Abstract
Background
Tourette syndrome (TS) is a childhood-onset neuropsychiatric disorder that is characterized by both motor and phonic tics. One half to two thirds of children with TS experience a reduction or complete resolution of tic symptoms during adolescence. At least one third of adults with TS have comorbid obsessive-compulsive disorder (OCD).
Objectives
To clarify the clinical course of tic and OCD symptoms in children with TS and determine if baseline clinical measurements in childhood are associated with future symptom severity in late adolescence and early adulthood.
Design
Prospective cohort study.
Setting
Yale Child Study Center tic and OCD outpatient specialty clinic.
Participants
Forty-six children with TS who received a structured clinical evaluation prior to age 14 years.
Main Outcome Measures
Expert-rated tic and OCD symptom severity at follow-up interview an average of 7.6 years later (range, 3.8-12.8 years).
Results
Eighty-five percent of subjects reported a reduction in tic symptoms during adolescence. Only increased tic severity in childhood was associated with increased tic severity at follow-up. The average age at worst-ever tic severity was 10.6 years. Forty-one percent of patients with TS reported at one time experiencing at least moderate OCD symptoms. Worst-ever OCD symptoms occurred approximately 2 years later than worst-ever tic symptoms. Increased childhood IQ was strongly associated with increased OCD severity at follow-up.
Conclusion
Obsessive-compulsive disorder symptoms in children with TS became more severe at a later age and were more likely to persist than tic symptoms.
Tourette Syndrome (TS) is a childhood-onset neuropsychiatric disorder that is characterized by both motor and phonic tics. In TS, tics typically begin at age 5 or 6 years and reach their peak severity between 10 and 12 years of age.1-3 One half to two thirds of children with TS experience a substantial decrease or complete remission of tics by the end of adolescence.2,3 However, the continuation of tics into adulthood can have serious consequences that may include self-injurious tics and those that cause social unease, such as coprolalia.1 Currently, no clinical measures are known to predict reliably which children will continue to express tics in adulthood.
Motor and vocal tics, the most prominent feature and diagnostic sine qua non of TS, are often neither the first nor the most impairing symptoms that patients with TS endure. In clinical populations, TS frequently co-occurs with obsessive-compulsive disorder (OCD), attention-deficit/hyperactivity disorder (ADHD), and other behavioral, emotional, and learning disorders. In 1 study, 65% of patients with TS in late adolescence regarded their behavioral problems (including ADHD and OCD) and learning difficulties to have had an equal or greater impact on functioning than did the tics themselves.1
We conducted this study to clarify the clinical course of tic symptoms and to extend our knowledge of the course of OCD symptoms in patients with TS. We also wanted to assess prospectively whether baseline clinical measurements in children with TS were associated with adult outcome with regard to severity of tic and OCD symptoms. Our a priori hypotheses were that (1) increased severity of tic symptoms and (2) a diagnosis of ADHD in children with TS would be associated with increased tic severity at follow-up and that (3) increased severity of OCD symptoms and (4) a higher IQ in childhood would be associated with increased OCD symptom severity at follow-up.
Methods
Subjects
The 46 subjects included in this study were previously evaluated at the Yale Child Study Center Tic Disorder Clinic (New Haven, Conn) and had previously participated in magnetic resonance imaging studies in childhood.4-6 Eligible subjects (1) had a previous diagnosis of TS, (2) underwent magnetic resonance imaging and a detailed evaluation prior to 14 years of age (time 1), and (3) were older than 16 years at follow-up (time 2). Exclusionary criteria in these earlier studies included a history of seizure, head trauma with loss of consciousness, ongoing or past substance abuse, or an IQ lower than 80. Parental written informed consent and subject assent were obtained at both time 1 and time 2. Compensation was provided for participation at both points under the guidelines of the Human Investigations Committee at Yale University, New Haven.
From an eligible sample of 64 subjects evaluated at time 1, 46 subjects elected to participate. Reasons for nonparticipation included subject refusal to participate in follow-up interview (n = 14) or inability to locate subjects (n = 4). Demographic measurements did not differ statistically significantly between participating and nonparticipating subjects as assessed during initial evaluation at time 1 (Table). However, there was a noticeably higher proportion of cases with comorbid OCD and a lower proportion of cases of ADHD among participating subjects compared with nonparticipating subjects at time 1.
Table.
Demographic Comparison Between Participating and Nonparticipating Patients With Tourette Syndrome*
| Characteristic | Participants | Nonparticipants | P Value |
|---|---|---|---|
| Sample size | 46 | 18 | |
| Age, y, mean ± SD | 11.42 ± 1.59 | 10.91 ± 0.97 | .21 |
| Men, No. (%) | 36 (78) | 13 (72) | .61 |
| Right handedness, No. (%) | 38 (83) | 16 (89) | .53 |
| IQ, mean ± SD | 111 ± 14.7 | 111 ± 11.3 | >.99 |
| OCD, No. (%) | 17 (37) | 3 (17) | .12 |
| ADHD, No. (%) | 9 (20) | 6 (33) | .28 |
| YGTSS7 score, mean ± SD | 19.9 ± 8.45 | 21.5 ± 6.45 | .47 |
| CY-BOCS8 score, mean ± SD | 2.11 ± 3.30 | 2.56 ± 5.29 | .65 |
Abbreviations: ADHD, attention-deficit/hyperactivity disorder; CY-BOCS, Children's Yale-Brown Obsessive Compulsive Scale; OCD, obsessive-compulsive disorder; YGTSS, Yale Global Tic Severity Scale.
There were no significant differences (P<.05) between any of the variables examined between participating and nonparticipating subjects using χ2 or t tests.
Interview Procedure at Time 1
Assessment at time 1, when subjects were younger than 14 years, included current and worst-ever measures using the Yale Global Tic Severity Scale (YGTSS) and Children's Yale-Brown Obsessive Compulsive Scale (CY-BOCS).7,8 IQ testing was performed using the Kaufman Brief Intelligence Test.9,10 The Schedule for Tourette and Other Behavioral Syndromes, which includes the Kiddie-Schedule for Affective Disorders and Schizophrenia Epidemiologic Present and Lifetime Version for diagnosis in children and more detailed sections on TS and OCD, was used to screen for comorbid psychiatric illnesses.11-13 Neuropsychiatric diagnoses were established using a best-estimate consensus procedure involving 2 child psychiatrists (B.S.P. and J.F.L.), who reviewed all available clinical and research data.14
Interview Procedure at Time 2
Assessments included current and worst-ever YGTSS and CY-BOCS ratings. Screening for any comorbid psychiatric conditions was conducted with the Structured Clinical Interview for DSM-IV Axis I Disorders.15 Additional assessments included a thorough medication history, specific inquiry about ADHD symptoms, and ages at worst-ever tic and OCD symptoms. Overall psychosocial functioning was rated using the Global Assessment Scale after the follow-up interview.16 Eight clinical evaluations relied solely on information provided by a parent who lived in the same home as the research subject.
Data Analysis
All statistical procedures were performed using SPSS version 12.0.17 Histograms were generated to examine the distribution of YGTSS and CY-BOCS scores at time 2. Because of the large number of subjects with no current tic or OCD symptoms at time 2 (YGTSS and CY-BOCS scores = 0), YGTSS and CY-BOCS scores were not normally distributed. To avoid the nonnormality problem for these outcome scales, we transformed them into ordinal groupings prior to hypothesis analysis to maintain gradations in symptom severity while also maintaining ordinal groupings of roughly equal subject number. The ordinal groupings for YGTSS score (range, 0-50) at follow-up used in these analyses were 0 (n = 15), 1 through 9 (n = 11), 10 through 19 (n = 10), and 20 or higher (n = 10). These groupings roughly correspond to absence of tics (YGTSS score, 0), minimal tic symptoms (YGTSS score, 1-9), mild tic symptoms (YGTSS score, 11-19), and moderate to severe tic symptoms (YGTSS score, ≥20). For CY-BOCS score at follow-up (range, 0-40), groupings for ordinal logistic regression were 0 (n = 24), 1 through 9 (n = 10), and greater than or equal to 10 (n = 9). These groupings roughly correspond to absence of OCD symptoms (CY-BOCS score, 0), sub-clinical OCD symptoms (CY-BOCS score, 1-9), and OCD symptoms of clinical significance (CY-BOCS score, ≥10).
The ordinal logistic regression module of SPSS 12.0 with a logit link was used for statistical analyses. This statistical package relies on the proportional odds model for ordinal logistic regression. Ordinal groupings for YGTSS and CY-BOCS ratings were used as the dependent variable in the analyses. For a priori hypothesis testing, YGTSS, CY-BOCS, and IQ scores or ADHD diagnosis at time 1 were entered as the independent variables. Each of these independent variables was then entered into individual ordinal logistic regression models. Additionally, for each ordinal logistic regression model, age at time 1 and interval between time 1 and time 2 were entered as additional covariates. Using a Bonferroni correction, the threshold for statistical significance was set at P<.01 to account for our 4 a priori hypotheses. All P values were 2 tailed.
Results
The 46 subjects were first interviewed at a mean ± SD age of 11.4 ± 1.6 years (range, 7.5-13.9 years). Demographic characteristics of subjects at time 1 are represented in the Table. At time 2, the patients with TS were at a mean ± SD age of 19.0 ± 1.8 years (range, 16.0-22.8 years). The mean ± SD duration between the initial and follow-up interviews was 7.6 ± 1.9 years (range, 3.8-12.8 years).
Current Tic Status
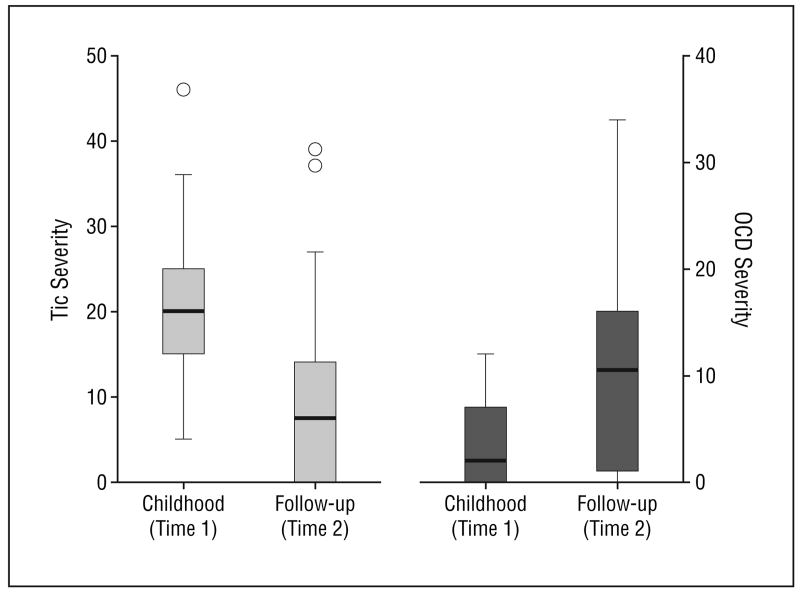
At time 2, when the patients with TS were 16 to 23 years of age, tic symptoms for the majority of patients were minimal or absent. On average, the mean ± SD total tic score of the YGTSS at time 2 was 10.0 ± 10.3 (median, 7.5; actual range, 0-39, with a possible range of 0-50). Fifteen patients (33%) reported being entirely tic free during the week before the follow-up interview, and only 10 patients (22%) were judged to have a moderate or marked level of tic severity based on their interview data (YGTSS score, ≥20 but <40). At time 1, all 46 subjects had tics of at least moderate severity (YGTSS score, ≥20). Figure 1 demonstrates a box plot comparing tic severity between time 1 and time 2 in our study sample.
Figure 1.
Box plots comparing tic and obsessive-compulsive disorder (OCD) symptom severity at the time of initial assessment in childhood (time 1) and follow-up in early adulthood (time 2). Tic symptom severity scores were measured by the Yale Global Tic Severity Scale7 and are reported for all 46 subjects with Tourette syndrome. The OCD symptom severity scores were measured using the Children's Yale-Brown Obsessive Compulsive Scale8 (CY-BOCS) and are reported for those 19 patients with Tourette syndrome with lifetime worst-ever OCD symptom severity scores that were at least in the moderate-severity range (CY-BOCS score, ≥10) as assessed at time 2.
Current Status of OCD Symptoms
Nineteen subjects with TS reported at some point experiencing at least mild lifetime OCD symptoms (worst-ever, CY-BOCS score, ≥10). The mean ± SD OCD severity score at follow-up was 10.8 ± 9.2 (median, 13; actual range, 0-34, with a possible range of 0-40) compared with 4.1 ± 4.2 (median, 2; actual range, 0-12) at baseline in childhood (Figure 1).
Severity and Timing of Tics and OCD Symptoms During the Worst-Ever Period
The mean ± SD worst-ever tic severity score was 31.6 ± 7.7 (range, 15-48) at a mean ± SD age of 10.6 ± 2.6 years (range, 6-19 years). Contrary to previous reports, the level of worst-ever tic severity was not significantly associated with the patient's age during the worst-ever period (Pearson correlation coefficient, r = 0.008; P = .96).5
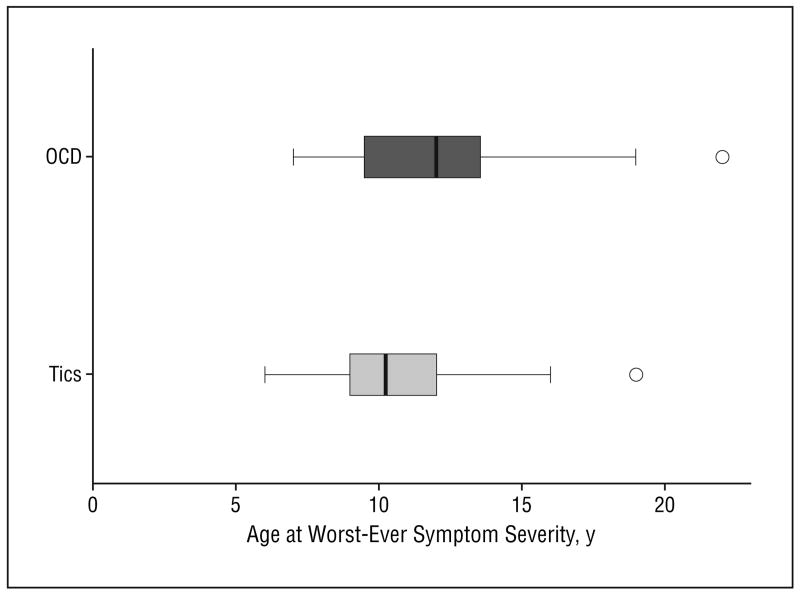
In the 19 subjects who had a lifetime history of OCD symptoms, the mean ± SD age at worst-ever OCD symptoms was 12.5 ± 3.9 (range, 7-22). Figure 2 depicts a box plot comparing age at worst-ever tic and OCD symptoms within our study sample. The mean ± SD worst-ever OCD severity score reported at follow-up was 23.3 ± 6.6 (range, 13-34).
Figure 2.
Box plot representing age when tic and obsessive-compulsive disorder (OCD) symptoms were at their worst. Age is represented for all 46 subjects with Tourette syndrome in this study sample. Age at worst-ever OCD symptoms were reported at follow-up for just those 19 patients with Tourette syndrome with lifetime worst-ever OCD symptom severity scores that were at least in the moderate-severity range (Children's Yale-Brown Obsessive Compulsive Scale score, ≥10).
Comorbid Conditions and Medication Use at Follow-Up
At the follow-up interview, 10 subjects had a lifetime history of ADHD symptoms. One subject had a history of ADHD symptoms but was clinically asymptomatic by the time 1 interview. Fifteen subjects (33%) had experienced a lifetime major depressive episode by the time 2 follow-up interview. Ten experienced their first depressive episode in the interval between the initial interview and the follow-up interview. Only 1 subject currently had depression. Two cases (4.3%) of bipolar disorder and 1 case of schizophrenia emerged at the follow-up interview.
At time 2, 6 subjects (13%) were using antipsychotics or α2-agonists (guanfacine hydrochloride or clonidine hydrochloride) (medication to treat tics), compared with 23 subjects (50%) at time 1. Five subjects (11%) were taking antipsychotics at follow-up (4 atypical antipsychotics, 1 typical) compared with 10 subjects (22%) at time 1 (9 typical antipsychotics, 1 atypical). Four subjects at both time 1 and time 2 were using selective serotonin reuptake inhibitors (fluvoxetine, sertraline hydrochloride, or clomipramine hydrochloride), although only 1 subject was taking a selective serotonin reuptake inhibitor at both times.
Childhood Clinical Measures Associated With Tic Severity at Follow-Up
Consistent with 1 of our a priori hypotheses, increased tic severity at time 1 was associated with increased tic severity at time 2 (odds ratio [OR], 1.11 [95% confidence interval (CI), 1.03-1.20]; P = .004; Nagelerke R2 = 0.19). Therefore, for every 10-point increase in the YGTSS tic severity rating in childhood, subjects were 2.8-fold more likely to have moderate or severe tics in early adulthood. In other words, a child who had marked tics (YGTSS score, ≥20) was 2.8-fold more likely to have marked to severe tics in adulthood compared with a child who had tics of only modest severity (YGTSS score, ≥10 but <20). Worst-ever tic severity measured at time 1 was not associated with tic severity at time 2 (OR, 1.05 [95% CI, 0.96-1.15]; P = .32). An ADHD diagnosis at time 1 (OR, 0.52 [95% CI, 0.14-1.95]; P = .33) was also not associated with follow-up tic severity.
Childhood Clinical Measures Associated With Increased OCD Severity at Follow-Up
In contrast, OCD symptom severity at time 1 (OR, 1.04 [95% CI, 0.86-1.25]; P = .70) was not associated with future OCD severity. However, higher IQ score measured at time 1 (OR, 1.11 [95% CI, 1.04-1.19]; P = .001; R2 = 0.46) was significantly associated with increased future OCD symptom severity. For every 10-point increase in childhood IQ, subjects were 2.8-fold more likely to express OCD symptoms at follow-up. Thus, the overall risk of developing clinically significant OCD symptoms (Y-BOCS score, ≥10) by early adulthood was 8-fold higher in a child with an IQ of 120 compared with a child with an IQ of 100.
Comment
This study is, to our knowledge, the first prospective longitudinal study of patients with TS that assessed both tic and OCD symptoms systematically at both initial evaluation and follow-up in early adulthood. On average, subjects in the cohort experienced their worst-ever tics at 10 to 11 years of age, and most experienced a marked attenuation of tic severity during adolescence. Slightly less than one quarter (22%) continued to experience mild or greater tic symptoms (YGTSS scores, ≥10) at follow-up, while nearly one third were in complete remission of tic symptoms at follow-up. The only childhood clinical measure significantly associated with increased tic severity at follow-up was increased tic severity at the time of baseline assessment during childhood (<14 years). This finding stands in contrast to those of previous studies, which did not detect an association of childhood tic severity with the severity of tics later in life.1
The time when OCD symptoms were at their worst was approximately 2 years later than when tic symptoms were at their worst. The majority of individuals who had OCD symptoms at follow-up reported the onset of their OCD symptoms in late childhood or early adolescence, after the time of their initial evaluation. Increased childhood IQ was strongly associated with the future development of OCD symptoms in children with TS. This result is consistent with previous studies in the general population that have associated increased childhood IQ with the development of OCD in adulthood.3
This study has several limitations. It reports findings in a clinically referred population of children with TS whose overall illness severity and rates of comorbid illnesses are likely to be greater than in children who have TS in the general population. Furthermore, approximately one quarter of eligible subjects did not participate in the follow-up evaluation. Although participating and nonparticipating subjects did not differ significantly in any demographic or clinical characteristics at the time of initial evaluation, we cannot exclude the possibility that self-selection bias affected our findings at follow-up. In addition, because at baseline we excluded children with an IQ lower than 80, our finding that higher IQ is associated with worse future OCD symptoms in patients with TS cannot be interpreted as indicating that that those with IQs lower than 80 are not at risk of developing OCD.
Despite these limitations, we can draw 4 clinical conclusions from the findings in this study. First, tics typically diminish in severity during adolescence, even for those children who have relatively severe tic symptoms. This is welcome information for parents seeking an evaluation and treatment for TS and consistent with previous reports.2 Given that tic symptoms are likely to decrease spontaneously during adolescence, support and tolerance from family, teachers, peers, and health care professionals may either reduce or eliminate the need for exposure to medications.1,2 Second, the severity of tics in late childhood was associated, though only modestly so, with future tic severity in late adolescence and early adulthood. Additional work is needed to identify these high-risk individuals. Genetic studies and performance on focused neuropsychological testing as well as magnetic resonance imaging volumetric measurements may hold promise. The predictive value of perinatal risk factors also needs to be evaluated prospectively. Third, the presence of childhood ADHD symptoms was not associated with the presence or severity of adulthood tic or OCD symptoms. Fourth, OCD symptoms on average reached their peak severity 2 years later than tics. Thus, educating patients and their parents about the possibility of developing clinically significant OCD symptoms at a point later in childhood and adolescence, even if OCD symptoms are not evident at the time of initial evaluation, may be important. This may be especially important for bright children who have tics because IQ in childhood in this sample and other samples has been most predictive of increased severity of OCD symptoms in later adolescence, regardless of OCD symptoms in childhood.11 Periodic monitoring for OCD symptoms after the initial evaluation by experts in tic disorders and OCD may be warranted, given the importance that OCD symptoms have in contributing to later psychosocial functioning.1
Acknowledgments
Funding/Support: This study was supported in part by grants MH49351 (Dr Leckman), MH01232 (Dr Peterson), MH59139 (Dr Peterson), MH30929, and RR06022 from the National Institute of Mental Health, Rockville, Md; a grant from the Tourette Syndrome Association, New York, NY; the Suzanne Crosby Murphy Endowment at Columbia University, New York; the Smart Family Foundation, Wilton, Conn; Jay and Jean Kaiser; Eric Brooks; and the Chasanoff Family at the Yale University Child Study Center as well as gifts from anonymous donors.
We thank our many collaborators at Yale including Robert King, MD, Diane Findley, PhD, Lily Katsovich, MS, and Heidi Grantz, MSW, for their encouragement and substantial contributions in following up these families and participating in the initial assessment of these individuals. We thank Robert Schultz, PhD, for providing IQ measurements in the children of this sample. We thank Hanna Stevens, MD, PhD, and Andres Martin, MD, MPH, for their contributions toward the final manuscript of this article.
Footnotes
Author Contributions: Dr Zhang was the statistical expert for this study. He can be reached at Department of Epidemiology and Public Health, Yale University School of Medicine, 60 College St, New Haven, CT 06520-8034 (heping.zhang@yale.edu).
Contributor Information
Michael H. Bloch, Yale Child Study Center, the General Clinical Research Center, and the Departments of Pediatrics and Epidemiology and Public Health, Yale University School of Medicine, New Haven, Conn.
Bradley S. Peterson, Division of Child and Adolescent Psychiatry and the Magnetic Resonance Imaging Unit, New York State Psychiatric Institute and Columbia University College of Physicians and Surgeons, New York.
Lawrence Scahill, Yale Child Study Center, the General Clinical Research Center, and the Departments of Pediatrics and Epidemiology and Public Health, Yale University School of Medicine, New Haven, Conn.
Jessica Otka, Yale Child Study Center, the General Clinical Research Center, and the Departments of Pediatrics and Epidemiology and Public Health, Yale University School of Medicine, New Haven, Conn.
Lily Katsovich, Yale Child Study Center, the General Clinical Research Center, and the Departments of Pediatrics and Epidemiology and Public Health, Yale University School of Medicine, New Haven, Conn.
Heping Zhang, Yale Child Study Center, the General Clinical Research Center, and the Departments of Pediatrics and Epidemiology and Public Health, Yale University School of Medicine, New Haven, Conn.
James F. Leckman, Yale Child Study Center, the General Clinical Research Center, and the Departments of Pediatrics and Epidemiology and Public Health, Yale University School of Medicine, New Haven, Conn.
References
- 1.Erenberg G, Cruse RP, Rothner AD. The natural history of Tourette's syndrome: a follow-up study. Ann Neurol. 1987;22:383–385. doi: 10.1002/ana.410220317. [DOI] [PubMed] [Google Scholar]
- 2.Leckman JF, Zhang H, Vitale A, et al. Course of tic severity in Tourette syndrome: the first two decades. Pediatrics. 1998;102:14–19. doi: 10.1542/peds.102.1.14. [DOI] [PubMed] [Google Scholar]
- 3.Peterson BS, Pine DS, Cohen P, Brook JS. Prospective, longitudinal study of tic, obsessive-compulsive, and attention-deficit/hyperactivity disorder in an epidemiological sample. J Am Acad Child Adolesc Psychiatry. 2001;40:685–695. doi: 10.1097/00004583-200106000-00014. [DOI] [PubMed] [Google Scholar]
- 4.Peterson BS, Skudlarski P, Anderson AW, et al. A functional magnetic resonance imaging study of tic suppression in Tourette syndrome. Arch Gen Psychiatry. 1998;55:326–333. doi: 10.1001/archpsyc.55.4.326. [DOI] [PubMed] [Google Scholar]
- 5.Peterson BS, Thomas P, Kane MJ, et al. Basal ganglia volumes in patients with Gilles de la Tourette syndrome. Arch Gen Psychiatry. 2003;60:415–424. doi: 10.1001/archpsyc.60.4.415. [DOI] [PubMed] [Google Scholar]
- 6.Peterson BS, Skudlarski P, Anderson AW, et al. Regional brain and ventricular volumes in Tourette's syndrome. Arch Gen Psychiatry. 2001;58:427–440. doi: 10.1001/archpsyc.58.5.427. [DOI] [PubMed] [Google Scholar]
- 7.Leckman JF, Riddle MA, Hardin MT, et al. The Yale Global Tic Severity Scale: initial testing of a clinician-rated scale of tic-severity. J Am Acad Child Adolesc Psychiatry. 1989;28:566–573. doi: 10.1097/00004583-198907000-00015. [DOI] [PubMed] [Google Scholar]
- 8.Scahill L, Riddle MA, McSwiggin-Hardin M, et al. Children's Yale-Brown Obsessive Compulsive Scale: reliability and validity. J Am Acad Child Adolesc Psychiatry. 1997;36:844–852. doi: 10.1097/00004583-199706000-00023. [DOI] [PubMed] [Google Scholar]
- 9.Grossman I, Kaufman AS, Grossman D. Correlations of scores on the Kaufman short neuropsychological assessment procedure and the Kaufman Adolescent and Adult Intelligence Test for a hospitalized depressed sample. Percept Mot Skills. 1993;77:1055–1058. doi: 10.2466/pms.1993.77.3.1055. [DOI] [PubMed] [Google Scholar]
- 10.Naugle RI, Chelune GJ, Tucker GD. Validity of the Kaufman Brief Intelligence Test. Psychol Assess. 1993;5:182–186. [Google Scholar]
- 11.Ambrosini P, Metz C, Prabucki K, Lee JC. Videotape reliability of the third revised edition of the K-SADS. J Am Acad Child Adolesc Psychiatry. 1989;28:723–728. doi: 10.1097/00004583-198909000-00013. [DOI] [PubMed] [Google Scholar]
- 12.Kaufman J, Birmaher B, Brent D, et al. Schedule for affective disorders and schizophrenia for school-age children—present and lifetime version (K-SADS-PL): initial reliability and validity data. J Am Acad Child Adolesc Psychiatry. 1997;36:980–988. doi: 10.1097/00004583-199707000-00021. [DOI] [PubMed] [Google Scholar]
- 13.Pauls DL, Hurst CR. Schedule for Tourette and Other Behavioral Syndromes. New Haven, Conn: Yale University Child Study Center; 1996. [Google Scholar]
- 14.Leckman JF, Sholomskas D, Thompson WD, et al. Best estimate of lifetime psychiatric diagnosis. Arch Gen Psychiatry. 1982;39:879–883. doi: 10.1001/archpsyc.1982.04290080001001. [DOI] [PubMed] [Google Scholar]
- 15.Endicott J, Spitzer RL. A diagnostic interview: the schedule for affective disorders and schizophrenia. Arch Gen Psychiatry. 1978;35:837–844. doi: 10.1001/archpsyc.1978.01770310043002. [DOI] [PubMed] [Google Scholar]
- 16.Endicott J, Spitzer RL, Fleiss JL, Cohen J. The Global Assessment Scale. Arch Gen Psychiatry. 1976;33:766–771. doi: 10.1001/archpsyc.1976.01770060086012. [DOI] [PubMed] [Google Scholar]
- 17.Statistical Product and Service Solutions [computer program]. Version 13.0 for Windows. Chicago, Ill: SPSS Inc; 2004. [Google Scholar]