Abstract
Within-session decreases in instrumental responding to obtain food, consistent with habituation, have been reliably demonstrated in adults and children. This study tested the hypothesis that within-session decreases in instrumental responding for food are due to habituation rather than satiation. Thirty-eight 8–12 year old children performed a computer- based operant task to earn points toward access to potato chips for 20 minutes, and for chocolate candies for the final six minutes of the session. Portion size of the food reinforcer (75 vs 225 kcal) and food consumption (consumption/no consumption) were varied during the first 20 minutes. There was no difference in the rate of response decrease between the two portion size conditions. Both the consumption and non-consumption groups showed response decelerations during the first 20 minutes, with responses in the consumption group decreasing at a faster rate. When the novel food was presented, participants in all conditions recovered responding. Although satiation may contribute to reductions in motivated responding for food when food is consumed, habituation provides a more complete explanation for the results observed in this study.
Keywords: habituation, satiation, food, eating, obesity, instrumental responding, humans
Habituation is a basic, neurobiological process by which responses to stimuli decrease with repeated exposures (Groves & Thompson, 1970). This is ubiquitous among species and can be applied to both reflexive physiological responses and complex behavioral processes (McSweeney & Swindell, 1999; McSweeney, Swindell, & Weatherly, 1996). There has been a series of habituation studies in humans which show that the salivary responses to food cues habituate after repeated presentations, and dishabituate or recover when a new food stimulus is presented (Myers Ernst & Epstein, 2002; Temple, Kent, Giacomelli, Paluch, Roemmich, & Epstein, 2006; Wisniewski, Epstein, & Caggiula, 1992). These studies have used both olfactory (Epstein, Saad, Handley, Roemmich, Hawk, & McSweeney, 2003; Temple et al., 2006) and taste (Epstein, Caggiula, Rodefer, Wisniewski, & Mitchell, 1993; Epstein & Paluch, 1997; Myers & Epstein, 1997) stimuli, which involve little or no energy intake. Animal research has also been conducted using very small portions of taste stimuli to study habituation of mouthing responses (Swithers-Mulvey, Miller, & Hall, 1991; Swithers-Mulvey, Mishu, & Hall, 1992). Thus, habituation to food cues can occur in the absence of food consumption and satiation, defined as the physiological and psychological factors that result in meal termination.
Habituation theory has also been applied to changes in motivated responding for reinforcers. Studies in animals and in humans have shown that instrumental responding for food is reduced over repeated presentations (Epstein et al., 2003; Myers Ernst & Epstein, 2002; Temple, 2007). In addition, responding for food recovers when a novel food is available (Epstein et al., 2003; Myers Ernst & Epstein, 2002; Temple et al., 2006), which is characteristic of habituation (Thompson & Spencer, 1966). These paradigms to assess within session changes in motivated responding for food may also involve simultaneous food consumption, which raises the question whether the decreased responding observed in these studies is due to habituation or satiation.
Based on an extensive literature review, McSweeney and Murphy (Mcsweeney & Murphy, 2000) argue that within-session decreases in instrumental responding for food are due to habituation, not satiation. One of the most powerful arguments against the satiation hypothesis is that if cessation of motivated responding were due to satiation, then presentation of additional food would not result in a resumption of responding. However, research reliably shows that presentation of a novel food after responding has ceased leads to recovery of responding (Epstein et al., 2003; Temple et al., 2006; Wisniewski et al., 1992), consistent with habituation. A satiation theory also would suggest that providing more energy, either by providing more energy dense reinforcers or a larger portion of a reinforcer, would result in a more rapid decrease in responding, as a sense of fullness would be achieved faster. However, studies have shown that using larger or more energy dense food stimuli results in either no difference, or sometimes an increase, in the rate of responding (Epstein et al., 1993; Fuhrer, 1977; Pitman, Ottenweller, & Natelson, 1990). Finally, because satiation is achieved after consuming food, satiation theory would argue that decreases in responding for food would not occur in the absence of food consumption, but habituation theory would predict that responding for food should decrease with sensory exposure to food cues, even in the absence of food consumption (McSweeney & Murphy, 2000).
The purpose of this study was to demonstrate that reduction in instrumental responding for food is due to habituation rather than satiation. We conducted three experimental manipulations. The first manipulation varied the portion size of the food stimulus that was earned. If satiation was important in response reduction, it would be expected that responses should decline faster for the large portion (225 kcal) group in comparison to the small portion (75 kcal) group. Habituation theory would predict no differences as a function of energy consumed or, perhaps, an increase in responses in the larger portion group (McSweeney & Roll, 1998; Weatherly, McSweeney, & Swindell, 2004). The second manipulation was the presentation of a novel food stimulus after responses to the original stimulus had decreased. If response reductions are due to satiation, and the subjects could earn as much food as they wished, then presenting more food should not result in a resumption of responding for food. However, recovery of responding after presentation of a novel stimulus is a defining characteristic of habituation. The third manipulation compared responses for food between individuals who were allowed to consume the food as it was earned and those who were exposed to sensory cues from the food but told that they had to wait until the end of the experiment to consume it. If satiation is required for response reduction, than the non-consumption group should show no decrease in responding for food over time. However, habituation theory would predict that a decrease in responding to sensory cues from food stimuli can be observed even in the absence of consumption of the food reinforcer (McSweeney & Murphy, 2000).
Methods
Participants
Participants were 20 females and 18 male, non-obese (<95th Body Mass Index, BMI Percentile) (Kuczmarski et al., 2002) 8 to 12 year-old children recruited from local newspaper advertisements and from the laboratory database. Compensation for completing the study was $20.00 US dollars. Potential participants were excluded if they had one or more of the following: medications or conditions that may suppress appetite or alter responding to food cues (e.g., methylphenidate, ADHD, diabetes, upper respiratory illness); current psychological disorder or developmental disability. Inclusion criteria included rating the test foods as moderately liked (>3 on a 5-point Likert-type scale of liking). Participants were randomized into one of four conditions based on portion size (small or large), and on within-session consumption (consumption vs. no consumption).
Procedure
Parents of potential participants were screened over the telephone to ensure initial eligibility to study protocol. If children were eligible, a 90-minute visit to the University at Buffalo’s Behavioral Medicine Laboratory was scheduled between the hours of 2:00PM - 5:30PM. The parents were told that their children could not eat or drink anything, except for water, for at least three hours prior and not to consume the study foods which were potato chips and chocolate for 24 hours prior to test appointment. Upon arrival to the laboratory, parents and children read and signed informed consent and assent forms, and assisted in completing a same-day dietary recall. Parents filled out a demographics questionnaire while the children completed a food preference questionnaire, food liking and hunger scales. After questionnaires and scales were complete, parents were escorted to the waiting area and instructions on how to perform the computer generated task were provided to the participants. Participants were shown how much food could be earned for one point. Depending upon the portion size condition, participants were either shown 75 kcal (14g) or 225 kcal (42g) portions of Wavy Lay’s Potato Chips™ as the habituation stimulus or 75 kcal (15g) or 225 kcal (45g) portions of M&M Chocolate Candies™ as the recovery stimulus. Participants in the Consumption condition were instructed that they could consume the food as soon as it was earned while participants in the Non-Consumption condition were told that they had to wait to consume food earned until the entire task was completed (Table 1). Water was provided ad libitum and both conditions could earn and consume as much or as little of the food stimuli as they wanted. When a point was earned the experimenter delivered the food stimulus into the experimental room and placed it on a table next to the computer station. Participants were able to earn points towards portions of potato chips for the first 20-minutes of the task and portions of M&M candies™ for the last six-minute session of the task. An activity station that included age-appropriate magazines (National Geographic©, Kids Discover© and Disney Adventures©), cross words, word searches, mazes and word jumbles was set up so that when children no longer wished to respond for food, they could engage in non-contingent activities. This reduces the potential for responding simply out of boredom. They were told that they could go back and forth between the activities and the computer task as much as they wanted. After the participants completed the computer task, hunger was reassessed and they completed a modified Dutch Eating Behavior Questionnaire designed to measure dietary restraint in children (Hill & Pallin, 1998). Finally participants and parents were debriefed and children were measured for height and weight. All procedures were conducted in accordance with NIH Guidelines for the ethical conduct of experiments in human participants and with the approval of the University at Buffalo Institutional Review Board.
Table 1.
Table showing study design and experimental groups.
| Consumption Condition | Portion Size | Trials 1 – 10 “Habituation” | Trials 11 – 13 “Recovery” |
|---|---|---|---|
| Consumed Food During Experiment (n = 19) | 75 kcal (n = 9) |
Potato chips | M&Ms |
| 225 kcal (n = 10) | |||
| Consumed Food at the End of the Experiment (n=19) | 75 kcal (n = 9) |
||
| 225 kcal (n = 10) | |||
The Laboratory Environment
The laboratory is designed specifically for food experiments and is constructed with a fresh air delivery system. The laboratory rooms are in negative pressure, so the exhaust has greater cubic feet per minute than the supply, and the air circulates ~10 times/h, or new air is circulated once every 6 min in the experimental room (1385 ft3) and the experiment room is also equipped with a Carbon HEPA Filtration System (Carbon, Permanganate, Zeolite) which filters at a rate of 225ft3/min to assist in removing lingering odors.
Measures
Instrumental Responding Task
An operant computer generated task using a Variable Interval 120s reinforcement schedule (VI 120) was used to measure motivated responding to food stimuli. The task consisted of a square that flashes red every time a response was made on a mouse button, and another square that flashes green when a point is earned. The habituation phase was 20 minutes, broken into of ten, 2-minute time blocks where participants could earn points towards portions of potato chips. The recovery phase was six minutes, broken into three, 2-minute time blocks where participants could earn portions of chocolate candies. These times were imposed to keep consistency among participants in the number of food portions available, but from the point of view of the participant, responding was continuous. The pattern of responses over trials served as a primary outcome measure.
Food Hedonics and Hunger
Liking of study foods assessed by a 5-point Likert-type scale, anchored by “do not like” (1) and “like very much” (5). A 40-item food questionnaire which included the study foods was administered to ensure reliability of liking of foods presented. Pre-experimental hunger was assessed using a 5-point Likert-type scale anchored by “not very hungry” (1) and “very hungry” (5).
Same-day Food Recall
Participants, with the help of their parent, were asked to recall all foods and beverages that had been eaten on the day of the experiment. The participant was guided through the interview by the experimenter who asked about time of day, portion sizes, and whether foods were completed. This measure was used to ensure that the study foods were not consumed the day of experiment, that the child adhered to not consuming food or drinking beverages except water at least 3 hours prior to scheduled appointment, and to control for potential differences in energy intake prior to the experiment. Total energy consumption (in Kcal) was calculated using Nutritionist V nutrient analysis software (I, 2000).
Dietary Restraint
Dietary restraint was evaluated using the Dutch Eating Behavior Questionnaire designed for children (Hill & Pallin, 1998). Examples of questions included on the restraint scale are “I have tried to lose weight”; “I try not to eat between meals because I want to be thinner”.
Demographics
An experimenter designed demographics questionnaire was used to assess highest education level, annual household income, race, and ethnicity.
Anthropometrics
Height (cm) and weight (lbs) were measured in light clothing with shoes removed after the participant had voided using a digital stadiometer (Digi-Kit, North Bend, WA) and digital Tanita™ weight scale (Tanita Corporation of America, Inc., Arlington Heights, IL). These measures were used to calculate BMI (Kg/m2).
Analytic Plan
Participant characteristics, food hedonics, and baseline hunger were compared using an analysis of variance (ANOVA) with consumption (consumption vs. no consumption) and portion size (small or large) as between subject variables for continuous variables and Chi-Square tests for dichotomous variables. Changes in the pattern of instrumental responding were analyzed using a mixed ANOVA with access to food and portion size as between subject factors and trials 1 – 13 as the within subjects factor. In order to distinguish between effects during the habituation and recovery phases, separate ANOVA analyses were also done with trials 1 – 10 (habituation) and trials 10 – 13 (recovery) as the within subjects factors. Analyses were conducted using SYSTAT software (Systat Software, 2002).
Results
Subject characteristics were shown in Table 2. There were no differences among the different conditions for any of the characteristics that were examined (all p > 0.05). The racial and ethnic composition of the sample included 75% non-Hispanic Caucasian, 13% African American, 3% African American and Hispanic, 3% American Indian or Alaskan Native, 3% Asian and 3% reported other. All groups had equivalent baseline hunger (F (1, 34) = 0.47, p = 0.50).
Table 2.
Participant characteristics by consumption and portion groups
| Consumption | Non-Consumption | |||||||
|---|---|---|---|---|---|---|---|---|
| Small Portion | Large Portion | Small Portion | Large Portion | |||||
| M | SD | M | SD | M | SD | M | SD | |
| Age (years) | 10.5 | 1.5 | 10.7 | 1.7 | 10.7 | 1.6 | 10.6 | 1.6 |
| BMI Percentile | 45.9 | 36.6 | 52.0 | 30.6 | 47.0 | 34.3 | 42.4 | 24.1 |
| Dietary Restraint | 3.1 | 2.1 | 4.4 | 1.8 | 4.8 | 3.2 | 3.9 | 3.6 |
| Potato Chip Liking | 4.7 | 0.5 | 4.6 | 0.5 | 4.2 | 0.7 | 4.2 | 0.6 |
| M&M Liking | 4.1 | 1.4 | 4.8 | 0.4 | 4.2 | 1.1 | 4.6 | 0.7 |
| Same-Day Intake (kcal) | 928 | 233 | 826 | 309 | 891 | 327 | 844 | 341 |
| M | F | M | F | M | F | M | F | |
| Sex | 4 | 5 | 4 | 6 | 5 | 4 | 5 | 5 |
| N | % | N | % | N | % | N | % | |
| Race: | ||||||||
| Caucasian | 7 | 78 | 7 | 70 | 5 | 56 | 9 | 90 |
| African American | 2 | 22 | 2 | 20 | 2 | 22 | 0 | 0 |
| Asian | 0 | 0 | 0 | 0 | 1 | 11 | 0 | 0 |
| Hispanic | 0 | 0 | 1 | 10 | 0 | 0 | 0 | 0 |
| Other | 0 | 0 | 0 | 0 | 1 | 11 | 1 | 10 |
| Education: | ||||||||
| High School | 2 | 22 | 1 | 10 | 4 | 44 | 2 | 20 |
| College or | 7 | 78 | 9 | 90 | 5 | 56 | 8 | 80 |
| Income: | ||||||||
| < $50,000 | 3 | 38 | 3 | 33 | 4 | 45 | 2 | 22 |
| $50,000 – $70,000 | 2 | 24 | 2 | 22 | 3 | 33 | 2 | 22 |
| > $70,000 | 3 | 38 | 4 | 45 | 2 | 22 | 5 | 66 |
When examining the pattern of responding across all trials, there was a two-way interaction between consumption condition and trials (F (12, 402) = 2.51, p = 0.003) and a main effect of consumption condition (F (1, 34) = 6.49, p = 0.02) with participants in the consumption group showing a more rapid decrease in instrumental responses than those who had delayed access to food. There was no three-way interaction among portion size, consumption condition, and trials (F (12, 402) = 0.84, p = 0.61), no two-way interaction between portion size and trials (F (12, 402) = 0.57, p = 0.87) or portion size and consumption condition (F (1, 34) = 0.34, p = 0.57), and no main effect of portion size (F (1, 34) = 0.14, p = 0.72).
When examining the habituation and recovery phases alone, the portion of food presented had no effect on the pattern of responding for food. The ANOVA showed an interaction between consumption and trials (F (9, 306) = 2.63, p = 0.006; Figure 1A) and a main effect of consumption of snack foods on number of responses (F (1, 34) = 7.29, p = 0.01) for trials 1 – 10. However, there was no interaction among portion size, consumption condition, and trials (F (9, 306) = 1.04; p = 0.41); no interaction between portion size and trials (F (9, 306) = 0.53, p = 0.85; Figure 1B) and no interaction between portion size and consumption (F (1, 34) = 0.50, p = 0.48). As was mentioned above, participants in the consumption group showed a more rapid decrease in instrumental responses than those who had delayed access to food. Despite differences in the rate of habituation, separate analyses on each group showed that the group who consumed food (F (1, 18) = 13.48, p = 0.002) and the group that had delayed access to food (F (1, 18) = 5.82, p = 0.03) both showed a decrease in responding between trial 1 – trial 10. There was a significant effect of trials on responding on trials 10 – 13 (F (3, 102) = 19.97, p < 0.001). There was no interaction between trials and portion size (F (3, 102) = 0.31, p = 0.82; Figure 1A) or consumption (F (3, 102) = 1.76, p = 0.16; Figure 1B), and no three-way interaction among trials, portion size, and consumption on responding from trials 10 – 13 (F (3, 102) = 0.17; p = 0.91). Linear contrasts showed significant increase in responding between trials 10 and 11 (F (4, 34) = 7.85, p < 0.001), and significant decreases between trials 11 and 12 (F (4, 34) = 18.10, p < 0.001) and trials 11 and 13 (F (4, 34) = 15.16, p < 0.001) for all conditions.
Figure 1.
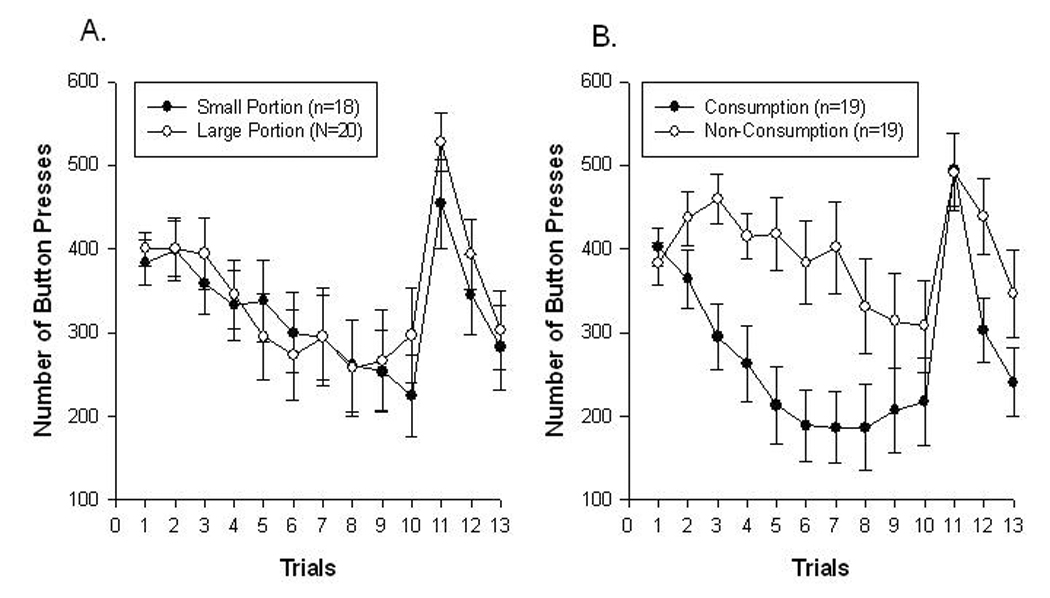
Mean ± SEM number of responses for potato chips (habituation; trials 1–10) and M&M’s (recovery; trials 11–13) for individuals A. who consumed the food throughout the duration of the experiment (black circles) and those who had to delay consumption until the end (white circles) and B. participants assigned to small (75 kcal; black circles) and large (225 kcal; white circles) portions of food. The habituation phase is shown to the left of the dashed line and the recovery phase is to the right of the dashed line in both figures.
Discussion
This study tested the hypothesis that within session changes in motivated responding for food in children are due to habituation and not satiation. The first test of this hypothesis was the comparison of motivated responding for either 75 kcal food portions or 225 kcal food portions, and our results showed portion size had no effect on the rate of change in instrumental responding, despite the fact that children in the large portion group earned a reinforcer that was three times greater than those in the small group. Our findings are consistent with previous work showing that larger or more energy dense reinforcers do not increase the rate of decrease in responding for food (Roll, McSweeney, Johnson & Weatherly, 1995; Weatherly et al. 2004). Roll and colleagues (1995, Experiment 1) showed that rates of decrease in motivated responding for food did not differ as a function of energy density, consistent with research from our laboratory showing no difference in the rate of habituation in humans when energy density was varied (Epstein et al., 1993). When sucrose concentration was varied, slower decreases in responding were observed for higher concentrations (Melville, Rue, Rybiski, & Weatherly, 1997), the opposite of what would be expected if satiation was the best explanation for the reduction in responding. In addition, when the magnitude of a food reinforcer was changed (either halved or doubled) within the session, increasing the amount of reinforcer slowed down the rate of response deceleration compared with the baseline condition (Weatherly et al., 2004).
Recovery in responding after presentation of a novel stimulus is a defining characteristic of habituation (Thompson & Spencer, 1966). A second test of the hypothesis involved the presentation of a novel food (M&Ms) after responding had significantly decreased for the original food (potato chips). If the satiation hypothesis were correct, then presentation of additional food would not result in a resumption of responding. However, we observed an increase in responding for all subjects, independent of the previous conditions, which is consistent with research that shows presentation of a novel food after responding has ceased leads to recovery of responding (Epstein et al., 2003; Temple et al., 2006; Wisniewski et al., 1992).
The third test of our hypothesis was to compare responses in individuals who consumed food throughout the duration of the experiment with those who received the food, but were not allowed to eat until the end of the experiment. A decrease in responding during the exposure to sensory cues without food consumption during the initial twenty minute period is predicted by habituation but not by satiation theory. Previous studies have demonstrated that habituation to sensory cues related to food, such as olfactory (Epstein et al., 2003; Temple et al., 2006) and gustatory (Epstein et al., 1993; Epstein & Paluch, 1997; Myers & Epstein, 1997) stimuli, occurs in the absence of eating. Food consumption involves the integration of multiple sensory stimuli, including visual, olfactory, and gustatory signals as well as cognitive and post-ingestive cues that may accelerate decreases in responding. The group that did not consume food was exposed only to visual and olfactory cues from the food stimuli throughout the duration of the experiment. Previous studies have shown that neuronal and physiological responses habituate to visual stimuli as well as to a combination of visual and olfactory stimuli (Epstein et al., 2003; Holsen, Zarcone, Thompson, Brooks, Anderson, Ahluwalia, Nollen, & Savage, 2005; Temple et al., 2006). Imaging studies have found that viewing pictures of food leads to neural activation in brain regions associated with taste, reward, and memory (Holsen et al., 2005; LaBar, Gitelman, Parrish, Kim, Nobre, & Mesulam, 2001; Simmons, Martin, & Barsalou, 2005). The integration of responses in these brain regions allow the individual to assess the salience of the stimulus and, if properly motivated, initiate behaviors involved in food consumption (Holsen et al., 2005). Once eating behaviors have been initiated, neural responding to the food stimulus habituates (Holsen et al., 2005). It is possible, as our data suggest, that habituation of neuronal responses to food drives habituation of behavioral responses for food as well, even in the absence of food consumption (LaBar et al., 2001).
Though subjects did habituate to food cues without eating the food, consumption did increase the rate of reduction in responding. One explanation for this effect is that both satiation and habituation contributed to this reduction when food was consumed. A steeper rate of reduction in responding after food consumption would be predicted by satiation theory (Bizo, Bogdanov & Killeen, 1998; DeMarse et al, 1999). One interesting way to test the independent effects of sensory stimulation that could lead to habituation versus energy repletion that could lead to satiation is in experiments that combine sensory taste stimuli and energy placed in the stomach that bypasses oral stimulation. In two studies, animals showed a reduction in response when only oral sensory stimuli were presented, but a more rapid reduction in responding when oral sensory stimuli were presented along with large gastric infusions (Roll et al., 1995, Experiment 3; Swithers-Mulvey & Hall, 1993). These results suggest that in paradigms in which oral sensory and gastric fill are presented simultaneously, both contribute to the within session reductions in operant responding. Another explanation for difference in the rate of decrease between eaten and uneaten foods is sensory specific satiety, defined as a decrease in hedonic ratings of foods eaten to satiety compared with uneaten foods (Rolls, Rolls, Rowe, & Sweeney, 1981). There is a large body of literature showing that as a food is consumed, it becomes less pleasurable and eating rate for that food declines, but that uneaten foods retain high hedonic ratings and are readily consumed when presented after another food has been eaten to satiety (Rolls, 1985; Rolls, 1986; Rolls et al., 1981; Rolls, Van Duijvenvoorde, Rolls, 1984). Although this study examined motivated responding for food and not hedonic ratings of food, it is possible that a decrease in food hedonics when the food was eaten contributed to a faster decline in motivation to obtain the food than when the food was not consumed. Similarly, the increase in responding when a novel food was presented is also consistent with sensory specific satiety and, thus, higher hedonic ratings for the novel food may have contributed to increased responding.
This study demonstrates that within-session changes in the pattern of responding for food can generally be attributed to habituation, consistent with findings in animal models as well as previous studies in humans. While the decreases in the rate of responding in the consumption condition could have been influenced in part by satiation, the decreases in responding to sensory stimuli from food in the non-consumption condition, the lack of effect of different portions sizes, and the recovery of responding when a novel food was presented are best understood by habituation. The observation that motivated responding for food can be decreased in the absence of energy intake, suggests that finding ways to promote habituation to food, such as presentation of food odors or pictures of food prior to eating, may reduce energy intake. Stimulation of the sensory systems, in particular olfactory and gustatory systems, has been applied to weight loss treatment. For example, a study by Hirsch and Gomez showed that inhalation of odors prior to eating, in the absence of any instructions about dieting or energy restriction, resulted in a significantly amount of weight loss relative to a control group given no odors (Hirsch & Gomez, 1995). Although the precise mechanism is not known, one possibility is that sensory stimulation facilitates weight loss by accelerating the rate of habituation to food cues.
Acknowledgements
This research was supported by a grant from the NICHD (HD044725) to LHE.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bizo LA, Bogdanov SV, Killeen PR. Satiation causes within-session decreasese in instrumental responding. Journal of Experimental Psychology: Animal Behavior Processes. 1998;24:439–452. [PubMed] [Google Scholar]
- DeMarse TB, Killeen PR, Baker D. Satiation, capacity, and within-session responding. Journal of Experimental Analysis of Behavior. 1999;72:407–423. doi: 10.1901/jeab.1999.72-407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein LH, Caggiula AR, Rodefer JS, Wisniewski L, Mitchell SL. The effects of calories and taste on habituation of the human salivary response. Addictive Behaviors. 1993;18:179–185. doi: 10.1016/0306-4603(93)90048-e. [DOI] [PubMed] [Google Scholar]
- Epstein LH, Paluch RA. Habituation of facial muscles responses to repeated food stimuli. Appetite. 1997;29:213–224. doi: 10.1006/appe.1997.0102. [DOI] [PubMed] [Google Scholar]
- Epstein LH, Saad FG, Handley EA, Roemmich JN, Hawk LW, McSweeney FK. Habituation of salivation and motivated responding for food in children. Appetite. 2003;41:283–289. doi: 10.1016/s0195-6663(03)00106-5. [DOI] [PubMed] [Google Scholar]
- First Data Bank I. Nutritionist V. San Bruno, CA: First Data Bank; 2000. [Google Scholar]
- Fuhrer MJ. Effects of stimulus intensity on the habituation of flexor withdrawal activity mediated by the functionally transected human spinal cord. Physiological Psychology. 1977;5:321–326. [Google Scholar]
- Groves PM, Thompson RF. Habituation: A dual-process theory. Psychological Reviews. 1970;77:419–450. doi: 10.1037/h0029810. [DOI] [PubMed] [Google Scholar]
- Hill AJ, Pallin V. Dieting awareness and low self-worth: related issues in 8-year-old girls. International Journal of Eating Disorders. 1998;24:405–413. doi: 10.1002/(sici)1098-108x(199812)24:4<405::aid-eat7>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- Hirsch AR, Gomez R. Weight reduction through inhalation of odorants. Journal of Neurological &, Orthopaedic Medicine and Surgery. 1995;16:28–31. [Google Scholar]
- Holsen LM, Zarcone JR, Thompson TI, Brooks WM, Anderson MF, Ahluwalia JS, Nollen NL, Savage CR. Neural mechanisms underlying food motivation in children and adolescents. Neuroimage. 2005;27:669–676. doi: 10.1016/j.neuroimage.2005.04.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuczmarski RJ, Ogden CL, Guo SS, Grummer-Strawn LM, Flegal KM, Mei Z, et al. 2000 CDC Growth Charts for the United States: methods and development. Vital Health Statistics. 2002;11:1–190. [PubMed] [Google Scholar]
- LaBar KS, Gitelman DR, Parrish TB, Kim YH, Nobre AC, Mesulam MM. Hunger selectively modulates corticolimbic activation to food stimuli in humans. Behavioral Neuroscience. 2001;115:493–500. doi: 10.1037/0735-7044.115.2.493. [DOI] [PubMed] [Google Scholar]
- McSweeney FK, Murphy ES. Criticisms of the satiety hypothesis as an explanation for within-session decreases in responding. Journal of the Experimental Analysis of Behavior. 2000;74:347–361. doi: 10.1901/jeab.2000.74-347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McSweeney FK, Roll JM. Do animals satiate or habituate to repeatedly presented reinforcers? Psychonomic Bulletin Reviews. 1998;5:428–442. [Google Scholar]
- McSweeney FK, Swindell S. Behavioral economics and within-session changes in responding. Journal of the Experimental Analysis of Behavior. 1999;72:355–371. doi: 10.1901/jeab.1999.72-355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McSweeney FK, Swindell S, Weatherly JN. Within-session changes in responding during concurrent schedules with different reinforcers in the components. Journal of the Experimental Analysis of Behavior. 1996;66:369–390. doi: 10.1901/jeab.1996.66-369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melville CL, Rue HC, Rybiski LR, Weatherly JN. Altering reinforcer variety or intensity changes the within-session decrease in responding. Learning & Motivation. 1997;28:609–621. [Google Scholar]
- Myers Ernst M, Epstein LH. Habituation of responding for food in humans. Appetite. 2002;38:224–234. doi: 10.1006/appe.2001.0484. [DOI] [PubMed] [Google Scholar]
- Myers MD, Epstein LH. The effect of dietary fat on salivary habituation and satiation. Physiology & Behavior. 1997;62:155–161. doi: 10.1016/s0031-9384(97)00028-0. [DOI] [PubMed] [Google Scholar]
- Pitman DL, Ottenweller JE, Natelson BH. Effect of stressor intensity on habituation and sensitization of glucocorticoid responses in rats. Behavioral Neuroscience. 1990;104:28–36. doi: 10.1037//0735-7044.104.1.28. [DOI] [PubMed] [Google Scholar]
- Roll JM, McSweeney FK, Johnson KS, Weatherly JN. Satiety contributes little to within-session decreases in responding. Learning & Motivation. 1995;26:324–341. [Google Scholar]
- Rolls BJ. Experimental analysis of the effects of variety in a meal on human feeding. American Journal of Clinical Nutrition. 1985;42:932–939. doi: 10.1093/ajcn/42.5.932. [DOI] [PubMed] [Google Scholar]
- Rolls BJ. Sensory-specific satiety. Nutrition Reviews. 1986;44:93–101. doi: 10.1111/j.1753-4887.1986.tb07593.x. [DOI] [PubMed] [Google Scholar]
- Rolls BJ, Rolls ET, Rowe EA, Sweeney K. Sensory specific satiety in man. Physiology & Behavior. 1981;27:137–142. doi: 10.1016/0031-9384(81)90310-3. [DOI] [PubMed] [Google Scholar]
- Rolls BJ, Van Duijvenvoorde PM, Rolls ET. Pleasantness changes and food intake in a varied four-course meal. Appetite. 1984;5:337–348. doi: 10.1016/s0195-6663(84)80006-9. [DOI] [PubMed] [Google Scholar]
- Simmons WK, Martin A, Barsalou LW. Pictures of appetizing foods activate gustatory cortices for taste and reward. Cerebral Cortex. 2005;15:1602–1608. doi: 10.1093/cercor/bhi038. [DOI] [PubMed] [Google Scholar]
- Swithers-Mulvey SE, Hall WG. Integration of oral habituation and gastric signals in decerebrate rat pups. American Journal of Physiology Regulatory Integrative & Comparative Physiology. 1993;265:216–219. doi: 10.1152/ajpregu.1993.265.1.R216. [DOI] [PubMed] [Google Scholar]
- Swithers-Mulvey SE, Miller GL, Hall WG. Habituation of oromotor responding to oral infusions in rat pups. Appetite. 1991;17:55–67. doi: 10.1016/0195-6663(91)90084-6. [DOI] [PubMed] [Google Scholar]
- Swithers-Mulvey SE, Mishu KR, Hall WG. Oral habituation in rat pups is in the brain stem. Physiology & Behavior. 1992;51:639–642. doi: 10.1016/0031-9384(92)90189-9. [DOI] [PubMed] [Google Scholar]
- Systat Software. Systat 10.2. SYSTAT Software Inc.; Richmond, CA: 2002. [Google Scholar]
- Temple JL, Kent KM, Giacomelli AM, Paluch RA, Roemmich JN, Epstein LH. Habituation and recovery of salivation and motivated responding for food in children. Appetite. 2006;46:280–284. doi: 10.1016/j.appet.2006.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson RF, Spencer WA. Habituation: A model phenomenon for the study of neural substrates of behavior. Psychological Reviews. 1966;73:13–43. doi: 10.1037/h0022681. [DOI] [PubMed] [Google Scholar]
- Weatherly JN, McSweeney FK, Swindell S. Within-session rates of responding when reinforcer magnitude is changed within the session. Journal of General Psychology. 2004;131:5–16. doi: 10.3200/GENP.131.1.5-17. [DOI] [PubMed] [Google Scholar]
- Wisniewski L, Epstein LH, Caggiula AR. Effect of food change on consumption, hedonics, and salivation. Physiology & Behavior. 1992;52:21–26. doi: 10.1016/0031-9384(92)90428-5. [DOI] [PubMed] [Google Scholar]


