Abstract
Aldosterone, a neurohormone known to affect electrolytes, has recently been implicated as playing a major role in the progression of heart failure, particularly in patients with systolic dysfunction. Major clinical trials designed to analyze clinical outcomes using an aldosterone antagonist have been done in two groups with heart failure. The first was the Randomized Aldactone Evaluation Study, which was done in symptomatic chronic advanced heart failure patients and showed that an aldosterone antagonist, spironolactone, reduced mortality significantly compared with placebo. Very few of these patients were on standard therapy with beta blockade. Another study, the Eplerenone Post myocardial infarction Heart failure Efficacy and SUrvival Study (EPHESUS), done in post-myocardial infarction patients with heart failure, demonstrated a significant reduction in mortality and hospitalizations for patients randomized to the aldosterone antagonist eplerenone. These trial results provide the background for aldosterone antagonist therapy in chronic advanced heart failure patients as well as post-myocardial infarction heart failure patients with reduced ejection.
Keywords: aldosterone, eplerenone, heart failure
Aldosterone has been known for about 50 years as a neurohormone that has a significant impact on metabolism. More recently aldosterone has been implicated as possibly playing a role in the progression of heart failure, particularly in patients with systolic dysfunction. This review will highlight the role of the aldosterone antagonist eplerenone in patients with heart failure.
Pathophysiology of heart failure
Heart failure is a complex syndrome with one million hospitalizations annually in the US. It is estimated that 5 million Americans have a diagnosis of heart failure, with the numbers increasing mostly in the elderly. The cost of caring for heart failure is approximately US$30 billion per year. Heart failure is the result of ventricular remodeling, changes in size, shape, structure, and function of the left ventricle that can be triggered by myocardial ischemia and/or myocardial infarction usually in the presence of vascular risk factors, or can become manifested in a more prolonged latent phase in patients with hypertension who develop ventricular hypertrophy. Pathways in ischemic, hypertensive/hypertrophic, or idiopathic cardiomyopathy can lead to systolic or diastolic dysfunction with eventual signs and symptoms of heart failure. Current understanding of the pathophysiology of heart failure involves the activation of a number of neurohormonal systems in response to some form of cardiac injury.
The predominant systems that become upregulated include the renin angiotensin aldosterone system (RAAS), the sympathetic nervous system, and the arginine vasopressin system. Neurohormones expressed from activation of these systems play a significant role in the development and progression of heart failure. These neurohormones can have a direct impact on the ventricular remodeling process by several mechanisms including ischemia, apoptosis, and alteration of gene expression. Additionally, some neurohormones (norepinephrine) can have direct toxic effects on myocytes. Thus the contemporary treatment of heart failure, specifically in patients with systolic dysfunction, involves intensively antagonizing these neurohormonal systems. (Eichhorn and Bristow 1996). Specific agents that target the RAAS are angiotensin converting enzyme inhibitors, angiotensin receptor blockers, and aldosterone antagonists. Agents that impact the sympathetic nervous system are the beta blockers.
The renin angiotensin aldosterone system
Physicians have known about the RAAS for some time. The system is active both at the tissue level as well as systemically. The rate-limiting enzyme in the activation of this system is renin which acts to cleave a peptide, angiotensinogen, which is secreted from the liver, into an inactive substance angiotensin 1. This protein is subsequently acted upon by a ubiquitous enzyme, angiotensin converting enzyme (ACE), which converts angiogtension 1 to the active neurohormone angiotensin II. Additionally, ACE plays an important role in the breakdown of bradykinin. Angiotensin II mediates a number of adverse parameters that have a negative impact on ventricular function and also result in the augmentation of the remodeling process. In order for angiotensin II to exert these effects there must be interaction with an angiotensin receptor.
These receptors have recently been identified and most of our information relates to the AT1 receptor which mediates vascular smooth muscle constriction, abnormal cell growth, and the release of aldosterone. In addition to its electrolyte and metabolic effects including sodium retention, water retention, potassium excretion, and magnesium excretion, aldosterone appears to play a very important role in the pathophysiology of heart failure (Figure 1). Aldosterone promotes the development of myocardial fibrosis which can facilitate the remodeling process and lead to the progression of heart failure as well as the progression of symptoms. Additionally, aldosterone decreases norepinephrine uptake and has a negative effect on endothelial function and increases PAI 1 levels which can lead to an increase in thrombosis. This can promote ischemic events which can lead to arrhythmias, some of which can result in sudden cardiac death which is frequently seen in patients with heart failure, particularly those with systolic dysfunction.
Figure 1.
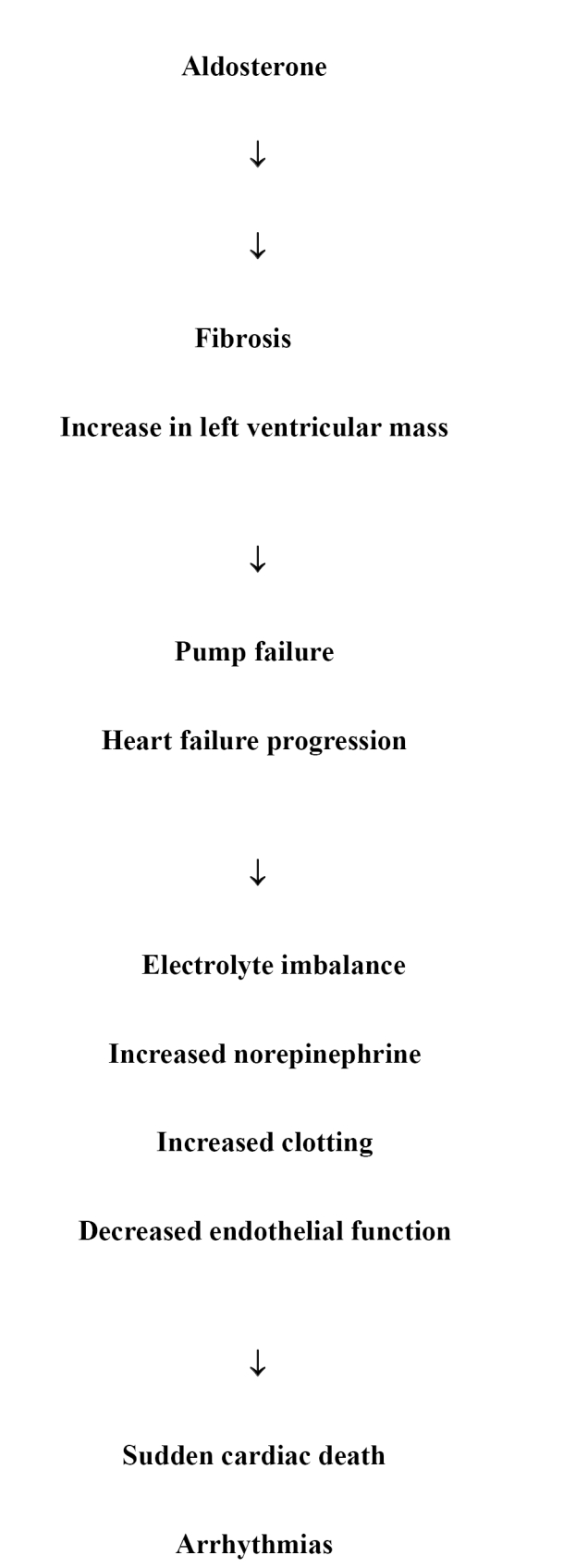
Pathophysiology of aldosteronone in heart failure.
It was thought that pharmacologic therapy directed at decreasing neurohormonal activation by impacting the RAAS would have an effect on aldosterone. However, treatment with either an ACE inhibitor alone or with an angiotensin receptor blocker alone or the two classes of agents combined does not decrease aldosterone levels (McKelvie et al 1999). A direct correlation of aldosterone levels and mortality was shown in the CONSENSUS trial. None of the patients enrolled in this trial received an aldosterone antagonist and elevation in aldosterone levels correlated with mortality (Swedberg et al 1990).
Clinical trial data
The first study that indicated a potential role of an aldosterone antagonist in patients with systolic heart failure was from the RALES or Randomized Aldactone Evaluation study (Pitt et al 1999). In this trial 1663 patients with advanced heart failure and an ejection fraction below 35%, all of whom were on an ACE inhibitor and loop diuretic, were randomized to receive the non-selective aldosterone antagonist spironolactone at 25 mg per day vs placebo. The primary endpoint for this trial was total mortality. The majority of the patients in this study had a diagnosis of advanced heart failure, that is New York Heart Association (NYHA) Class III or IV. Mean ejection fraction in this trial was 25%. Patients had ischemic (55%) or non-ischemic (45%) etiology and were treated appropriately. It should be noted that this study was implemented prior to beta blockers becoming standard therapy for these types of patients. Only 10% of the patients in this trial were also on a beta blocker. Mortality was significantly affected by the addition of spironolactone with a relative risk reduction of 30%, which was highly statistically significant and resulted in early termination of the trial. Serious hyperkalemia was a major disadvantage, with a relatively large number of hospitalizations and deaths from hyperkalemia following publication of the trial (Juurlink et al 2004). Additionally, a variety of side effects commonly seen with non-selective aldosterone antagonism were noted.
A compound with a similar structure to spironolactone but with more mineralocorticoid specificity and consequently fewer anti-androgenic and progestational actions is eplerenone. This aldosterone antagonist was approved for hypertension but had not been available for clinical practice. Because of the positive data available from small trials that noted the impact of fibrosis in the remodeling process of the left ventricle particularly following a myocardial infarction, it was hypothesized that giving post-infarction patients with heart failure an agent that seemed to decrease the amount of myocardial fibrosis might prove to be beneficial regarding clinical outcomes.
Each year more 1 million Americans suffer a myocardial infarction. Of these individuals approximately 40% exhibit left ventricular systolic dysfunction. Many of these patients remain asymptomatic; however 22% of men and 46% of women who have experienced a myocardial infarction will be disabled with heart failure within 6 years.
Standard therapy for patients who have suffered a myocardial infarction and who have developed clinical heart failure or who manifest left ventricular systolic dysfunction has been treatment with beta blockers and ACE inhibitors. Despite this therapy, mortality and morbidity remains high in these patients. Additionally, patients who have had a myocardial infarction tend to undergo a remodeling process relatively early following their infarction. With this background a new trial was designed using eplerenone as an intervention in patients post-myocardial infarction in order to reduce mortality and morbidity following a myocardial infarction with systolic left ventricular dysfunction. This trial, the Eplerenone Post myocardial infarction Heart failure Efficacy and SUrvival Study (EPHESUS), randomized 6632 post-myocardial infarction patients with ejection fractions below 40% and signs and symptoms of heart failure to either eplerenone at a dose of 25–50 mg daily vs placebo (Pitt et al 2003). All patients were treated with standard therapy including ACE inhibitors and beta blockers. The primary endpoint for this trial was total mortality and cardiovascular mortality or cardiovascular hospitalization. Secondary endpoints included cardiovascular mortality and total mortality or total hospitalizations. A cardiovascular hospitalization was defined as a hospitalization for myocardial infarction, stroke, heart failure, or a ventricular arrhythmia. The study was designed to continue until there were 1012 deaths. Events were adjudicated by a blinded endpoint adjudication committee and the trial was periodically reviewed by a data safety monitoring board.
Baseline characteristics of patients in this trial were somewhat different from patients in chronic heart failure trials in that ejection fraction (mean value 33%) was higher and many patients were relatively asymptomatic during the 3- to 14-day window for starting the study drug. The patients were very well treated, with 86% of the study cohort being assigned to an ACE inhibitor or an angiotensin receptor blocker. Three quarters of the patients were on beta blockade, 60% on diuretics. Forty-five percent of the patients underwent some form of revascularization that included thrombolytic therapy, percutaneous coronary angioplasty, or coronary artery bypass graft surgery. The results of the study showed a small decrease in systolic and diastolic blood pressure at the end of the trial. Slight but significantly higher serum potassium and serum creatinine were also noted. Mortality was reduced, with a relative risk reduction of 15% (p = 0.008). Separation of the Kaplan Meier curves began at 3 months from randomization. The relative risk of cardiovascular mortality and/or hospitalization was also significantly improved in the eplerenone-assigned patients, by 13% (p = 0.002). Again the Kaplan Meier curves showed separation at 3 months and continued to separate during the study. A very important secondary endpoint analyzed sudden cardiac death in this group of patients. This has been a difficult area to treat in the post-infarction patient. However, in EPHESUS a relative risk reduction of sudden cardiac death of 21% was noted favoring the eplerenone-assigned group (p = 0.009). Hospitalizations for heart failure were also significantly reduced during the trial. A post hoc analysis looking at 30-day events again favored assignment to eplerenone, with an all cause mortality reduction of 31% and a cardiovascular mortality reduction of 32% (Pitt et al 2005). Sudden cardiac death was reduced 37% during this time frame. In analyzing the patients with ejection fractions below 30%, all-cause mortality was reduced by 43% and sudden cardiac death by 50% at 30 days. The mechanisms and benefit of these outcomes in post-myocardial infarction patients may relate to attenuation or reversal of the remodeling process which can occur very rapidly post myocardial infarction. Myocardial fibrosis has been suggested to play a major role in the development of the remodeling process in the post-infarction patient. The stimulus for this increased fibrosis is an increase in aldosterone. By selectively antagonizing aldosterone, the remodeling process is most likely attenuated or reversed, thereby leading to a reduction in cardiovascular events. Adverse events in the EPHESUS trial were quite small, with similarities to placebo. Potassium was relatively stable during the trial with a 1.6% absolute increase in serious hyperkalemia defined as potassium greater than 6.0 meq/L. Importantly, hypokalemia was prevented with this strategy.
Recommendations for monitoring serum potassium with aldosterone antagonists include obtaining a serum potassium level at 1 week and 1 month after initiating therapy and then obtain a potassium level at each visit. If potassium is less than 5 mmol/L at 1 month, uptitration will be performed. If potassium is 5.0–5.4 mmol/L, the dose will be maintained; if the level is 5.5–6.0 mmol/L, the dose will be decreased; and if the level is greater than 6.0 mmol/L, the drug will be discontinued.
It should be recognized that eplerenone and spironolactone manifest certain pharmacologic differences. Spironolactone is metabolized with a high degree of first-pass metabolism and its half-life for metabolites is over 16 hours. Additionally, it is highly protein bound (Overdich and Merkus 1987). It is affected by the presence of food. Eplerenone, on the other hand, does not undergo first-pass metabolism, has a shorter half life of 4–6 hours, does not possess active metabolites, is less than 50% protein bound, and is not affected by food intake. Drug interactions with statins and digoxin are not noted with eplerenone. Another major difference between spironolactone and eplerenone is cost. Since spironolactone is generic, the estimated cost of eplerenone is substantial. However, a recent study evaluated the cost benefit related to survival following a myocardial infarction complicated by heart failure. This study showed that compared with placebo, eplerenone was cost effective in increasing years of life (Weintraub et al 2005). The incidence of gyneocomastia (9% of men treated with spironolactone) is lower with eplereonone (<1%), attributed to its higher affinity for the mineralocorticoid receptor.
Summary
Aldosterone appears to play a pivotal role in congestive heart failure. Important effects of this neurohormone include electrolyte and fluid shifts including potassium and magnesium loss and well as sodium retention. The neurohormone potentiates catecholamines and can promote ventricular arrhythmias. Prothrombotic effects are well known with aldosterone, and cellular effects on endothelial dysfunction and vascular inflammation and injury have also been noted. Perhaps one of the most important aspects of this neurohormone is its ability to increase myocardial fibrosis. One of the primary roles of the aldosterone antagonist eplerenone is probably some attenuation of the stimulus for myocardial fibrosis thus resulting in improved outcomes (Pitt et al 1999).
Acknowledgments
Mrs Valerie Craig for assistance with manuscript preparation.
Disclosures
AM is on the Speakers Bureau for Pfizer and has received research grants from Pfizer.
References
- Eichhorn EJ, Bristow MR. Medical therapy can improve the biological properties of the chronic heart: A new era in the treatment of heart failure. Circulation. 1996;94:2285–96. doi: 10.1161/01.cir.94.9.2285. [DOI] [PubMed] [Google Scholar]
- Juurlink DN, Mamdahi MM, Los DS, et al. Rates of hyperkalemia after publication of the Randomized Aldactone Evaluation Study. N Eng J Med. 2004;352:543–51. doi: 10.1056/NEJMoa040135. [DOI] [PubMed] [Google Scholar]
- McKelvie RS, Yusuf S, Pericak D, et al. Comparison of candesartan, enalapril and their combination in congestive heart failure: Randomized evaluation of strategies for left ventricular dysfunction (RESOLVD) pilot study: The Resolved Pilot Study Investigators. Circulation. 1999;100:1056–64. doi: 10.1161/01.cir.100.10.1056. [DOI] [PubMed] [Google Scholar]
- Overdich HJ, Merkus FW. The metabolism in biopharmaceuticals of spironolactone in man. Rev Drug Metab Drug Interact. 1987;5:273–302. doi: 10.1515/dmdi.1987.5.4.273. [DOI] [PubMed] [Google Scholar]
- Pitt B, Remme W, Zannad F, et al. Eplerenone a selective aldosterone blocker, in patients with left ventricular dysfunction after myocardial infarction. N Engl J Med. 2003;348:1309–21. doi: 10.1056/NEJMoa030207. [DOI] [PubMed] [Google Scholar]
- Pitt B, White H, Nicolau J, et al. Eplerenone reduces mortality 30 days after randomization following acute myocardial infarction in patients with left ventricular systolic dysfunction and heart failure. J Am Coll Cardiol. 2005;46:425–31. doi: 10.1016/j.jacc.2005.04.038. [DOI] [PubMed] [Google Scholar]
- Pitt B, Zannad F, Remme WJ, et al. The effect of spironolactone on morbidity and mortality in patients with severe heart failure. N Engl J Med. 1999;341:709–17. doi: 10.1056/NEJM199909023411001. [DOI] [PubMed] [Google Scholar]
- Swedberg K, Eneroth P, Kjekshus J, et al. Hormones regulating cardiovascular function in Patients with Severe Congestive Heart Failure and their relation to mortality. Consensus Trial Study Group. Circulation. 1990;82:1730–6. doi: 10.1161/01.cir.82.5.1730. [DOI] [PubMed] [Google Scholar]
- Weintraub WS, Zhang Z, Mahoney EM, et al. Cost effectiveness of eplerenone compared with placebo in patients with myocardial infarction complicated by left ventricular dysfunction and heart failure. Circulation. 2005;111:1106–13. doi: 10.1161/01.CIR.0000157146.86758.BC. [DOI] [PubMed] [Google Scholar]


