Abstract
Several prior imaging studies of healthy adults have correlated volumes of the hippocampus and amygdala with measures of general intelligence (IQ), with variable results. In this study, we assessed correlations between volumes of the hippocampus and amygdala and full-scale IQ scores (FSIQ) using a method of image analysis that permits detailed regional mapping of this correlation throughout the surface contour of these brain structures. We delineated the hippocampus and amygdala in high-resolution magnetic resonance images of the brain from 34 healthy individuals. We then correlated FSIQ with overall volumes and with the surface morphologies of each of these structures. Hippocampus volumes correlated significantly and inversely with FSIQ independently of gender, age, socioeconomic status, and whole brain volume. Left and right hippocampus volumes correlated respectively with verbal and performance IQ subscales. Higher IQs were significantly associated with large inward deformations of the surface of the anterior hippocampus bilaterally. These findings suggest that a smaller anterior hippocampus contributes to an increased efficiency of neural processing that subserves overall intelligence.
Keywords: Neuroimaging, Intelligence, Cognition, Neuropsychology, Hippocampus, Amygdala
1. Introduction
Individual differences in general intelligence (IQ) have been attributed largely to differences in the total volumes of the brain and of gray (GM) and white matter (WM) (McDaniel, 2005; Pennington et al., 2000; Posthuma et al., 2002). One large study of healthy adults indicated a weak but significant positive correlation between measures of full-scale IQ (FSIQ) and the volumes of frontal and temporal lobes (Flashman, Andreasen, Flaum, & Swayze, 1998). Consistent with these findings, recent studies using voxel-based morphometry have shown an association of FSIQ with the density of GM and WM in regions of the frontal, parietal, temporal, and occipital lobes that subserve verbal and performance abilities (Haier, Jung, Yeo, Head, & Alkire, 2004; Thompson et al., 2001). In addition, a vast number of animal and human studies have suggested that mesolimbic structures, including the hippocampus and amygdala, also play an important role in learning, memory, and cognition (Bechara, Damasio, & Damasio, 2003; Burns, Everitt, & Robbins, 1999; Everitt et al., 1999; Fried, Cameron, Yashar, Fong, & Morrow, 2002; Fried et al., 2001; Jones-Gotman, 1986; Kahn et al., 2002; Squire, Stark, & Clark, 2004). Moreover, the volume of the hippocampus has been shown to correlate positively and significantly with IQ in healthy adults (Andreasen, Flaum, Swayze, O’Leary, et al., 1993), although another more recent study using voxel-based morphometry failed to demonstrate any correlation of IQ with volumes of GM or WM volume in mesolimbic structures (Haier, et al., 2004). Differences in the methods of image analysis may have contributed to the discrepancies between these findings.
To understand better the role of the hippocampus and amygdala in supporting general intellectual abilities, we used magnetic resonance imaging (MRI) to measure the volumes of these structures in 34 healthy adults, and we assessed whether the volumes and the surface morphologies of these structures correlated with FSIQ. Given the clinical and preclinical evidence that the hippocampus and amygdala subserve learning and memory functions, as well as the important contributions of these functions to overall intelligence, we hypothesized that volume and surface morphology of the hippocampus and amygdala would correlate significantly with FSIQ.
2. Methods and materials
2.1. Subject recruitment and characterization
We recruited healthy adult participants selected randomly from a telemarketing database of households and from fliers distributed in the New Haven community (Peterson et al., 2000). Subjects were contacted initially through an introductory letter, followed by screening telephone calls. Exclusionary criteria included an IQ below 80, history of head trauma with loss of consciousness, substance abuse, and seizure disorder. The Schedule for Affective Disorders and Schizophrenia (Endicott & Spitzer, 1978) was administered to all participants. We excluded from participation any individual who met DSM-IV criteria for a current axis I or II disorder (American Psychiatric Association, 1994).
Participants included 34 adults (15 women and 19 men) aged 18–57 (mean 31.5 ± 11.1) years. They were primarily from households of upper-middle-class socioeconomic status (SES) (mean 49.1 ± 12.4, possible range 0–64) (Hollingshead, 1975), and all were medication-free. Written, informed consent was obtained for all participants after they received a full description of the study.
2.2. MRI scanning
High-resolution T1-weighted magnetic resonance images (MRI) were acquired using a single 1.5-T scanner (GE Signa; General Electric, Milwaukee, Wis.). Head positioning was standardized using cantho-meatal landmarks. Brain scans were acquired using a sagittal 3-D volume spoiled gradient echo sequence with repetition time = 24 ms, echo time = 5 ms, 45° flip angle, frequency encoding superior/inferior, no wrap, 256 × 192 matrix, field of view = 30 cm, 2 excitations, slice thickness = 1.2 mm, and 124 contiguous slices encoded for sagittal slice reconstruction, with voxel dimensions of 1.17 × 1.17 × 1.2 mm.
2.3. Neuroanatomical measures
The amygdala and hippocampus were manually traced on Sun Ultra 10 workstations using ANALYSE 7.5 software (Biomedical Imaging Resource, Mayo Foundation, Rochester, MN); analysts were blind to participant characteristics and hemisphere (images were randomly flipped left–right prior to analysis). Large-scale variations in image intensity were removed, and images were reformatted to standardize head flexion, rotation, and tilt prior to region definition (Peterson et al., 2001; Sled & Evans, 1998). Axial slices were oriented parallel to the anterior and posterior commissures; sagittal slices were oriented parallel to standard midline landmarks.
Methods used in this study for defining the amygdala and hippocampus have been described previously (Kates, Abrams, Kaufmann, Breiter, & Reiss, 1997). Briefly, the anterior extent of the amygdala coincided with the most anterior slice in which the anterior commissure was seen to cross the midline. The transition between the amygdala and hippocampus was determined by drawing a straight horizontal line connecting the inferior horn of the lateral ventricle with the amygdaloid sulcus or, when the sulcus was not clearly identifiable, by a straight horizontal line connecting the inferior horn of the lateral ventricle with the surface on the uncus (Watson et al., 1992). The most posterior slice was the last in which the crus of the fornix and the fimbria of the hippocampal formation could be delineated. Interrater reliabilities of the morphometric measurements (intraclass correlation coefficients using two-way random effects (Arndt, Cohen, Alliger, Swayze, & Andreasen, 1991) were found to be .91 and .92 for the right and left hippocampus and .88 and .89 for the left and right amygdala, respectively).
Whole brain volume (WBV) was included as a covariate in the statistical analysis to control for scaling effects within the brain (Arndt et al., 1991). This measure included total cerebral tissue (TCT) (the total volume of all gray and white matter), ventricular cerebrospinal fluid (CSF), and CSF spaces within the brain (cisterns, fissures, and cortical sulci). CSF was incorporated into WBV using a connected components analysis (the ANALYZE subroutine “delete holes”) to minimize any effects of age-related cortical atrophy in measures from older subjects (Fig. 1).
Fig. 1.
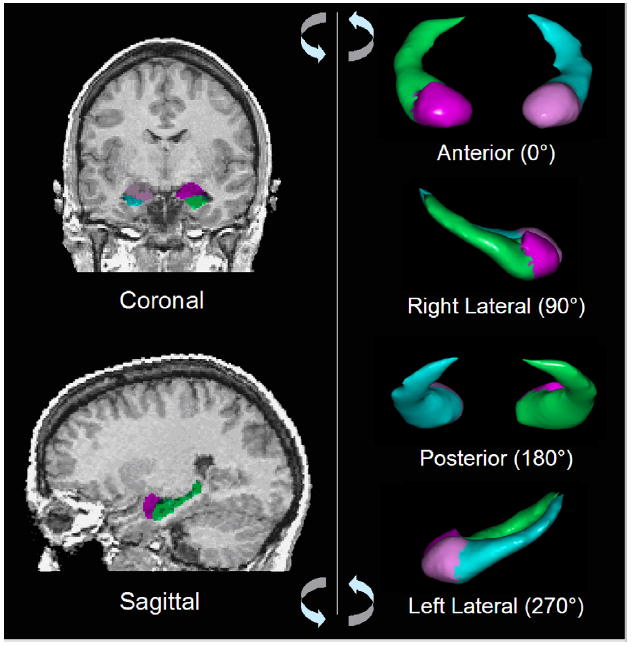
Manual definition of the amygdala and hippocampus. Left: coronal (top) and sagittal (bottom) brain slices through amygdala (red/purple) and hippocampus (green/blue). Right: three-dimensional volume rendering with 90° degree rotations from anterior to left lateral view. (For interpretation of the references in color in this figure legend, the reader is referred to the web version of this article.)
2.4. Cognitive measures
FSIQ was estimated by prorating two performance (block design and object assembly) and three verbal (information, digit span, and vocabulary) age-scaled subtest scores of the Wechsler Adult Intelligence Scale-Revised (WAIS-R.) (Wechsler, 1981). Typically these measures exhibit more than 97% correlation with full-scale IQ based on all 11 subtests of the WAIS-R. These estimates indicated that the 34 participants were of above-average intelligence (Performance IQ = 120.3 ± 15.9; Verbal IQ = 128.1 ± 17.4; FSIQ = 127.2 ± 15.0). Two participants failed to complete the battery of IQ subtests: one subject was lacking the block design and the other the object assembly.
2.5. Statistical analyses
We calculated partial correlation coefficients to assess the associations of amygdala and hippocampus volumes with IQ measures adjusted for WBV, age, gender, and SES. Secondary exploratory analyses to test for regional and hemispheric specificity were conducted by comparing within each hemisphere the partial correlation coefficients calculated for the amygdala and hippocampus (e.g., correlations for the left amygdala were compared with correlations for the left hippocampus). The hemispheric specificity of the findings was assessed by comparing correlation coefficients for a given region across hemispheres (e.g., correlations for left amygdala were compared with correlations for the right amygdala). Correlation coefficients were compared with statistical t-tests for intercorrelated variables (Cohen, Cohen, West, & Aiken, 2003).
Our a priori hypothesis was that hippocampus volumes would correlate significantly with full-scale IQ scores. Post-hoc analyses were conducted to explore which cognitive subprocesses (IQ subtests) contributed the most to the hypothesized association of volumes with IQ. Additional exploratory analyses were conducted and included assessment of the associations of IQ measures with WBV, TCT, and total cortical gray matter. Statistical analyses were performed using SPSS version 9.0 (SPSS Inc., Chicago, ILL), and all p-values were 2-sided.
2.6. Surface analysis
We used multiple variable linear regression to assess whether focal deviations in surface contours of the amygdala and hippocampus in our sample were associated with FSIQ compared with canonical templates. First, the cerebrums of all subjects were coregistered to the cerebrum of a single representative healthy subject (the template brain) using a rigid-body similarity transformation (3 translations, 3 rotations, and a global scale); these parameters of transformation were estimated while maximizing mutual information (Wells, Viola, Atsumi, Nakajima, & Kikinis, 1996) across the gray-scale values of the brains compared with that of the template. The amygdala and hippocampus of each subject were then individually rigidly coregistered to the corresponding structures of the template brain to further improve registration. Point-to-point correspondences between surfaces of the subject and template amygdala and hippocampus were then identified using a method of registering gray-scale values based on fluid dynamics (described elsewhere) (Bansal, Staib, Whiteman, Wang, & Peterson, 2005). Finally, we calculated the signed Euclidean distances between the corresponding points of the structures for each subject and the template brain, with positive and negative values indicating, respectively, the presence of an outward or inward surface deformation. To control for the effects of covariates (age, sex) on surface morphology, we performed an analysis using multivariate, linear regression (Rosner, 1995) at each point on the reference surface: di = β0 + β1 × FSIQ + β2 × Age + β3 × Sex; ∀i = 1, … , 32, where di was the set of signed Euclidean distances from the surface of the reference structure. We computed the correlation between the distances and FSIQ, and we evaluated the p-value of this correlation using a Student’s t-test. Color-coded p-values were then displayed across the entire surface of the reference structure (Fig. 3).
Fig. 3.
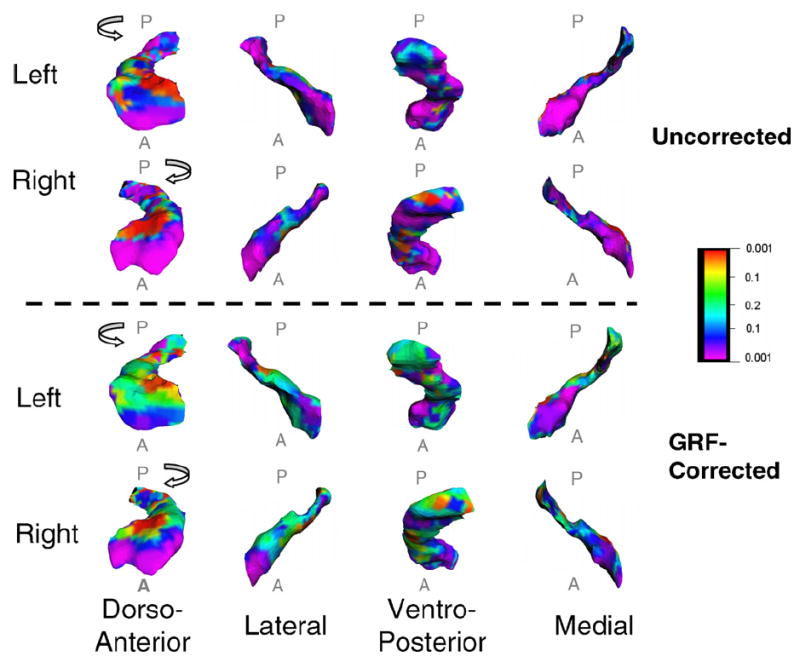
Correlations of hippocampus surface morphology with IQ. A color map of the hippocampus that shows significant, GRF-corrected correlations (p ≤ .05) of inward (purple) and outward (red) deformations of the hippocampus surface with IQ. A, anterior; P, posterior.
We used the theory of Gaussian Random Fields (GRFs) (Adler, 1981) to compute the corrected p-value that appropriately accounts for the multiple correlations computed at each voxel on the surfaces of each structure (Bansal, Staib, Xu, Zhu, & Peterson, 2006). We first computed at each point a Student’s t-statistic, which was then converted to a z-statistic from the Gaussian distribution. Next, for the random field f defined on the surface, we calculated the expected value of the Euler Characteristic (EC) by appropriately smoothing the random field. The expected EC then determined the points on the surface where the z-statistic differed from zero at a prespecified level of significance (p = .05 − .0001), thereby identifying locations of statistically significant correlations of local morphology with IQ at the surface of each structure (Taylor & Adler, 2003).
3. Results
3.1. Amygdala
We detected no significant correlation of FSIQ or its subtests with volumes of the amygdala using either overall volume or analyses of the surface of this structure (Table 1).
Table 1.
Correlation analyses: Statistically significant correlation coefficients of left and right amygdala (“Amyg”) and hippocampus (“Hipp”) volumes with cognitive measures, controlling (using covariates) for gender, age, whole brain volume (WBV), and socioeconomic status (SES)
| Cognitive measures | Left
|
Right
|
Total
|
|||
|---|---|---|---|---|---|---|
| Amyg | Hipp | Amyg | Hipp | Amyg | Hipp | |
| WAIS-R (n = 32) | ||||||
| FSIQ | .057 | −.429* | .140 | −.472** | .106 | −.486** |
| VIQ | .082 | −.414* | .276 | −.342 | .191 | −.413* |
| PIQ | .036 | −.294 | −.062 | −.550** | −.013 | −.446** |
| Information | .012 | −.367* | .200 | −.450* | .112 | −.439* |
| Vocabulary | .212 | −.205 | .343 | −.065 | .299 | −.153 |
| Digit span | .118 | −.375* | .151 | −.650** | .146 | −.543** |
| Block design | .149 | .038 | −.039 | −.128 | .062 | −.041 |
| Object assembly | −.090 | −.247 | −.180 | −.468** | −.146 | −.377* |
Significance values are indicated as follows:
p ≤ .05;
p ≤ .01;
psychometric measurements: WAIS-R: Full-Scale IQ (FSIQ), Verbal IQ (VIQ), Performance IQ (PIQ), and the subtests of VIQ (information, vocabulary, digit span) and PIQ (block design, object assembly).
3.2. Hippocampus
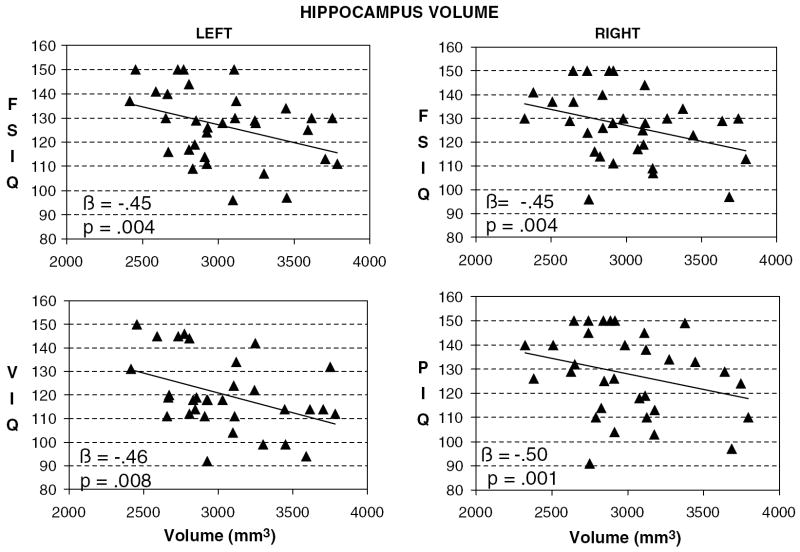
We detected a statistically significant inverse correlation of overall hippocampus volume (summed across the left and right sides) with FSIQ (r = −.49, p < .007). Secondary exploratory analyses showed that this correlation of IQ with hippocampus volume was present bilaterally [left; r = −.43, p < .02 and right; r = −.47, p < .01]. VIQ and PIQ, the two components of FSIQ, each contributed to this correlation with FSIQ. VIQ correlations with hippocampus volumes were significant only in the left hemisphere, whereas PIQ correlations were significant only in the right hemisphere (Table 1). The hemispheric specificity of the PIQ correlation was supported in a comparison of correlation coefficients of PIQ with hippocampus volumes across hemispheres [t = 2.1, p < .05], whereas a comparison of the correlation coefficients of VIQ with hippocampus volumes across hemispheres was not significant [t = −.50, p = .62]. Comparing the correlation coefficients of IQ with hippocampus or amygdala volumes within the same hemisphere generally demonstrated that these findings were specific to the hippocampus (i.e., they were significant in the hippocampus but not in the amygdala, and these correlations of volume with FSIQ differed significantly across regions) both in the left (t = 2.5, p < .03) and right hemisphere (t = 2.6, p < .02) (Fig. 2).
Fig. 2.
Correlations of hippocampus volumes with IQ. FSIQ, full-scale IQ; VIQ, verbal IQ; PIQ, performance IQ; β, standardized regression coefficient for multiple linear regression models, with volumes adjusted for age, gender, and whole brain volume (n = 32).
Surface analyses revealed that IQ correlated strongly and inversely with surface morphology over extensive portions of the anterior aspect of the hippocampus bilaterally, indicating that higher IQ accompanied an inward deformation, or a localized reduction in volume, of the surface of the structure (Figs. 3 and 4). IQ correlated with much smaller areas of surface morphology in scattered portions of the posterior hippocampus.
Fig. 4.
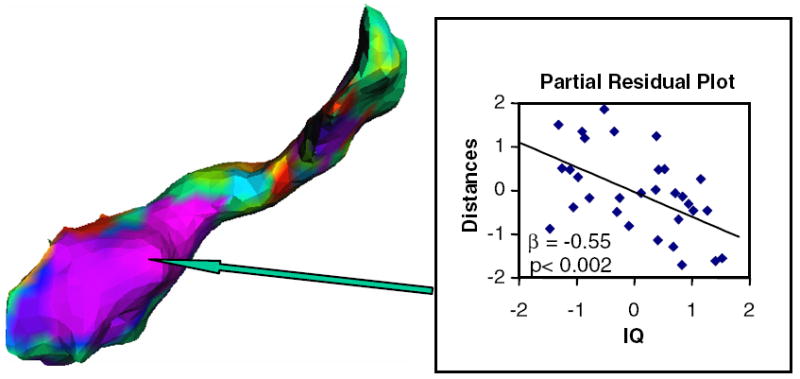
Scatterplot of hippocampus surface morphology with IQ. Medial view of right hippocampus: a color map of the significant correlations of IQ with deformations in its surface contour correlating inversely with IQ. The box shows the scatter plot of residuals of IQ values and distances associated with the point on the surface of the hippocampus that is indicated by the arrow. β, standardized regression coefficient for a multiple linear regression model in which distance from the template hippocampus was entered as the dependent variable, IQ was entered as the independent variable, and age, gender, and whole brain volume were covariates.
3.3. Assessment of possible confounds
WBV was included in the correlation analyses as a covariate to control for scaling effects within the brain. WBV, indeed, correlated significantly with regional volumes in this data set [left amygdala r = .31, p = .02; left hippocampus r = .37, p = .006; right amygdala r = .33, p = .01; right hippocampus r = .43, p = .001], consistent with the presence of generalized scaling effects in the amygdala and hippocampus. Inclusion of WBV in the analyses, however, could conceivably have destabilized the regression equations, thereby introducing spurious correlations of regional volumes with measures of cognition. We therefore assessed all correlations using the same covariates as before, but excluding WBV. The results were unchanged.
Additionally, we assessed the correlations of IQ and its subtests with WBV and total cerebral volume (TCV), defined as total cerebral gray and white matter combined, to ensure that previously reported positive correlations of IQ with overall brain size were not driving the observed associations of IQ with hippocampus volumes; none of the IQ measures correlated significantly with WBV or TCV. Sequentially partialing out other covariates, such as age, gender, and SES, did not affect the overall trend of correlations of volumes with FSIQ.
4. Discussion
The major finding of this study was a significant inverse correlation of FSIQ with volumes of the hippocampus bilaterally. Furthermore, post-hoc analyses suggest that this correlation was attributable to the correlation of VIQ with volumes of the left hippocampus and the correlation of PIQ with volumes of the right hippocampus. These brain-behavior correlations were independent of the effects of gender, age, SES, and WBV. Finally, higher IQ was strongly associated with bilateral inward deformations in the surface contour of the anterior hippocampus (Figs. 3 and 4), which we attribute to locally reduced volumes, possibly within the dentate gyrus and cornu ammonis, which constitute most of the volume of the anterior hippocampus (Amunts et al., 2005; Duvernoy, 1988).
Statistical tests for intercorrelated variables indicated that all of these correlations of IQ scores with hippocampus volumes differed significantly from the correlations of IQ with amygdala volumes, which themselves were not statistically significant. Thus, the morphological correlates of IQ in these regions were highly specific to the hippocampus. In addition, significant correlations of VIQ and PIQ with left and right hippocampus volumes, respectively, suggested that the contributions of the hippocampus to intelligence within each hemisphere may be specific to particular cognitive domains, with more verbal functions based within the left hemisphere and more visuospatial (i.e., performance-based) functions located within the right hemisphere (Table 1).
4.1. Regional and hemispheric specialization
Historically, postulates of hemispheric and regional specialization of human intellectual abilities have been based on the specificity of cognitive deficits that accompany differing types and locations of brain lesions. For example, left hemisphere lesions in parietotemporal cortices tend to impair verbal abilities, whereas right hemisphere lesions there tend to impair visuospatial abilities (Milner, 1971). The validity of the claim for regional and hemispheric specificity of functional deficits associated with brain lesions has received increasing support more recently from studies of functional deficits caused by small seizure foci or by surgically induced lesions in the mesial-temporal lobe (Jones-Gotman, 1986; Katz et al., 1989; Loring & Meador, 2001; Sass et al., 1990; Sass et al., 1991; Trenerry et al., 1993). Similar hemispheric specialization of medial-temporal lobe structures in subserving verbal and nonverbal tasks has been demonstrated in functional MRI studies (Kelley et al., 1998; Kirchhoff, Wagner, Maril, & Stern, 2000; Wagner et al., 1998) and PET studies (Haier et al., 1992), both of which showed selective activation of the right hippocampus during visuospatial tasks. Hemispheric specialization has also been suggested in magnetoencephalography studies that show activation of the left hippocampus during word recognition and activation of the right hippocampus during visual recognition tasks (Breier, Simos, Zouridakis, & Papanicolaou, 1999; Papanicolaou et al., 2002). Consistent with this specialization of function across cerebral hemispheres in the mesial temporal lobe, we found that PIQ correlated significantly and more strongly with hippocampus volume in the right than in the left hemisphere. Although VIQ did correlate significantly with hippocampus volumes in the left hemisphere, comparing across hemispheres the correlation coefficients for VIQ with hippocampus volumes did not provide evidence for hemispheric specificity. Thus, our findings supported prior claims for a relative specialization of the right hippocampus in visuospatial processing, but they did not provide strong support for claims of specialization of the left hippocampus in verbal processing. Our comparisons of the correlation coefficients of IQ with hippocampus and amygdala volumes within the same hemisphere, however, did generally demonstrate that the correlations of IQ with regional volumes were specific to the hippocampus.
The inverse correlations of IQ with surface morphology of the anterior hippocampus suggests that the functions that the anterior hippocampus subserves may play a particularly important role in supporting other, more general, cognitive processes within the central nervous system. The anterior hippocampus is thought to encode spatial and temporal relationships among sensory experiences (Agster, Fortin, & Eichenbaum, 2002; Bast & Feldon, 2003; Huerta, Sun, Wilson, & Tonegawa, 2000; Shors, 2004), which the posterior hippocampus then consolidates for storage in long-term memory (Kandel, 2001; Strange & Dolan, 1999). The anterior hippocampus works within a distributed network that includes the prefrontal cortex to encode these temporal relationships into a serial ordering of events (Agster et al., 2002; Beiser & Houk, 1998; Eichenbaum, 2000; Shapiro & Eichenbaum, 1999). The serial order of sensory experience in humans may contribute to the prominent role that the anterior hippocampus is thought to play in indexing novelty, in detecting change, and in exploring new environments (Dolan & Fletcher, 1997; Strange & Dolan, 1999; Strange, Fletcher, Henson, Friston, & Dolan, 1999; Tulving, Markowitsch, Craik, Habib, & Houle, 1996).
4.2. Possible ultrastructural determinants
We cannot identify definitively the neurobiological mechanisms or the ultrastructural features of the hippocampus that account for smaller regional and subregional volumes within its anterior portions accompanying enhanced cognitive abilities. However, a growing body of evidence suggests that the developmental processes of neurogenesis, apoptosis, and dendritic pruning are promising candidates for the processes that contribute to the morphological correlations with IQ (Thompson et al., 2001). Neurogenesis in perinatal and early adult life, modulated by environmental stimulation, increases volume in the dentate gyrus of the hippocampus in mammals and primates (Kempermann, Kuhn, & Gage, 1997; Kornack & Rakic, 1999; van Praag, Kempermann, & Gage, 2000). During early postnatal life, apoptosis (Simonati, Rosso, & Rizzuto, 1997; Williams & Rakic, 1988) and pruning of both redundant neuronal processes and non-functional dendritic synapses (Herschkowitz, 1988; Huttenlocher, 1979; Huttenlocher, De Courten, Garey, & van der Loos, 1982; Purves & Lichtman, 1980) are probably the most significant determinants of regional volumes. By eliminating redundant neuronal processes, dendritic branches, and supernumerary synaptic spines, pruning supports learning and development of psychomotor skills (Bock & Braun, 1998; Bock & Braun, 1999; Nixdorf-Bergweiler, Wallhausser-Franke, & DeVoogd, 1995; Rakic, Bourgeois, & Goldman-Rakic, 1994; Rausch & Scheich, 1982; Rollenhagen & Bischof, 1994; Wallhausser & Scheich, 1987). Moreover, human postmortem studies of the cerebral cortex have demonstrated a 40–50% decrease in synaptic density in frontal and parietal cortices between infancy and late adolescence (Huttenlocher, 1979; Huttenlocher, 1984; Huttenlocher & de Courten, 1987). Anatomical imaging studies have demonstrated age-related reductions in gray matter in these regions throughout childhood and adolescence (Sowell, Thompson, Holmes, Jernigan, & Toga, 1999; Sowell et al., 1999), the period when cognitive abilities develop most rapidly (Sowell, Delis, Stiles, & Jernigan, 2001; Sowell, Thompson, Tessner, & Toga, 2001). Brain enlargement, potentially caused by faulty synaptic pruning or apoptosis, has been associated with neuropsychiatric disorders such as autism and some forms of mental retardation (Fiala, Spacek, & Harris, 2002; Hazlett et al., 2005; Schumann et al., 2004). Given the importance of synaptic pruning to the developmental and plastic processes that support normal cognitive functioning, we hypothesize that a smaller anterior hippocampus accompanies higher IQs in our sample because this region contains simplified and functionally more efficient neuronal networks, with redundant neuronal collateral connections having been eliminated successfully through pruning.
4.3. Relation to prior studies
The inverse correlations that we detected between hippocampus volumes and IQ differ from the positive correlations of hippocampus volumes with IQ that were detected in a previous study of 67 healthy adults (Andreasen, Flaum, Swayze, O’Leary, et al., 1993). This discrepancy is likely attributable to the vastly differing imaging methods used in the two studies. The prior study used brain images with low-resolution and poor contrast, and gaps between slices excluded more than a third of the hippocampus from measurement, perhaps missing the anterior hippocampus, which our analyses indicated contributed most strongly to the inverse correlations of overall hippocampus volumes with IQ. Moreover, brain images in the prior study were not reformatted to a standard orientation, and measurement reliability (r = .53) was unacceptably poor. The images used in the present study, on the other hand, included the entire hippocampus, they provided excellent tissue contrast and high-resolution, brain positioning was standardized, and consequently the reliability and validity of our morphological measurements were high. Finally, the findings of our study are consistent with a recent meta-analysis that showed an association of enhanced memories and cognitive abilities with smaller hippocampus volumes in a young adult population (van Petten, 2004).
4.4. Limitations
Our finding of an inverse correlation of hippocampus volumes with IQ does not necessarily indicate a direct causal link between a smaller hippocampus and greater intelligence. The inverse correlation between these measures could instead conceivably arise from increased volumes of the hippocampus in individuals who have lower IQs—perhaps hypertrophy of the hippocampus, for example, represents a long-term plastic response to the presence of suboptimal performance of a larger, more distributed neural system that subserves higher cognitive abilities. Alternatively, the inverse correlation could arise from the correlation of both volumes and cognitive measures with a third, unknown variable. Finally, the generalizability of our findings may be limited, as they may be specific to adults with above-average intelligence, who were overrepresented in our sample.
5. Conclusion
Findings of the present study suggest that the hippocampus, but not the amygdala, contributes significantly to the neural processes underlying human intellectual abilities. Extensive clinical and preclinical studies of the hippocampus suggest that developmental and plastic influences on neural pruning within the hippocampus increase its functional efficiency. These influences seem to be the most likely explanation for the inverse correlations of volume with IQ that we observed. This association with enhanced intellectual abilities is most prominent in the anterior aspect of the hippocampus.
Acknowledgments
We thank Isabel Muzzio and Christoph Wiedenmayer for their helpful comments on this manuscript. This work was supported in part by NIMH grants MH59139, MH068318, K02-74677, and MH16434; in part by grants from the Tourette Syndrome Association and the National Alliance for Research in Schizophrenia and Affective Disorders (NARSAD); and in part by the Suzanne Crosby Murphy Endowment at Columbia University and the Thomas D. Klingenstein and Nancy D. Perlman Family Fund.
References
- Adler RJ. The geometry of random fields. New York: J Wiley; 1981. [Google Scholar]
- Agster KL, Fortin NJ, Eichenbaum H. The hippocampus and disambiguation of overlapping sequences. Journal of Neuroscience. 2002;22(13):5760–5768. doi: 10.1523/JNEUROSCI.22-13-05760.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 4. Washington, DC: American Psychiatric Association; 1994. [Google Scholar]
- Amunts S, Kedo O, Kindler M, Pieperhoff P, Mohlberg H, Shah NJ, et al. Cytoarchitechtonic mapping of the human amygdala, hippocampal region and enthorhinal cortex: Intersubject variability and probability maps. Anatomy and Embryology. 2005;210:343–352. doi: 10.1007/s00429-005-0025-5. [DOI] [PubMed] [Google Scholar]
- Andreasen NC, Flaum M, Swayze VW, O’Leary DS, et al. Intelligence and brain structure in normal individuals. American Journal of Psychiatry. 1993;150(1):130–134. doi: 10.1176/ajp.150.1.130. [DOI] [PubMed] [Google Scholar]
- Arndt S, Cohen G, Alliger RJ, Swayze VW, 2nd, Andreasen NC. Problems with ratio and proportion measures of imaged cerebral structures. Psychiatry Research. 1991;40(1):79–89. doi: 10.1016/0925-4927(91)90031-k. [DOI] [PubMed] [Google Scholar]
- Bansal R, Staib LH, Xu D, Zhu H, Peterson BS. Statistical analysis of brain surfaces using Gaussian random fields on 2D manifolds. IEEE Transactions on Medical Imaging. 2006 doi: 10.1109/TMI.2006.884187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bansal R, Staib LH, Whiteman R, Wang YM, Peterson BS. ROC-based assessments of 3D cortical surface-matching algorithms. Neuroimage. 2005;24(1):150–162. doi: 10.1016/j.neuroimage.2004.08.054. [DOI] [PubMed] [Google Scholar]
- Bast T, Feldon J. Hippocampal modulation of sensorimotor processes. Progress in Neurobiology. 2003;70(4):319–345. doi: 10.1016/s0301-0082(03)00112-6. [DOI] [PubMed] [Google Scholar]
- Bechara A, Damasio H, Damasio AR. Role of the amygdala in decision-making. Annals of the New York Academy of Sciences. 2003;985:356–369. doi: 10.1111/j.1749-6632.2003.tb07094.x. [DOI] [PubMed] [Google Scholar]
- Beiser DG, Houk JC. Model of cortical-basal ganglionic processing: Encoding the serial order of sensory events. Journal of Neurophysiology. 1998;79(6):3168–3188. doi: 10.1152/jn.1998.79.6.3168. [DOI] [PubMed] [Google Scholar]
- Bock J, Braun K. Differential emotional experience leads to pruning of dendritic spines in the forebrain of domestic chicks. Neural Plasticity. 1998;6(3):17–27. doi: 10.1155/NP.1998.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bock J, Braun K. Blockade of N-methyl-D-aspartate receptor activation suppresses learning-induced synaptic elimination. Proceedings of the National Academy of Sciences of the United States of America. 1999;96(5):2485–2490. doi: 10.1073/pnas.96.5.2485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breier JI, Simos PG, Zouridakis G, Papanicolaou AC. Lateralization of cerebral activation in auditory verbal and non-verbal memory tasks using magneto encephalography. Brain Topography. 1999;12(2):89–97. doi: 10.1023/a:1023458110869. [DOI] [PubMed] [Google Scholar]
- Burns LH, Everitt BJ, Robbins TW. Effects of excitotoxic lesions of the basolateral amygdala on conditional discrimination learning with primary and conditioned reinforcement. Behavioural Brain Research. 1999;100(1–2):123–133. doi: 10.1016/s0166-4328(98)00119-3. [DOI] [PubMed] [Google Scholar]
- Cohen J, Cohen E, West S, Aiken L, editors. Applied multiple regression/correlation analysis for the behavioral sciences. 3. Vol. 1. Mahwah, New Jersey: Lawrence Erlbaum Asssociates, Publishers; 2003. [Google Scholar]
- Dolan RJ, Fletcher PC. Dissociating prefrontal and hippocampal function in episodic memory encoding. Nature. 1997;388(6642):582–585. doi: 10.1038/41561. [DOI] [PubMed] [Google Scholar]
- Duvernoy HM. An atlas of atlas of applied anatomy. Munich: JF Bergman Verlag; 1988. [Google Scholar]
- Eichenbaum H. A cortical-hippocampal system for declarative memory. Nature Reviews Neuroscience. 2000;1(1):41–50. doi: 10.1038/35036213. [DOI] [PubMed] [Google Scholar]
- Endicott J, Spitzer R. A diagnostic interview: The schedule for Affective disorders and schizophrenia. Archives of General Psychiatry. 1978;35:837–844. doi: 10.1001/archpsyc.1978.01770310043002. [DOI] [PubMed] [Google Scholar]
- Everitt BJ, Parkinson JA, Olmstead MC, Arroyo M, Robledo P, Robbins TW. Associative processes in addiction and reward. The role of amygdala-ventral striatal subsystems. Annals of the New York Academy of Sciences. 1999;877:412–438. doi: 10.1111/j.1749-6632.1999.tb09280.x. [DOI] [PubMed] [Google Scholar]
- Fiala JC, Spacek J, Harris KM. Dendritic spine pathology: Cause or consequence of neurological disorders? Brain Research–Brain Research Reviews. 2002;39(1):29–54. doi: 10.1016/s0165-0173(02)00158-3. [DOI] [PubMed] [Google Scholar]
- Flashman LA, Andreasen NC, Flaum M, Swayze VW. Intelligence and regional volumes in normal controls. Intelligence. 1998;25(3):149–160. [Google Scholar]
- Fried I, Cameron KA, Yashar S, Fong R, Morrow JW. Inhibitory and excitatory responses of single neurons in the human medial temporal lobe during recognition of faces and objects. Cerebral Cortex. 2002;12(6):575–584. doi: 10.1093/cercor/12.6.575. [DOI] [PubMed] [Google Scholar]
- Fried I, Wilson CL, Morrow JW, Cameron KA, Behnke ED, Ackerson LC, et al. Increased dopamine release in the human amygdala during performance of cognitive tasks. Nature Neuroscience. 2001;4(2):201–206. doi: 10.1038/84041. [DOI] [PubMed] [Google Scholar]
- Haier RJ, Jung RE, Yeo RA, Head K, Alkire MT. Structural brain variation and general intelligence. Neuroimage. 2004;23(1):425–433. doi: 10.1016/j.neuroimage.2004.04.025. [DOI] [PubMed] [Google Scholar]
- Haier RJ, Siegel BV, Jr, MacLachlan A, Soderling E, Lottenberg S, Buchsbaum MS. Regional glucose metabolic changes after learning a complex visuospatial/motor task: A positron emission tomographic study. Brain Research. 1992;570(12):134–143. doi: 10.1016/0006-8993(92)90573-r. [DOI] [PubMed] [Google Scholar]
- Hazlett HC, Poe M, Gerig G, Smith RG, Provenzale J, Ross A, et al. Magnetic resonance imaging and head circumference study of brain size in autism: Birth through age 2 years. Archives of General Psychiatry. 2005;62(12):761366–761376. 1366. doi: 10.1001/archpsyc.62.12.1366. [DOI] [PubMed] [Google Scholar]
- Herschkowitz N. Brain development in the fetus, neonate and infant. Biology of the Neonate. 1988;54(1):1–19. doi: 10.1159/000242818. [DOI] [PubMed] [Google Scholar]
- Hollingshead AB. Four-factor index of social status. New Haven, Conn: Yale University Press; 1975. [Google Scholar]
- Huerta PT, Sun LD, Wilson MA, Tonegawa S. Formation of temporal memory requires NMDA receptors within CA1 pyramidal neurons. Neuron. 2000;25(2):473–480. doi: 10.1016/s0896-6273(00)80909-5. [DOI] [PubMed] [Google Scholar]
- Huttenlocher PR. Synaptic density in human frontal cortex–developmental changes and effects of aging. Brain Research. 1979;163(2):195–205. doi: 10.1016/0006-8993(79)90349-4. [DOI] [PubMed] [Google Scholar]
- Huttenlocher PR. Synapse elimination and plasticity in developing human cerebral cortex. American Journal of Mental Deficiency. 1984;88(5):488–496. [PubMed] [Google Scholar]
- Huttenlocher PR, de Courten C. The development of synapses in striate cortex of man. Human Neurobiology. 1987;6(1):1–9. [PubMed] [Google Scholar]
- Huttenlocher PR, De Courten C, Garey LJ, van der Loos H. Synaptic development in human cerebral cortex. International Journal of Neurology. 1982;17:144–154. [PubMed] [Google Scholar]
- Jones-Gotman M. Right hippocampal excision impairs learning and recall of a list of abstract designs. Neuropsychologia. 1986;24(5):659–670. doi: 10.1016/0028-3932(86)90005-9. [DOI] [PubMed] [Google Scholar]
- Kahn I, Yeshurun Y, Rotshtein P, Fried I, Ben-Bashat D, Hendler T. The role of the amygdala in signaling prospective outcome of choice. Neuron. 2002;33(6):983–994. doi: 10.1016/s0896-6273(02)00626-8. [DOI] [PubMed] [Google Scholar]
- Kandel ER. The molecular biology of memory storage: A dialog between genes and synapses. Bioscience Reports. 2001;21(5):565–611. doi: 10.1023/a:1014775008533. [DOI] [PubMed] [Google Scholar]
- Kates WR, Abrams MT, Kaufmann WE, Breiter SN, Reiss AL. Reliability and validity of MRI measurement of the amygdala and hippocampus in children with fragile X syndrome. Psychiatry Research. 1997;75(1):31–48. doi: 10.1016/s0925-4927(97)00019-x. [DOI] [PubMed] [Google Scholar]
- Katz A, Awad IA, Kong AK, Chelune GJ, Naugle RI, Wyllie E, et al. Extent of resection in temporal lobectomy for epilepsy. II. Memory changes and neurologic complications. Epilepsia. 1989;30(6):763–771. doi: 10.1111/j.1528-1157.1989.tb05336.x. [DOI] [PubMed] [Google Scholar]
- Kelley WM, Miezin FM, McDermott KB, Buckner RL, Raichle ME, Cohen NJ, et al. Hemispheric specialization in human dorsal frontal cortex and medial temporal lobe for verbal and nonverbal memory encoding. Neuron. 1998;20(5):927–936. doi: 10.1016/s0896-6273(00)80474-2. [DOI] [PubMed] [Google Scholar]
- Kempermann G, Kuhn HG, Gage FH. More hippocampal neurons in adult mice living in an enriched environment. Nature. 1997;386(6624):493–495. doi: 10.1038/386493a0. [DOI] [PubMed] [Google Scholar]
- Kirchhoff BA, Wagner AD, Maril A, Stern CE. Prefrontal-temporal circuitry for episodic encoding and subsequent memory. Journal of Neuroscience. 2000;20(16):6173–6180. doi: 10.1523/JNEUROSCI.20-16-06173.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kornack DR, Rakic P. Continuation of neurogenesis in the hippocampus of the adult macaque monkey. Proceedings of the National Academy of Sciences of the United States of America. 1999;96(10):5768–5773. doi: 10.1073/pnas.96.10.5768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loring DW, Meador KJ. Cognitive and behavioral effects of epilepsy treatment. Epilepsia. 2001;8:24–32. [PubMed] [Google Scholar]
- McDaniel M. Big-brained people are smarter: A meta-analysis of the relationship between in vivo brain volume and intelligence. Intelligence. 2005;33:337–346. [Google Scholar]
- Milner B. Interhemispheric differences in the localization of psychological processes in man. British Medical Bulletin. 1971;27(3):272–277. doi: 10.1093/oxfordjournals.bmb.a070866. [DOI] [PubMed] [Google Scholar]
- Nixdorf-Bergweiler BE, Wallhausser-Franke E, DeVoogd TJ. Regressive development in neuronal structure during song learning in birds. Journal of Neurobiology. 1995;27(2):204–215. doi: 10.1002/neu.480270207. [DOI] [PubMed] [Google Scholar]
- Papanicolaou AC, Simos PG, Castillo EM, Breier JI, Katz JS, Wright AA. The hippocampus and memory of verbal and pictorial material. Learning & Memory. 2002;9(3):99–104. doi: 10.1101/lm.44302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pennington BF, Filipek PA, Lefly D, Chhabildas N, Kennedy DN, Simon JH, et al. A twin MRI study of size variations in the human brain. Journal of Cognitive Neuroscience. 2000;12(1):223–232. doi: 10.1162/089892900561850. [DOI] [PubMed] [Google Scholar]
- Peterson BS, Leckman JF, Tucker D, Scahill L, Staib L, Zhang H, et al. Preliminary findings of antistreptococcal antibody titers and basal ganglia volumes in tic, obsessive-compulsive, and attention deficit/hyperactivity disorders. Archives of General Psychiatry. 2000;57(4):364–372. doi: 10.1001/archpsyc.57.4.364. [DOI] [PubMed] [Google Scholar]
- Peterson BS, Staib L, Scahill L, Zhang H, Anderson C, Leckman JF, et al. Regional brain and ventricular volumes in Tourette syndrome. Archives of General Psychiatry. 2001;58(5):427–440. doi: 10.1001/archpsyc.58.5.427. [DOI] [PubMed] [Google Scholar]
- Posthuma D, De Geus EJC, Baaré WFC, Hilleke E, Pol H, Khan RS, et al. The association between brain volume and intelligence is of genetic origin. Nature Neuroscience. 2002;5:83–84. doi: 10.1038/nn0202-83. [DOI] [PubMed] [Google Scholar]
- Purves D, Lichtman JW. Elimination of synapses in the developing nervous system. Science. 1980;210(4466):153–157. doi: 10.1126/science.7414326. [DOI] [PubMed] [Google Scholar]
- Rakic P, Bourgeois JP, Goldman-Rakic PS. Synaptic development of the cerebral cortex: Implications for learning, memory, and mental illness. Progress in Brain Research. 1994;102:227–243. doi: 10.1016/S0079-6123(08)60543-9. [DOI] [PubMed] [Google Scholar]
- Rausch G, Scheich H. Dendritic spine loss and enlargement during maturation of the speech control system in the mynah bird (Gracula religiosa) Neuroscience Letters. 1982;29(2):129–133. doi: 10.1016/0304-3940(82)90341-x. [DOI] [PubMed] [Google Scholar]
- Rollenhagen A, Bischof HJ. Spine morphology of neurons in the avian forebrain is affected by rearing conditions. Behavioral & Neural Biology. 1994;62(2):83–89. doi: 10.1016/s0163-1047(05)80029-9. [DOI] [PubMed] [Google Scholar]
- Rosner B. Fundamentals of Biostatistics. 4. Belmont, California: Wadsworth; 1995. [Google Scholar]
- Sass KJ, Lencz T, Westerveld M, Novelly RA, Spencer DD, Kim JH. The neural substrate of memory impairment demonstrated by the intracarotid amobarbital procedure. Archives of Neurology. 1991;48(1):48–52. doi: 10.1001/archneur.1991.00530130056020. [DOI] [PubMed] [Google Scholar]
- Sass KJ, Spencer DD, Kim JH, Westerveld M, Novelly RA, Lencz T. Verbal memory impairment correlates with hippocampal pyramidal cell density. Neurology. 1990;40(11):1694–1697. doi: 10.1212/wnl.40.11.1694. [DOI] [PubMed] [Google Scholar]
- Schumann CM, Hamstra J, Goodlin-Jones BL, Lotspeich LJ, Kwon H, Buonocore MH, et al. The amygdala is enlarged in children but not adolescents with autism; the hippocampus is enlarged at all ages. J Neurosci. 2004;24(28):6392–6401. doi: 10.1523/JNEUROSCI.1297-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapiro ML, Eichenbaum H. Hippocampus as a memory map: Synaptic plasticity and memory encoding by hippocampal neurons. Hippocampus. 1999;9(4):365–384. doi: 10.1002/(SICI)1098-1063(1999)9:4<365::AID-HIPO4>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- Shors TJ. Memory traces of trace memories: Neurogenesis, synaptogenesis and awareness. Trends in Neuroscience. 2004;27(5):250–256. doi: 10.1016/j.tins.2004.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simonati A, Rosso T, Rizzuto N. DNA fragmentation in normal development of the human central nervous system: A morphological study during corticogenesis. Neuropathology & Applied Neurobiology. 1997;23(3):203–211. [PubMed] [Google Scholar]
- Sled JGZA, Evans AC. A nonparametric method for automatic correction of intensity nonuniformity in MRI data. IEEE Transactions on Medical Imaging. 1998;17(1):87–97. doi: 10.1109/42.668698. [DOI] [PubMed] [Google Scholar]
- Sowell ER, Delis D, Stiles J, Jernigan TL. Improved memory functioning and frontal lobe maturation between childhood and adolescence: A structural MRI study. Journal of the International Neuropsychological Society. 2001;7(3):312–322. doi: 10.1017/s135561770173305x. [DOI] [PubMed] [Google Scholar]
- Sowell ER, Thompson PM, Holmes CJ, Batth R, Jernigan TL, Toga AW. Localizing age-related changes in brain structure between childhood and adolescence using statistical parametric mapping. Neuroimage. 1999;9(6 Pt 1):587–597. doi: 10.1006/nimg.1999.0436. [DOI] [PubMed] [Google Scholar]
- Sowell ER, Thompson PM, Holmes CJ, Jernigan TL, Toga AW. In vivo evidence for postadolescent brain maturation in frontal and striatal regions. Nature Neuroscience. 1999;2(10):859–861. doi: 10.1038/13154. [DOI] [PubMed] [Google Scholar]
- Sowell ER, Thompson PM, Tessner KD, Toga AW. Mapping continued brain growth and gray matter density reduction in dorsal frontal cortex: Inverse relationships during postadolescent brain maturation. Journal of Neuroscience. 2001;21(22):8819–8829. doi: 10.1523/JNEUROSCI.21-22-08819.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Squire LR, Stark CE, Clark RE. The medial temporal lobe. Annual Review of Neuroscience. 2004;27:279–306. doi: 10.1146/annurev.neuro.27.070203.144130. [DOI] [PubMed] [Google Scholar]
- Strange B, Dolan R. Functional segregation within the human hippocampus. Molecular Psychiatry. 1999;4(6):508–511. doi: 10.1038/sj.mp.4000593. [DOI] [PubMed] [Google Scholar]
- Strange BA, Fletcher PC, Henson RN, Friston KJ, Dolan RJ. Segregating the functions of human hippocampus. Proceedings of the National Academy of Sciences of the United States of America. 1999;96(7):4034–4039. doi: 10.1073/pnas.96.7.4034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor E, Adler RJ. Euler characteristic for Gaussian fields on manifolds. Annals of Probability. 2003;31:533–563. [Google Scholar]
- Thompson PM, Cannon TD, Narr KL, van Erp T, Poutanen VP, Huttunen M, et al. Genetic influences on brain structure. Nature Neuroscience. 2001;4(12):1253–1258. doi: 10.1038/nn758. [DOI] [PubMed] [Google Scholar]
- Trenerry MR, Jack CR, Jr, Ivnik RJ, Sharbrough FW, Cascino GD, Hirschorn KA, et al. MRI hippocampal volumes and memory function before and after temporal lobectomy. Neurology. 1993;43(9):1800–1805. doi: 10.1212/wnl.43.9.1800. [DOI] [PubMed] [Google Scholar]
- Tulving E, Markowitsch HJ, Craik FE, Habib R, Houle S. Novelty and familiarity activations in PET studies of memory encoding and retrieval. Cereberal Cortex. 1996;6(1):71–79. doi: 10.1093/cercor/6.1.71. [DOI] [PubMed] [Google Scholar]
- van Petten C. Relationships between hippocampal volume and memory ability in healthy individuals across the lifespan: Review and meta-analysis. Neuropsychologia. 2004;42:1394–1413. doi: 10.1016/j.neuropsychologia.2004.04.006. [DOI] [PubMed] [Google Scholar]
- van Praag H, Kempermann G, Gage FH. Neural consequences of environmental enrichment. Nature Reviews Neuroscience. 2000;1(3):191–198. doi: 10.1038/35044558. [DOI] [PubMed] [Google Scholar]
- Wagner AD, Schacter DL, Rotte M, Koutstaal W, Maril A, Dale AM, et al. Building memories: Remembering and forgetting of verbal experiences as predicted by brain activity. Science. 1998;281(5380):1188–1191. doi: 10.1126/science.281.5380.1188. comment. [DOI] [PubMed] [Google Scholar]
- Wallhausser E, Scheich H. Auditory imprinting leads to differential 2-deoxyglucose uptake and dendritic spine loss in the chick rostral forebrain. Brain Research. 1987;428(1):29–44. doi: 10.1016/0165-3806(87)90080-0. [DOI] [PubMed] [Google Scholar]
- Wechsler D, editor. Wechsler Adult Intelligence Scale-Revised. New York: Psychological Corporation; 1981. [Google Scholar]
- Watson C, Andermann F, Gloor P, Jones-Gotman M, Peters T, Evans A, et al. Anatomic basis of amygdaloid and hippocampal volume measurement by magnetic resonance imaging. Neurology. 1992;42(9):1743–1750. doi: 10.1212/wnl.42.9.1743. [DOI] [PubMed] [Google Scholar]
- Wells WM, Viola P, Atsumi H, Nakajima S, Kikinis R. Multi-modal volume registration by maximization of mutual information. Medical Image Analysis. 1996;1(1):31–51. doi: 10.1016/s1361-8415(01)80004-9. [DOI] [PubMed] [Google Scholar]
- Williams RW, Rakic P. Elimination of neurons from the rhesus monkey’s lateral geniculate nucleus during development. Journal of Comparative Neurology. 1988;272(3):424–436. doi: 10.1002/cne.902720310. [DOI] [PubMed] [Google Scholar]