Abstract
AIMS
Oral L-arginine supplementation has been used in several studies to improve endothelium-dependent, nitric oxide (NO)-mediated vasodilation. L-Arginine treatment is hampered by extensive presystemic elimination due to intestinal arginase activity. In contrast, L-citrulline is readily absorbed and at least in part converted to L-arginine. The aim of our study was to assess this metabolic conversion and its subsequent pharmacodynamic effects.
METHODS
In a double-blind, randomized, placebo-controlled cross-over study, 20 healthy volunteers received six different dosing regimes of placebo, citrulline, and arginine. Pharmacokinetic parameters (Cmax, Tmax, Cmin, AUC) were calculated after 1 week of oral supplementation. The ratio of plasma L-arginine over asymmetric dimethylarginine, an endogenous inhibitor of nitric oxide synthase (arginine/ADMA ratio), urinary cyclic guanosine monophosphate (cGMP) and nitrate excretion rates, and flow-mediated vasodilation (FMD) was measured to assess pharmacodynamic effects.
RESULTS
L-Citrulline dose-dependently increased AUC and Cmax of plasma L-arginine concentration more effectively than L-arginine (P < 0.01). The highest dose of citrulline (3 g bid) increased the Cmin of plasma L-arginine and improved the L-arginine/ADMA ratio from 186 ± 8 (baseline) to 278 ± 14 [P < 0.01, 95% confidence interval (CI) 66, 121]. Moreover, urinary nitrate and cGMP were increased from 92 ± 10 to 125 ± 15 µmol mmol−1 creatinine (P = 0.01, 95% CI 8, 58) and from 38 ± 3.3 to 50 ± 6.7 nmol mmol−1 creatinine (P = 0.04, 95% CI 0.4, 24), respectively. No treatment improved FMD over baseline. However, pooled analysis of all FMD data revealed a correlation between the increase of arginine/ADMA ratio and improvement of FMD.
CONCLUSION
Our data show for the first time that oral L-citrulline supplementation raises plasma L-arginine concentration and augments NO-dependent signalling in a dose-dependent manner.
WHAT IS ALREADY KNOWN ABOUT THIS SUBJECT
L-Arginine is a semiessential amino acid that is converted to nitric oxide (NO) by NO synthase (NOS).
NO improves endothelial function by elevating cyclic guanosine monophosphate.
However, oral L-arginine treatment in humans is hampered by extensive metabolism.
WHAT THIS STUDY ADDS
Oral L-citrulline supplementation raises plasma L-arginine concentration and augments NO-dependent signalling in a dose-dependent manner.
L-Citrulline may thus be an alternative to L-arginine in patients with impaired NOS activity.
Keywords: asymmetric dimethylarginine, flow-mediated vasodilation, L-arginine, L-citrulline, nitric oxide
Introduction
The three isoforms of nitric oxide synthase (NOS), neuronal NOS (nNOS, NOS 1), inducible NOS (iNOS, NOS 2) and endothelial (eNOS, NOS 3), convert l-arginine to nitric oxide (NO) and l-citrulline [1]. NO is a vasoactive compound that induces vasodilation of arterial and venous blood vessels. In endothelial cells, l-arginine is transported via the cell membrane by cationic amino acid transporters that are colocalized with eNOS [2]. The Michaelis–Menten constant (Km) for eNOS is ∼3 µM l-arginine [3]. This is at least one order of magnitude lower than the normal plasma concentrations of l-arginine, which are usually in the range 60–140 µM [4]. Nevertheless, oral supplementation with l-arginine has been shown to enhance NO-mediated vasodilation in several clinical studies [5, 6], but not in all [7, 8]. One possible explanation for this ‘arginine paradox’ is the presence of an endogenous inhibitor of NOS, which may shift the steep part of the substrate–activity curve of NOS towards higher l-arginine levels [9]. Asymmetric dimethylarginine (ADMA) is an endogenous inhibitor of all three isoforms of NOS and it is circulating at low µM concentrations in humans [9]. The ratio of l-arginine over ADMA (arginine/ADMA ratio) is one determinant of NO production by NOS [10]. Once produced, NO activates soluble guanylyl cyclase (sGC) in smooth muscle cells, which leads to elevated intracellular cyclic guanosine monophosphate (cGMP). In human blood vessels this mechanism results in vasodilation [11]. This process is essential for endothelial function, and disturbed NO production in the human endothelium contributes to endothelial dysfunction [1, 9, 12].
The semiessential amino acid l-arginine is part of the human diet and only 5–15% of plasma arginine originate from de novo synthesis [4, 13]. After oral administration, l-arginine is subject to extensive presystemic and systemic elimination, i.e. by bacteria in the gut and arginases in the gut and liver, respectively [14]. The non-essential amino acid l-citrulline is not subject to presystemic elimination but to systemic metabolism. l-Citrulline is converted to l-argininosuccinate by argininosuccinate synthase and subsequently to l-arginine by argininosuccinate lyase [15]. It may therefore serve as an l-arginine precursor [16].
The aim of this study was to investigate the pharmacokinetic (PK) and pharmacodynamic (PD) effects of different oral doses of l-arginine and l-citrulline in humans subjects with impaired NO elaboration secondary to elevated ADMA concentrations.
Methods
Subjects
Twenty healthy, non-obese volunteers (13 male, 7 female) were included in this study. They were recruited from a group of 168 clinically healthy humans screened for fasting plasma ADMA concentration. Subjects were eligible if they had ADMA concentrations within the highest quartile of the distribution of the screened population. All participants had normal clinical history and physical examination, 12-lead electrocardiogram, haematological and biochemical screen. Diabetes, obesity, hypertension, cardiovascular disease, liver or kidney disease, current infections or smoking were exclusion criteria. None of the volunteers received any drugs that might alter amino acid or vitamin status, and dietary habits were kept constant during the study. A history of hormone replacement therapy (HRT) was known in two female participants. HRT was stopped 21 days prior to receiving the first dose of study drug. Written informed consent was obtained from all participants. The study protocol was approved by the Ethics Committee of the Hamburg Board of Physicians, and the investigation was conducted in accordance with the Declaration of Helsinki.
Study design
In a randomized, double-blind, placebo-controlled cross-over design participants received either l-citrulline 0.75 g twice daily, l-citrulline 1.5 g twice daily, l-citrulline 3 g twice daily, l-arginine immediate-release (IR) 1.0 g tid, l-arginine sustained-release (SR) 1.6 g twice daily, or placebo for 7 days each. The study periods were separated by wash-out phases of 1 week, and the sequence of the medications was randomly chosen in each participant. On day 7 of each medication phase, venous blood samples were drawn from an antecubital vein for PK analyses at 0, 0.5, 1, 2, 4, 6, 8, 12, 16 and 24 h. On day 7 only a single dose, equivalent to half of the total daily dose, was administered. The twice daily or three times daily dosing was administered on days 1 through 6. At baseline and on day 7 (at 4 h after dosing) additional blood and urine samples were collected for ADMA plasma concentrations and urinary nitrate and cGMP excretion rates, respectively (Figure 1). Finally, at baseline and at 4 h after dosing on day 7, endothelial function was assessed by flow-mediated vasodilation (FMD) testing of the brachial artery as detailed below.
Figure 1.
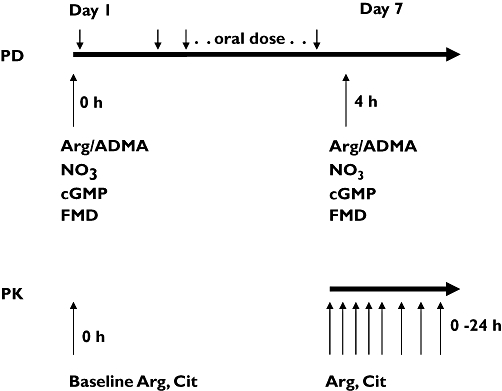
Study design
Biochemical analyses
Plasma l-arginine and l-citrulline concentrations were determined by liquid chromatography (LC)-tandem mass spectrometry (MS) analysis as described previously [17]. Briefly, a 50-µl aliquot of plasma was spiked with stable isotope-labelled l-citrulline and l-arginine, which served as internal standards. Protein was precipitated with 100 µl of methanol, filtrated through a 0.22-µm hydrophilic membrane (Multiscreen HTSTM; Millipore, Molsheim, France), derivatized with butanolic HCl (1 N, 65°C, 17 min) and analysed by LC-tandem MS. Quantification was performed by selected reaction monitoring of the respective daughter ions of analytes and internal standards (Waters, Eschborn, Germany). Plasma ADMA was analysed by enzyme-linked immunosorbent assay (ELISA), as previously described [18]. Urinary nitrate levels were determined by GC-MS as described elsewere [10]. Urinary cGMP was analysed by ELISA [11]. Urinary excretion rates of nitrate and cGMP were corrected for creatinine excretion.
Pharmacokinetic analyses
PK parameters (Cmax, Tmax, Cmin, AUC) were calculated for each dose of l-arginine and l-citrulline after 1 week of oral supplementation. After l-citrulline supplementation, PK parameters were calculated for l-arginine and l-citrulline plasma concentrations, whereas PK parameters were calculated only for l-arginine concentrations after l-arginine supplementation. Areas under the plasma concentration–time curve (AUC) were calculated for up to 24 h. To account for the circadian rhythms of endogenous l-arginine and l-citrulline concentrations, plasma concentrations following l-arginine and l-citrulline administration at each time point were corrected for individual baseline and placebo data prior to calculation of Cmax, Tmax, Cmin and AUC values. Even for corrected data, calculation of half-life was still not possible. All PK calculations were performed using WinNonlin (v. 5.0; Pharsight Corp., Mountain View, CA, USA).
Vascular function testing
Methods of assessing endothelium-dependent vasodilation followed the principles set by the International Brachial Artery Reactivity Task Force [19]. Endothelial function was assessed in the volunteers' right arm in a quiet, temperature-controlled room (22°C) by high-resolution ultrasound (12 MHz linear array transducer; Siena, Siemens, Germany). Longitudinal scans of the brachial artery were obtained approximately 5 cm proximal of the antecubital fossa. The transmit focus zone was set at the depth of the anterior wall. Anatomical landmarks and snapshot images were used to assess FMD in the same vessel section on each study day and at each time point. A view of a 5-cm longitudinal section of the brachial artery was recorded for time periods of 30 s at baseline and during peak reactive hyperaemia (60 s after deflation of a blood pressure cuff previously inflated to 50 mmHg above the volunteer's systolic blood pressure for 5 min). Each 30-s recording was digitalized (Vascular Imager 4.1.3; Medical Imaging Applications LLC, IA, USA) at a rate of 10 high-resolution frames per second (= 300 frames per recording), by using specialized software (Brachial Analyser 4.1.3; Medical Imaging Applications LLC). FMD was calculated as the percent change in diameter 1 min after cuff release relative to the baseline diameter before cuff release. Ultrasound studies and image analysis were performed separately by independent investigators in an observer-blinded fashion. The mean intraindividual coefficient of variation of the arterial diameter at the baseline measurements obtained on the six separate study days was 4.65%.
Statistical analyses
All data are given as mean ± SEM, together with 95% confidence intervals for the mean differences (CI). Statistical comparisons were made by Student's t-test (two-tailed) for paired data. Statistical analysis was performed with SPSS (release 10 for Windows; Chicago, IL, USA).
Results
Baseline characteristics of subjects investigated are given in Table 1. All participants were apparently healthy White nonsmokers. Oral l-citrulline supplementation increased the plasma concentrations of l-citrulline (Table 2) and l-arginine in a dose-dependent manner (Figure 2). Oral l-arginine did not alter the plasma concentrations of l-citrulline (data not shown), but increased plasma l-arginine concentrations (Table 2). The change in l-arginine AUC was about as pronounced after oral l-citrulline administration at a dose of 0.75 g twice daily as after a twofold higher dose of oral l-arginine SR (1.6 g bid) and a twofold higher total daily dose of l-arginine IR (1.0 g tid). The higher doses of oral l-citrulline induced dose-dependent elevations of l-arginine Cmax and AUC (Table 2). The peak plasma arginine concentration was significantly increased for l-citrulline administration at a dose of 1.5 g twice daily compared with l-arginine SR (P < 0.01, 95% CI for difference between mean values 17, 43 µmol l−1) and at a dose of 3 g twice daily compared with l-arginine SR (P < 0.01, 95% CI 84, 116 µmol l−1) and with l-arginine IR (P < 0.01, 95% CI 43, 84 µmol l−1). The l-arginine AUC was significantly increased after l-citrulline administration at a dose of 1.5 g twice daily compared with l-arginine SR (P < 0.01, 95% CI 49, 214 µmol h l−1) and at a dose of 3 g twice daily compared with l-arginine SR (P < 0.01, 95% CI 497, 721 µmol h l−1) and with l-arginine IR (P < 0.01, 95% CI 475, 723 µmol h l−1). [Correction added after online publication 13 September 2007: Units of measurement corrected]
Table 1.
Groups' baseline characteristics
| Subjects investigated (n = 20) | ||
|---|---|---|
| Mean | 95% CI | |
| Age (years) | 57 | 52, 61 |
| Gender (n) | 7 females (35%) | |
| Smoker (n) | 0 (0%) | |
| Body mass index (kg m−2) | 25.6 | 24, 27 |
| Blood pressure (mmHg) | ||
| Systolic | 130 | 125, 135 |
| Diastolic | 83 | 78, 87 |
| Blood lipids (mg dl−1) | ||
| Total cholesterol | 226 | 211, 242 |
| LDL-cholesterol | 137 | 122, 154 |
| HDL-cholesterol | 65 | 58, 73 |
| Triglycerides | 116 | 84, 146 |
| Asymmetric dimethylarginine (µmol l−1) | 0.60 | 0.56, 0.63 |
| Blood glucose (mg dl−1) | 85 | 79, 92 |
| Blood urea nitrogen (mg dl−1) | 17 | 15, 19 |
| Serum creatinine (mg dl−1) | 0.92 | 0.84, 0.99 |
LDL, Low-density lipoprotein; HDL, high-density lipoprotein.
Table 2a.
Kinetic parameters of arginine in human plasma after 1 week of oral supplementation with either citrulline or arginine‡
| Compound | Dose (mg) | Cmax (µmol l−1) | Tmax (h) | Cmin (µmol l−1) | AUC (µmol h l−1) |
|---|---|---|---|---|---|
| Citrulline | 750 bid | 54 ± 5 | 2.3 ± 0.7 | 19 ± 4 | 271 ± 38 |
| Citrulline | 1500 bid | 79 ± 8* | 1.6 ± 0.3 | 21 ± 4 | 421 ± 65* |
| Citrulline | 3000 bid | 149 ± 42*† | 1.4 ± 0.1 | 45 ± 5*† | 898 ± 67*† |
| Arginine SR | 1600 bid | 49 ± 6 | 3.7 ± 1.3§ | 19 ± 4 | 289 ± 50 |
| Arginine lR | 1000 tid | 84 ± 9 | 0.7 ± 0.1 | 10 ± 3 | 283 ± 51 |
P < 0.01 vs. arginine sustained-release (SR).
P < 0.01 vs. arginine immediate-release (IR).
P = 0.03 vs. arginine IR.
Kinetic parameters are calculated for baseline-placebo corrected data. Data are given as mean ± SEM. bid, twice daily.
Figure 2.
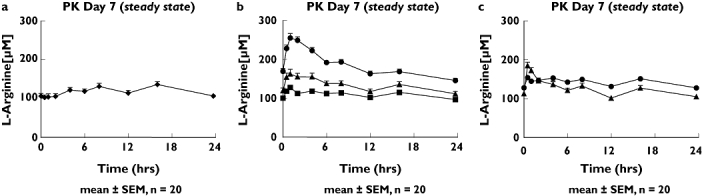
Plasma concentrations of L-arginine at steady state (mean ± SEM, n = 17 for arginine immediate-release (IR) and n = 20 for all others). (A) Placebo (♦) curve. (B) After 1 week of 0.75 (▪), 1.5 (▴) and 3 g (•) twice-daily citrulline supplementation. (C) After 1 week of 1.0 g (▴) tid arginine IR and 1.6 g (•) bid arginine sustained-release supplementation
Table 2b.
Kinetic parameters of citrulline in human plasma after 1 week of oral supplementation with either citrulline or arginine‡¶
| Compound | Dose (mg) | Cmax (µmol l−1) | Tmax (h) | Cmin (µmol l−1) | AUC (µmol h l−1) |
|---|---|---|---|---|---|
| Citrulline | 750 bid | 163 ± 14 | 0.7 ± 0.1 | 9 ± 2 | 288 ± 35 |
| Citrulline | 1500 bid | 350 ± 38* | 0.8 ± 0.1 | 6 ± 1 | 566 ± 47* |
| Citrulline | 3000 bid | 864 ± 45*† | 0.7 ± 0.1 | 9 ± 2 | 1486 ± 78*† |
P < 0.01 vs. citrulline 750 bid.
P < 0.01 vs. citrulline 1500 bid.
Kinetic parameters are calculated for baseline–placebo corrected data. Data are given as mean ± SEM.
Kinetic parameters of citrulline in human plasma after arginine supplementation were not available (no increase of citrulline in human plasma over baseline). Cmax, Maximal plasma concentration; Tmax, time of reach Cmax; Cmin, minimal plasma concentration.
Both 1.6 g l-arginine SR and 3 g l-citrulline improved the plasma l-arginine/ADMA ratio from 171 ± 7 to 232 ± 14 (P < 0.01, 95% CI 36, 91) and from 186 ± 8 to 278 ± 14 (P < 0.01, 95% CI 66, 121), respectively (Figure 3a). Other treatments were ineffective. Only the highest dose of l-citrulline significantly increased urinary excretion of nitrate and cGMP from 92 ± 10 to 125 ± 15 µmol mmol−1 creatinine (P = 0.01, 95% CI 8, 58; Figure 3b) and from 38 ± 3.3 to 50 ± 6.7 nmol mmol−1 creatinine (P = 0.04, 95% CI 0.4, 24; Figure 3c), respectively. Neither blood urea nitrogen nor serum creatinine was altered by active treatment. Baseline arterial diameter in the first treatment period was 4.8 ± 0.1 mm. None of the treatments was associated with a significant change in baseline arterial diameter (all P > 0.05). Baseline FMD in the first treatment period was 6.9 ± 1.0%. None of the treatments significantly improved FMD (Figure 3d). However, analysis of pooled data over all treatments revealed a correlation between mean changes of FMD and mean changes of plasma l-arginine/ADMA ratio (Pearson's correlation, r = 0.92, P = 0.01, Figure 4).
Figure 3.
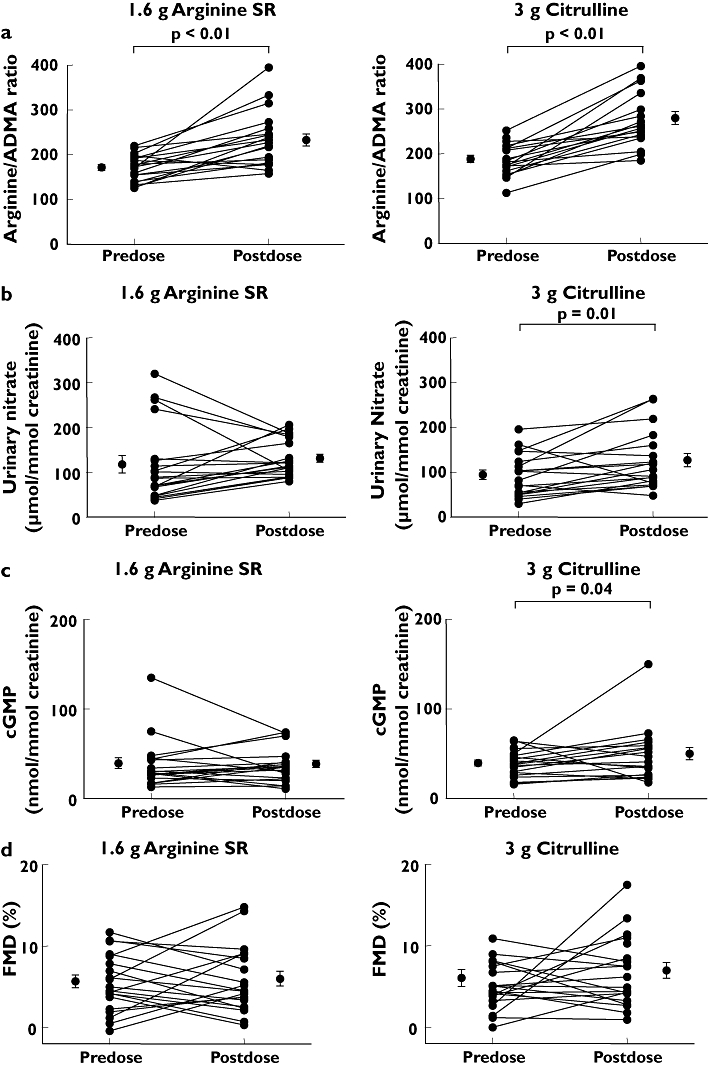
Change in pharmacodynamic parameters after 1 week of 1.6 g bid arginine sustained-release (SR) and 3 g bid citrulline in 20 healthy subjects. Change in (A) L-arginine/asymmetric dimethylarginine ratio, (B) urinary nitrate, (C) urinary cyclic guanosine monophosphate and (D) flow-mediated vasorelaxation (FMD). Individual changes and changes of the mean ± SEM are illustrated. Only statistically significant P-values are given
Figure 4.
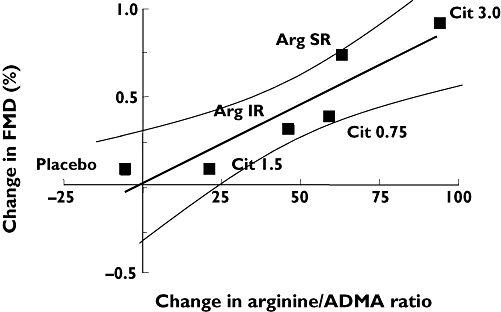
Cumulative data over all treatments. Mean change of flow-mediated vasorelaxation (FMD) is correlated with the mean change of L-arginine/asymmetric dimethylarginine ratio (Pearson's correlation, r = 0.92, P = 0.01, 95% confidence intervals indicated)
Discussion
The major finding of our study is that oral administration of l-citrulline efficiently increases l-arginine plasma concentrations in healthy human. After 1 week of oral supplementation, l-citrulline 0.75 g twice daily increased Cmax for plasma l-arginine and AUC for plasma l-arginine to the same extent as did l-arginine SR 1.6 twice daily and l-arginine IR 1.0 g tid (Table 2). Moreover, higher doses of l-citrulline dose-dependently elevated Cmax and AUC for plasma l-arginine. Trough plasma concentrations of l-arginine were also dose-dependently elevated by l-citrulline. They were significantly higher after l-citrulline 3 g twice daily than after l-arginine IR and l-arginine SR (P < 0.01, Table 2). These findings strongly suggest that oral l-citrulline is at least as efficient in improving plasma l-arginine concentrations in man as is oral administration of l-arginine.
Oral supplementation with l-arginine has been used in a variety of clinical conditions, including hypercholesterolaemia, coronary artery disease, congestive heart failure, peripheral arterial disease, sickle cell disease, and in elderly humans [5–9, 20], in attempts to improve NO-mediated vascular function. Metabolic data from experimental and human studies suggest that after oral administration, l-arginine is extensively metabolized by arginase in the gut wall and liver [14, 21]. This may limit its bioavailability as a substrate for NOS and subsequent effect on vascular function. l-Citrulline has been suggested as a precursor of l-arginine [16, 22], because it can be converted in a two-step enzymatic reaction into l-arginine. A recent small clinical study has suggested that oral l-citrulline may actually lead to higher elevations of plasma l-arginine concentrations than administration of l-arginine itself [23]. Our present data add further evidence by showing that one-half the dosage strength of l-citrulline results in similar plasma l-arginine AUCs compared with oral l-arginine SR and IS (Table 2). Our observation that l-arginine concentrations were increased in peripheral venous blood suggests that l-arginine derived from orally administered l-citrulline is systemically converted to l-arginine, presumably by the kidney and other tissues, including the vasculature [24].
In an experimental study using stable isotope-labelled l-arginine and MS analysis, we were able to show that only a minute proportion of oral l-arginine (approximately 1% of the dose) was being utilized as a substrate of NOS [25]. Metabolic studies using the same technology in man have demonstrated that extensive metabolism of l-arginine occurs in the intestinal tract [21]. This, in combination with a very short half-life of about 1 h [11], may have contributed to the negligible effect of IR l-arginine on any of the PK and PD parameters measured in the present study. Besides using a SR formulation of l-arginine, i.e. l-arginine SR [26], l-citrulline administration may thus be an elegant way of prolonging the exposure of the vasculature to elevated concentrations of plasma l-arginine. In our healthy study population, l-citrulline supplementation was well tolerated and no related side-effects were observed. Nevertheless, in patients with elevated l-citrulline concentrations, e.g. renal failure [27], the efficacy and side-effects of this supplementation should be investigated.
The second aim of our study was to investigate whether these PK findings translate into PD effects. Plasma l-arginine is one important source of l-arginine substrate for NOS [13], because l-arginine is readily taken up from plasma into endothelial cells by the y+ transport system for cationic amino acids [2], which is colocalized with eNOS in caveloae [28]. The ratio of l-arginine over the endogenous NOS inhibitor ADMA is one predictor for the substrate availability for NOS [10, 29]. Thus, treatment-induced elevation of plasma l-arginine concentrations can be expected to increase the l-arginine/ADMA ratio. On the other hand, high concentrations of l-arginine are known to inhibit dimethylarginine dimethylaminohydrolase (DOAH), the enzyme responsible for ADMA catabolism, which would increase ADMA concentrations [30]. However, the relatively low dose of l-arginine investigated in our study, i.e. a maximum of 3.2 g day−1, did not increase ADMA plasma concentrations in participants.
An improved arginine/ADMA ratio would enhance the conversion of l-arginine to NO and subsequently increase urinary excretion of the major urinary metabolite of NO, nitrate. In fact, the l-arginine/ADMA ratio was elevated after 1 week of oral supplementation with l-arginine SR or l-citrulline 3 g (Figure 3a). Other treatments, including l-arginine IR or lower doses of l-citrulline, did not elicit significant changes in the ratio. l-Citrulline 3 g was more efficient in increasing Cmax, Cmin and AUC for plasma l-arginine over baseline placebo than l-arginine SR or IR. This could explain why l-citrulline 3 g was the only treatment to enhance urinary nitrate excretion significantly (Figure 3b). Urinary nitrate excretion as a measure of systemic NO production is highly confounded by other, i.e. dietary, sources, which serves as another explanation why only the highest dose of l-citrulline appeared effective [10, 25]. Also, only l-citrulline 3 g increased urinary excretion of cGMP (Figure 3c). cGMP is the product of sGC in vascular smooth muscle and other cells, which is activated upon stimulation by NO. The increased NO production observed after the highest dose of l-citrulline resulted in increased urinary excretion of cGMP. Both urinary nitrate and cGMP have previously been used as markers of systemic NO production and bioactivity [6, 11, 25]. Thus, NO was not only produced, but also bioactive, in the present study.
The third question this study set out to answer was whether increased bioavailability of l-arginine resulted in an improvement of endothelium-dependent vasodilation as a surrogate marker of NO-mediated physiological effects. There was no significant improvement of endothelium-dependent vasodilation by any of the treatments after 1 week of administration (Figure 3d). Although disappointing, this was only a secondary goal of the present study, and there are several possible explanations for this negative result. First, in a previous study in patients with severe intermittent claudication in which intravenous infusions of l-arginine (8 g bid) were used for 3 weeks [31], FMD was enhanced in a time-dependent manner. After 1 week, there was only a slight tendency for improvement which was in the range of 1–2% absolute change in FMD; this response to l-arginine treatment built up to a significant improvement after 3 weeks, which was also associated with a significantly prolonged claudication distance. Thus, the absolute difference in FMD in the present study corresponds well to the change observed in the previous study after the same treatment period, and one can therefore speculate that a significant improvement of endothelialfunction may occur if treatment with l-arginine or l-citrulline is prolonged.
In previous studies, l-citrulline and l-arginine administration has resulted in improved endothelium-dependent vasodilation [5, 6, 31], and has not [7, 8]. In one study, oral administration of 5 g tid l-arginine for 2 weeks failed to increase further acetylcholine-induced forearm blood flow [7]. Importantly, a recent placebo-controlled study has suggested possible harm in 153 post-myocardial infarction (MI) patients treated with oral l-arginine (3 g tid) vs. placebo [32]. However, those results are discordant with another randomized, placebo-controlled study, in which 792 post-MI patients clearly benefited from l-arginine supplementation (3 g tid) [33], and the causal relationship between the excess number of deaths observed in the former study and the l-arginine administration has been questioned [34]. One reason for these contradictory findings of l-arginine supplementation studies could be that unselected patients have been included. Selection of patients in whom l-arginine-derived NO production is impaired secondary to elevated ADMA concentrations may increase the chance of identifying patients that respond positively to l-arginine or l-citrulline administration [35]. Indeed, restoration of plasma l-arginine concentrations was found to reverse the endothelial dysfunction attributable to high ADMA in some clinical and experimental studies [5, 31, 36, 37]. This suggestion is further supported by our finding of a correlation between the mean change in l-arginine/ADMA ratio and the mean change in FMD when data from all treatments were pooled (Figure 4). This supports the assumption that the change of FMD, at least in part, depends on the l-arginine/ADMA ratio, and therefore corroborates previous findings by our group [36, 38] and others [6, 39].
Finally, although the subjects included in the present study had been selected according to their comparatively high ADMA concentrations, the mean ADMA concentration in study participants was 0.6 ± 0.1 µM, which is still within the normal range [40]. High ADMA concentrations have been associated with endothelial dysfunction and vascular disease [20, 41, 42], and competition with high ADMA has been suggested to explain the so-called ‘l-arginine paradox’[9]. Therefore, it is not surprising that endothelial function was normal in our apparently healthy study population. Baseline FMD was 6.9 ± 1.0%, a value which is at the lower margin, but still within the range previously reported for healthy individuals [31]. This may be another explanation why neither l-citrulline nor l-arginine treatment resulted in significant improvement of endothelial function within the short treatment period of 1 week.
In conclusion, our results provide a rationale for larger, prospective clinical studies with longer treatment periods to investigate the effects of oral l-citrulline supplementation on endothelial function in patients with endothelial dysfunction and vascular disease.
Acknowledgments
The excellent technical assistance of M. Kastner, A. Steenpass and E. Silberhorn is gratefully acknowledged. This study was supported by Angiogenix Inc., Burlingame, CA, USA.
Competing interest: William Spickler was the chief medical officer of Angsagenix, Inc. the sponsor of this study.
Ranier H. Böger has received funds for research and fees for consulting from Angiogenix, Inc. a manufacturer of l-citrulline tablets.
Supplementary material
Alternative presentation of data from Figure 4. Changes of flow-mediated vasorelaxation (FMD) vs. L-arginine/asymmetric dimethylarginine ratio (mean ± SEM)
References
- 1.Naseem KM. The role of nitric oxide in cardiovascular diseases. Mol Aspects Med. 2005;26:33–65. doi: 10.1016/j.mam.2004.09.003. [DOI] [PubMed] [Google Scholar]
- 2.Closs EI, Simon A, Vekony N, Rotmann A. Plasma membrane transporters for arginine. J Nutr. 2004;134(10 Suppl.):2752S–2759S. doi: 10.1093/jn/134.10.2752S. [DOI] [PubMed] [Google Scholar]
- 3.Pollock JS, Förstermann U, Mitchell JA, Warner TD, Schmidt HH, Nakane M, Murad F. Purification and characterization of particulate endothelium-derived relaxing factor synthase from cultured and native bovine aortic endothelial cells. Proc Natl Acad Sci USA. 1991;88:10480–4. doi: 10.1073/pnas.88.23.10480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cynober LA. Plasma amino acid levels with a note on membrane transport: characteristics, regulation, and metabolic significance. Nutrition. 2002;18:761–6. doi: 10.1016/s0899-9007(02)00780-3. [DOI] [PubMed] [Google Scholar]
- 5.Bode-Böger SM, Muke J, Surdacki A, Brabant G, Böger RH, Frölich JC. Oral 1-arginine improves endothelial function in healthy individuals older than 70 years. Vasc Med. 2003;8:77–81. doi: 10.1191/1358863x03vm474oa. [DOI] [PubMed] [Google Scholar]
- 6.Piatti PM, Monti LD, Valsecchi G, Magni F, Setola E, Marchesi F, Galli-Kienle M, Pozza G, Alberti KG. Long-term oral L-arginine administration improves peripheral and hepatic insulin sensitivity in type 2 diabetic patients. Diabetes Care. 2001;24:875–80. doi: 10.2337/diacare.24.5.875. [DOI] [PubMed] [Google Scholar]
- 7.Walker HA, McGing E, Fisher I, Böger RH, Bode-Böger SM, Jackson G, Ritter JM, Chowienczyk PJ. Endothelium-dependent vasodilation is independent of the plasma L-arginine/ADMA ratio in men with stable angina: lack of effect of oral L-arginine on endothelial function, oxidative stress and exercise performance. J Am Coll Cardiol. 2001;38:499–505. doi: 10.1016/s0735-1097(01)01380-8. [DOI] [PubMed] [Google Scholar]
- 8.Blum A, Hathaway L, Mincemoyer R, Schenke WH, Kirby M, Csako G, Waclawiw MA, Panza JA, Cannon RO., 3rd Effects of oral 1-arginine on endothelium-dependent vasodilation and markers of inflammation in healthy postmenopausal women. J Am Coll Cardiol. 2000;35:271–6. doi: 10.1016/s0735-1097(99)00553-7. [DOI] [PubMed] [Google Scholar]
- 9.Böger RH. Asymmetric dimethylarginine, an endogenous inhibitor of nitric oxide synthase, explains the ‘L-arginine paradox’ and acts as a novel cardiovascular risk factor. J Nutr. 2004;134:2842S–7S. doi: 10.1093/jn/134.10.2842S. [DOI] [PubMed] [Google Scholar]
- 10.Tsikas D, Sandmann J, Savva A, Luessen P, Böger RH, Gutzki FM, Mayer B, Frölich JC. Assessment of nitric oxide synthase activity in vitro and in vivo by gas chromatography-mass spectrometry. J Chromatogr B Biomed Sci Appl. 2000;742:143–53. doi: 10.1016/s0378-4347(00)00142-0. [DOI] [PubMed] [Google Scholar]
- 11.Bode-Böger SM, Böger RH, Galland A, Tsikas D, Frölich JC. L-arginine-induced vasodilation in healthy humans: pharmacokinetic–pharmacodynamic relationship. Br J Clin Pharmacol. 1998;46:489–97. doi: 10.1046/j.1365-2125.1998.00803.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vallance P, Chan N. Endothelial function and nitric oxide: clinical relevance. Heart. 2001;85:342–50. doi: 10.1136/heart.85.3.342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Böger RH, Bode-Böger SM. The clinical pharmacology of L-arginine. Annu Rev Pharmacol Toxicol. 2001;41:79–99. doi: 10.1146/annurev.pharmtox.41.1.79. [DOI] [PubMed] [Google Scholar]
- 14.Morris SM., Jr Enzymes of arginine metabolism. J Nutr. 2004;134:2743S–7S. doi: 10.1093/jn/134.10.2743S. [DOI] [PubMed] [Google Scholar]
- 15.Curis E, Nicolis I, Moinard C, Osowska S, Zerrouk N, Benazeth S, Cynober L. Almost all about citrulline in mammals. Amino Acids. 2005;29:177–205. doi: 10.1007/s00726-005-0235-4. [DOI] [PubMed] [Google Scholar]
- 16.Waugh WH, Daeschner CW, Files BA, McConnell ME, Strandjord SE. Oral citrulline as arginine precursor may be beneficial in sickle cell disease: early phase two results. J Natl Med Assoc. 2001;93:363–71. [PMC free article] [PubMed] [Google Scholar]
- 17.Chace DH, Kalas TA, Naylor EW. Use of tandem mass spectrometry for multianalyte screening of dried blood specimens from newborns. Clin Chem. 2003;49:1797–817. doi: 10.1373/clinchem.2003.022178. [DOI] [PubMed] [Google Scholar]
- 18.Schulze F, Wesemann R, Schwedhelm E, Sydow K, Albsmeier J, Cooke JP, Böger RH. Determination of asymmetric dimethylarginine (ADMA) using a novel ELISA assay. Clin Chem Lab Med. 2004;42:1377–83. doi: 10.1515/CCLM.2004.257. [DOI] [PubMed] [Google Scholar]
- 19.Corretti MC, Anderson TJ, Benjamin EJ, Celermajer D, Charbonneau F, Creager MA, Deanfield J, Drexler H, Gerhard-Herman M, Herrington D, Vallance P, Vita J, Vogel R. Guidelines for the ultrasound assessment of endothelial-dependent flow-mediated vasodilation of the brachial artery: a report of the International Brachial Artery Reactivity Task Force. J Am Coll Cardiol. 2002;39:257–65. doi: 10.1016/s0735-1097(01)01746-6. [DOI] [PubMed] [Google Scholar]
- 20.Böger RH. Asymmetric dimethylarginine (ADMA): a novel risk marker in cardiovascular medicine and beyond. Ann Med. 2006;38:126–36. doi: 10.1080/07853890500472151. [DOI] [PubMed] [Google Scholar]
- 21.Castillo L, deRojas TC, Chapman TE, Vogt J, Burke JF, Tannenbaum SR, Young VR. Splanchnic metabolism of dietary arginine in relation to nitric oxide synthesis in normal adult man. Proc Natl Acad Sci USA. 1993;90:193–7. doi: 10.1073/pnas.90.1.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Urschel KL, Shoveller AK, Uwiera RR, Pencharz PB, Ball RO. Citrulline is an effective arginine precursor in enterally fed neonatal piglets. J Nutr. 2006;136:1806–13. doi: 10.1093/jn/136.7.1806. [DOI] [PubMed] [Google Scholar]
- 23.Kuhn KP, Harris PA, Cunningham GR, Robbins IM, Lawson WE, Summar ML, Christman BW. Oral citrulline effectively elevates plasma arginine levels for 24 hours in normal volunteers. Circulation. 2002;106:II1–766S. [Google Scholar]
- 24.Romero MJ, Platt DH, Caldwell RB, Caldwell RW. Therapeutic use of citrulline in cardiovascular disease. Cardiovasc Drug Rev. 2006;24:275–90. doi: 10.1111/j.1527-3466.2006.00275.x. [DOI] [PubMed] [Google Scholar]
- 25.Böger RH, Tsikas D, Bode-Böger SM, Phivthong-Ngam L, Schwedhelm E, Frölich JC. Hypercholesterolemia impairs basal nitric oxide synthase turnover rate: a study investigating the conversion of L-[guanidino-15N2]-arginine to 15N-labeled nitrate by gas chromatography-mass spectrometry. Nitric Oxide. 2004;11:1–8. doi: 10.1016/j.niox.2004.07.008. [DOI] [PubMed] [Google Scholar]
- 26.Böger GI, Rudolph T, Maas R, Schwedhelm E, Dumbardze E, Bierend A, Benndorf R, Böger RH. Improvement of endothelium-dependent vasodilation by simvastatin is enabled by combination with L-arginine in patients with elevated ADMA levels. J Am Coll Cardiol. 2007;49:2274–82. doi: 10.1016/j.jacc.2007.02.051. [DOI] [PubMed] [Google Scholar]
- 27.Kielstein JT, Böger RH, Bode-Böger SM, Schaffer J, Barbey M, Koch KM, Frölich JC. Asymmetric dimethylarginine plasma concentrations differ in patients with end-stage renal disease: relationship to treatment method and atherosclerotic disease. J Am Soc Nephrol. 1999;10:594–600. doi: 10.1681/ASN.V103594. [DOI] [PubMed] [Google Scholar]
- 28.Li C, Huang W, Harris MB, Goolsby JM, Venema RC. Interaction of the endothelial nitric oxide synthase with the CAT-1 arginine transporter enhances NO release by a mechanism not involving arginine transport. Biochem J. 2005;386:567–74. doi: 10.1042/BJ20041005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Böger RH, Vallance P, Cooke JP. Asymmetric dimethylarginine (ADMA): a key regulator of nitric oxide synthase. Atheroscler Suppl. 2003;4:1–3. doi: 10.1016/s1567-5688(03)00027-8. [DOI] [PubMed] [Google Scholar]
- 30.Wang J, Sim AS, Wang XL, Wilcken DE. L-arginine regulates asymmetric dimethylarginine metabolism by inhibiting dimethylarginine dimethylaminohydrolase activity in hepatic (HepG2) cells. Cell Mol Life Sci. 2006;63:2838–46. doi: 10.1007/s00018-006-6271-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Böger RH, Bode-Böger SM, Thiele W, Creutzig A, Alexander K, Frölich JC. Restoring vascular nitric oxide formation by L-arginine improves the symptoms of intermittent claudication in patients with peripheral arterial occlusive disease. J Am Coll Cardiol. 1998;32:1336–44. doi: 10.1016/s0735-1097(98)00375-1. [DOI] [PubMed] [Google Scholar]
- 32.Schulman SP, Becker LC, Kass DA, Champion HC, Terrin ML, Forman S, Ernst KV, Kelemen MD, Townsend SN, Capriotti A, Hare JM, Gerstenblith G. L-arginine therapy in acute myocardial infarction: the Vascular Interaction With Age in Myocardial Infarction (VINTAGE MI) randomized clinical trial. JAMA. 2006;295:58–64. doi: 10.1001/jama.295.1.58. [DOI] [PubMed] [Google Scholar]
- 33.Bednarz B, Jaxa-Chamiec T, Maciewjewski P, Szpajer M, Janik K, Gniot J, Kawka Urbanek T, Drozdowska D, Gessek J, Laskowski H. Efficacy and safety of oral L-arginine in acute myocardial infarction. Results of multicenter, randomized, placebo-controlled ARAMI pilot trial. Kardiol Pol. 2005;62:421–6. [PubMed] [Google Scholar]
- 34.Abumrad NN, Barbul A. Arginine therapy for myocardial infarction. J Am Med Assoc. 2006;295:1238–2139. doi: 10.1001/jama.295.18.2138-b. [DOI] [PubMed] [Google Scholar]
- 35.Böger RH. Asymmetric dimethylarginine (ADMA) modulates endothelial function—therapeutic implications. Vasc Med. 2003;8:149–51. doi: 10.1191/1358863x03vm501ed. [DOI] [PubMed] [Google Scholar]
- 36.Sydow K, Schwedhelm E, Arakawa N, Bode-Böger SM, Tsikas D, Hornig B, Frölich JC, Böger RH. ADMA and oxidative stress are responsible for endothelial dysfunction in hyperhomocyst(e)inemia: effects of L-arginine and B vitamins. Cardiovasc Res. 2003;57:244–52. doi: 10.1016/s0008-6363(02)00617-x. [DOI] [PubMed] [Google Scholar]
- 37.Böger RH, Bode-Böger SM, Phivthong-ngam L, Brandes RP, Schwedhelm E, Mugge A, Böhme M, Tsikas D, Frölich JC. Dietary L-arginine and alpha-tocopherol reduce vascular oxidative stress and preserve endothelial function in hypercholesterolemic rabbits via different mechanisms. Atherosclerosis. 1998;141:31–43. doi: 10.1016/s0021-9150(98)00145-2. [DOI] [PubMed] [Google Scholar]
- 38.Perticone F, Sciacqua A, Maio R, Perticone M, Maas R, Böger RH, Tripepi G, Sesti G, Zoccali C. Asymmetric dimethylarginine, L-arginine, and endothelial dysfunction in essential hypertension. J Am Coll Cardiol. 2005;46:518–23. doi: 10.1016/j.jacc.2005.04.040. [DOI] [PubMed] [Google Scholar]
- 39.Yilmaz MI, Saglam M, Caglar K, Cakir E, Ozgurtas T, Sonmez A, Eyileten T, Yenicesu M, Acikel C, Oguz Y, Ozcan O, Bozlar U, Erbil K, Aslan I, Vural A. Endothelial functions improve with decrease in asymmetric dimethylarginine (ADMA) levels after renal transplantation. Transplantation. 2005;80:1660–6. doi: 10.1097/01.tp.0000183750.22675.be. [DOI] [PubMed] [Google Scholar]
- 40.Schulze F, Maas R, Freese R, Schwedhelm E, Silberhorn E, Böger RH. Determination of a reference value for N(G), N(G) -dimethyl-L-arginine in 500 subjects. Eur J Clin Invest. 2005;35:622–6. doi: 10.1111/j.1365-2362.2005.01561.x. [DOI] [PubMed] [Google Scholar]
- 41.Böger RH, Bode-Böger SM, Szuba A, Tangphao O, Tsao PS, Chan JR, Blaschke TF, Cooke JP. Asymmetric dimethylarginine: a novel risk factor for endothelial dysfunction. Its role in hypercholesterolemia. Circulation. 1998;98:1842–7. doi: 10.1161/01.cir.98.18.1842. [DOI] [PubMed] [Google Scholar]
- 42.Cooke JP. ADMA—the Über-marker? Circulation. 2004;109:1813–8. doi: 10.1161/01.CIR.0000126823.07732.D5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


