Abstract
AIM
To determine the frequency of CYP2D6 poor metabolizers (PMs) in a Faroese patient group medicated with amitriptyline (AT) and to investigate plasma concentrations of AT and metabolites in relation to CYP2D6.
METHODS
CYP2D6 phenotype and genotype were determined in 23 Faroese patients treated with AT. Plasma concentrations of AT and metabolites were determined by high-performance liquid chromatography and investigated in relation to CYP2D6 activity.
RESULTS
Of the 23 patients phenotyped and genotyped, five (22%) (95% confidence interval 7.5, 43.7) were CYP2D6 PMs. No difference was found in AT daily dosage between PMs (median 25 mg day−1; range 5–80) and extensive metabolizers (EMs) (median 27.5 mg day−1; range 10–100). The (E)-10-OH-nortriptyline (NT)/dose concentrations were higher in EMs than in PMs and the NT/(E)-10-OH-NT and AT/(E)-10-OH-AT ratios were higher in PMs compared with EMs. The log sparteine metabolic ratio correlated positively with the NT/(E)-10-OH-NT ratio (rs = 0.821; P < 0.0005) and the AT/(E)-10-OH-AT ratio (rs = 0.605; P < 0.006).
CONCLUSION
A high proportion of CYP2D6 PMs was found in a Faroese patient group medicated with AT. However, similar doses of AT and concentrations of AT and NT were noted in EMs and PMs, probably due to varying doses and indications for AT treatment.
WHAT IS ALREADY KNOWN ABOUT THIS SUBJECT
The metabolisms of amitriptyline (AT) to (E)-10-hydroxyamitriptyline and of nortripyline (NT) to (E)-10-hydroxynortriptyline are catalysed by CYP2D6.
A correlation between the sparteine metabolic ratio and the NT/(E)-10-hydroxynortriptyline and the AT/(E)-10-hydroxyamitriptyline ratios, respectively, has been observed in patients in treatment with the same dose of AT or NT.
The frequency of CYP2D6 poor metabolizers (PMs) is 15% (twofold compared with other Whites) among healthy Faroese.
This frequency has not been investigated in Faroese patients in AT treatment and the consequences of the CYP2D6 PM phenotype for dose and plasma concentrations of AT and metabolites are not known in these patients.
WHAT THIS STUDY ADDS
In patients treated with different daily dosages (5–100 mg) of AT a correlation between the sparteine metabolic ratio and the NT/(E)-10-hydroxynortriptyline and AT/(E)-10-hydroxyamitriptyline ratios was observed.
A high proportion (22%) of CYP2D6 PMs in a Faroese patient group medicated with AT was observed.
However, similar doses of AT and concentrations of AT and NT were noted in extensive metabolizers and in PMs.
Keywords: amitriptyline, CYP2D6 polymorphism, Faroe Islands, nortriptyline, the Faroese population
Introduction
For more than 40 years tricyclic antidepressants (TCA) have been used in the treatment of depression [1]. Despite the introduction of newer antidepressants, usually better tolerated and less toxic, TCA are still commonly used [1, 2]. The CYP2D6 polymorphism is an important determinant of TCA metabolism [2], and 7–10% of Europeans are CYP2D6 poor metabolizers (PMs) [3]. We have recently shown an increased occurrence of CYP2D6 PMs (15%) in the Faroese population [4]. Genetic studies of this island population show differences from other White populations [5, 6].
Amitriptyline (AT) is the most widely used TCA [7]. Both AT and its active metabolite nortripyline (NT) undergo benzylic hydroxylation, largely by CYP2D6 [8–10]. The hydroxylation results in two geometric metabolites, the major being (E)-10-OH and the minor (Z)-10-OH, each forming two enantiomers [7, 11–13]. The hydroxylated NT metabolites are active and the pharmacological profiles differ between the geometric isomers [10, 14–16]. Furthermore, the hydroxylation of NT by CYP2D6 is highly stereoselective, mainly forming (–)-(E)-10-OH-NT [7, 10, 11, 17, 18]. Several studies [8, 13, 19, 20] have shown a significant correlation between total clearance of NT via E-10 hydroxylation and the activity of CYP2D6.
The aims of this study were to determine the frequency of CYP2D6 PMs in a Faroese patient group medicated with a CYP2D6 substrate (AT) and to investigate plasma concentrations of AT and metabolites in relation to CYP2D6.
Methods
General practitioners in the Faroe Islands were asked to forward an invitation to their patients receiving TCA. Twenty-three unrelated AT treated patients (14 female and nine male) aged 31–84 years (median 60 years) were included in the study. Their daily dose varied from 5 to 100 mg day−1 (median 25 mg day−1) AT (Amitriptyline ‘DAK’; Nycomed, Denmark or Saroten®, Lundbeck, Denmark). All 23 patients were CYP2D6 phenotyped and genotyped. In order to phenotype for CYP2D6 the participants were given an oral dose of 100 mg sparteine sulphate pentahydrate (The Central Pharmacy, Odense University Hospital, Odense, Denmark) followed by urine collection for 12 h. The total volume was recorded and 10-ml aliquots were stored at −80°C until analysis. Sparteine, 2,3- and 5,6- didehydrosparteine in urine were assayed by gas chromatography as described previously [21]. For the analysis of sparteine and metabolites the coefficient of variation (CV%) was <15%. The metabolic ratio (MR) was expressed as sparteine to 2,3- and 5,6- didehydrosparteine concentrations. Patients having an MR ≥20 were phenotyped as CYP2D6 PM [22]. Further a 10-ml blood sample was drawn from each patient. DNA was isolated and CYP2D6*3, *4, *6 and *9 genotype analyses were performed as previously described [4].
Under steady-state conditions for AT, plasma levels of AT and relevant metabolites were determined by a modified version of a high-performance liquid chromatography method described previously [23]. The CV% for the AT analysis was <10%. For practical reasons the timing of sampling relative to dosing varied. Plasma concentrations of AT and/or metabolites were determined in 21 of the 23 patients (Table 1). The concentrations were too low to measure in two patients due to low dosages (5 and 10 mg day−1), and these patients were excluded from data analyses concerning AT plasma concentrations.
Table 1.
Median, range and statistical inferences of the differences in plasma concentrations of amitriptyline and metabolites in the CYP2D6 extensive (n = 17) and poor metabolizers (n = 4) among 21 Faroese patients in treatment with amitriptyline
| EM | PM | Geometric mean ratio (PM/EM) (95% CI) | P-value | |
|---|---|---|---|---|
| CAT+NT/dose* | 4.4 (1.1–18.2) | 6.5 (5.7–10.6) | 1.69 (0.75–3.82) | 0.191 |
| NT/dose* | 1.49 (0.41–5.26) | 3.42 (2.22–4.87) | 2.21 (0.94–5.20) | 0.066 |
| (E)-10-OH-NT/dose* | 3.59 (1.61–8.71) | 1.00 (0.95–1.94) | 0.32 (0.19–0.53) | 0.0005 |
| NT/(E)-10-OH-NT | 0.34 (0.22–1.05) | 3.07 (1.49–5.10) | 7.04 (3.92–12.63) | 0.0005 |
| AT/dose*† | 2.4 (1.20–14.32) | 3.89 (1.73–5.74) | 1.28 (0.54–3.01) | 0.556 |
| (E)-10-OH-AT/dose*‡ | 0.76 (0.25–1.65) | 0.41 (0.16–0.71) | 0.56 (0.25–1.22) | 0.136 |
| AT/(E)-10-OH-AT§ | 4.0 (1.7–13.7) | 10.7 (5.8–14.1) | 2.16 (1.06–4.42) | 0.036 |
nm mg−1 day−1.
EM (n = 16), PM (n = 4).
EM (n = 17), PM (n = 3).
EM (n = 16), PM (n = 3). Different n's are used as AT or metabolites were not quantifiable in all samples. EM, Extensive metabolizer; PM, poor metabolizer; NT, nortripyline; AT, amitriptyline.
No patients were taking any substrates known to be CYP2D6 inhibitors. Written, informed consent was obtained from all patients on the basis of verbal and written information in Faroese. The study was approved by the Faroese Ethics Committee.
Statistical analyses were performed using SPSS 14.0 (SPSS Inc., Chicago, IL, USA). All parameters were transformed by natural logarithm to approach a Gaussian distribution before statistical analysis. An unpaired t-test was used to analyse differences between PMs and extensive metabolizers (EMs). Geometric ratios of means with 95% confidence intervals (CI), calculated by GraphPad QuickCalcs (GraphPad Software, San Diego, CA, USA), and P-values are presented. The correlations presented were tested using the Spearman rank correlations test.
Results
Of the 23 patients, five (22%) (95% CI 7.5, 43.7) were phenotyped as CYP2D6 PMs. These were also characterized as PMs by CYP2D6 genotype analyses, as all had the CYP2D6*4/*4 genotype. The results from pheno- and genotype analyses were in concordance. Hence, AT administration did not interfere with phenotype assignment with sparteine.
The frequency of CYP2D6 PMs found in this study (22%) was not statistically significantly higher (P < 1.000; χ2 test) than that previously observed among healthy Faroese (15%) [4], but it substantially exceeded the prevalence observed in other populations.
In one of five PMs the AT + NT plasma concentration (454 nm) exceeded the recommended plasma concentrations (AT + NT) during AT treatment (130–325 nm) [24]. No difference was found in AT daily dosage between PMs (median 25 mg day−1; range 5–80) and EMs (median 27.5 mg day−1; range 10–100).
The differences in AT and metabolite concentrations and ratios between PMs and EMs are presented in Table 1. The median and range of the NT/(E)-10-OH-NT ratio in patients carrying 0 (n = 4), one (n = 7) or two (n = 10) functional CYP2D6 alleles were 3.07 (1.49–5.10), 0.53 (0.27–1.05) and 0.28 (0.22–0.82), respectively. The values for those not carrying any functional allele were statistically significantly higher compared with the ratios for patients with one or two functional alleles. Regarding the AT/(E)-10-OH-AT ratio, the median and range values for patients carrying 0 (n = 3), one (n = 6) and two (n = 10) functional CYP2D6 alleles were 10.69 (5.75–14.10), 4.61 (2.70–9.51) and 3.98 (1.67–13.69), respectively. The ratios differed significantly between patients carrying no functional alleles and patients with two functional alleles.
The log sparteine MR correlated positively with the NT/(E)-10-OH-NT ratio (rs = 0.821; P < 0.0005) and positively with the AT/(E)-10-OH-AT ratio (rs = 0.605; P < 0.006) (Figure 1).
Figure 1.
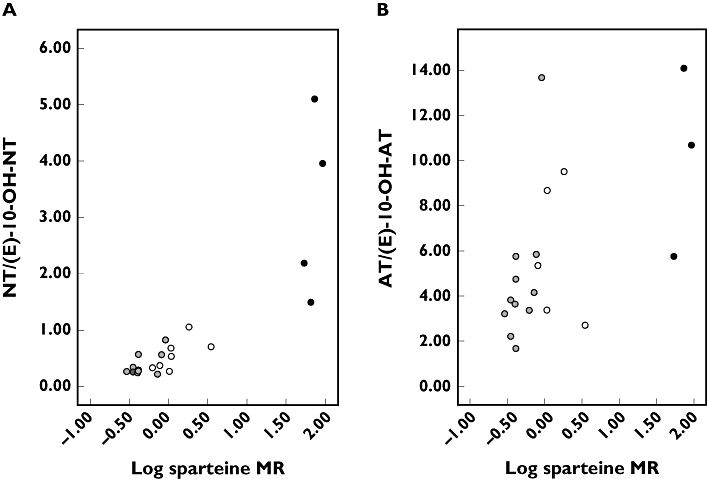
Scatter plots. (A) Correlation between the log sparteine metabolic ratio (MR) and the steady-state nortriptyline/(E)-10-hydroxynortriptyline ratio in 21 patients treated with 10–100 mg amitriptyline (AT) daily. (B) Correlation between the log sparteine MR and the steady-state amitriptyline/(E)-10-hydroxyamitriptyline ratio in 21 patients treated with 10–100 mg AT) daily. (2 functional CYP2D6 alleles, ( ); 1 functional CYP2D6 allele, (○); 0 functional CYP2D6 allele, (•))
); 1 functional CYP2D6 allele, (○); 0 functional CYP2D6 allele, (•))
Discussion
Despite the relatively small number of participants in this study (n = 23), an even higher frequency of CYP2D6 PMs was observed (22%) compared with the previous investigation (15%) in healthy Farose (n = 309) [4]. In the light of a previous study of the CYP2C19 polymorphism in the isolated islands of Vanuatu [25], where the frequency of PMs was greatly increased, the isolated geographical position of the Faroe Islands might represent an interesting factor in the explanation for the high CYP2D6 PM frequency observed.
The high frequency of CYP2D6 PMs presumably results in a large part of the population being at risk of adverse effects when taking CYP2D6 substrates, especially TCA, because of their narrow therapeutic indices [26]. All patients were receiving low dosages (5–100 mg day−1) of AT compared with the recommended daily dosages (150–200 mg day−1 oral). This observation is in concordance with the previous finding that outpatients generally are receiving lower dosages compared with hospitalized patients [27]. In the present study only one PM had an AT + NT plasma concentration exceeding the recommended range. Hence, this study indicates that the remaining PMs are not at any particular risk of side-effects. However, one could speculate that if the patients were receiving recommended daily dosages, the AT + NT plasma concentrations would probably exceed the recommended range in the other PMs also. The very wide range of doses and, to some degree, the low doses used in the patients is probably related to different indications for taking AT, e.g. depression, slight to moderate pain, etc. In a more homogeneous group, e.g. hospitalized AT patients all suffering from major depression, toxic plasma concentrations and side-effects would presumably be expected in PMs.
The correlation between sparteine MR and the NT/(E)-10-OH-NT ratio has been observed in several other studies among different populations and has been confirmed in Faroese patients in treatment with AT. Further, this study has confirmed the correlation between NT hydroxylation to (E)-10-OH-NT and CYP2D6 activity.
In conclusion, we have found a high proportion of CYP2D6 PMs, but similar doses of AT and concentrations of AT and NT in EMs and PMs (Table 1), probably due to varying doses and indications for AT treatment.
Acknowledgments
This study was supported by ‘Apotekerfonden af 1991’. Professor Philippe Grandjean's comments on a previous version of this study are greatly appreciated. We thank the general practitioners in the Faroe Islands and the Faroese Chief Pharmaceutical Officer for assistance.
References
- 1.Barbui C, Hotopf M. Amitriptyline v. the rest: still the leading antidepressant after 40 years of randomised controlled trials. Br J Psychiatry. 2001;178:129–44. doi: 10.1192/bjp.178.2.129. [DOI] [PubMed] [Google Scholar]
- 2.Brosen K. Some aspects of genetic polymorphism in the biotransformation of antidepressants. Therapie. 2004;59:5–12. doi: 10.2515/therapie:2004003. [DOI] [PubMed] [Google Scholar]
- 3.Alván G, Becthel P, Islius L, Gundert-Remy U. Hydrolylation polymorphisms of debrisoquine and mephenytoin in European population. Eur J Clin Pharmacol. 1990;39:533–7. doi: 10.1007/BF00316090. [DOI] [PubMed] [Google Scholar]
- 4.Halling J, Petersen MS, Damkier P, Nielsen F, Grandjean P, Weihe P, Lundgren S, Lundblad MS, Brosen K. Polymorphism of CYP2D6, CYP2C19, CYP2C9 and CYP2C8 in the Faroese population. Eur J Clin Pharmacol. 2005;61:491–7. doi: 10.1007/s00228-005-0938-1. [DOI] [PubMed] [Google Scholar]
- 5.Santer R, Kinner M, Steuerwald U, Kjaergaard S, Skovby F, Simonsen H, Shaiu W-L, Chen T-T, Schneppenheim R, Schaub J. Molecular genetic basis and prevalence of glycogen storage disease type IIIA in the Faroe Islands. Eur J Hum Gen. 2001;9:388–91. doi: 10.1038/sj.ejhg.5200632. [DOI] [PubMed] [Google Scholar]
- 6.Schwartz M, Sorensen N, Brandt NJ, Hogdall E, Holm T. High incidence of cystic fibrosis on the Faroe Islands: a molecular and genealogical study. Hum Genet. 1995;95:703–6. doi: 10.1007/BF00209491. [DOI] [PubMed] [Google Scholar]
- 7.Breyer-Pfaff U. The metabolic fate of amitriptyline, nortriptyline and amitriptylinoxide in man. Drug Metab Rev. 2004;36:723–46. doi: 10.1081/dmr-200033482. [DOI] [PubMed] [Google Scholar]
- 8.Mellstrom B, Bertilsson L, Sawe J, Schulz HU, Sjoqvist F. E- and Z-10-hydroxylation of nortriptyline: relationship to polymorphic debrisoquine hydroxylation. Clin Pharmacol Ther. 1981;30:189–93. doi: 10.1038/clpt.1981.147. [DOI] [PubMed] [Google Scholar]
- 9.Balant-Gorgia AE, Schulz P, Dayer P, Balant L, Kubli A, Gertsch C, Garrone G. Role of oxidation polymorphism on blood and urine concentrations of amitriptyline and its metabolites in man. Arch Psychiatr Nervenkr. 1982;232:215–22. doi: 10.1007/BF02141782. [DOI] [PubMed] [Google Scholar]
- 10.Nordin C, Bertilsson L. Active hydroxymetabolites of antidepressants. Emphasis on E-10-hydroxy-nortriptyline. Clin Pharmacokinet. 1995;28:26–40. doi: 10.2165/00003088-199528010-00004. [DOI] [PubMed] [Google Scholar]
- 11.Breyer-Pfaff U, Pfandl B, Nill K, Nusser E, Monney C, Jonzier-Perey M, Baettig D, Baumann P. Enantioselective amitriptyline metabolism in patients phenotyped for two cytochrome P450 isozymes. Clin Pharmacol Ther. 1992;52:350–8. doi: 10.1038/clpt.1992.155. [DOI] [PubMed] [Google Scholar]
- 12.Bertilsson L, Alexanderson B. Stereospecific hydroxylation of nortriptyline in man in relation to interindividual differences in its steady-state plasma level. Eur J Clin Pharmacol. 1972;4:201–5. [Google Scholar]
- 13.Gram LF, Brosen K, Kragh-Sorensen P, Christensen P. Steady-state plasma levels of E- and Z-10-OH-nortriptyline in nortriptyline-treated patients: significance of concurrent medication and the sparteine oxidation phenotype. Ther Drug Monit. 1989;11:508–14. doi: 10.1097/00007691-198909000-00003. [DOI] [PubMed] [Google Scholar]
- 14.Nilvebrant L, Nordin C. Affinity of nortriptyline and its E-10-hydroxy metabolite for muscarinic receptors. Pharmacol Toxicol. 1991;68:64–7. doi: 10.1111/j.1600-0773.1991.tb01210.x. [DOI] [PubMed] [Google Scholar]
- 15.Nordin C, Krijzer F. Antidepressant and anxiolytic profiles of E-10-hydroxynortriptyline on electrocorticograms of rats. Neuropsychobiology. 1996;34:44–8. doi: 10.1159/000119290. [DOI] [PubMed] [Google Scholar]
- 16.Bertilsson L, Mellstrom B, Sjoqvist F. Pronounced inhibition of noradrenaline uptake by 10-hydroxymetabolites of nortriptyline. Life Sci. 1979;25:1285–92. doi: 10.1016/0024-3205(79)90393-x. [DOI] [PubMed] [Google Scholar]
- 17.Dahl ML, Nordin C, Bertilsson L. Enantioselective hydroxylation of nortriptyline in human liver microsomes, intestinal homogenate, and patients treated with nortriptyline. Ther Drug Monit. 1991;13:189–94. doi: 10.1097/00007691-199105000-00001. [DOI] [PubMed] [Google Scholar]
- 18.Dahl-Puustinen ML, Perry TL Jr, Dumont E, von Bahr C, Nordin C, Bertilsson L. Stereoselective disposition of racemic E-10-hydroxynortriptyline in human beings. Clin Pharmacol Ther. 1989;45:650–6. doi: 10.1038/clpt.1989.86. [DOI] [PubMed] [Google Scholar]
- 19.Nordin C, Siwers B, Benitez J, Bertilsson L. Plasma concentrations of nortriptyline and its 10-hydroxy metabolite in depressed patients relationship to the debrisoquine hydroxylation metabolic ratio. Br J Clin Pharmacol. 1985;19:832–5. doi: 10.1111/j.1365-2125.1985.tb02723.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dahl ML, Bertilsson L, Nordin C. Steady-state plasma levels of nortriptyline and its 10-hydroxy metabolite: relationship to the CYP2D6 genotype. Psychopharmacol (Berl) 1996;123:315–9. doi: 10.1007/BF02246640. [DOI] [PubMed] [Google Scholar]
- 21.Vinks A, Inaba T, Otton SV, Kalow W. Sparteine metabolism in Canadian Causcasians. Clin Pharmacol Ther. 1982;31:23–9. doi: 10.1038/clpt.1982.4. [DOI] [PubMed] [Google Scholar]
- 22.Brosen K, Otton SV, Gram LF. Sparteine oxidation polymorphism in Denmark. Acta Pharmacol Toxicol. 1985;57:357–60. doi: 10.1111/j.1600-0773.1985.tb00058.x. [DOI] [PubMed] [Google Scholar]
- 23.Nielsen KK, Brosen K. High-performance liquid chromatography of clomipramine and metabolites in human plasma and urine. Ther Drug Monit. 1993;15:122–8. doi: 10.1097/00007691-199304000-00010. [DOI] [PubMed] [Google Scholar]
- 24.Baumann P, Hiemke C, Ulrich S, Eckermann G, Gaertner I, Gerlach M, Kuss HJ, Laux G, Muller-Oerlinghausen B, Rao ML, Riederer P, Zernig G. Arbeitsge-meinschaft fur Neuropsychopharmakologie und Pharmakopsychiatrie—Therapeutic Drug Monitoring Group. Pharmacopsychiatry. 2004;37:243–65. doi: 10.1055/s-2004-832687. [DOI] [PubMed] [Google Scholar]
- 25.Kaneko A, Lum JK, Yaviong L, Takahashi N, Ishizaki T, Bertilsson L, Kobayakawa T, Bjorkman A. High variable frequencies of CYP2C19 mutations: medical consequences of poor drug metabolism in Vanuatu and other Pacific islands. Pharmacogenetics. 1999;9:581–90. [PubMed] [Google Scholar]
- 26.Steimer W, Müller B, Leucht S, Kissling W. Pharmacogenetics: a new diagnostic tool in the management of antidepressive drug therapy. Clin Chim Acta. 2001;308:33–41. doi: 10.1016/s0009-8981(01)00423-5. [DOI] [PubMed] [Google Scholar]
- 27.Rosholm JU, Hallas J, Gram LF. Outpatient utilization of antidepressants: a prescription database analysis. J Affect Disord. 1993;27:21–8. doi: 10.1016/0165-0327(93)90092-x. [DOI] [PubMed] [Google Scholar]


