 492 COPD patients who met the eligibility criteria. The mean age at cohort entry was 73 years, 61.5% of patients were male and patients were followed on average for 2.4 years. A total of 3040 patients had three or more COPD exacerbations in the year prior to cohort entry and were followed on average for 1.5 years. The baseline characteristics of the study patients are summarized in Table 1. Among study patients, we observed that 22.9% had at least one treatment episode with theophyllines during the study follow-up and 63.7% had at least one treatment episode with ICS. The most commonly prescribed theophyllines were theophylline in long-acting formulation (84.0%) and the average daily dose of theophyllines was 346 mg (SD 204). The prescribed ICS were fluticasone (50.9%), beclomethasone (39.4%) and budesonide (17.2%), and the average daily dose of ICS was 818 µg (SD 554) beclomethasone equivalent. COPD patients had, on average, a high comorbidity score of 8.9 (SD 4.1). A total of 37.9% of patients received at least one prescription of oral corticosteroids, 29.0% had at least one visit to an ED for COPD and 19.2% were hospitalized for COPD in the year prior to cohort entry.
Table 1.
Characteristics of all chronic obstructive pulmonary disease study patients and patients having three exacerbations or more in the year prior to cohort entry
| Variables | All patients, n = 36 492 | Patients with ≥3 exacerbations in year prior to cohort entry, n = 3040 |
|---|---|---|
| Male (%) | 22 430 (61.5) | 1915 (63.0) |
| Age at cohort entry (years), mean ± SD | 73.1 ± 8.5 | 73.0 ± 8.0 |
| Income, n (%) | ||
| Poor to average | 22 828 (62.6) | 1803 (59.3) |
| Other | 13 664 (37.4) | 1237 (40.7) |
| Follow-up (years), mean ± SD | 2.4 ± 1.9 | 1.5 ± 1.6 |
| Year of cohort entry, n (%) | ||
| 1996 | 10 461 (26.7) | 903 (29.7) |
| 1997 | 7 116 (19.5) | 561 (18.5) |
| 1998 | 6 991 (19.2) | 574 (18.9) |
| 1999 | 6 092 (16.7) | 497 (16.4) |
| 2000 | 5 832 (16.0) | 505 (16.6) |
| ≥1 episode(s) of treatment during follow-up, n (%) | ||
| Theophylline | 8 371 (22.9) | 865 (28.5) |
| ICS | 23 262 (63.7) | 1736 (57.1) |
| LABA | 3 015 (8.3) | 239 (7.9) |
| LABA plus ICS | 5 738 (15.7) | 492 (16.2) |
| Theophylline plus ICS | 9 200 (25.2) | 1117 (36.7) |
| In the year prior to cohort entry | ||
| COC index, mean ± SD | 0.45 ± 0.29 | 0.43 ± 0.26 |
| Comorbidity score, mean ± SD | 8.9 ± 4.1 | 11.4 ± 3.8 |
| Prescriptions of oral corticosteroids, n (%) | ||
| 0 | 22 667 (62.1) | 109 (3.6) |
| 1 | 7 047 (19.3) | 197 (6.5) |
| 2 | 2 939 (8.1) | 365 (12.0) |
| ≥3 | 3 842 (10.5) | 2368 (77.9) |
| Emergency department visits COPD, n (%) | ||
| 0 | 25 895 (71.0) | 902 (29.7) |
| 1 | 7 814 (21.4) | 848 (27.9) |
| ≥2 | 2 783 (7.6) | 1290 (42.4) |
| Hospitalizations for COPD, n (%) | ||
| 0 | 29 468 (80.8) | 1376 (45.3) |
| ≥1 | 7 024 (19.2) | 1664 (54.7) |
| In the 3 months prior to cohort entry | ||
| ≥1 visit(s) to a respiratory physician, n (%) | 6 311 (17.3) | 911 (30.0) |
| Number of filled prescriptions of antibiotics for COPD, mean ± SD | 0.5 ± 0.9 | 1.1 ± 1.3 |
| Number of doses of SABA per day, mean ± SD | 3.2 ± 2.2 | 4.3 ± 2.7 |
| Number of doses of ipratropium bromide per day, mean ± SD | 2.1 ± 2.3 | 3.4 ± 2.8 |
COPD, Chronic obstructive pulmonary disease; COC, continuity of care; ICS, inhaled corticosteroids; LABA, long-acting β2-agonists; SABA, short-acting β2>-agonists.
Similar socio-demographic characteristics were observed among patients with three or more exacerbations in the year prior to cohort entry. In this subgroup of patients, the most commonly prescribed theophylline was also in the long-acting formulation (89%) and the average daily dose was 377 mg (SD 206). The average daily dose of ICS was 1001 µg (SD 579) beclomethasone equivalent. However, these patients appeared to have severe COPD, because a larger proportion of them had prescriptions of oral corticosteroids (77.9% filled more than three prescriptions), an ED visit for COPD (70.3%) and hospitalization for COPD (54.7% had at least one hospitalization) in the year prior to cohort entry. Furthermore, they used more doses of SABA and ipratropium bromide per day and filled more antibiotics for COPD before their entry in the cohort.
The Table 2 shows the characteristics of all study patients according to compared treatment regimens. Patients using theophyllines alone or in addition to ICS had a slightly higher score of continuity of care than other patients. Patients using ICS had slightly more comorbidity than patients using other treatment regimens. Furthermore, on average, patients treated with theophyllines alone or in addition to ICS used more SABA prior to treatment initiation than those treated with other drug regimens. Patients treated with LABA alone or in addition to ICS used more ipratropium bromide and were more likely to visit a respiratory physician prior to treatment initiation than patients treated with other drug regimens. Similar patterns were observed among patients with three or more exacerbations in the year prior to cohort entry (Table 3), but they used more SABA, ipratropium bromide and antibiotics for COPD prior to treatment initiation than other patients.
Table 2.
Selected patients' characteristics according to treatment regimens (36 492 patients)
| Variables | Theophyllines | ICS | LABA | LABA + ICS | Theophyllines + ICS |
|---|---|---|---|---|---|
| Number of episode of treatment | 19 613 | 58 916 | 5623 | 10 697 | 21 760 |
| Duration of episode (days), mean ± SD | 188 ± 273 | 115 ± 239 | 93 ± 163 | 185 ± 237 | 172 ± 269 |
| Male (%) | 67.1 | 60.6 | 64.2 | 65.1 | 66.7 |
| Age at cohort entry (years), mean ± SD | 72.5 ± 8.0 | 73.0 ± 8.5 | 71.7 ± 7.8 | 71.2 ± 7.9 | 72.5 ± 7.9 |
| Income (%) | |||||
| Poor | 63.8 | 63.2 | 57.3 | 60.8 | 64.3 |
| Other | 36.2 | 36.8 | 42.7 | 39.3 | 35.7 |
| Number of COPD exacerbations per patient in the year prior to cohort entry, mean ± SD | 0.9 ± 1.2 | 0.8 ± 1.1 | 0.8 ± 1.1 | 0.8 ± 1.1 | 1.0 ± 1.3 |
| In the 3 months prior to an episode of treatment | |||||
| ≥1 visit to a respiratory physician, % | 16.0 | 14.0 | 24.2 | 27.9 | 17.9 |
| Number of filled prescriptions of antibiotics for COPD, mean ± SD | 0.5 ± 1.0 | 0.5 ± 0.9 | 0.6 ± 1.0 | 0.7 ± 1.1 | 0.7 ± 1.0 |
| Number of doses of SABA per day, mean ± SD | 3.5 ± 2.6 | 3.1 ± 2.2 | 2.9 ± 2.7 | 3.3 ± 2.8 | 3.9 ± 2.5 |
| Number of doses of ipratropium bromide per day, mean ± SD | 2.5 ± 2.7 | 2.1 ± 2.3 | 3.0 ± 2.8 | 3.1 ± 2.8 | 2.6 ± 2.7 |
COPD, Chronic obstructive pulmonary disease; ICS, inhaled corticosteroids; LABA, long-acting β2-agonists; SABA, short-acting β2-agonists.
Table 3.
Patients' characteristics according to treatment regimen, among patients with three exacerbations or more in the year prior to cohort entry (3040 patients)
| Variables | Theophyllines | ICS | LABA | LABA + ICS | Theophyllines + ICS |
|---|---|---|---|---|---|
| Number of episode of treatment | 1676 | 3535 | 398 | 883 | 2202 |
| Duration of episode (days), mean ± SD | 76 ± 159 | 162 ± 233 | 67 ± 129 | 171 ± 227 | 161 ± 251 |
| Male (%) | 69.0 | 61.6 | 65.3 | 62.0 | 66.7 |
| Age at cohort entry (years), mean ± SD | 72.2 ± 7.8 | 73.6 ± 8.0 | 72.1 ± 7.2 | 71.4 ± 7.5 | 72.3 ± 7.4 |
| Income (%) | |||||
| Poor | 59.2 | 61.7 | 59.0 | 60.8 | 63.3 |
| Other | 40.8 | 38.3 | 41.0 | 39.2 | 36.7 |
| Number of COPD exacerbations per patient in the year prior to cohort entry, mean ± SD | 3.7 ± 1.0 | 3.7 ± 1.0 | 3.6 ± 0.9 | 3.6 ± 0.9 | 3.7 ± 1.0 |
| In the 3 months prior to an episode of treatment | |||||
| ≥1 visit to a respiratory physician, % | 24.5 | 23.5 | 32.4 | 36.1 | 26.4 |
| Number of filled prescriptions of antibiotics for COPD, mean ± SD | 1.0 ± 1.2 | 1.0 ± 1.2 | 1.0 ± 1.4 | 1.1 ± 1.3 | 1.1 ± 1.2 |
| Number of doses of SABA per day, mean ± SD | 4.4 ± 3.0 | 4.0 ± 2.7 | 3.6 ± 3.0 | 3.8 ± 2.7 | 4.8 ± 2.9 |
| Number of doses of ipratropium bromide per day, mean ± SD | 3.5 ± 2.9 | 3.2 ± 2.6 | 3.7 ± 3.0 | 3.8 ± 2.9 | 3.8 ± 2.9 |
COPD, Chronic obstructive pulmonary disease; ICS, inhaled corticosteroids; LABA, long-acting β2-agonists; SABA, short-acting β2-agonists.
Table 4 displays the crude rate of moderate to severe COPD exacerbations; this rate was found to be higher among patients with two adjuvant therapies (users of theophyllines plus ICS or users of LABA plus ICS) than patients with only one adjuvant therapy. For example, among users of theophyllines in monotherapy the rate of filled prescriptions of oral corticosteroids was 72 per 100 patient-years (4432 filled prescriptions divided by 6156 patient-years) and was lower than the rate among users of theophyllines plus ICS, which was of 118 filled prescriptions of oral corticosteroids per 100 patient-years.
Table 4.
Crude rate of moderate to severe COPD exacerbations during the study follow-up according to treatment regimens
| All patients | ≥3 moderate to severe exacerbations in the year prior to cohort entry | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Treatment | Patient-years | Rate* of OCS prescriptions | Rate* of ED visits for COPD | Rate* of hospitalizations for COPD | Rate * of exacerbations† | Patient-years | Rate* of OCS prescriptions | Rate* of ED visits for COPD | Rate* of hospitalizations for COPD | Rate* of exacerbations† |
| Theophyllines | 6 156 | 72 | 39 | 32 | 84 | 350 | 271 | 126 | 102 | 273 |
| ICS | 30 413 | 75 | 30 | 21 | 77 | 1568 | 237 | 85 | 57 | 213 |
| LABA | 1 426 | 89 | 30 | 24 | 91 | 73 | 261 | 68 | 52 | 230 |
| LABA + ICS | 5 414 | 126 | 33 | 26 | 112 | 415 | 292 | 68 | 49 | 233 |
| Theophyllines + ICS | 10 283 | 118 | 46 | 33 | 114 | 974 | 305 | 106 | 75 | 268 |
Rate per 100 patient-years.
An exacerbation was defined either as a prescription of oral corticosteroids filled, a visit to an ED for COPD or hospitalization for COPD. Only one single exacerbation was considered if more than one marker of exacerbation (prescription of oral corticosteroids, ED visits for COPD or hospitalizations for COPD) occurred within a period of 15 days. COPD, Chronic obstructive pulmonary disease; ED, emergency department; ICS, inhaled corticosteroids; LABA, long-acting β2 -agonists; OCS, oral corticosteroids.
Among patients with three or more exacerbations in the year prior to cohort entry, the rate of moderate to severe COPD exacerbations was higher among patients treated with theophyllines (alone or in addition to ICS).
The results of Poisson regression analysis presented in Table 5 show that all users of theophyllines were found to be significantly (7%) more likely to have a moderate to severe COPD exacerbation than users of ICS [adjusted rate ratio (RR) 1.07, 95% confidence interval (CI) 1.04, 1.10]. However, in this model, users of theophyllines were found to be significantly (11%) less likely to have moderate to severe COPD exacerbations than users of LABA (adjusted RR 0.89, 95% CI 0.84, 0.95). A similar association was observed when theophyllines were added to ICS and compared with LABA plus ICS (adjusted RR 0.89, 95% CI 0.87, 0.92). From this Poisson regression model it was also observed that women were significantly less likely than men to have an exacerbation, that for every increase of one unit in the score of comorbidity the risk of exacerbations increased by 5% (crude RR 1.05; 95% CI 1.046,1.050), but this association was not found to be statistically significant when we adjusted for other covariables (adjusted RR 1.001; 95% CI 0.999, 1.003), whereas patients with markers of disease severity were more likely to have an exacerbation. Patients had an increased risk of having an exacerbation as they increased their daily doses of SABA taken in the 3 months prior to an episode of treatment (13% increase for two to three doses and 32% increase for more than three doses). Also, patients were more likely to have an exacerbation if they used two or more doses of ipratropium bromide per day (24% for two to three doses and 34% for more than three doses) or filled antibiotic prescriptions for COPD in the 3 months prior to an episode of treatment.
Table 5.
Crude and adjusted rate ratios of moderate to severe COPD exacerbations comparing theophyllines, ICS and LABA
| All patients | Patients with ≥3 exacerbations in the year prior to cohort entry | |||||
|---|---|---|---|---|---|---|
| Crude RR | Adjusted* RR | 95% CI | Crude RR | Adjusted* RR | 95% CI | |
| Theophyllines/ICS | 1.09 | 1.07 | 1.04, 1.10 | 1.28 | 1.28 | 1.19, 1.38 |
| Theophyllines/LABA | 0.92 | 0.89 | 0.84, 0.95 | 1.19 | 1.07 | 0.90, 1.26 |
| Theophyllines + ICS/LABA + ICS | 1.02 | 0.89 | 0.87, 0.92 | 1.15 | 1.02 | 0.95, 1.10 |
| Female vs. male | 0.88 | 0.94 | 0.93, 0.96 | 0.93 | 0.96 | 0.92, 1.00 |
| Age (5 years' difference) | 1.01 | 1.02 | 1.02, 1.03 | 0.97 | 0.99 | 0.98, 1.00 |
| Socio-economic status (poor/others) | 0.97 | 0.99 | 0.98, 1.01 | 0.99 | 1.01 | 0.97, 1.05 |
| COC index (0.1 difference) | 0.98 | 1.00 | 0.99, 1.00 | 0.99 | 0.99 | 0.99, 1.00 |
| Comorbidity score (1 difference) | 1.05 | 1.00 | 1.00, 1.00 | 0.99 | 1.00 | 0.99, 1.00 |
| Year of cohort entry (1997 as the reference) | ||||||
| 1996 | 1.08 | 1.00 | 0.98, 1.02 | 1.04 | 1.00 | 0.95, 1.06 |
| 1998 | 1.05 | 0.96 | 0.94, 0.98 | 1.02 | 1.01 | 0.95, 1.07 |
| 1999 | 0.94 | 0.85 | 0.83, 0.88 | 0.87 | 0.89 | 0.84, 0.95 |
| 2000 | 0.93 | 0.80 | 0.77, 0.82 | 0.91 | 0.91 | 0.85, 0.97 |
| Markers of disease severity in the year prior to cohort entry | ||||||
| Prescriptions of oral corticosteroids (0 reference): | ||||||
| 1 | 1.76 | 1.39 | 1.36, 1.42 | |||
| 2 | 2.44 | 1.66 | 1.61, 1.71 | |||
| ≥3 | 4.09 | 2.37 | 2.32, 2.44 | †1.38 | †1.32 | 1.26, 1.39 |
| Emergency department visits COPD, y/n | 1.99 | 1.24 | 1.22, 1.26 | 0.96 | 0.97 | 0.93, 1.01 |
| Hospitalizations for COPD, y/n | 2.08 | 1.12 | 1.10, 1.15 | 1.13 | 1.13 | 1.09, 1.18 |
| Markers of disease severity in the 3 months prior to an episode of treatment | ||||||
| Visit to a respiratory physician, y/n | 1.72 | 1.22 | 1.20, 1.25 | 1.23 | 1.09 | 1.04, 1.14 |
| Number of filled prescriptions of antibiotics for | ||||||
| COPD (0 reference): | ||||||
| 1 | 1.81 | 1.51 | 1.49, 1.54 | 1.29 | 1.23 | 1.18, 1.29 |
| 2 | 2.51 | 1.78 | 1.74, 1.83 | 1.50 | 1.40 | 1.33, 1.48 |
| ≥3 | 3.25 | 2.01 | 1.95, 2.07 | 1.79 | 1.60 | 1.51, 1.70 |
| Doses of SABA per day (<2 reference): | ||||||
| 2–3 | 1.33 | 1.13 | 1.10, 1.17 | 1.27 | 1.26 | 1.17, 1.36 |
| >3 | 2.02 | 1.32 | 1.29, 1.35 | 1.45 | 1.30 | 1.22, 1.38 |
| Doses of ipratropium bromide per day (<2 reference): | ||||||
| 2–3 | 1.48 | 1.24 | 1.21, 1.27 | 1.07 | 0.96 | 0.90, 1.03 |
| >3 | 2.12 | 1.34 | 1.31, 1.36 | 1.23 | 1.00 | 0.95, 1.05 |
Adjusted for all variables in the table.
The reference was less than three exacerbations in the year prior to cohort entry. COPD, Chronic obstructive pulmonary disease; COC, continuity of care; ICS, inhaled corticosteroids; LABA, long-acting β2 -agonists; SABA, short-acting β2 -agonists; RR, rate ratio; CI, confidence interval.
Among patients with three or more COPD exacerbations in the year prior to cohort entry, ICS were associated with a reduced risk of COPD exacerbations compared with theophyllines (adjusted RR 0.78, 95% CI 0.72, 0.84; Table 3). No other significant difference was found for the other treatment comparisons.
Poisson regression models were performed to investigate the effect of the daily dose of theophyllines and ICS on the rate of exacerbations. The dose was not found to be significantly associated with the risk of exacerbations when theophyllines were prescribed in monotherapy, but was when theophyllines were added to ICS (adjusted RR 0.87, 95% CI 0.80, 0.94, 201–300 mg vs.≤200 mg; adjusted RR 0.78, 95% CI 0.74, 0.83, >300 mg vs.≤200 mg). On the other hand, the dose of ICS (≤1000 vs. >1000 µg per day in beclomethasone equivalent) was not found to be significantly associated with the risk of exacerbations when ICS were given in monotherapy (adjusted RR 0.99, 95% CI 0.96, 1.0) but was when ICS were given in addition to theophyllines (adjusted RR 1.04, 95% CI 1.01, 1.07).
Discussion
In the entire cohort, COPD patients treated with theophyllines (either alone or in addition to ICS) were found to be less likely to have moderate to severe COPD exacerbations than patients treated with LABA (either alone or in addition to ICS). However, users of theophyllines, particularly those with frequent past COPD exacerbations, were found to be more at risk of exacerbations than users of ICS.
A review of 20 RCTs concluded that oral theophyllines improve lung function because they increase central respiratory drive, respiratory muscle performance, arterial blood gas tensions and ventilatory capacity [29]. This systematic review also reported that patients preferred theophyllines to placebo and concluded that theophyllines remain an important option in the management of COPD, but their benefits have to be weighed against their risk of adverse effects [29]. Although many RCTs have shown that theophyllines are relatively weak bronchodilators, an advantage of theophyllines is that their systemic administration may have effects on small airways, may reflect in a reduction of the hyperinflation, a reduction in dyspnoea and an improvement in exercise performance [30]. Theophyllines can also improve mucociliary clearance, and this beneficial action may complement their effects on bronchoconstriction and respiratory muscle dysfunction in patients with chronic bronchitis [12, 13]. Theophyllines are also nonselective phosphodiesterase (PDE) inhibitors. This effect may account in part for their efficacy [31]. Moreover, theophyllines may increase responsiveness to ICS, avoid steroid resistance and allow ICS to suppress chronic inflammation in COPD patients [32]. This might explain why theophyllines added to ICS were more effective then LABA added to ICS in reducing the rate of exacerbations. However, RCTs have shown that patients treated with LABA [33, 34] or LABA plus ICS [35] have a significant greater improvement in lung function [forced expiratory volume in 1 s (FEV1), forced vital capacity and morning peak expiratory flow], a higher percentage of symptom-free (cough, wheezing and shortness of breath) days and use less rescue medications (salbutamol or albuterol) than patients treated with theophyllines [33, 34, 36] or theophyllines plus ICS [35].
Our study is the first to investigate the association between theophyllines and moderate to severe COPD exacerbations as the primary outcome. To our knowledge, only one RCT has evaluated and compared the frequency of moderate to severe COPD exacerbations as a secondary efficacy outcome between formoterol and oral, slow-release theophylline [33]. In this RCT, the mean percentage of patients receiving additional therapy for COPD exacerbations (corticosteroids, antibiotics or oxygen) during follow-up was lower among patients receiving theophyllines (20%) than among those receiving 12 µg of inhaled formoterol (32%) or 24 µg of inhaled formoterol (23%). The number of COPD-related hospitalizations was also lower among the theophylline group (n = 6) than in the 12 µg inhaled formoterol group (n = 10), but was similar to the number found in the 24 µg inhaled formoterol group (n = 5). In our study, we found a 11% reduction in the risk of moderate to severe exacerbations comparing users of theophyllines with users of LABA.
Our results on the effectiveness of ICS in reducing moderate to severe exacerbations, particularly among patients with frequent past exacerbations, confirm the results of previous RCTs [10, 11]. In the ISOLDE trial, Burge et al. found a significant reduction of 25% in the rate of exacerbations comparing users of fluticasone propionate (ICS) with placebo (P = 0.026) among patients with moderate to severe COPD. In our study, we found a 22% reduction in the risk of moderate to severe exacerbations comparing users of ICS with users of theophyllines among patients with frequent past exacerbations. Our data support the recommendations of the GOLD treatment guidelines indicating that ICS should be considered in patients with moderate to severe COPD who experience frequent exacerbations [5].
This study has several strengths, namely a large sample size, a cohort representative of the population of COPD patients, a long follow-up of up to 7 years and details about pharmacological treatments taken during all these years. It also avoids recall bias by the use of administrative database. On the other hand, the study has some limitations inherent in the use of administrative databases. First, the diagnosis of COPD was not confirmed with spirometric measures (FEV1). Consequently, we assumed that patients had COPD at cohort entry if they received at least one medical service for COPD and filled at least six bronchodilator prescriptions in the year preceding cohort entry. However, given that bronchodilator treatments are also commonly used for asthma, all patients who received a medical service for asthma in the year preceding cohort entry were excluded. A previous validation study has shown that the RAMQ databases were accurate in distinguishing COPD from asthma [25]. Second, the diagnosis for hospitalizations and emergency visits were based on the diagnoses recorded in the RAMQ and MED-ECHO databases, and were not validated with the medical chart. Third, the exposure to treatment was based on dispensed prescriptions, which might not correspond exactly to the intake of the medications. Fourth, we censored patients with a hospital stay of >30 days because in the RAMQ database there is no information on prescriptions dispensed in hospital and it is thus not possible to measure drug use. We also censored patients who initiated a continuous therapy with oral steroids, because it would have been difficult to evaluate our primary outcome, COPD exacerbations, which is defined, at least in part, on the use of oral corticosteroids. However, a hospital stay of >30 days or the beginning of continuous oral steroids therapy might reflect the development of more severe disease. To investigate whether censoring of patients had an impact on the study results, a sensitivity analysis was conducted in which censoring was not applied. The uncensored analysis gave similar results to the censored analysis presented in this study, revealing the robustness of the results (data available on request). Finally, we cannot rule out completely the presence of confounding due to unmeasured variables such as cigarette smoking and clinical measures of disease severity. However, in order to minimize residual confounding patients were compared who had equal numbers of adjuvant therapies. Patients with theophyllines only were compared with ICS or LABA only and patients using theophyllines plus ICS were compared with patients using LABA plus ICS. Moreover, we adjusted in the analysis for the use of SABA and ipratropium bromide in the 3 months prior to each treatment episode.
Lastly, theophylline is associated with adverse gastrointestinal effects as well as serious cardiovascular (tachycardia and arrhythmias) and central nervous system side-effects attributed to nonselective inhibition of PDE, even at therapeutic doses [31]. In this study, we observed that 86% of theophylline users renewed their prescription at least once during follow-up. Moreover, theophylline users filled 11 prescriptions per year, on average, suggesting that they tolerated their medication well and that our cohort might be over-represented by patients who tolerate theophyllines well. Moreover, the average daily dose of theophyllines used by study patients did not exceed the maximun recommanded daily dose of 800 mg [5].
Theophyllines, as the only adjuvant therapy or in addition to ICS, were found to be more effective than LABA in avoiding moderate to severe exacerbations. However, among patients with frequent past exacerbations, ICS were found to be more effective than theophyllines in avoiding moderate to severe exacerbations, and no significant difference was observed between theophyllines and LABA. Theophyllines are much less expensive medications than ICS and LABA, and oral formulations have been shown to increase patient adherence compared with inhaled formulations in asthma [37]. In conclusion, theophyllines could be seen as an effective therapy in selected COPD patients who tolerate this medication well. Further study, based on outcomes that can measure overall morbidity, will be required to evaluate whether the benefits observed in this study outweigh the potential risk of adverse reactions of theophyllines.
Acknowledgments
We thank Ms Labrie-Pelletier from the RAMQ and Louise Légaré (Ministère de la Santé et des Services Sociaux du Québec) for her assistance with the data. M-C.C. has received a doctoral research scholarship from the Fonds de la Recherche en Santé du Québec (FRSQ). L.B. is the recipient of a salary award form the Fonds de la recherche en santé du Québec (FRSQ). She has also received grant support and consulting fee from AstraZeneca Canada Inc, AstraZeneca Wilmington, DE, GlaxoSmithKline Canada Inc and Amgen Canada Inc. L.B. and M.F.B. are co-chairs of the AstraZeneca Endowement Pharmaceutical Chair in respiratory health. M.F.B. has received grant support from Bayer Canada Inc, GlaxoSmithKline Canada Inc, Merck Frosst Inc, and AstraZeneca Canada Inc. She has also received honoraria for providing continuing education by Pfizer, GSK, AstraZeneca, and BI. C.L. is recipient of a New Investigator salary support grant from the Canadian Institutes for Health Research (CIHR). Catherine Lemiere has also received consulting fees and speaker fees from AstraZeneca, GlaxoSmithKline, Altana Pharma, Merck Frosst and Novartis. This study was funded through a research grant provided by the Québec Lung Association.
References
- 1.The Center for Chronic Disease Prevention and Control, Health Canada, Canadian Institute for Health Information. Respiratory Disease In Canada. Ottawa: Health Canada; 2003. [Google Scholar]
- 2.Michaud CM, Murray CJ, Bloom BR. Burden of disease – implications for future research. JAMA. 2001;285:535–9. doi: 10.1001/jama.285.5.535. [DOI] [PubMed] [Google Scholar]
- 3.Murray CJ, Lopez AD. Alternative projections of mortality and disability by cause 1990–2020: Global Burden of Disease Study. Lancet. 1997;349:1498–504. doi: 10.1016/S0140-6736(96)07492-2. [DOI] [PubMed] [Google Scholar]
- 4.Burrows B, Earle RH. Course and prognosis of chronic obstructive lung disease. A prospective study of 200 patients. N Engl J Med. 1969;280:397–404. doi: 10.1056/NEJM196902202800801. [DOI] [PubMed] [Google Scholar]
- 5.Pauwels RA, Buist AS, Calverley PM, Jenkins CR, Hurd SS. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease. NHLBI/WHO Global Initiative for Chronic Obstructive Lung Disease (GOLD) Workshop summary. Am J Respir Crit Care Med. 2001;163:1256–76. doi: 10.1164/ajrccm.163.5.2101039. [DOI] [PubMed] [Google Scholar]
- 6.Vestbo J, Sorensen T, Lange P, Brix A, Torre P, Viskum K. Long-term effect of inhaled budesonide in mild and moderate chronic obstructive pulmonary disease: a randomised controlled trial. Lancet. 1999;353:1819–23. doi: 10.1016/s0140-6736(98)10019-3. [DOI] [PubMed] [Google Scholar]
- 7.Burge PS, Calverley PM, Jones PW, Spencer S, Anderson JA, Maslen TK. Randomised, double blind, placebo controlled study of fluticasone propionate in patients with moderate to severe chronic obstructive pulmonary disease: the ISOLDE trial. BMJ. 2000;320:1297–303. doi: 10.1136/bmj.320.7245.1297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lung Health Study Research Group. Effect of inhaled triamcinolone on the decline in pulmonary function in chronic obstructive pulmonary disease. N Engl J Med. 2000;343:1902–9. doi: 10.1056/NEJM200012283432601. [DOI] [PubMed] [Google Scholar]
- 9.Markham A, Faulds D. Theophylline. A review of its potential steroid sparing effects in asthma. Drugs. 1998;56:1081–91. doi: 10.2165/00003495-199856060-00018. [DOI] [PubMed] [Google Scholar]
- 10.Murciano D, Auclair MH, Pariente R, Aubier M. A randomized, controlled trial of theophylline in patients with severe chronic obstructive pulmonary disease. N Engl J Med. 1989;320:1521–5. doi: 10.1056/NEJM198906083202304. [DOI] [PubMed] [Google Scholar]
- 11.Mahon JL, Laupacis A, Hodder RV, McKim DA, Paterson NA, Wood TE, Donner A. Theophylline for irreversible chronic airflow limitation: a randomized study comparing n of 1 trials to standard practice. Chest. 1999;115:38–48. doi: 10.1378/chest.115.1.38. [DOI] [PubMed] [Google Scholar]
- 12.Wanner A. Effects of methylxanthines on airway mucociliary function. Am J Med. 1985;79:16–21. doi: 10.1016/0002-9343(85)90082-8. [DOI] [PubMed] [Google Scholar]
- 13.Ziment I. Theophylline and mucociliary clearance. Chest. 1987;92:38S–43S. doi: 10.1378/chest.92.1_supplement.38s. [DOI] [PubMed] [Google Scholar]
- 14.Vaz Fragoso CA, Miller MA. Review of the clinical efficacy of theophylline in the treatment of chronic obstructive pulmonary disease. Am Rev Respir Dis. 1993;147:S40–S47. doi: 10.1164/ajrccm/147.6_Pt_2.S40. [DOI] [PubMed] [Google Scholar]
- 15.Van Andel AE, Reisner C, Menjoge SS, Witek TJ. Analysis of inhaled corticosteroid and oral theophylline use among patients with stable COPD from 1987 to 1995. Chest. 1999;115:703–7. doi: 10.1378/chest.115.3.703. [DOI] [PubMed] [Google Scholar]
- 16.Blais L, Bourbeau J, Sheehy O, LeLorier J. Inhaled corticosteroids in COPD: determinants of use and trends in patient persistence with treatment. Can Respir J. 2004;11:27–32. doi: 10.1155/2004/289420. [DOI] [PubMed] [Google Scholar]
- 17.Pauwels RA, Lofdahl CG, Laitinen LA, Schouten JP, Postma DS, Pride NB, Ohlsson SV. Long-term treatment with inhaled budesonide in persons with mild chronic obstructive pulmonary disease who continue smoking. European Respiratory Society Study on Chronic Obstructive Pulmonary Disease. N Engl J Med. 1999;340:1948–53. doi: 10.1056/NEJM199906243402503. [DOI] [PubMed] [Google Scholar]
- 18.Paggiaro PL, Dahle R, Bakran I, Frith L, Hollingworth K, Efthimiou J. Multicentre randomised placebo-controlled trial of inhaled fluticasone propionate in patients with chronic obstructive pulmonary disease. International COPD Study Group. Lancet. 1998;351:773–80. doi: 10.1016/s0140-6736(97)03471-5. [DOI] [PubMed] [Google Scholar]
- 19.Bourbeau J, Rouleau MY, Boucher S. Randomised controlled trial of inhaled corticosteroids in patients with chronic obstructive pulmonary disease. Thorax. 1998;53:477–82. doi: 10.1136/thx.53.6.477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.George J, Kong DC, Thoman R, Stewart K. Factors associated with medication nonadherence in patients with COPD. Chest. 2005;128:3198–204. doi: 10.1378/chest.128.5.3198. [DOI] [PubMed] [Google Scholar]
- 21.Régie de l'assurance maladie du Québec. Statistiques annuelles. Québec: Régie de l'assurance maladie du Québec, Health Ministry, Government of Québec; 1999. [Google Scholar]
- 22.Tamblyn R, Lavoie G, Petrella L, Monette J. The use of prescription claims databases in pharmacoepidemiological research: the accuracy and comprehensiveness of the prescription claims database in Quebec. J Clin Epidemiol. 1995;48:999–1009. doi: 10.1016/0895-4356(94)00234-h. [DOI] [PubMed] [Google Scholar]
- 23.Pilote L, Lavoie F, Ho V, Eisenberg MJ. Changes in the treatment and outcomes of acute myocardial infarction in Quebec, 1988–1995. CMAJ. 2000;163:31–6. [PMC free article] [PubMed] [Google Scholar]
- 24.Martel MJ, Rey E, Beauchesne MF, Perreault S, Lefebvre G, Forget A, Blais L. Use of inhaled corticosteroids during pregnancy and risk of pregnancy induced hypertension: nested case–control study. BMJ. 2005;330:230. doi: 10.1136/bmj.38313.624352.8F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McKnight J, Scott A, Menzies D, Bourbeau J, Blais L, Lemiere C. A cohort study showed that health insurance databases were accurate to distinguish chronic obstructive pulmonary disease from asthma and classify disease severity. J Clin Epidemiol. 2005;58:206–8. doi: 10.1016/j.jclinepi.2004.08.006. [DOI] [PubMed] [Google Scholar]
- 26.Von Korff M, Wagner EH, Saunders K. A chronic disease score from automated pharmacy data. J Clin Epidemiol. 1992;45:197–203. doi: 10.1016/0895-4356(92)90016-g. [DOI] [PubMed] [Google Scholar]
- 27.Bice TW, Boxerman SB. A quantitative measure of continuity of care. Med Care. 1977;15:347–9. doi: 10.1097/00005650-197704000-00010. [DOI] [PubMed] [Google Scholar]
- 28.O'Donnell DE, Aaron S, Bourbeau J, Hernandez P, Marciniuk D, Balter M, Ford G, Gervais A, Goldstein R, Hodder R, Maltais F, Road J. Canadian Thoracic Society recommendations for management of chronic obstructive pulmonary disease – 2003. Can Respir J. 2003;10(Suppl. A):11A–65A. doi: 10.1155/2003/567598. [DOI] [PubMed] [Google Scholar]
- 29.Ram FS, Jardin JR, Atallah A, Castro AA, Mazzini R, Goldstein R, Lacasse Y, Cendon S. Efficacy of theophylline in people with stable chronic obstructive pulmonary disease: a systematic review and meta-analysis. Respir Med. 2005;99:135–44. doi: 10.1016/j.rmed.2004.10.009. [DOI] [PubMed] [Google Scholar]
- 30.Chrystyn H, Mulley BA, Peake MD. Dose – response relation to oral theophylline in severe chronic obstructive airways disease. BMJ. 1988;297:1506–10. doi: 10.1136/bmj.297.6662.1506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vignola AM. PDE4 inhibitors in COPD – a more selective approach to treatment. Respir Med. 2004;98:495–503. doi: 10.1016/j.rmed.2003.12.012. [DOI] [PubMed] [Google Scholar]
- 32.Barnes PJ. Theophylline: new perspectives for an old drug. Am J Respir Crit Care Med. 2003;167:813–8. doi: 10.1164/rccm.200210-1142PP. [DOI] [PubMed] [Google Scholar]
- 33.Rossi A, Kristufek P, Levine BE, Thomson MH, Till D, Kottakis J, Della CG. Comparison of the efficacy, tolerability, and safety of formoterol dry powder and oral, slow-release theophylline in the treatment of COPD. Chest. 2002;121:1058–69. doi: 10.1378/chest.121.4.1058. [DOI] [PubMed] [Google Scholar]
- 34.Broseghini C, Testi R, Polese G, Tosatto R, Rossi A. A comparison between inhaled salmeterol and theophylline in the short-term treatment of stable chronic obstructive pulmonary disease. Pulm Pharmacol Ther. 2005;18:103–8. doi: 10.1016/j.pupt.2004.10.006. [DOI] [PubMed] [Google Scholar]
- 35.Cazzola M, Matera MG, Santangelo G, Vinciguerra A, Rossi F, D'Amato G. Salmeterol and formoterol in partially reversible severe chronic obstructive pulmonary disease: a dose–response study. Respir Med. 1995;89:357–62. doi: 10.1016/0954-6111(95)90008-x. [DOI] [PubMed] [Google Scholar]
- 36.Di Lorenzo G, Morici G, Drago A, Pellitteri ME, Mansueto P, Melluso M, Norrito F, Squassante L, Fasolo A. Efficacy, tolerability, and effects on quality of life of inhaled salmeterol and oral theophylline in patients with mild-to-moderate chronic obstructive pulmonary disease. SLMT02 Italian Study Group. Clin Ther. 1998;20:1130–48. doi: 10.1016/s0149-2918(98)80109-4. [DOI] [PubMed] [Google Scholar]
- 37.Dorais M, Blais L, Chabot I, LeLorier J. Treatment persistence with leukotriene receptor antagonists and inhaled corticosteroids. J Asthma. 2005;42:385–93. doi: 10.1081/JAS-63007. [DOI] [PubMed] [Google Scholar]


