Abstract
Acute alcohol administration decreases overall brain glucose metabolism, which serves as a marker of brain activity. The behavioral effects of alcohol however, are likely to reflect not only changes in regional brain activity but also on the patterns of brain functional organization. Here we assessed the effects of a moderate dose of alcohol on the patterns of brain activity and cerebral differentiation. We measured brain glucose metabolism in 20 healthy controls with PET and FDG during baseline and during alcohol intoxication (0.75 g/kg). We used the coefficient of variation (CV) to assess changes in brain metabolic homogeneity, which we used as a marker for cerebral differentiation. We found that alcohol decreased the CV in the brain and this effect was independent of the decrements in overall glucose metabolism. Our study revealed marked disruption in brain activity during alcohol intoxication including decreases in global and regional brain differentiation, a loss of right versus left brain metabolic laterality and a shift in the predominance of activity from cortical to limbic brain regions. The widespread nature of the changes induced by a moderate dose of alcohol is likely to contribute to the marked disruption of alcohol on behavior, mood, cognition and motor activity.
Keywords: Imaging, PET, heterogeneity, addiction, coefficient of variation
1. Introduction
Alcohol is believed to exert its effects by affecting multiple neurotransmitter systems including facilitation of GABA, dopamine, opiate and serotonin neurotransmission and inhibition of glutamate activity (Koob et al., 1998). While it is postulated that the reinforcing effects of alcohol are due to its activation of dopaminergic and opiate pathways and that its anxiolitic effects are due to its GABAergic effects, the changes in brain function that are responsible for the state of alcohol intoxication are poorly understood. This state is typically characterized by both symptoms of excitement and sedation, changes in mood and an overall decrease in motor and cognitive abilities (Lukas et al., 1986; Peterson et al., 1990).
Imaging studies assessing the effects of acute alcohol administration on regional brain metabolism or on cerebral blood flow (CBF) have assessed the effects of intoxication on increases or decreases in absolute or normalized activity in specific brain regions (Volkow et al., 1988; De Wit et al., 1990; Volkow et al., 1990; Tiihonen et al., 1994; Ingvar et al., 1998; Wang et al., 2000). These studies have shown that acute alcohol administration significantly reduces brain glucose metabolism -- an effect that is most pronounced in the occipital cortex and in cerebellum. In contrast studies assessing the effect of acute alcohol in CBF have shown that it increases CBF in multiple brain regions including the prefrontal cortex where the increases are linked to alcohol’s reinforcing effects (Tiihonen et al., 1994). However, the mechanisms underlying the marked disruptive effects of alcohol on human behavior are likely to reflect not only its ability to decrease or increase the activity of a given brain region but also its effects on the patterns of brain functional organization. This is relevant because it is possible that the effects of alcohol are due to its ability to decrease the degree of differentiation within or among the various brain regions -- an effect that we postulate will result in a decrease in regional variability in metabolism. We recently proposed the coefficient of variation analysis to assess regional and brain metabolic variability (Volkow et al., 2002). The purpose of this study was to assess if acute alcohol administration changed regional brain metabolic variability. For this purpose we compared regional brain metabolism in 20 healthy controls tested at baseline (no stimulation) and after an acute moderate dose of alcohol using PET and FDG. Variability in metabolism was assessed using the coefficient of variation. The results on the effects of alcohol on absolute brain metabolic activity were published as part of the study that compared the metabolic changes induced by alcohol from those induced by benzodiazepines (Wang et al., 2000).
2. Methods
2.1. Subjects
Twenty right-handed healthy subjects, 10 females (mean 36.1±8 years, age range 21–50) and 10 males (mean 40.6±8 years, age range 25–53) with comparable educational and socioeconomic background participated in this study. Subjects received a complete physical, neurological and psychiatric examination as well as routine laboratory tests including urine toxicology. Subjects with medical, neuropsychiatric illnesses, treated with any prescription drugs, history of substance abuse including alcohol or who consumed more than five 12 ounce-cans of beer or more than 5 drinks of one ounce hard liquor per week and/or 2 packs of cigarette per day were excluded. None of the females were using oral contraceptives or female hormone supplements and all were premenopausal. Subjects were instructed to refrain from alcohol drinking and to discontinue any over-the-counter medication one-week prior to the scan. Written informed consents were obtained from each participant after the nature of the experiment was fully explained. Studies were approved by the IRB at Brookhaven National Laboratory.
2.2. Experimental design
Subjects received two PET scans with FDG on two separate days within 1 week of each other. For the females, the studies were done in the mid-luteal phase (16–22 days after the onset of menstruation). On the days of the scans subjects were asked to refrain from smoking, consuming caffeine and/or glucose containing drinks and food for at least 4 hours prior to the study. On the first day, subjects drank a placebo (100 ml of diet noncaffeinated soda) over a 40 minutes period given 40–50 minutes prior to FDG administration. On the second day, subjects drank a mixture of 95% alcohol (0.75 g/kg) with diet soda added up to 100 ml for 40 minutes given 40–50 minutes prior to FDG. The subjects were blind to the drug received. To avoid confounds from circadian variability (Bartlett et al., 1988) the two scans for a given subject were done at the same time of day (±1 hour). Blood alcohol concentration was measured prior to and 20, 40, 55, 80, 100, 140 and 160 min after the initiation of alcohol administration using the enzymatic assay of Lloyd (Lloyd el al., 1978).
2.3. PET scanning
The subjects were placed in the scanner with their eyes open and their ears unplugged in a dimly lit room with minimal noise. A nurse remained with the subjects to ensure that they kept their eyes open throughout the study. Two intravenous lines were inserted and maintained with saline and heparin. Arterialized venous blood was obtained from a catheter placed in a dorsal hand vein. The hand was pre-warmed to 48°C in a heating box. The other catheter was in the antecubital region of the opposite arm for tracer injection. PET scans were performed with a Siemens HR+ tomograph (resolution 4.5x4.5x4.5 mm full width half-maximum, 63 slices). An individualized headholder was used to position the subjects with the aid of two orthogonal laser beams, one that was placed at corner of canthus and the other parallel to the sagittal plane. After recording a 10-minute transmission scan for photon attenuation correction, a 20-minute emission scan was obtained beginning 35 minutes after injection of 150–185 MBq (4–5 mCi) of FDG. Arterialized venous blood samples were obtained to measure plasma radioactivity and plasma glucose concentration. Metabolic images were computed as described previously (Wang et al., 1994). Each image was co-registered in the Talairach space via SPM99, and the SPM brain mask was used to extract the brain.
2.4. Statistics
CV – a measure of functional heterogeneity
We adopted the coefficient of variation (CV) as a measure brain functional heterogeneity/homogeneity. The CV is the ratio of the standard deviation and the mean. The advantage of the CV measure lies in that it is dimensionless and unaffected by the unit/magnitude of the mean. This is particularly relevant in the image studies where different images are of different signal strength, including those for the same subject, under similar conditions, and in adjacent time periods. Depending on the domain volume, we define the brain functional heterogeneity measure at three levels –global (whole brain), hemispheric (left versus right hemisphere), and regional (within a given area without using predefined anatomical selection and instead relying on the voxel analysis).
Previously, in order to evaluate the regional CV we had partitioned the entire cortex into small adjacent and non-overlapping cortical regions of similar sizes (Volkow et al, 2002). However, one shortcoming of this approach is that the resulting CV map is patchy and discontinuous. Consequently we have developed a new method that provides with a smooth CV map in order to assess the regional CV. The regional CV is defined at the voxel level. For each voxel, a small neighborhood is selected and the CV based on this neighborhood volume is defined to be the CV of the designated voxel. The resulting CV map is continuous and can be analyzed on the voxel level via SPM in the same fashion as the analysis of the metabolic image. We have written a program to calculate the local CV using cubical neighborhoods. The neighborhood size was set to 27 voxels in total (3x3x3) with the designated voxel in the center. The program partitions the entire brain, the gray matter, the white matter, and the CSF using the corresponding masks in SPM99. To assess the effects of alcohol we measured the CV in the gray and the white matter and filtered the CSF. Note that CV is invariant to the metabolic measure – be it the absolute or the normalized (divided by the global mean) metabolic values. The CV value is expressed as a percentage (i.e. CV of 34 is indeed CV = 34%).
Statistical Analyses
Differences in CV and in absolute metabolism between baseline and alcohol intoxication were tested with paired-sample t tests at the voxel level via SPM99. For global and laterality comparisons significance was set at P < 0.01 and for voxel comparisons at P < 0.001. To assess if alcohol-induced changes in CV were secondary to alcohol-induced changes in metabolism we measured the Pearson’s product moment correlation between these variables. Comparisons were performed for the global, the hemispheric and the regional effects.
3. Results
3.1. Brain homogeneity
Global and Hemispheric CV
The CV values for the entire brain when the subjects were on baseline and when they were under alcohol intoxication are shown on Table 1. Alcohol significantly decreased the CV (t = 2.91, df =19; P = 0.008). That is, alcohol rendered the brain more homogeneous.
Table 1.
Mean and standard deviation of the coefficient of variation (%) and the absolute metabolism for the entire brain (CSF excluded).
| Coefficient of Variation | Absolute Metabolism | |
|---|---|---|
| Baseline | 23.43 (s = 1.09) | 31.70 (s = 4.95) |
| Alcohol | 22.74 (s = 1.07) | 25.26 (s = 3.90) |
| % Change | −2.94 (P = 0.008) | −20.32 (P < 0.0001) |
The mean and standard deviation of the CV values for the left and the right hemispheres during baseline and during alcohol intoxication are displayed in Table 2. At baseline the left cortex was slightly less homogeneous than the right but the difference was not statistically significant. The comparison of the baseline versus alcohol condition showed that on left hemisphere the CV decreased during alcohol intoxication (right: t = 2.66, df = 19, P = 0.015; left: t = 3.14, df = 19, P = 0.005). The magnitude of decrease on the right hemisphere was not as significant as the decrease on the left hemispheres.
Table 2.
Mean and standard deviation (in parentheses) of the coefficient of variation (%), the absolute metabolism, and the relative metabolism for the hemispheres (CSF excluded).
| Coefficient of Variation | Absolute Metabolism | Relative Metabolism | ||||
|---|---|---|---|---|---|---|
| Left | Right | Left | Right | Left | Right | |
| Baseline | 23.47
(0.96) |
23.35
(1.23) |
31.51
(4.98) |
31.86
(4.95) |
0.886
(0.019) |
0.896
(0.017) |
| Alcohol | 22.73
(1.02) |
22.68
(1.19) |
25.15
(3.90) |
25.35
(3.92) |
0.888
(0.017) |
0.895
(0.022) |
| % Change | −3.15
(P = 0.003) |
−2.87
(P = 0.008) |
−20.18
(P < 0.0001) |
−20.43
(P < 0.0001) |
+0.23
N.S. |
−0.11
N.S. |
We also compared the volume of voxels with significant CV changes (increase or decrease) due to alcohol intoxication at the 0.001 significance level between the left and the right hemispheres (Table 3). At the significance level of 0.001, we found that for the right hemisphere, the volume of decrease was significantly larger than the volume of increase while the opposite was true for the left hemisphere. The volume of increase for the left was significantly larger than that of the right while the converse was true for the volume of decrease. Overall, by combining both hemispheres, we found that the volume of decrease significantly exceeded the volume of increase.
Table 3.
Volume of changes (number of voxels) and percentage (in parenthesis)), from baseline to alcohol, at the significance level of 0.001 for the coefficient of variation and the relative metabolism for the hemispheres and the entire brain (CSF excluded).
| Coefficient of Variation | Relative Metabolism | |||||
|---|---|---|---|---|---|---|
| Left | Right | Both | Left | Right | Both | |
| Increased | 4955 (3.4) | 2034 (1.4) | 6989 (4.8) | 10207 (7.0) | 2586 (1.8) | 12793 (8.7) |
| Decreased | 3227 (2.2) | 5011 (3.4) | 8238 (5.6) | 4534 (3.1) | 2494 (1.7) | 7028 (4.8) |
Regional CV
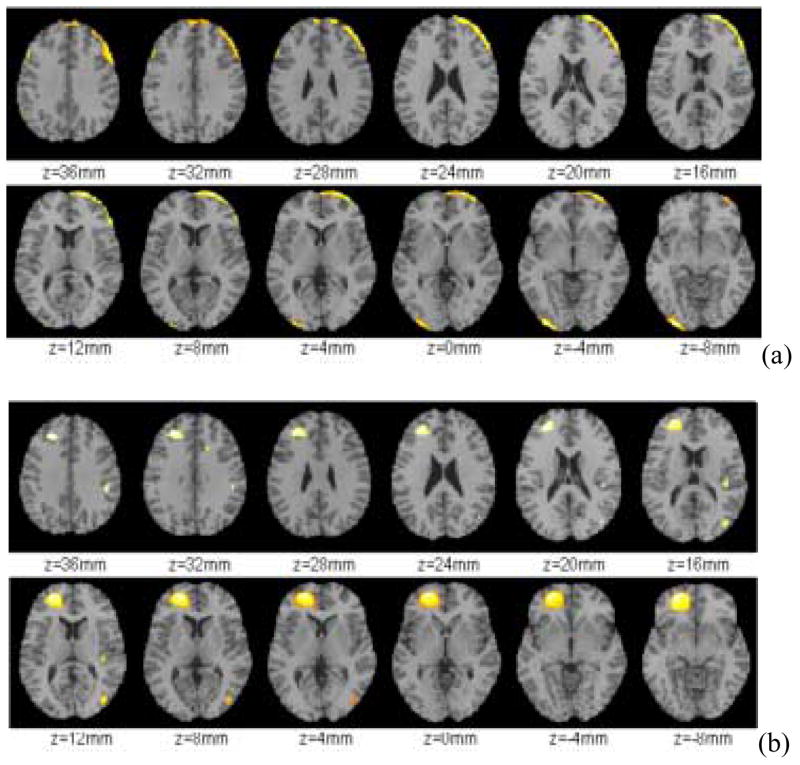
Analysis of the voxel CV maps revealed that the right frontal cortex significantly decreased the CV and this effect was observed throughout most of the right frontal lobe (Figure 1a). The superior left parietal cortex as well as part of the left occipital cortex and posterior left cerebellum also showed a significant decrease in CV (Figure 1a). In contrast increases in CV were noted in the left anterior cingulate gyrus (CGA), the lower part of the middle temporal gyrus (area 22) and the left rectal gyrus. (Figure 1b).
Figure 1.
(a) Brain regions that showed decreases (Baseline > Alcohol) and (b) increases (Baseline < Alcohol) in CV during alcohol intoxication (Significance level α = 0.001, 2-sided).
3.2. Brain metabolism
Global and Hemispheric Metabolism
The global average metabolic level, as predicted, reduced significantly under alcohol intoxication (t = 7.86, df =19; P < 0.001) (Table 1). In comparing the left and right hemispheres, we found that at baseline, the right hemisphere is more active than the left for both the absolute metabolism (Right mean = 31.86, SD = 4.95 micromol/100g/min; Left mean = 31.51, SD = 4.98 micromol/100g/min; t = 2.67, df =19, P = 0.015) and the relative metabolism (Right mean = 0.896 SD = 0.017; Left mean = 0.886, SD = 0.019; t = 2.83, df =19, P = 0.011). However, the difference between the hemispheres disappeared during alcohol intoxication – the two hemispheres became equally active (Absolute: Right mean = 25.35, SD = 3.92 micromol/100g/min, Left mean = 25.15, SD = 3.90 micromol/100g/min; Relative: Right mean = 0.895, SD = 0.022; Left: mean = 0.888, SD = 0.017).
To further study the differential effect of alcohol on the hemispheres, we compared the volume of voxels that had significant changes in relative metabolism (increase or decrease) due to alcohol intoxication at the 0.001 significance level (Table 3). For the right hemisphere, the volume of decrease is significantly larger than the volume of increase while the opposite is true for the left hemisphere. In fact, half of the left hemispheres had a significantly increase in relative metabolism.
Regional Metabolism
To asses which areas of the brain showed the largest decrements and which ones the least changes in metabolism we assessed the effects of alcohol intoxication on relative metabolism. The SPM analysis of relative metabolism showed that the most accentuated decrements occurred in the posterior of the brain in the areas corresponding to the occipital cortex and posterior cerebellum (Figure 2a). The SPM analysis of relative increases showed that this occurred mostly in limbic brain regions namely left and right caudate, left putamen, left temporal insula, ventral striatum (including nucleus accumbens), anterior cingulate, amygdala and mesencephalon (including red nucleus) (Figure 2b). Relative increases were also observed in the left precentral gyrus (Broadman areas 1,2,3) and in Broadman area 6 (Figure 2b).
Figure 2.
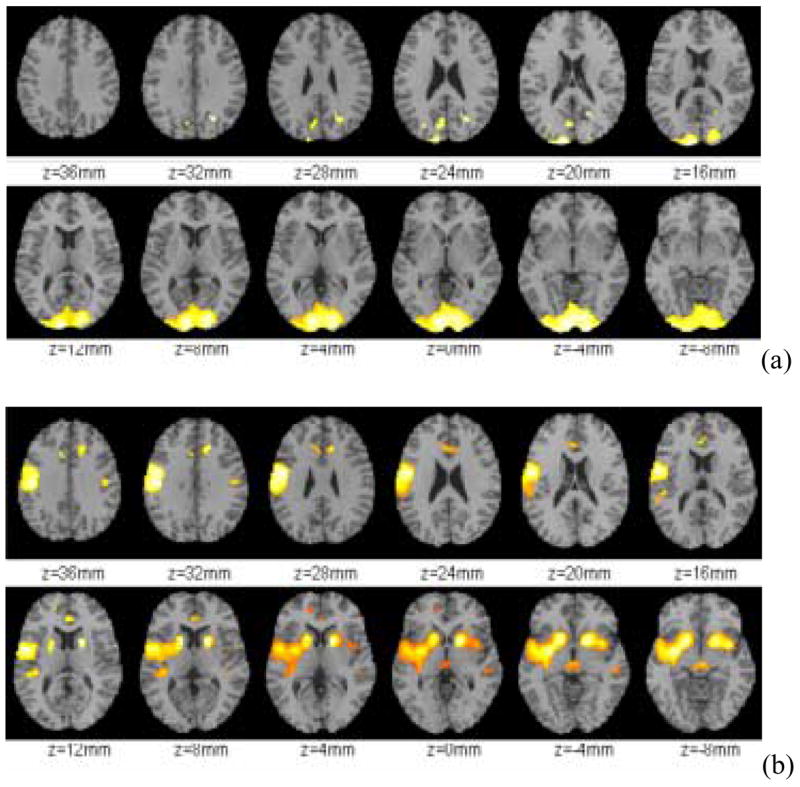
Brain regions that showed (a) decreases (Baseline > Alcohol) and (b) increases (Baseline < Alcohol) in relative metabolism due to alcohol intoxication (Significance level α = 0.001, 2-sided).
3.3. Correlations between alcohol induced changes in CV and in relative metabolism
The correlation analyses between the alcohol-induced change in global CV and in global metabolism was not significant (P = 0.122) (Figure 3). The voxel-wise correlations between the changes in CV and the changes in relative metabolism from baseline to alcohol showed very few significant correlation. Figure 4 shows the correlation map; negative correlations are shown in blue and positive ones in yellow. The few significant correlations were mostly negative and occurred at the brain external boundaries or at the boundaries between white and gray matter, which most likely reflect the boundary effect (lower metabolism and higher CV).
Figure 3.
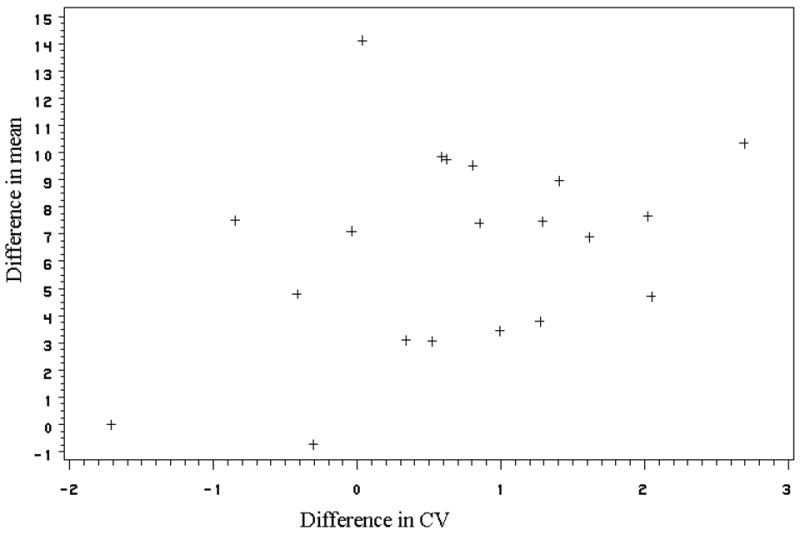
Scatter plot of changes in global CV versus changes in global metabolism from baseline to alcohol. These changes were not significantly correlated (p = 0.122).
Figure 4.
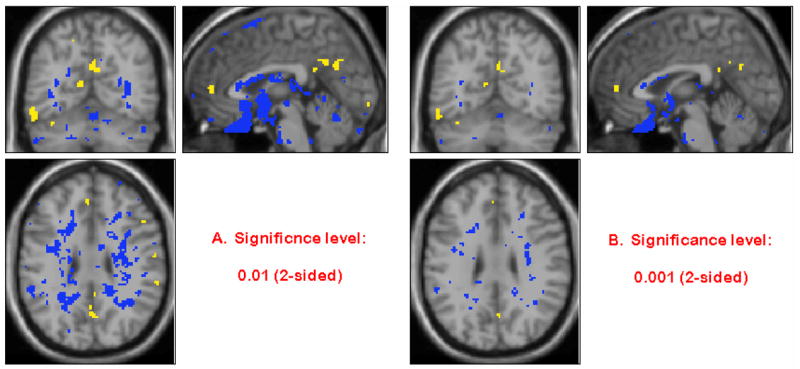
Correlations between changes in CV and changes in relative metabolism with blue and yellow colors representing the negative and positive correlations respectively (Significance level α = 0.01 and α = 0.001, 2-sided).
4. Discussion
4.1. Alcohol induced decreases in CV
The use of the CV is based on the assumption that the more heterogeneous a brain region the more differentiated it will be in its function. Thus the findings of decreases in CV during alcohol intoxication are consistent with an overall decrease in functional differentiation during alcohol intoxication both at a global and a regional level. Previous studies had shown a decrease in the patterns of electroencephalographic differentiation of the brain both at rest and during activation under the influence of alcohol (Stenberg et al., 1994; Wendt et al., 1994). Increases in brain homogeneity from alcohol administration could result from a dampening of the most active brain areas with less effect to the areas that are less active. Alternatively since brain regions are highly interconnected deactivation of one region due to alcohol intoxication could result in disruption of neural networks and thus the deactivation of related brain regions on the network. This in turn could modify heterogeneity in brain activity at the global level. However, it should be noted that while there was an overall decrease in CV this was not a homogeneous response and some brain areas had an increase in CV with alcohol intoxication. The changes in CV were not correlated with the changes in metabolism, which indicates that they reflect different information with respect to alcohol’s effects on increasing or decreasing glucose consumption. Statistically this is plausible because the mean and variance describe two aspects of the population characteristics. In case of normal distribution, the sample mean and the sample variance are stochastically independent.
4.2. Laterality effects
In this study we show a laterality effect for alcohol’s effects in CV and in normalized metabolism. We found that the volume of decreases in CV for the right hemisphere was significantly larger than that of the left hemisphere. This could be interpreted as indicating that alcohol renders the right hemisphere more homogeneous than the left. Similarly analyses of the normalized metabolic measure reveals that the ratio of the volumes of decrease in relative metabolism was significantly larger in the right hemisphere than in the left. In contrast the relative metabolic increases were greater in the left than in the right hemisphere. We interpret this to indicate that during alcohol intoxication the activity of the right hemisphere is decreased to a greater extent than that of the left. This finding is consistent with the literature showing that alcohol’s effects tend to be larger for the right than for the left hemisphere (Wendt et al., 1994; Levin et al., 1998). However, alcohol’s disrupting effects on laterality do not appear to be limited to the right hemisphere but adversely affect the hemispheric specialization that is present during sobriety (Wendt and Risberg, 2001). Moreover, in this case the effects of alcohol were to blur the laterality in the metabolic measures by equalizing the activity of both hemispheres.
4.3. CV regional effects
The areas that showed the largest decrements in CV were the left parietal and the right frontal regions. We interpret this as an indication of enhanced internal regional connectivity, which could result from decreased functional connectivity with brain regions that send projections to the left parietal and right frontal cortex. In fact studies using transcranial magnetic stimulation have given evidence that alcohol disrupts the connectivity in the right frontal and left parietal cortices (Kahkonen et al., 2001). Moreover, alcohol induced enhancement of intracortical inhibition and suppression of intracortical facilitation was shown by a transcranial magnetic stimulation study that used alcohol doses equivalent to those in the current study (Ziemann et al., 1995). These effects were related to alcohol-induced potentiation of gamma-aminobutyric acid (GABA) neurotransmission in the cortex. Interestingly the right frontal cortex, which was the area most sensitive to alcohol induced decrements in CV, is the area of the brain where alcohol-induced increases in blood flow, have been associated with its reinforcing effects (Tiihonen et al., 1994). Also the right frontal cortex in addition to the left parietal cortex are two of areas that are most affected by chronic effects of alcohol (Volkow et al., 1992). The higher sensitivity of these two brain regions to the disorganizing effects of alcohol intoxication may make them more vulnerable to alcohol’s long-term effects.
The areas showing increases in CV with alcohol intoxication were much smaller than those showing decreases and included the left CGA, the lower part of the middle temporal gyrus (area 22) and the left rectal gyrus. The increased CV could reflect a heterogeneous effect of alcohol in those brain regions or it could reflect facilitation of input from projecting regions. If alcohol affects the region differentially, one could predict that the region would become more heterogeneous under alcohol influence. In the case of the CGA there is evidence that alcohol targets only certain neurons but not others (Alexandrov et al., 2001), which could provide an explanation for this pattern of response.
4.4. Metabolic regional effects
The changes in absolute and relative metabolism are consistent with previous findings documenting significant reduction in absolute whole brain and regional metabolism (Wang et al., 2000). They also replicate previous findings showing that alcohol induces relative decreases in occipital cortex and cerebellum while it induced relative increases in basal ganglia. The largest decrements in metabolism have been consistently reported in the occipital cortex, which is likely to reflect the very high concentration of GABA receptors. One would predict that the high sensitivity of the occipital cortex to the inhibiting effects of alcohol in metabolism is likely to make visual functions particularly vulnerable to alcohol effects. In fact recent fMRI studies showed that alcohol reduced visual cortical activation by photic stimulation by 33% (Levin et al., 1988).
A very interesting pattern of areas emerged when we analyzed the relative increases in activity. To start with the effects were greater for left hemispheric regions. The regions with relative increases except for the precentral gyrus, have been associated with the brain reward circuits (CGA, striatum including nucleus accumbens, amygdala, insula and mesencephalon). A similar pattern of activation of reward areas by alcohol was reported using PET and CBF measures (Ingvar et al., 1998). Increases in activity in CGA, caudate, nucleus accumbens, amygdala, insula and mesencephalon have been associated with the reinforcing effects of drugs of abuse in drug abusers (Breiter et al., 1997; Stein et al., 1998). The relative increases in metabolism induced by alcohol could indicate areas that are less sensitive to alcohol and hence when normalized by the changes in the rest of the brain appear higher. However, this change in the pattern of activity also implies a shift in the relative predominance that a given brain region has on the overall pattern of brain activity. Thus under intoxication one could postulate that the activity of reward centers in the brain predominates over that of activity in most of the cortical areas. More detailed studies are required to assess the contribution of the pattern of low cortical and relative high limbic activity on the behavioral and cognitive changes during intoxication.
In summary this study shows that during intoxication there is not only a marked reduction in whole brain glucose metabolism but also a marked modification of the regional distribution of brain function. The right hemisphere and the posterior regions in the brain become less prominent whereas the limbic regions become relatively more active. The decrease in CV, which we interpret as a decrease in brain homogeneity provides evidence that during alcohol intoxication there is also a reduction in the functional organization of the brain.
Acknowledgments
This research was supported in part by the DOE (OBER) (DE-ACO2-98CH10886), NIAAA (AA 09481) and NIH (3 M01 RR010710-06S1 and 2 P30 AG08051-11). We wish to thank David Schlyer and Robert Carciello for Cyclotron operations; Donald Warner for PET operations; Colleen Shea, Victor Garza, Robert MacGregor, David Alexoff and Payton King for radiotracer preparation and analysis; Pauline Carter, Paula Cervany, Noelwah Netusil and Naome Pappas for patient care; and Tsz Yan Lam for programming support.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Alexandrov YI, Grinchenko YV, Shevchenko DG, Averkin RG, Matz VN, Laukka S, Korpusova AV. A subset of cingulate cortical neurones is specifically activated during alcohol-acquisition behaviour. Acta Physiologica Scandinavica. 2001;171:87–97. doi: 10.1046/j.1365-201X.2001.00787.x. [DOI] [PubMed] [Google Scholar]
- 2.Bartlett EJ, Brodie JD, Wolf AP, Christman DR, Laska E, Meissner M. Reproducibility of cerebral glucose metabolic measurements in resting human subjects. Journal of Cerebral Blood Flow & Metabolism. 1988;8:502–512. doi: 10.1038/jcbfm.1988.91. [DOI] [PubMed] [Google Scholar]
- 3.Breiter HC, Gollub RL, Weisskoff RM, Kennedy DN, Makris N, Berke JD, Goodman JM, Kantor HL, Gastfriend DR, Riorden JP, Mathew RT, Rosen BR, Hyman SE. Acute effects of cocaine on human brain activity and emotion. Neuron. 1997;19:591–611. doi: 10.1016/s0896-6273(00)80374-8. [DOI] [PubMed] [Google Scholar]
- 4.De Wit H, Metz J, Wagner N, Cooper M. Behavioral and subjective effects of ethanol: relationship to cerebral metabolism using PET. Alcoholism Clinical Experimental Research. 1990;14:482–489. doi: 10.1111/j.1530-0277.1990.tb00508.x. [DOI] [PubMed] [Google Scholar]
- 5.Ingvar M, Ghatan PH, Wirsen-Meurling A, Risberg J, Von Heijne G, Stone-Elander S, Ingvar DH. Alcohol activates the cerebral reward system in man. Journal of Studies on Alcohol. 1998;59:258–69. doi: 10.15288/jsa.1998.59.258. [DOI] [PubMed] [Google Scholar]
- 6.Kahkonen S, Kesaniemi M, Nikouline VV, Karhu J, Ollikainen M, Holi M, Ilmoniemi RJ. Ethanol modulates cortical activity: direct evidence with combined TMS and EEG. Neuroimage. 2001;14:322–328. doi: 10.1006/nimg.2001.0849. [DOI] [PubMed] [Google Scholar]
- 7.Koob GF, Roberts AJ, Schulteis G, Parsons LH, Heyser CJ, Hyytia P, Merlo-Pich E, Weiss F. Neurocircuitry targets in ethanol reward and dependence. Alcoholism: Clinical & Experimental Research. 1998;22:3–9. [PubMed] [Google Scholar]
- 8.Levin JM, Ross MH, Mendelson JH, Kaufman MJ, Lange N, Maas LC, Mello NK, Cohen BM, Renshaw PF. Reduction in BOLD fMRI response to primary visual stimulation following alcohol ingestion. Psychiatry Research. 1998;82:135–146. doi: 10.1016/s0925-4927(98)00022-5. [DOI] [PubMed] [Google Scholar]
- 9.Lloyd BP, Burrin P, Smythe P, Alberti KGMM. Enzymatic fluorometric continuous flow assays for blood glucose, lactate, pyruvate, alanine, glycerol, and 3-hydroxybutrate. Clinical Chemistry. 1978;24:1724–1729. [PubMed] [Google Scholar]
- 10.Lukas SE, Mendelson JH, Benedikt RA, Jones B. EEG, physiologic and behavioral effects of ethanol administration. NIDA Research Monograph. 1986;67:209–14. [PubMed] [Google Scholar]
- 11.Peterson JB, Rothfleisch J, Zelazo PD, Pihl RO. Acute alcohol intoxication and cognitive functioning. Journal of Studies on Alcohol. 1990;51:114–122. doi: 10.15288/jsa.1990.51.114. [DOI] [PubMed] [Google Scholar]
- 12.Stein EA, Pankiewicz J, Harsch HH, Cho JK, Fuller SA, Hoffmann RG, Hawkins M, Rao SM, Bandettini PA, Bloom AS. Nicotine-induced limbic cortical activation in the human brain: a functional MRI study. American Journal of Psychiatry. 1998;155:1009–1015. doi: 10.1176/ajp.155.8.1009. [DOI] [PubMed] [Google Scholar]
- 13.Stenberg G, Sano M, Rosen I, Ingvar DH. EEG topography of acute ethanol effects in resting and activated normals. Journal of Studies on Alcohol. 1994;55:645–656. doi: 10.15288/jsa.1994.55.645. [DOI] [PubMed] [Google Scholar]
- 14.SPM99. Institute of Neurology, Wellcome Department of Cognitive Neurology.
- 15.Tiihonen J, Kuikka J, Hakola P, Paanila J, Airaksinen J, Eronen M, Hallikainen T. Acute ethanol-induced changes in cerebral blood flow. American Journal of Psychiatry. 1994;151:1505–158. doi: 10.1176/ajp.151.10.1505. [DOI] [PubMed] [Google Scholar]
- 16.Volkow ND, Hitzemann R, Wolf AP, Logan J, Fowler JS, Christman D, Dewey SL, Schlyer D, Burr G, Vitkun S, Hirschowitz J. Acute effects of ethanol on regional brain glucose metabolism and transport. Psychiatry Research. 1990;35:39–48. doi: 10.1016/0925-4927(90)90007-s. [DOI] [PubMed] [Google Scholar]
- 17.Volkow ND, Mullani N, Gould L, Adler SS, Guynn RW, Overall JE, Dewey S. Effects of acute alcohol intoxication on cerebral blood flow measured with PET. Psychiatry Research. 1988;24:201–209. doi: 10.1016/0165-1781(88)90063-7. [DOI] [PubMed] [Google Scholar]
- 18.Volkow ND, Zhu W, Felder CA, Mueller K, Welsh TF, Wang GJ, de Leon MJ. Changes in brain functional homogeneity in subjects with Alzheimer’s disease. Psychiatry Research. 2002;114:39–50. doi: 10.1016/s0925-4927(01)00130-5. [DOI] [PubMed] [Google Scholar]
- 19.Wang GJ, Volkow ND, Franceschi D, Fowler JS, Thanos PK, Scherbaum N, Pappas NR, Wong CT, Hitzemann RJ, Felder CA. Regional brain metabolism during alcohol intoxication. Alcoholism: Clinical & Experimental Research. 2000;24:822–829. [PubMed] [Google Scholar]
- 20.Wendt PE, Risberg J. Ethanol reduces rCFB activation of left dorsolateral prefrontal cortex during a verbal fluency task. Brain and Language. 2001;77:197–215. doi: 10.1006/brln.2000.2434. [DOI] [PubMed] [Google Scholar]
- 21.Wendt PE, Risberg J, Stenberg G, Rosen I, Ingvar DH. Ethanol reduces asymmetry of visual rCBF responses. Journal of Cerebral Blood Flow & Metabolism. 1994;14:963–973. doi: 10.1038/jcbfm.1994.129. [DOI] [PubMed] [Google Scholar]
- 22.Ziemann U, Lonnecker S, Paulus W. Inhibition of human motor cortex by ethanol. A transcranial magnetic stimulation study. Brain. 1995;118:1437–1446. doi: 10.1093/brain/118.6.1437. [DOI] [PubMed] [Google Scholar]