Abstract
Objectives
Abnormalities in volumes of the amygdala have been reported previously in adolescents and adults with bipolar disorder (BD). Several studies have reported reduced volumes in adolescents with BD; however, both decreases and increases in volumes have been reported in adults with BD. Understanding of potential developmental contributions to these disturbances in morphology of the amygdala has been limited by the absence of longitudinal data in persons with BD. Here we use a within-subject longitudinal design to investigate whether amygdala volume abnormalities persist in adolescents and young adults with BD over a time interval of approximately 2 years.
Methods
Participants included 18 adolescents and young adults: 10 participants with BD I and 8 healthy comparison participants. Amygdala volumes were measured on high-resolution magnetic resonance imaging scans acquired twice for each subject over intervals of approximately 2 years. Amygdala volumes were the dependent measures in a mixed-model statistical analysis to compare amygdala volumes between groups over time while covarying for total brain volume.
Results
Amygdala volumes were significantly smaller in adolescents and young adults with BD compared with healthy participants (p = 0.018). The effect of time was not significant.
Conclusions
Although the sample size is modest, this study provides preliminary evidence to support the presence of decreased amygdala volumes in adolescents and young adults with BD that persist during this developmental epoch.
Keywords: adolescents, amygdala, bipolar disorder, magnetic resonance imaging
The amygdala has long been implicated in the pathophysiology of mood disorders. Early observations of bipolar-type mood symptoms associated with medial temporal seizure foci prefigured the notion that abnormalities in the morphology and activity of the amygdala might contribute to the symptoms of primary bipolar disorder (BD) (1, 2). Abnormal emotional processing in individuals with BD, such as difficulty recognizing emotions depicted by facial expressions, as well as the biasing of behavioral responses towards stimuli that are congruent with the acute mood state, further implicated the amygdala in adults with BD (3–6). Functional neuroimaging studies in adults provide evidence of excessive amygdala activity in research participants with BD compared with healthy comparison (HC) participants in both resting states and while performing emotional processing tasks (3, 7, 8). The divergent levels of amygdala activity detected between groups may relate to underlying differences in volume of the amygdala that have been observed in structural imaging studies. While many studies of amygdala volumes in adults with BD have reported differences between individuals with BD and HC participants, these findings have been highly variable, including both increases and decreases in volume of the amygdala in adults with BD (9–13).
The few extant morphological studies of the amygdala in adolescents with BD have yielded more consistent findings than have the adult studies. Several groups have reported smaller amygdala volumes in adolescents with BD compared with HC adolescents (13–16), suggesting that smaller amygdala volumes may be present in at least a subset of individuals who manifest symptoms of BD in adolescence. Our previous cross-sectional study reported reduced amygdala volumes in both adolescents and adults with BD (13). Although inferring longitudinal trajectories from cross-sectional data such as these must be done cautiously, our findings did suggest that morphological abnormalities in persons with BD might manifest as early as the teenage years and persist into adulthood (13). A subsequent cross-sectional study of adolescents with BD suggested that although reduced amygdala volumes are evident early in adolescence, progressive enlargement with age might produce larger volumes by later adolescence or early adulthood in persons with BD (15). We undertook a longitudinal study of amygdala volumes in adolescents and young adults with BD to assess whether reduced volumes of the amygdala persist during the adolescent/early adult period. To the best of our knowledge, this is the first report of amygdala volumes in BD studied within a prospective, longitudinal framework.
Patients and methods
Subjects
Participants included 10 outpatients with BD I (50% female) who were between 10 and 21 years of age (mean 15.0 years ± 4.0 SD) at the time of the initial scan. Rescanned on average 2.5 ± 0.4 SD years after their first scans, they were between 12 and 23 years old (mean 17.5 years ± 3.9 SD) at the time of the second scan. The HC group consisted of eight adolescents and young adults without DSM-IV Axis I diagnosis in themselves or in their first-degree relatives; they were between 11 and 19 years of age (mean 15.3 years ± 2.8 SD) at the time of the initial scan. Rescanned on average 2.0 ± 0.6 SD years after their first scan, they were 13–20 years old (mean 17.4 years ± 2.8 SD) at the second scan (Table 1). The participants with BD are a subset of the participants with BD described previously (13); however, here they are compared with a different sample of HC participants for whom longitudinal data are available. All subjects were without a history of other neurologic disorders, loss of consciousness for longer than 5 min, and significant medical illness, with the exception of one patient with hypothyroidism.
Table 1.
Age and sex of the subjects
| Variable | Bipolar
(n = 10) |
Healthy controls
(n = 8) |
p-value |
|---|---|---|---|
| Age at time 1, (mean ± SD) | 15.0 ± 4.0 | 15.3 ± 2.8 | NS |
| Age at time 2, (mean ± SD) | 17.5 ± 3.9 | 17.4 ± 2.8 | NS |
| Interval between scans (mean ± SD) | 2.5 ± 0.4 | 2.0 ± 0.6 | NS |
| Sex, n (%) | |||
| Female | 5 (50) | 6 (75) | NS |
| Male | 5 (50) | 2 (25) |
NS = not significant.
The revised Schedule for Affective Disorders and Schizophrenia for School-Age Children-Present and Lifetime Version (KSAD-PL) (17) confirmed the presence or absence of DSM-IV Axis I disorders in participants 18 years of age and under. Interviews were administered separately to the subject and a parent (or maternal grandmother for one participant with BD). The Structured Clinical Interview for DSM-IV Axis I Disorders (SCID) (Version 2.0) (18) confirmed the presence or absence of diagnosis in participants over 18 years of age. Final DSM-IV diagnoses were established by the consensus of clinical and structured interviews. All participants in the index group met criteria for BD I (see Table 2 for proband characteristics). Six participants with BD (60%) met criteria for rapid cycling at the time of the first scan. Three additional participants with BD met criteria for rapid cycling at the time of the second scan, although two participants that previously met criteria for rapid cycling did not do so at the time of the second scan. Therefore, a total of seven participants (70%) met criteria for rapid cycling at the time of the second scan. All participants with BD were symptomatic at the time of the initial scan and exhibited varying degrees of depressive and manic symptomatology, with the exception of one adolescent who did not display sufficient symptomatology to meet criteria for an acute mood episode at first scan but was symptomatic at the time of the second scan. Three participants were euthymic at second scan. Co-morbidities (Table 2) included attention-deficit hyperactivity disorder (ADHD), oppositional defiant disorder, social phobia, post-traumatic stress disorder, learning disorder not otherwise specified and developmental coordination disorder. Co-morbid diagnoses did not change over the duration of the study. One participant had a history of alcohol dependence (criteria not met for > 1 year prior to the first and 3 years prior to the second scan) and other substance abuse. All BD participants reported at least one first- or second-degree relative with a mood-spectrum disorder that included BD, major depressive disorder, or alcohol abuse, although a positive family history was not required for study entry. Five participants with BD (50%) were free of medication at the initial scan. At the second scan seven participants with BD (70%) were not taking psychotropic medications. All five participants who were not taking psychotropic medication at the time of both scans had been exposed to either lithium or valproic acid or other psychotropic medications. There was no systematic pattern for medication usage in the interval between the first and second scans. Medications at time of scanning are presented in Table 2. HC participants were unmedicated at first scan, and one was taking birth control pills at second scan.
Table 2.
Clinical characteristics of the patient group
| Patient no. | Sex | Age at first scan (years) | Age at second scan (years) | Interval between scans (years) | Rapid cycling | Medications at scan 1 | Medications at scan 2 | Comorbid diagnoses |
|---|---|---|---|---|---|---|---|---|
| 1 | Female | 11.7 | 14.3 | 2.6 | Scans 1 & 2 | None | None | Social phobia |
| 2 | Female | 15.8 | 18.6 | 2.8 | Scan 2 | None | Birth control pills | None |
| 3 | Female | 16.6 | 18.8 | 2.2 | Scans 1 & 2 | None | Birth control pills | None |
| 4 | Female | 17.2 | 20.2 | 3.0 | Scan 2 | Carbamazepine, levothyroxine | None | Oppositional defiant disorder, post-traumatic stress disorder, hypothyroidism |
| 5 | Female | 21.5 | 23.6 | 2.1 | Scan 1 | None | None | History of alcohol dependence, hallucinogen and marijuana abuse |
| 6 | Male | 10.7 | 12.7 | 2.0 | Scans 1 & 2 | Lithium | None | Oppositional defiant disorder |
| 7 | Male | 11.7 | 14.0 | 2.2 | Scan 2 | VPA, olanzapine, methylphenidate, clonidine | Olanzapine, methylphenidate | ADHD, developmental coordination disorder |
| 8 | Male | 12.0 | 14.6 | 2.5 | Scan 1 | VPA, nortriptyline | VPA, nortriptyline, methylphenidate | Learning disorder NOS |
| 9 | Male | 12.2 | 15.6 | 3.3 | None | Lithium, risperidone, sertraline | Lithium, VPA, risperidone, sertraline, atomoxetine | Learning disorder NOS |
| 10 | Male | 20.8 | 23.0 | 2.2 | Scans 1 & 2 | None | None | None |
VPA = valproic acid; ADHD = Attention-deficit hyperactivity disorder; NOS = not otherwise specified.
After complete description of the study, written informed consent was provided by all participants 18 years and older. For subjects under 18 years of age, written informed consent was provided by a guardian and written informed assent was provided by the participant. These procedures were in accordance with the human investigation committees of the Yale University School of Medicine and the Department of Veterans Affairs.
Magnetic resonance image acquisition and processing
Magnetic resonance imaging scans were obtained using a single 1.5-T scanner (GE Signa; General Electric, Milwaukee, WI, USA). Head positioning was standardized using canthomeatal landmarks. Images were obtained using a 3-dimensional sagittal spoiled gradient echo sequence (repetition time, 24 ms; echo time, 5 ms; flip angle, 45°; frequency encoding superior/inferior; no wrap; 256 × 192 matrix; field of view, 30 cm; 2 excitations; slice thickness, 1.2 mm; and 124 contiguous slices).
Morphometric measures were performed on Linux workstations. The brains were first stripped using BET [Brain Extraction Tool (19)] which is included in the FSL package [Oxford Centre for Functional Magnetic Resonance Imaging of the Brain (FMRIB) Software Library, Oxford University, http://www.fmrib.ox.ac.uk/fsl] and then segmented into gray matter, white matter and cerebrospinal fluid using FAST [FMRIB Automated Segmentation Tool (20) – also part of FSL]. The total brain volume was computed by adding the gray and white matter volumes. The amygdala volumes were defined by means of manual tracing by operators blind to diagnosis using a custom tool modified for this application (21) with anatomical boundaries in accordance with those described previously (13, 22, 23). In brief, the amygdala was defined from the coronal slice at the plane of the anterior commissure (anterior border) to the slice on which the uncinate gyrus formed a loop and turned laterally to abut the amygdala and where CSF was present between the amygdala and hippocampus (posterior border). White matter or CSF defined the inferior and lateral borders. The medial border was marked by the medial margin of the temporal lobe, and the medial superior border by the entorhinal sulcus. The superior border was defined with a line from the inferior aspect of the entorhinal sulcus in the direction of the circular sulcus of the insula to the temporal stem. Initial tracings in the coronal-oblique plane (perpendicular to the anterior commissure–posterior commissure plane) were confirmed in orthogonal views. Inter-rater intraclass correlation coefficients for these procedures assessed on 10 scans were 0.91 for the right amygdala and 0.96 for the left amygdala. All traces were checked by an expert (RKF).
Statistical analyses
All data were analyzed using SAS, version 9.1 (SAS Institute Inc., Cary, NC, USA). Amygdala volumes were tested for normality using Kolmogorov–Smirnov test statistics and normal probability plots; all were approximately normal in their distribution. Volumes of the amygdala from the measurements of two raters who had high agreement were averaged for each hemisphere and time. The primary statistical mixed model tested whether the BD and HC groups differed in amygdala volume and whether time influenced this difference. The amygdala volume represented the dependent variable, group (BD and HC) was included as a fixed effect, and hemisphere (left and right) and time (scan 1 and scan 2) were included as within-subject explanatory factors. This model allowed for testing of all 2- and 3-way interactions among group, hemisphere, and time. In this model, subject was used as the clustering factor and total brain volume was included as a continuous covariate to control for scaling effects. The above analyses were considered statistically significant for p < 0.05. Least squares means (ls means), and standard errors (SE) were calculated in the mixed model for regional volumes and plotted to interpret the effects of diagnosis.
Results
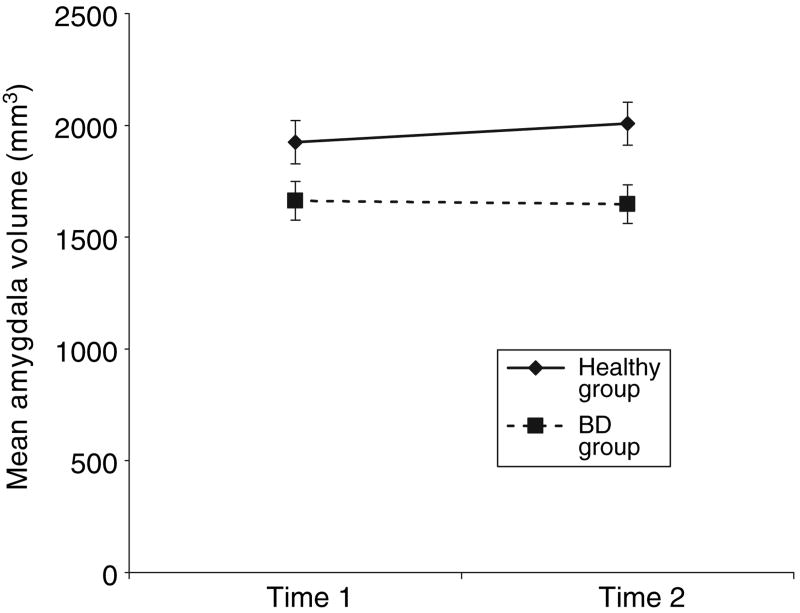
The BD and HC groups did not differ significantly in age at first or second scan or in the length of the interval between scans (Table 1). Overall amygdala volumes were significantly smaller in participants with BD compared with the HC participants (group effect: F1,15.2 = 7.00, p = 0.018) (Fig. 1). Group differences were present at both time 1 (HC amygdala volume ls mean ± SEM = 1925 mm3 ± 97, BD = 1663 ± 86, F1,21.2 = 4.07, p = 0.056) and time 2 (HC amygdala volume ls mean ± SD = 2008 mm3 ± 96, BD = 1648 ± 86, F1,21.2 = 7.84, p = 0.011). A significant overall effect of total brain volume (F1,19.7 = 10.23, p = 0.005) indicated that general scaling within the brain contributed to the inter-individual variance in regional volume. No significant main effects of time or side, or group-by-time and group-by-side interactions were detected (all p > 0.37). However, a time-by-side interaction was significant (F1,32 = 4.52, p = 0.041), deriving from small, non-significant increases in volume of the left amygdala in the HC group and small, non-significant decreases in volume of the right amygdala in the BD group over time.
Fig. 1.
Amygdala mean volume values (±SE) for the healthy adolescent comparison group (n = 8) and the bipolar disorder (BD) group (n = 10). Mean values are adjusted for total brain volume and hemisphere. The effect of diagnosis was significant at p = 0.018.
Discussion
We detected reduced volumes of the amygdala bilaterally in adolescents and young adults with BD compared with HC participants, both at the time of initial scan and after a time interval of approximately 2 years. This finding is consistent with previous reports, from cross-sectional studies, of reduced amygdala volume in adolescents and adults with BD (12, 14–16). It extends our previous cross-sectional study (13) by suggesting that morphological disturbances of the amygdala are relatively stable over a 2-year period in adolescents and young adults with BD.
We cannot infer the disturbances in specific cellular elements that might have contributed to the macroscopic abnormalities in volume detected in these data. Reduced volumes could derive from any number of cellular disturbances, including reductions in the numbers or sizes of neurons or their processes, alterations in packing density, or disturbances in the numbers or processes of glial cells (24–27). Indeed, recent reports suggest the presence of reduced glial cell numbers, particularly reduced numbers of oligodendrocytes, in the amygdala of adults with mood disorders (24, 25).
Possible behavioral consequences of disturbances in the morphology of the amygdala in persons with BD may include deficits in adaptive responses to emotional and social stimuli (28–34). Similar to individuals with amygdala lesions (33), individuals with BD are impaired in their ability to recognize specific facial emotions (3–5), and preliminary evidence suggests that these deficits are associated with abnormal functional activity in the amygdala of adults with BD (3, 8). While abnormal functioning of basal ganglia structures has been reported in adolescents with BD (35–37), functional abnormalities specifically in the amygdala of adolescents with BD have not been reported. Therefore, whether these early structural abnormalities of the amygdala are associated with functional disturbances during adolescence in persons with BD is currently unknown.
The detection of structural abnormalities in the amygdala during adolescence suggests that these morphological characteristics may serve as potential early biological markers of this disorder. However, studies earlier in childhood are needed to determine precisely when in ontogeny these abnormalities arise and whether indeed they are present at the time of onset of the illness or whether instead they are consequences of either chronic or recurrent illness (38) or of medication exposure. The presence of abnormalities in the amygdala during adolescence, a period of intense dynamic development of the orbitofrontal cortices and other regions connected to the amygdala that have been implicated in the pathophysiology of BD (39–44), raises the possibility that targeting those abnormalities with treatments could help in preventing the progression of abnormalities in cortico-limbic circuits (37). We did not detect medication effects on amygdala volumes, although this may be attributable to insufficient statistical power. Preliminary evidence does suggest that mood-stabilizing medications are associated with increased cortical gray matter in BD (45, 46) and that lithium and valproic acid treatment are associated with increased amygdala volume in pediatric BD (16). This suggests that future neuroimaging studies, combined with systematic treatment of pediatric BD patients, are warranted.
The findings differ from those of a previous study that noted abnormal increases in amygdala volume with age in adolescents and young adults with BD (15). However, differences between the two studies include the cross-sectional design and larger number of BD participants taking lithium or valproic acid in the previous study. The results of the present study, taken in the context of variable findings in volumes of the amygdala in adults with BD (9–13), suggest that reduced amygdala volumes could represent an early-onset morphological subtype of BD. The ability to generalize from the pediatric BD sample studied may be limited by the inclusion criteria requiring that participants meet full DSM-IV criteria for acute BD episodes, the presence of rapid-cycling subtype in a majority of BD participants (a characteristic of only a subset of patients with BD and one observed more commonly in children than in adults) and the relatively low rate of other comorbidities, such as ADHD, that have been reported to occur frequently in adolescents with BD (47). Heterogeneous features, such as a range in age from 10 to 21 during which time significant neurodevelopmental changes occur as well as a range of comorbid illnesses and medications in some of the subjects, could have diluted the power to detect effects that could be detected in a larger, more homogeneous sample. It is possible that one of these features, such as a particular medication contributed to the group differences observed.
In summary, this study provides preliminary evidence, in a limited subject sample, that volume reductions in the amygdala are stable over an interval of approximately 2 years in adolescents and young adults with BD. Larger longitudinal studies that include prepubescent subjects, as well as children at risk for developing the disorder, will be important for understanding the role of amygdala abnormalities in the development of BD.
Acknowledgments
The authors thank Kathleen Colonese for her expert care in coordinating the research, Ralitza Gueorguieva for her statistical consultation, Monique Jones for her assistance with the data, Cheryl Lacadie, Hedy Sarofin, and Terry Hickey for their technical expertise, and the research subjects for their participation.
Funding sources: The authors were supported by research grants from the Ethel F. Donaghue Women's Investigator Program at Yale (New Haven, CT) (HPB), National Alliance for Research in Affective Disorders and Schizophrenia (Great Neck, NY) (HPB), the Stanley Medical Research Institute (HPB), the Department of Veterans Affairs (HPB, JHK), the National Institute of Mental Health RO1MH69747, RO1MH070902 (HPB), and NIAAA KO5AA14906-01 (JHK).
Footnotes
The authors of this paper do not have any commercial associations that might pose a conflict of interest in connection with this manuscript.
References
- 1.Flor-Henry P. Schizophrenia-like reactions and affective psychoses associated with temporal lobe epilepsy: etiological factors. Am J Psychiatry. 1969;126:400–404. doi: 10.1176/ajp.126.3.400. [DOI] [PubMed] [Google Scholar]
- 2.Post RM, Uhde TW, Putnam FW, Ballenger JC, Berrettini WH. Kindling and carbamazepine in affective illness. J Nerv Ment Dis. 1982;170:717–731. doi: 10.1097/00005053-198212000-00002. [DOI] [PubMed] [Google Scholar]
- 3.Yurgelun-Todd DA, Gruber SA, Kanayama G, Killgore WD, Baird AA, Young AD. fMRI during affect discrimination in bipolar affective disorder. Bipolar Disord. 2000;2:237–248. doi: 10.1034/j.1399-5618.2000.20304.x. [DOI] [PubMed] [Google Scholar]
- 4.George MS, Huggins T, McDermut W, Parekh PI, Rubinow D, Post RM. Abnormal facial emotion recognition in depression: serial testing in an ultra-rapid-cycling patient. Behav Modif. 1998;22:192–204. doi: 10.1177/01454455980222007. [DOI] [PubMed] [Google Scholar]
- 5.Lembke A, Ketter TA. Impaired recognition of facial emotion in mania. Am J Psychiatry. 2002;159:302–304. doi: 10.1176/appi.ajp.159.2.302. [DOI] [PubMed] [Google Scholar]
- 6.Murphy FC, Sahakian BJ, Rubinsztein JS, et al. Emotional bias and inhibitory control processes in mania and depression. Psychol Med. 1999;29:1307–1321. doi: 10.1017/s0033291799001233. [DOI] [PubMed] [Google Scholar]
- 7.Drevets WC, Price JL, Bardgett ME, Reich T, Todd RD, Raichle ME. Glucose metabolism in the amygdala in depression: relationship to diagnostic subtype and plasma cortisol levels. Pharmacol Biochem Behav. 2002;71:431–47. doi: 10.1016/s0091-3057(01)00687-6. [DOI] [PubMed] [Google Scholar]
- 8.Lawrence NS, Williams AM, Surguladze S, et al. Subcortical and ventral prefrontal cortical neural responses to facial expressions distinguish patients with bipolar disorder and major depression. Biol Psychiatry. 2004;55:578–587. doi: 10.1016/j.biopsych.2003.11.017. [DOI] [PubMed] [Google Scholar]
- 9.Swayze VW, II, Andreasen NC, Alliger RJ, Yuh WTC, Ehrhardt JC. Subcortical and temporal structures in affective disorders and schizophrenia: a magnetic resonance imaging study. Biol Psychiatry. 1992;31:221–240. doi: 10.1016/0006-3223(92)90046-3. [DOI] [PubMed] [Google Scholar]
- 10.Strakowski SM, DelBello MP, Sax KW, et al. Brain magnetic resonance imaging of structural abnormalities in bipolar disorder. Arch Gen Psychiatry. 1999;56:254–260. doi: 10.1001/archpsyc.56.3.254. [DOI] [PubMed] [Google Scholar]
- 11.Altshuler LL, Bartzokis G, Grieder T, et al. An MRI study of temporal lobe structures in men with bipolar disorder or schizophrenia. Biol Psychiatry. 2000;48:147–162. doi: 10.1016/s0006-3223(00)00836-2. [DOI] [PubMed] [Google Scholar]
- 12.Pearlson GD, Barta PE, Powers RE, et al. Medial and superior temporal gyral volumes and cerebral asymmetry in schizophrenia versus bipolar disorder. Biol Psychiatry. 1997;41:1–14. doi: 10.1016/s0006-3223(96)00373-3. [DOI] [PubMed] [Google Scholar]
- 13.Blumberg HP, Kaufman J, Martin A, et al. Amygdala and hippocampal volumes in adolescents and adults with bipolar disorder. Arch Gen Psychiatry. 2003;60:1201–1208. doi: 10.1001/archpsyc.60.12.1201. [DOI] [PubMed] [Google Scholar]
- 14.DelBello MP, Zimmerman ME, Mills NP, Getz GE, Strakowski SM. Magnetic resonance imaging analysis of amygdala and other subcortical brain regions in adolescents with bipolar disorder. Bipolar Disord. 2004;6:43–52. doi: 10.1046/j.1399-5618.2003.00087.x. [DOI] [PubMed] [Google Scholar]
- 15.Chen BK, Sassi R, Axelson D, et al. Cross-sectional study of abnormal amygdala development in adolescents and young adults with bipolar disorder. Biol Psychiatry. 2004;56:399–405. doi: 10.1016/j.biopsych.2004.06.024. [DOI] [PubMed] [Google Scholar]
- 16.Chang KD, Karchemskiy A, Barnea-Goraly N, Garrett A, Simeonova D, Reiss AL. Decreased amygdalar volume in pediatric familial bipolar disorder. J Am Acad Child Adolesc Psychiatry. 2005;44:565–573. doi: 10.1097/01.chi.0000159948.75136.0d. [DOI] [PubMed] [Google Scholar]
- 17.Kaufman J, Birmaher B, Brent D, et al. Schedule for affective disorders and schizophrenia for school-age children – present and lifetime version (K-SADS-PL): initial reliability and validity. J Am Acad Child Adolesc Psychiatry. 1997;36:980–988. doi: 10.1097/00004583-199707000-00021. [DOI] [PubMed] [Google Scholar]
- 18.First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV Axis I & II Disorders (Version 2.0) New York, NY: New York State Psychiatric Institute; 1995. [Google Scholar]
- 19.Smith SM. Fast robust automated brain extraction. Hum Brain Mapp. 2002;17:143–155. doi: 10.1002/hbm.10062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang Y, Brady M, Smith S. Segmentation of brain MR images through a hidden Markov random field model and the expectation maximization algorithm. IEEE Trans Med Imaging. 2001;20:45–57. doi: 10.1109/42.906424. [DOI] [PubMed] [Google Scholar]
- 21.Papademetris X, Jackowski M, Rajeevan N, Constable RT, Staib LH. BioImage Suite: an integrated medical image analysis suite. IJ-2005 MICCAI Open-Source Workshop. http://hdl.handle.net/1926/37. [PMC free article] [PubMed]
- 22.Kates WR, Abrams MT, Kaufmann WE, Breiter S, Reiss AL. Reliability and validity of MRI measurement of the amygdala and hippocampus in children with fragile X syndrome. Psychiatry Res. 1997;75:31–48. doi: 10.1016/s0925-4927(97)00019-x. [DOI] [PubMed] [Google Scholar]
- 23.Schumann CM, Hamstra J, Goodlin-Jones BL, et al. The amygdala is enlarged in children but not adolescents with autism; the hippocampus is enlarged at all ages. J Neurosci. 2004;24:6392–6401. doi: 10.1523/JNEUROSCI.1297-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bowley MP, Drevets WC, Öngür D, Price JL. Low glial numbers in the amygdala in major depressive disorder. Biol Psychiatry. 2002;52:404–412. doi: 10.1016/s0006-3223(02)01404-x. [DOI] [PubMed] [Google Scholar]
- 25.Hamidi M, Drevets WC, Price JL. Glial reduction in amygdala in major depressive disorder is due to oligodendrocytes. Biol Psychiatry. 2004;55:563–569. doi: 10.1016/j.biopsych.2003.11.006. [DOI] [PubMed] [Google Scholar]
- 26.Tkachev D, Mimmack ML, Ryan MM, et al. Oligodendrocyte dysfunction in schizophrenia and bipolar disorder. Lancet. 2003;362:798–805. doi: 10.1016/S0140-6736(03)14289-4. [DOI] [PubMed] [Google Scholar]
- 27.Rajkowska G. Cell pathology in mood disorders. Semin Clin Neuropsychiatry. 2002;7:281–292. doi: 10.1053/scnp.2002.35228. [DOI] [PubMed] [Google Scholar]
- 28.Bechara A, Damasio H, Damasio AR, Lee GP. Different contributions of the human amygdala and ventromedial prefrontal cortex to decision-making. J Neurosci. 1999;19:5473–5481. doi: 10.1523/JNEUROSCI.19-13-05473.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Amaral DG. The primate amygdala and the neurobiology of social behavior: implications for understanding social anxiety. Biol Psychiatry. 2002;51:11–17. doi: 10.1016/s0006-3223(01)01307-5. [DOI] [PubMed] [Google Scholar]
- 30.Davis M, Whalen PJ. The amygdala: vigilance and emotion. Mol Psychiatry. 2001;6:13–34. doi: 10.1038/sj.mp.4000812. [DOI] [PubMed] [Google Scholar]
- 31.Morris JS, Frith CD, Perrett DI, et al. A differential neural response in the human amygdala to fearful and happy facial expressions. Nature. 1996;383:812–815. doi: 10.1038/383812a0. [DOI] [PubMed] [Google Scholar]
- 32.Anderson AK, Phelps EA. Expression without recognition: contributions of the human amygdala to emotional communication. Psychol Sci. 2000;11:106–111. doi: 10.1111/1467-9280.00224. [DOI] [PubMed] [Google Scholar]
- 33.Adolphs R, Tranel D, Damasio AR. The human amygdala in social judgment. Nature. 1998;393:470–474. doi: 10.1038/30982. [DOI] [PubMed] [Google Scholar]
- 34.Fudge JL, Powers JM, Haber SN, Caine ED. Considering the role of the amygdala in psychotic illness: a clinicopathological correlation. J Neuropsychiatry Clin Neurosci. 1998;10:383–394. doi: 10.1176/jnp.10.4.383. [DOI] [PubMed] [Google Scholar]
- 35.Chang K, Adleman NE, Dienes K, Simeonova DI, Menon V, Reiss A. Anomalous prefrontal-subcortical activation in familial pediatric bipolar disorder. A functional magnetic resonance imaging investigation. Arch Gen Psychiatry. 2004;61:781–792. doi: 10.1001/archpsyc.61.8.781. [DOI] [PubMed] [Google Scholar]
- 36.Blumberg HP, Martin A, Kaufman J, et al. Frontostriatal abnormalities in adolescents with bipolar disorder: preliminary observations using functional MRI. Am J Psychiatry. 2003;160:1345–1347. doi: 10.1176/appi.ajp.160.7.1345. [DOI] [PubMed] [Google Scholar]
- 37.Blumberg HP, Kaufman J, Martin A, Charney DS, Krystal JH, Peterson BS. Significance of adolescent neurodevelopment for the neural circuitry of bipolar disorder. Ann N Y Acad Sci. 2004;1021:376–383. doi: 10.1196/annals.1308.048. [DOI] [PubMed] [Google Scholar]
- 38.Ali SO, Denicoff KD, Altshuler LL, et al. Relationship between prior course of illness and neuroanatomic structures in bipolar disorder: a preliminary study. Neuropsychiatry Neuropsychol Behav Neurol. 2001;14:227–232. [PubMed] [Google Scholar]
- 39.Amaral DG, Price JL. Amygdalo-cortical projections in the monkey (Macaca fascicularis) J Comp Neurol. 1984;230:465–496. doi: 10.1002/cne.902300402. [DOI] [PubMed] [Google Scholar]
- 40.Morecraft RJ, Geula C, Mesulam MM. Cytoarchitecture and neural afferents of orbitofrontal cortex in the brain of the monkey. J Comp Neurol. 1992;323:341–358. doi: 10.1002/cne.903230304. [DOI] [PubMed] [Google Scholar]
- 41.Machado CJ, Bachevalier J. Non-human primate models of childhood psychopathology: the promise and the limitations. J Child Psychol Psychiatry. 2003;44:64–87. doi: 10.1111/1469-7610.00103. [DOI] [PubMed] [Google Scholar]
- 42.Ghashghaei HT, Barbas H. Pathways for emotion: interactions of prefrontal and anterior temporal pathways in the amygdala of the rhesus monkey. Neurosci. 2002;115:1261–1279. doi: 10.1016/s0306-4522(02)00446-3. [DOI] [PubMed] [Google Scholar]
- 43.Blumberg H, Stern E, Ricketts S, et al. Rostral and orbital prefrontal dysfunction in the manic state of bipolar disorder. Am J Psychiatry. 1999;156:1986–1988. doi: 10.1176/ajp.156.12.1986. [DOI] [PubMed] [Google Scholar]
- 44.Blumberg HP, Leung HC, Skudlarski P, et al. A functional magnetic resonance imaging study of bipolar disorder: state- and trait-related dysfunction in ventral prefrontal cortices. Arch Gen Psychiatry. 2003;60:601–609. doi: 10.1001/archpsyc.60.6.601. [DOI] [PubMed] [Google Scholar]
- 45.Blumberg HP, Krystal JH, Bansal R, et al. Preliminary evidence for effects of age, rapid-cycling and pharmacotherapy on ventral prefrontal cortex in bipolar disorder. Biol Psychiatry. doi: 10.1016/j.biopsych.2005.08.031. in press. [DOI] [PubMed] [Google Scholar]
- 46.Moore GJ, Bebchuk JM, Wilds IB, Chen G, Manji HK. Lithium-induced increase in human brain grey matter. Lancet. 2000;356:1241–1242. doi: 10.1016/s0140-6736(00)02793-8. [DOI] [PubMed] [Google Scholar]
- 47.Wozniak J, Biederman J, Richards JA. Diagnostic and therapeutic dilemmas in the management of pediatric-onset bipolar disorder. J Clin Psychiatry. 2001;14(Suppl):10–15. [PubMed] [Google Scholar]