Abstract
AIMS
Polyclonal antilymphocyte globulins (ALGs) are currently used in transplantation, but the sources of interindividual variability of their effect are poorly understood. No pharmacokinetic–pharmacodynamic (PK–PD) study of ALG is available. Moreover, the genetic polymorphism of FcγRIIIa, a receptor for the Fc portion of immunoglobulins involved in antibody-dependent cellular cytotoxicity (ADCC), may influence their concentration–effect relationship.
METHODS
Fourteen kidney transplant patients treated by horse ALG were included in a prospective, noncomparative study. A population two-compartment PK model including a time dependence of the central volume of distribution was developed. Total lymphocyte count was used as biomarker of effect. Concentration–effect data were described using a physiological indirect response model, combining concentration-dependent and -independent inhibitions of lymphocyte input into the circulation. In addition, six kidney transplant patients in whom ALG concentrations were not available were included retrospectively. All patients were genotyped for FCGR3A.
RESULTS
Both the PK and the PK–PD model described the data satisfactorily and showed high interindividual variability. Asymptotic T1/2-α and T1/2-β-values were 1.3 and 25 days, respectively. The concentration of ALG leading to a 50% inhibition of lymphocyte input (IC50) was lower in FCGR3A-V carriers than in FCGR3A-F/F patients (383 ± 199 vs. 593 ± 209 mg l−1, P = 0.008).
CONCLUSIONS
This is the first description of the ALG effect on lymphocyte count using PK–PD modelling. Our results show that part of the variability in their concentration–effect relationship may be explained by FcγRIIIa genetic polymorphism and therefore that horse ALG may deplete lymphocytes by ADCC.
WHAT IS ALREADY KNOWN ABOUT THIS SUBJECT
There is interindividual variability in the antilymphocyte globulin (ALG) effect, but there is no pharmacokinetic–pharmacodynamic study of this subject.
In addition, a time dependence of the pharmacokinetics of some therapeutic antibodies has been described.
ALGs may partly act by antibody-dependent cellular cytotoxicity (ADCC), but their mechanism of action in humans is not known.
WHAT THIS STUDY ADDS
Horse ALG pharmacokinetics can be described using a two-compartment model with time-dependent central volume of distribution.
After an initial concentration-independent lymphocyte depletion, the concentration–effect relationship can be described using a physiological indirect response model.
The genetic polymorphism of FcγRIIIa at position 158 may influence the ALG concentration–effect relationship and these polyclonal antibodies may therefore act by ADCC.
Keywords: antilymphocyte serum, dose–response relationship, IgG receptors, kidney transplantation, pharmacogenetics, pharmacokinetics
Introduction
Antilymphocyte globulins (ALGs) are polyclonal antibodies used in organ transplantation to prevent or treat acute allograft rejection. ALGs are obtained by immunizing animals (rabbits or horses) with human lymphoid cells (lymphoblasts, lymphocytes or thymocytes). Although ALGs have been in use for several decades, the interindividual variability in patient response remains poorly understood [1, 2]. Monitoring of drug effect is based on lymphocyte numeration, because ALG doses may be decreased when lymphopenia is obtained or the drug readministered to maintain it [3]. Although lymphocyte count is an established biomarker of ALG therapeutic effect, the study of its quantitative relationship with ALG concentrations has not been reported.
The mechanism of action of ALG is probably complex, but it eventually results in target cell destruction. Several mechanisms have been observed in vitro, including apoptosis, complement-dependent cytotoxicity (CDC) and also antibody-dependent cellular cytotoxicity (ADCC) in the presence of cytotoxic cells expressing receptors for the Fc portion of IgG (FcγR) [4]. The FCGR3A gene, which encodes FcγRIIIa, a FcγR expressed by macrophages and natural killer cells, displays a single nucleotide polymorphism at position 559 of the cDNA (G or T, rs number 17857127), which generates two FcγRIIIa allotypes, with a valine (V) or a phenylalanine (F) at position 158, respectively [5, 6]. Non-Hodgkin's lymphoma patients with the 158V allotype have a better response to rituximab, an anti-CD20 cytolytic therapeutic antibody, than patients with the 158F allotype [7–11].
FCGR3A polymorphism may therefore be a genetic factor influencing the effect of cytolytic therapeutic antibodies, although it remains to be demonstrated that this is also true for antibodies of animal origin (with an animal Fc portion). The aim of this study was to analyse the concentration–effect relationship of horse ALG in renal transplant patients, with the hypothesis that FCGR3A polymorphism may influence the ALG effect, as measured by lymphocyte depletion, and to explain part of the interindividual variability of its effect.
Methods
Patients
This study was based on both a prospective and a retrospective group of patients. The prospective group consisted of 14 kidney transplant patients included in a noncomparative study of horse ALG pharmacokinetics (PK) and pharmacodynamics (PD), recruited in four renal transplant centres (Caen, Poitiers, Reims and Tours, France). They had received a first cadaveric renal transplantation with a cold ischaemia time <48 h. The patients were not hyperimmunized (panel-reactive IgG antibodies <75%) and had never been treated with horse ALG. This prospective study was approved by the local ethics committee (CCPPRB of Tours) and all patients gave written informed consent, both for the clinical study and for FCGR3A genotyping.
The retrospective group was also studied to increase the number of patients and to validate our models. It consisted of six renal transplant recipients treated with the same horse ALG. They had been transplanted in the centre of Tours and gave written informed consent for FCGR3A genotyping. Patients' characteristics are shown in Table 1.
Table 1.
Characteristics of the patients
| Patient | Gender | Age (years) | Weight (kg) | Number of infusions | Total dose (mg) | Initial disease |
|---|---|---|---|---|---|---|
| Prospective group | ||||||
| P1 | F | 37 | 50 | 4 | 1600 | Vesico-ureteral reflux |
| P2 | M | 23 | 65 | 7 | 3300 | IgA nephropathy |
| P3 | M | 32 | 76 | 8 | 3900 | Tubulointerstitial nephropathy |
| P4 | M | 45 | 57 | 6 | 2700 | Nephroangiosclerosis |
| P5 | M | 52 | 67 | 7 | 3100 | Glomerulonephritis |
| P6 | M | 37 | 75 | 8 | 3800 | Focal and segmental hyalinosis |
| P7 | F | 41 | 71 | 5 | 2200 | Glomerulonephritis |
| P8 | M | 56 | 78 | 7 | 3400 | Congenital renal hypoplasia |
| P9 | M | 47 | 64 | 7 | 4200 | Type II diabetes |
| P10 | M | 53 | 66 | 9 | 4500 | Polycystic kidney disease |
| P11 | F | 41 | 52 | 12 | 4200 | Alport syndrome |
| P12 | F | 33 | 50 | 7 | 2600 | Alport's syndrome |
| P13 | M | 43 | 75 | 7 | 2400 | Glomerulonephritis |
| P14 | F | 52 | 67 | 7 | 3600 | Polycystic kidney disease |
| Mean (SD) | 42 (9) | 65 (10) | 7.2 (1.8) | 3250 (850) | ||
| Retrospective group | ||||||
| R1 | F | 66 | 54 | 8 | 3100 | Nephroangiosclerosis |
| R2 | M | 27 | 69 | 5 | 1900 | Tubulointerstitial nephropathy |
| R3 | F | 55 | 43 | 7 | 2400 | Polycystic kidney disease |
| R4 | M | 65 | 71 | 8 | 2700 | Type II diabetes |
| R5 | F | 41 | 59 | 5 | 1500 | Glomerulonephritis |
| R6 | F | 31 | 53 | 4 | 1900 | Type I diabetes |
| Mean (SD) | 48 (17) | 58 (10) | 6.2 (1.7) | 2250 (600) | ||
| P value* | NS | NS | NS | NS | P = 0.026 | |
Comparison between prospective and retrospective groups.
Treatment
Antilymphocyte globulin dosing regimen
In the prospective group, ALG (Lymphoglobuline®; Genzyme Imtix-SangStat, Lyon, France) was administered through a central venous catheter or an arteriovenous fistula. The first infusion was started within the first hour after patient entry in the recovery room and lasted for 24 h. The second infusion was started at least 12 h after the end of the first infusion and subsequent infusions over a period of 8–12 h, separated by a 24-h period. The initial dose was 10 mg kg−1 day−1 without exceeding 600 mg per infusion. After the first two infusions, the dose was halved if T-cell lymphopenia was achieved, with a minimum of 100 mg per infusion. T-cell lymphopenia was defined by one of the two following criteria: a CD3+ lymphocyte count of <20 cells mm−3 (measured using flow cytometry) or a total lymphocyte count of <200 cells mm−3. The conditions leading to ALG discontinuation were a platelet count of <50 000 cells mm−3 or a leucocyte count of <1500 mm−3. When severe adverse effects possibly related to the studied ALG were observed, they were replaced by Thymoglobuline® (Genzyme Imtix-SangStat), an ALG of rabbit origin. Patients in the retrospective group had been administered an initial dose of 10 mg kg−1. Subsequent doses had been adjusted to maintain a CD3+ count of <20 cells mm−3.
Concomitant immunosuppressive treatment
Concomitant immunosuppressive treatment consisted of ciclosporin (Neoral®; Novartis Pharmaceuticals, Basel, Switzerland), mycophenolate mofetil (Cellcept®; Roche, Neuilly sur-Seine, France) and corticosteroids. Ciclosporin was given at an initial dose of 8 mg kg−1 day−1, starting after the sixth infusion of ALG, if serum creatinine was <250 µmol l−1. A 48-h overlap between ciclosporin introduction and ALG discontinuation was maintained, in order to ensure adequate immunosuppression. The dose of ciclosporin was individually adjusted to reach trough concentrations of 150–250 ng ml−1. Mycophenolate mofetil was administered orally prior to transplantation, at an initial dose of 2 g day−1, subsequently adjusted according to clinical and haematological parameters. On the day of transplantation, 250 mg of methylprednisolone were infused before and after surgery, in the latter case before the start of ALG infusion. During the first week, 1 mg kg−1 day−1 of methylprednisolone or prednisolone were given before each ALG infusion. After day 7, the dose was progressively reduced. Before each ALG infusion, 5 mg of dexchlorpheniramine was administered.
Biological measurements
Antilymphocyte globulin serum concentrations
At the time of the first infusion, ALG serum concentrations were measured before and 12 h and 18 h after the start of the first infusion, on days 4, 6, 8, 10 and 14 before the beginning of each ALG infusion and on days 30, 60 and 90 after the end of the first infusion. The number of available serum samples was reduced if horse ALG had to be discontinued because of side-effects. The day 30 sample was missing for patients P1, P4 and P7. Days 60 and 90 samples were missing for patients P1, P4, P6 and P7. Serum concentrations of horse ALG were measured by Imtix-SangStat (Marcy l'Etoile, France) using an enzyme-linked immunosorbant assay (ELISA) method. Briefly, the ELISA plates were coated with a goat antibody directed against equine IgG and incubated with diluted patient's serum (1 : 500, 1 : 1500 and 1 : 4500). The presence of ALG was revealed by a goat antihorse IgG labelled with peroxidase. The lower limit of quantification was 0.3 ng ml−1.
To detect possible immunization against horse ALG, blood samples were drawn before the first infusion and on days 10, 14 and 30. Measurements of human IgG and IgM directed against ALG were done by an ELISA using goat antihuman IgG or IgM labelled with peroxidase.
Lymphocyte count
In both the prospective and retrospective groups, the total lymphocyte count was measured before transplantation, at the end of the first infusion and every day from day 2 to day 14. For patients P1, P4, P7 and P14, who were switched to another ALG during the course of the study, lymphocyte data were truncated, the first data point to be removed being the one measured after the first infusion of the other ALG.
FCGR3A genotyping
FCGR3A genotyping was performed using a real time allele-specific polymerase chain reaction assay based on SYBR Green fluorescence [12].
Pharmacokinetic and pharmacokinetic–pharmacodynamic modelling
Pharmacokinetic modelling
Models with one, two or three compartments and first-order elimination from the central compartment were tested. Compartmental PK parameters, i.e. volumes and clearances of central (Vc and CLc) and peripheral compartments (Vp and CLp), and half-lives (distribution and elimination, T1/2-α and T1/2-β) were estimated. Both individual and population approaches were used. Separate modelling of the infusion period (i.e. from the beginning of the first to the end of the last infusion) and the postinfusion period showed that some estimated PK parameters were time dependent: notably, Vc was smaller during the infusion period than after. A population PK model was therefore developed to take into account the decrease of Vc with time.
The interindividual variability in CLc, Vp and CLp was described using an exponential model as follows:
| (1) |
where θi is an individual parameter, θ is the mean (typical) parameter for the population and ηi is a random-effect parameter describing the interindividual variability. Volume of distribution of the central compartment at time t (Vc,t) was described as:
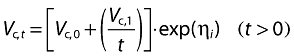 |
(2) |
where Vc,0 and Vc,1 are baseline and time-varying components of Vc, respectively. This equation describes a hyperbolic decrease in Vc with time. Other functions were tested (including exponential decrease), but they led to less satisfactory results.
In the long run, the parameters Vc,0, T1/2-α and T1/2-β reach an asymptotic value. The expression Vc,0 + (Vc,1/t) tends to Vc,0, and T1/2-α and T1/2-β tend to their ‘real’ values.
Pharmacokinetic–pharmacodynamic modelling
PK–PD modelling of patients from the prospective and the retrospective groups was performed using indirect-response models. These models are suitable when drugs act indirectly by inhibiting or stimulating the production or the loss of endogenous elements that are biomarkers of drug response [13]. Stimulation or inhibition functions commonly used in indirect-response modelling are driven by drug blood concentrations. Since ALG leads to lymphocyte depletion, its effect may be described either by inhibition of lymphocyte input in the blood or by stimulation of lymphocyte elimination from the blood. The PD data were better described using indirect-response models with inhibition of lymphocyte input.
At the beginning of the infusion period, especially in the 48 h following the initiation of the first infusion, lymphocyte depletion was massive and seemed independent of ALG serum concentration. This may be explained by a nonlinear relationship between the amount of ALG bound to target cells (i.e. the ‘effect site’ concentration) and ALG serum concentration. Therefore, an inhibition function independent of ALG plasma concentration but dependent on time was added during the infusion period. The final indirect-response model with inhibition of lymphocyte input was:
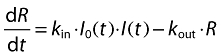 |
(3) |
where R is blood lymphocyte count, kin is the zero-order rate constant of the input of lymphocytes into the blood, kout is the first-order rate constant of their elimination. I0(t) and I(t) are, respectively, time- and concentration-dependent inhibition functions as follows:
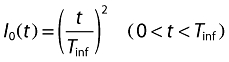 |
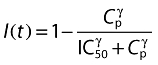 |
where IC50 is ALG blood concentration leading to 50% of maximum ALG-induced inhibition of input, γ is a shape factor and Tinf is the duration of the infusion period for each patient. At the beginning of the infusion period, I0(t) takes the value of 0, assuming a concentration-independent total lymphocyte depletion. During the infusion period, I0(t) increases towards a value of 1, following a quadratic function and, at the end of the infusion period, lymphocyte depletion is fully concentration dependent.
For the patients of the prospective group, the indirect response models used their own PK parameters. For the patients of the retrospective group, for whom no PK data were available, typical values of population PK parameters of the prospective group were used.
Modelling methods
The comparisons of the different PK and PK–PD models tested were made by visual inspection of plots of observed and predicted dependent variable (either concentration or effect) vs. time and of plots of residuals vs. predicted variable and vs. time, and analysis of coefficients of variation (CV) of parameter estimations, interindividual CV of the estimated parameters, residuals sums of squares and Akaike's information criteria (AIC). Population PK analysis was based on the FOCE method, using WinNonMix 2.0.1 (Pharsight Corp., Mountain View, CA, USA). Individual PK–PD modelling was performed with WinNonLin professional 4.1 (Pharsight Corp.).
Statistical analysis
Characteristics of the patients of the retrospective and the prospective groups were compared using Mann–Whitney test. The PD parameters estimated in F/F patients were compared with those estimated in V carriers (V/V + V/F) using Mann–Whitney test. The PD parameters estimated in the prospective and in the retrospective groups, in F/F patients as well as in V carriers, were compared with the same test. Statistical analyses were carried out using Instat (GraphPad Software, San Diego, CA, USA).
Results
A total of 155 ALG blood concentrations were available in the 14 patients of the prospective group. None of the patients developed IgG or IgM against horse ALG. The two-compartment PK model with a time-dependent Vc allowed a satisfactory description of ALG concentrations (Figures 1 and 2, Table 2). Asymptotic values of Vc (2.0 l) and T1/2-α (1.3 days) were reached in approximately 6 days, whereas asymptotic value of T1/2-β (25 days) was reached in 4 days. An important interindividual variability was observed (Table 2). No influence of demographic characteristics or FCGR3A genotype on PK parameters was observed (data not shown).
Figure 1.
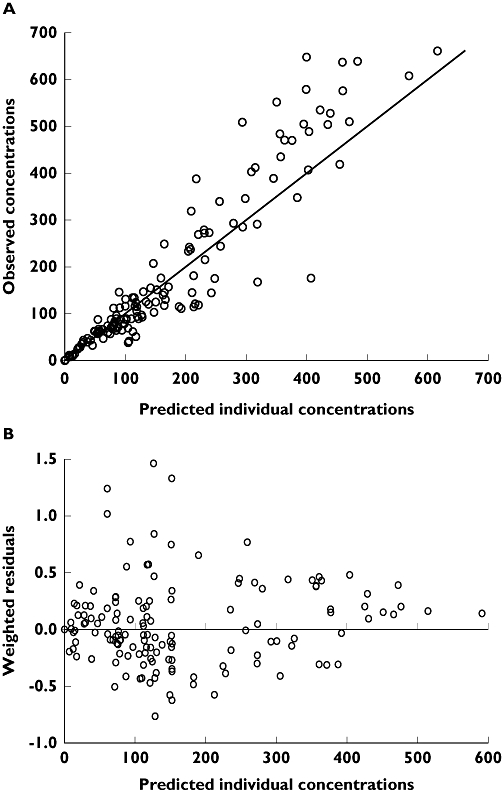
Individual model-predicted vs. observed concentrations (A) and weighted residuals vs. individual model-predicted concentrations (B)
Figure 2.
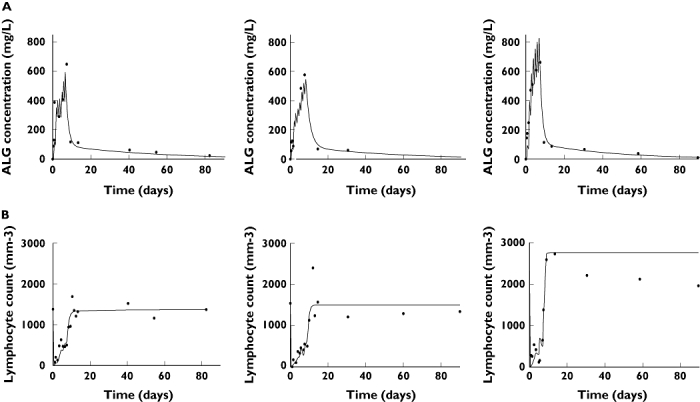
Observed (•) and model-predicted (lines) (A) antilymphocyte globulin (ALG) concentrations and (B) lymphocyte count, from left to right, in a V/V (P5), a V/F (P6) and a F/F (P9) patient. P5, P6 and P9 were administered 3100, 3800 and 4200 mg of horse ALG, respectively. The models used were: (A) a two-compartment model with first-order elimination and a time-varying central volume of distribution and (B) a physiological indirect response model with inhibition of input of lymphocytes in the circulation. This model combined concentration-dependent and -independent functions of inhibition of input
Table 2.
Estimated antilymphocyte globulin pharmacokinetic parameters using a two-compartment model with a time-varying component for Vc
| n = 14 | Parameter | Typical value | Precision of estimation (%)* | Interindividual CV (%) |
|---|---|---|---|---|
| Vc,t | (L.h) | 22.4 | 5.6 | 40 |
| Vc | (L) | 2.0 | 3.5 | 40 |
| CLc | (L.h−1) | 0.017 | 5.4 | 21 |
| Vp | (L) | 8.0 | 7.4 | 28 |
| CLp | (L.h−1) | 0.022 | 5.9 | 18 |
| T1/2-α | (days) | 1.3 | 6.0 | 35 |
| T1/2-β | (days) | 25.5 | 6.0 | 29 |
Vc and CLc are volume of distribution and clearance of the central compartment, respectively, Vc,t is the time-varying component of Vc, Vp and CLp are volume of distribution and clearance of the peripheric compartment, respectively, T1/2−α and T1/2−β are asymptotic distribution and elimination half-lives, respectively.
CV percentage, Standard error of estimate/mean × 100.
The indirect response model with inhibition of lymphocyte input gave a satisfactory description of lymphocyte count over time (Figure 1) in 10 patients, showing high interindividual variability. One patient was FCGR3A-V/V, two were F/F and seven were heterozygous (Table 3, Figure 3). The F/F patients had a significantly higher IC50 than V carriers (n = 10, P = 0.04, Table 3). In four patients, the PD parameters could not be estimated because of an insufficient number of lymphocyte counts; all were heterozygous. Patients in the retrospective group were similar to those in the prospective group, except for total dose, which was lower in the retrospective group (Table 1). In this group, three patients were F/F and three were V/F. No difference in estimated PD parameters was observed between the prospective and retrospective groups. A comparison of F/F and V carriers was therefore performed after pooling these two groups. The higher value of IC50 of F/F subjects compared with that of V carriers was confirmed (n = 17, P = 0.008, Table 3, Figure 3).
Table 3.
Estimated antilymphocyte globulin pharmacodynamic parameters using an indirect response model with inhibition of lymphocyte input
| Patient | Génotype FCGR3A | Kin(mm−3·h−1) | Kout(h−1) | IC50(mg·L−1) | gamma (mg·L−1) |
|---|---|---|---|---|---|
| Prospective group (n = 10) | |||||
| P2 | V/F | 8 476 | 3.94 | 302 | 2.9 |
| P3 | F/F | 6 912 | 3.04 | 412 | 6.0 |
| P5 | V/V | 6 902 | 5.05 | 359 | 2.8 |
| P6 | V/F | 6 941 | 4.62 | 409 | 4.9 |
| P9 | F/F | 9 924 | 3.59 | 674 | 4.3 |
| P10 | V/F | 12 146 | 10.64 | 225 | 0.5 |
| P11 | V/F | 3 415 | 3.03 | 276 | 2.9 |
| P12 | V/F | 4 796 | 2.46 | 332 | 5.9 |
| P13 | V/F | 5 251 | 3.75 | 140 | 1.1 |
| P14 | V/F | 10 013 | 11.11 | 371 | 3.6 |
| All genotypes | 7 478 (2683) | 5.12 (3.13) | 350 (142) | 3.5 (1.8) | |
| F/F patients | 8 418 (2130) | 3.32 (0.39) | 543 (186) | 5.1 (1.2) | |
| V-carriers | 7 243 (2880) | 5.57 (3.37) | 302 (87) | 3.1 (1.8) | |
| p value* | 0.71 | 0.40 | 0.04 | 0.18 | |
| Retrospective group (n = 6) | |||||
| R1 | F/F | 8 237 | 8.05 | 331 | 5.0 |
| R2 | F/F | 2 622 | 6.42 | 748 | 0.3 |
| R3 | F/F | 6 346 | 10.49 | 799 | 2.8 |
| R4 | V/F | 8 041 | 9.94 | 143 | 1.0 |
| R5 | V/F | 7 869 | 7.70 | 191 | 0.4 |
| R6 | V/F | 4 318 | 6.02 | 410 | 1.8 |
| All genotypes | 6 239 (2310) | 8.10 (1.81) | 437 (278) | 1.9 (1.8) | |
| F/F patients | 5 735 (2857) | 8.32 (2.05) | 626 (257) | 2.7 (2.4) | |
| V-carriers | 6 743 (2102) | 7.89 (1.97) | 248 (143) | 1.0 (0.7) | |
| All patients (n = 16) | |||||
| All genotypes | 7 013 (2546) | 6.24 (3.03) | 383 (199) | 2.9 (1.9) | |
| F/F patients | 6 808 (2716) | 6.32 (3.10) | 593 (209) | 3.7 (2.2) | |
| V-carriers | 7 106 (2597) | 6.21 (3.15) | 287 (100) | 2.5 (1.8) | |
| p value* | 0.91 | 0.99 | 0.008 | 0.39 | |
Results are expressed as mean (SD). Kin and kout are constants of input and output, respectively. IC50 is the concentration leading to 50% of lymphocyte input inhibition.
Values of IC50 estimated were not different between prospective and retrospective groups (p = 0.38).
Comparison between F/F patients and V carriers.
Figure 3.
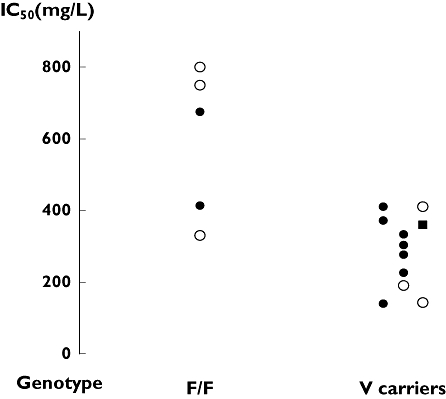
Estimated IC50 of 10 patients from the prospective group (F/F and V/F patients are represented by • and the V/V patient by ▪) and six patients from the retrospective group (○) according to their FCGR3A-158 genotype
Discussion
A limited number of studies on ALG pharmacokinetics are available in the literature and only a few of them have studied horse ALG. Most studies of rabbit ALG PK have used log-linear regression and reported median T1/2 values of around 14 days, with high interindividual variability [14–16]. Waller et al. reported mean T1/2-α and T1/2-β of 14 days and 30 days, respectively [17]. Compartmental PK analysis was applied in only one study [18], reporting mean T1/2-α and T1/2-β of 3 days and 18 days, respectively. In horse ALG, using log-linear regression, Schmidtke et al.[19] reported a median T1/2 of 12 days, whereas Filo et al.[20] reported a T1/2 ranging from 5 to 10 days. No compartmental modelling of horse ALG has been reported. In the present study, a satisfactory description of horse ALG concentrations was obtained using a population two-compartment model with first-order elimination from the central compartment and a time-varying central volume of distribution. Estimated asymptotic values of T1/2-α and T1/2-β were 1.3 and 25.5 days, with interindividual coefficients of variation of 35 and 29%, respectively. Estimated T1/2-β is therefore longer than that previously reported [19, 20], but these studies used log-linear regression. The estimated typical asymptotic value of Vc was 2.0 l, which is half of the plasma volume.
The time-varying Vc observed in the present study may be explained by changes in the binding of ALG to their targets, i.e. lymphocytes but also other cells [21], with time. In the initial part of the infusion period, target antigens are in excess and ALGs seem therefore to ‘disappear’ from the serum, leading to an apparent very high volume of distribution. Later, when target cells are lysed and/or target antigens are coated by ALGs, the apparent volume of distribution of ALG decreases towards its value in the absence of target antigen. Such a time dependence of PK parameters of therapeutic antibodies has not been reported before using a compartmental approach, even if changes in elimination T1/2 with time have been described. Studying multiple doses rabbit ALG, Guttmann et al.[16] indeed reported a T1/2 of 44 h during the first 24 h and a terminal T1/2 of 14 days. However, when infusions are frequent compared with drug T1/2, it may not be possible to differentiate the distribution phase form the terminal elimination phase without compartmental modelling.
The concentration–effect relationship was analysed using blood lymphocyte count, the biomarker used routinely to monitor the ALG effect. Using a physiological indirect response model, the best description was given by a model describing an inhibition of lymphocyte input into the circulation. This could appear paradoxical, since ALGs act by inducing lymphocyte lysis. However, cytolysis may not be intravascular: if it takes place in tissues (notably in secondary lymphoid organs), inhibition of production is plausible, since circulating lymphocytes are in transit from one tissue or organ to another. In this case, it would not be actual inhibition of input, but rather destruction taking place upstream from the circulation.
Our PK–PD model took into account two phases of lymphocyte depletion, one not concentration dependent and taking place at the beginning of the infusion period, and the other concentration dependent, taking place later. Because of the rapid and massive capture of ALGs by their targets, serum concentrations measured during the initial part of the infusion period cannot reflect the concentration in the biophase, i.e. at the site of action. Therefore, the rapid lymphocyte depletion observed at the beginning of the treatment cannot be described as a function of measured ALG concentrations. When an equilibrium between serum concentration and concentration in the biophase is obtained, a concentration–effect relationship can be recovered.
Rapid lymphocyte depletion was observed by Preville et al.[4, 22, 23] in the cynomolgus monkey after administration of rabbit ALG and it was found to take place primarily in peripheral lymphoid tissues. Apoptosis and CDC seem to occur at high ALG concentrations, whereas ADCC would occur at low ALG concentrations [4, 22, 23]. Our results with horse ALG in kidney transplant patients confirm this rapid lymphocyte depletion.
Our PK–PD model allowed estimation of IC50, a PD parameter which is an index of individual patient susceptibility to ALG. In the prospective group, the IC50 of FCGR3A-V carrier patients was significantly lower than that of F/F patients (Table 3, Figure 3), reflecting a higher potency of ALG in V carriers. To increase the number of patients and to validate the models, we chose to study also a retrospective group in whom ALG concentrations were simulated. The pooling of the two populations of patients confirmed the lower value of IC50 in V carriers compared with F/F patients (Table 3, Figure 3). In the representative examples displayed in Figure 2, one can observe that the F/F patient had a rapid recovery of his lymphocyte count despite higher dose and concentrations of ALG.
The better response of patients carrying the V allotype may be explained by the fact that IgG1 binds with higher affinity to the 158V allotype of FcγRIIIa than to the 158F allotype, as shown for rituximab [24, 25]. In our study, the significant difference in IC50 may therefore be explained by a higher affinity of horse IgG for the human FcγRIIIa-V allotype than for the FcγRIIIa-F allotype. Affinities of human IgG1, IgG3 and IgG4 for human FcγRIIIa 158V are higher than those for FcγRIIIa-158F. Such an influence is plausible in horse, but would be difficult to show in vitro, because of the existence of several (at least seven) IgG subclasses in the horse [26]. An influence of FCGR3A genotype on the clinical response of non-Hodgkin's lymphoma patients to rituximab is well known [27]. This genetic polymorphism may influence the effect of other monoclonal antibodies, such as infliximab [28] and antirhesus D monoclonal antibodies [29], which act, at least partly, by cytolysis. The quantitative influence of FCGR3A polymorphism on the concentration–effect relationship of rituximab was studied using an in vitro ADCC model [25]. Maximal lysis, which could be obtained by increasing antibody concentrations, did not differ between V/V and F/F donors, but the concentration leading to 50% of maximal lysis (EC50) was ≈ 4 times lower for V/V than for F/F donors. In the present study, we estimated an IC50 of ALG effect on lymphocyte count twice lower in V-carriers than in F/F patients. This may be explained by the fact that V-carriers were mostly heterozygotes. Overall, our results suggest that the horse ALG mechanism of action on depletion of lymphocytes may depend on ADCC, especially at distance from the treatment, and that V-carriers may be more susceptible to the effect of this polyclonal antibody.
In conclusion, we have studied the concentration–effect relationship of ALG in kidney transplant patients using lymphocyte count as a biomarker, and a physiological indirect response model. The estimated value of IC50, the parameter describing ALG potency, was found to be twice lower in FCGR3A-158V-carrier patients than in F/F patients. FCGR3A-158V carriers may therefore be more susceptible to ALG than FCGR3A-158 F/F patients. Larger studies are needed to confirm these results.
Acknowledgments
This study was supported by the Société Française de Pharmacologie et de Thérapeutique and the Société Francophone de Transplantation. We thank Genzyme Imtix-SangStat, Lyon, France for sharing the data with us and Chloé Charroing for her technical help.
References
- 1.Muller TF, Grebe SO, Neumann MC, Heymanns J, Radsak K, Sprenger H, Lange H. Persistent long-term changes in lymphocyte subsets induced by polyclonal antibodies. Transplantation. 1997;64:1432–7. doi: 10.1097/00007890-199711270-00010. [DOI] [PubMed] [Google Scholar]
- 2.Oertel M, Sack U, Kohlhaw K, Lehmann I, Emmrich F, Berr F, Hauss J, Schwarz R. Induction therapy including antithymocyte globulin induces marked alterations in T lymphocyte subpopulations after liver transplantation: results of a long-term study. Transpl Int. 2002;15:463–71. doi: 10.1007/s00147-002-0455-4. [DOI] [PubMed] [Google Scholar]
- 3.Abouna GM, al-Abdullah IH, Kelly-Sullivan D, Kumar MS, Loose J, Phillips K, Yost S, Seirka D. Randomized clinical trial of antithymocyte globulin induction in renal transplantation comparing a fixed daily dose with dose adjustment according to T cell monitoring. Transplantation. 1995;59:1564–8. [PubMed] [Google Scholar]
- 4.Genestier L, Fournel S, Flacher M, Assossou O, Revillard JP, Bonnefoy-Berard N. Induction of Fas (Apo-1, CD95)-mediated apoptosis of activated lymphocytes by polyclonal antithymocyte globulins. Blood. 1998;91:2360–8. [PubMed] [Google Scholar]
- 5.Koene HR, Kleijer M, Algra J, von Roos D, dem Borne AE, de Haas M. Fc gammaRIIIa-158V/F polymorphism influences the binding of IgG by natural killer cell Fc gammaRIIIa, independently of the Fc gammaRIIIa-48L/R./H phenotype. Blood. 1997;90:1109–14. [PubMed] [Google Scholar]
- 6.Wu J, Edberg JC, Redecha PB, Bansal V, Guyre PM, Coleman K, Salmon JE, Kimberly RP. A novel polymorphism of FcgammaRIIIa (CD16) alters receptor function and predisposes to autoimmune disease. J Clin Invest. 1997;100:1059–70. doi: 10.1172/JCI119616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cartron G, Dacheux L, Salles G, Solal-Celigny P, Bardos P, Colombat P, Watier H. Therapeutic activity of humanized anti-CD20 monoclonal antibody and polymorphism in IgG Fc receptor FcgammaRIIIa gene. Blood. 2002;99:754–8. doi: 10.1182/blood.v99.3.754. [DOI] [PubMed] [Google Scholar]
- 8.Weng WK, Levy R. Two immunoglobulin G fragment C receptor polymorphisms independently predict response to rituximab in patients with follicular lymphoma. J Clin Oncol. 2003;21:3940–7. doi: 10.1200/JCO.2003.05.013. [DOI] [PubMed] [Google Scholar]
- 9.Ghielmini M, Rufibach K, Salles G, Leoncini-Franscini L, Leger-Falandry C, Cogliatti S, Fey M, Martinelli G, Stahel R, Lohri A, Ketterer N, Wernli M, Cerny T, Schmitz SF. Single agent rituximab in patients with follicular or mantle cell lymphoma: clinical and biological factors that are predictive of response and event-free survival as well as the effect of rituximab on the immune system: a study of the Swiss Group for Clinical Cancer Research (SAKK) Ann Oncol. 2005;16:1675–82. doi: 10.1093/annonc/mdi320. [DOI] [PubMed] [Google Scholar]
- 10.Treon SP, Hansen M, Branagan AR, Verselis S, Emmanouilides C, Kimby E, Frankel SR, Touroutoglou N, Turnbull B, Anderson KC, Maloney DG, Fox EA. Polymorphisms in FcgammaRIIIA (CD16) receptor expression are associated with clinical response to rituximab in Waldenstrom's macroglobulinemia. J Clin Oncol. 2005;23:474–81. doi: 10.1200/JCO.2005.06.059. [DOI] [PubMed] [Google Scholar]
- 11.Kim DH, Jung HD, Kim JG, Lee JJ, Yang DH, Park YH, Do YR, Shin HJ, Kim MK, Hyun MS, Sohn SK. FcGRIIIa gene polymorphisms may correlate with response to frontline R-CHOP therapy for diffuse large B-cell lymphoma. Blood. 2006;11:11. doi: 10.1182/blood-2006-01-009480. [DOI] [PubMed] [Google Scholar]
- 12.Dall'Ozzo S, Andres C, Bardos P, Watier H, Thibault G. Rapid single-step FCGR3A genotyping based on SYBR Green I fluorescence in real-time multiplex allele-specific PCR. J Immunol Methods. 2003;277:185–92. doi: 10.1016/s0022-1759(03)00123-6. [DOI] [PubMed] [Google Scholar]
- 13.Jusko WJ, Ko HC. Physiologic indirect response models characterize diverse types of pharmacodynamic effects. Clin Pharmacol Ther. 1994;56:406–19. doi: 10.1038/clpt.1994.155. [DOI] [PubMed] [Google Scholar]
- 14.Bunn D, Lea CK, Bevan DJ, Higgins RM, Hendry BM. The pharmacokinetics of anti-thymocyte globulin (ATG) following intravenous infusion in man. Clin Nephrol. 1996;45:29–32. [PubMed] [Google Scholar]
- 15.Bieber CP, Griepp RB, Oyer PE, Wong J, Stinson EB. Use of rabbit antithymocyte globulin in cardiac transplantation. Relationship of serum clearance rates to clinical outcome. Transplantation. 1976;22:478–88. doi: 10.1097/00007890-197611000-00011. [DOI] [PubMed] [Google Scholar]
- 16.Guttmann RD, Caudrelier P, Alberici G, Touraine JL. Pharmacokinetics, foreign protein immune response, cytokine release, and lymphocyte subsets in patients receiving thymoglobuline and immunosuppression. Transplant Proc. 1997;29:24S–26S. [PubMed] [Google Scholar]
- 17.Waller EK, Langston AA, Lonial S, Cherry J, Somani J, Allen AJ, Rosenthal H, Redei I. Pharmacokinetics and pharmacodynamics of anti-thymocyte globulin in recipients of partially HLA-matched blood hematopoietic progenitor cell transplantation. Biol Blood Marrow Transplant. 2003;9:460–71. doi: 10.1016/s1083-8791(03)00127-7. [DOI] [PubMed] [Google Scholar]
- 18.Kakhniashvili I, Filicko J, Kraft WK, Flomenberg N. Heterogeneous clearance of antithymocyte globulin after CD34+-selected allogeneic hematopoietic progenitor cell transplantation. Biol Blood Marrow Transplant. 2005;11:609–18. doi: 10.1016/j.bbmt.2005.05.001. [DOI] [PubMed] [Google Scholar]
- 19.Schmidtke JR, Rynasiewicz J, Gifford RR, Sr, Ferguson RM, Najarian JS. In vivo elimination of equine antilymphocyte globulin by renal allograft recipients. Clin Immunol Immunopathol. 1980;15:409–14. doi: 10.1016/0090-1229(80)90052-5. [DOI] [PubMed] [Google Scholar]
- 20.Filo RS, Book B, Pescovitz MD, Milgrom ML, Leapman SB. Association of sensitization to horse antilymphocyte/thymocyte globulin with recipient age and decreased renal allograft survival rates. Transplant Proc. 1993;25:577–80. [PubMed] [Google Scholar]
- 21.Bonnefoy-Berard N, Vincent C, Revillard JP. Antibodies against functional leukocyte surface molecules in polyclonal antilymphocyte and antithymocyte globulins. Transplantation. 1991;51:669–73. doi: 10.1097/00007890-199103000-00024. [DOI] [PubMed] [Google Scholar]
- 22.Preville X, Flacher M, LeMauff B, Beauchard S, Davelu P, Tiollier J, Revillard JP. Mechanisms involved in antithymocyte globulin immunosuppressive activity in a nonhuman primate model. Transplantation. 2001;71:460–8. doi: 10.1097/00007890-200102150-00021. [DOI] [PubMed] [Google Scholar]
- 23.Michallet MC, Saltel F, Preville X, Flacher M, Revillard JP, Genestier L. Cathepsin-B-dependent apoptosis triggered by antithymocyte globulins: a novel mechanism of T-cell depletion. Blood. 2003;102:3719–26. doi: 10.1182/blood-2003-04-1075. [DOI] [PubMed] [Google Scholar]
- 24.Okazaki A, Shoji-Hosaka E, Nakamura K, Wakitani M, Uchida K, Kakita S, Tsumoto K, Kumagai I, Shitara K. Fucose depletion from human IgG1 oligosaccharide enhances binding enthalpy and association rate between IgG1 and FcgammaRIIIa. J Mol Biol. 2004;336:1239–49. doi: 10.1016/j.jmb.2004.01.007. [DOI] [PubMed] [Google Scholar]
- 25.Dall'Ozzo S, Tartas S, Paintaud G, Cartron G, Colombat P, Bardos P, Watier H, Thibault G. Rituximab-dependent cytotoxicity by natural killer cells: influence of FCGR3A polymorphism on the concentration–effect relationship. Cancer Res. 2004;64:4664–9. doi: 10.1158/0008-5472.CAN-03-2862. [DOI] [PubMed] [Google Scholar]
- 26.Wagner B. Immunoglobulins and immunoglobulin genes of the horse. Dev Comp Immunol. 2006;30:155–64. doi: 10.1016/j.dci.2005.06.008. [DOI] [PubMed] [Google Scholar]
- 27.Cartron G, Watier H, Golay J, Solal-Celigny P. From the bench to the bedside: ways to improve rituximab efficacy. Blood. 104:2635–42. doi: 10.1182/blood-2004-03-1110. [DOI] [PubMed] [Google Scholar]
- 28.Louis E, El Ghoul Z, Vermeire S, Dall'Ozzo S, Rutgeerts P, Paintaud G, Belaiche J, De Vos M, Van Gossum A, Colombel JF, Watier H. Association between polymorphism in IgG Fc receptor IIIa coding gene and biological response to infliximab in Crohn's disease. Aliment Pharmacol Ther. 2004;19:511–9. doi: 10.1111/j.1365-2036.2004.01871.x. [DOI] [PubMed] [Google Scholar]
- 29.Miescher S, Spycher MO, Amstutz H, De Haas M, Kleijer M, Kalus UJ, Radtke H, Hubsch A, Andresen I, Martin RM, Bichler J. A single recombinant anti-RhD IgG prevents RhD immunization: association of RhD-positive red blood cell clearance rate with polymorphisms in the FcgammaRIIA and FcgammaIIIA genes. Blood. 2004;103:4028–35. doi: 10.1182/blood-2003-11-3929. [DOI] [PubMed] [Google Scholar]


