Abstract
Nebivolol is a highly selective beta1-adrenergic blocker that also enhances nitric oxide bioavailability via the L-arginine-nitric oxide pathway, leading to vasodilation and decreased peripheral vascular resistance. It is marketed in Europe for the treatment of hypertension and heart failure and is currently being reviewed for use in the US by the Food and Drug Administration. Nebivolol appears to be well tolerated with an adverse event profile that is at least similar, if not better, than that of other beta-adrenergic blockers. Studies suggest that long-term therapy with nebivolol improves left ventricular function, exercise capacity, and clinical endpoints of death and cardiovascular hospital admissions in patients with stable heart failure. To date, it is one of the only beta-adrenergic blockers that have been exclusively studied in elderly patients. Additionally, the unique mechanism of action of nebivolol makes it a promising agent for treatment of chronic heart failure in high-risk patient populations, such as African Americans. This article will review the pharmacologic and pharmacokinetic properties of nebivolol as well as clinical studies assessing its efficacy for the treatment of heart failure.
Keywords: nebivolol, beta-adrenergic blockers, heart failure
Introduction
The pathophysiology of chronic heart failure involves a process of left ventricular remodeling, whereby molecular changes occur within the myocardium in response to mechanical stresses induced by underlying diseases, such as hypertension, ischemic heart disease, cardiomyopathies, and valvular abnormalities. Structural changes that occur, including left ventricular hypertrophy and/or dilation, typically result in decreased left ventricular diastolic or systolic function (Jessup and Brozena 2003; Opie et al 2006). This remodeling process is accelerated by the activation of a number of endogenous neurohormonal systems including, but not limited to, the sympathetic nervous system, which releases high levels of the adrenergic substance norepinephrine and stimulates the release of renin in the kidney (Jessup and Brozena 2003; Hunt et al 2005). The resultant increase in heart rate, contractility, peripheral vasoconstriction, and blood volume, as well as the direct toxic effects of norepinephrine on myocytes, increases cardiac workload and further impairs cardiac performance (Hunt et al 2005). Beta-adrenergic blockers, by suppressing the deleterious effects of norepinephrine have become routine therapy for the treatment of chronic heart failure (Hunt et al 2005; McMurray et al 2005; Swedberg et al 2005).
The benefits of beta-adrenergic blockers in the treatment of chronic heart failure are exclusive to those agents that have demonstrated a survival benefit in clinical trials and should not, therefore, be considered a class effect. In the US, three beta-adrenergic blockers are currently available for use in chronic heart failure based on evidence demonstrating a survival benefit: carvedilol, which blocks alpha1-, beta1-, and beta2-receptors; and sustained-release metoprolol succinate and bisoprolol, which both selectively block beta1-receptors (Packer et al 1996; CIBIS II Investigators 1999; Hjalmarson et al 1999; Packer et al 2001; Hunt et al 2005). Nebivolol is a third-generation beta-adrenergic blocker that has been marketed and used in Europe for the treatment of hypertension and heart failure (A. Menarini Pharmaceuticals 2005; McMurray et al 2005). It is currently under FDA review in the US for hypertension and it is anticipated that an indication for heart failure will be pursued in the near future.
Pharmacology
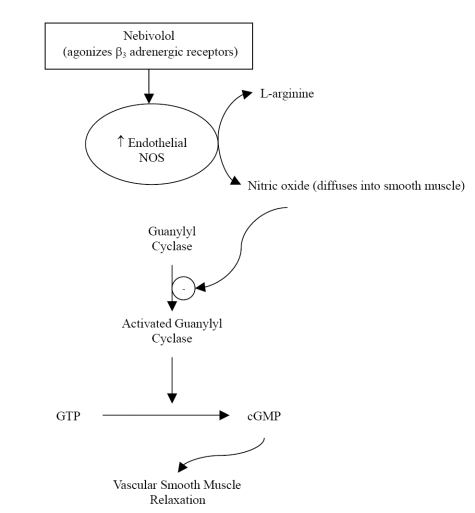
Nebivolol is a racemic mixture containing equal amounts of 2 isomers, d-nebivolol and l-nebivolol. D-nebivolol provides selective beta1-adrenergic receptor blockade while both d- and l-nebivolol cause nitricoxide-induced vasodilation (Cockcroft et al 1995; Van Neuten 1998). Nebivolol, which has no intrinsic sympathomimetic activity, is considered a highly selective beta1-adrenergic blocker due to its 321-fold higher affinity for human cardiac beta1-receptors versus beta2-receptors; it is also more selective for beta1-receptors than any other agent in its class (Brixius et al 2001; Bristow et al 2005). Unlike other beta-adrenergic blockers with vasodilatory properties, nebivolol has no alpha-blocking effects (Bowman et al 1994; Van Bortel et al 1997). The vasodilatory action of nebivolol is mediated via the L-arginine-nitric oxide pathway, whereby nitric oxide production by endothelial nitric oxide synthases is enhanced (Bowman et al 1994; Cockcroft et al 1995; Ignarro 2004). There is evidence to suggest that this mechanism is in part due to agonist activity of nebivolol at endothelial beta3-adrenergic receptors (Figure 1) (Gauthier et al 1998; Gosgnach et al 2001; Dessy et al 2005). This was recently tested and confirmed by Dessy and colleagues who established that nebivolol relaxation of human coronary microarteries that were precontracted with endothelin-1 was significantly inhibited by bupranolol, a beta1,2,3-receptor blocker, but not significantly inhibited by nadolol, a beta1,2-receptor blocker (Dessy et al 2005). Nitric oxide bioavailability may also be augmented with nebivolol treatment due to decreased inactivation by reactive oxygen entities (Janssen et al 2001; Cominacini et al 2003; Pasini et al 2005).
Figure 1.
The effect of nebivolol on the L-arginine-nitric-oxide pathway. Reprinted with permission Veverka A, Nuzum DS, Jolly JL. 2006. Nebivolol: a third-generation β-adrenergic blocker. Ann Pharmacother, 40:1353–60. Copyright ©2006. Harvey Whitney Books.
Abbreviations: NOS, nitric oxide synthase; GTP, guanosine triphosphate; cGMP, cyclic guanosine monophosphate.
This unique mechanism of nebivolol is particularly important due to the critical role of nitric oxide in the pathophysiology of cardiovascular diseases, including heart failure. In addition to causing vasodilation, nitric oxide also inhibits platelet aggregation, atherosclerosis and proliferation of vascular smooth muscle cells (Mason 2006). When endothelial dysfunction occurs, nitric oxide production and function is impaired, leading to increased peripheral resistance and a pro-thrombotic and pro-atherogenic environment (Panza et al 1990; Moncada and Higgs 1993; Kinlay et al 2001; Mason 2006). To distinguish the vasodilatory action of nebivolol from other beta-adrenergic blockers that selectively inhibit beta1-receptors, Lekakis and colleagues tested the effect of nebivolol and atenolol on flow-mediated dilation of the brachial artery (Lekakis et al 2005). Following 4 weeks of drug therapy, patients treated with nebivolol had significantly increased flow-mediated dilation, while those treated with atenolol had no change compared to baseline.
The increased bioavailability of nitric oxide with nebivolol treatment may prove to be particularly useful for treating African American patients with cardiovascular disease. It has been proposed that decreased endothelial nitric oxide bioavailability may be more prevalent as an underlying cause of cardiovascular disease in this patient subgroup, and previous studies of African American patients with heart failure have shown a favorable response to therapies that increase nitric oxide availability (Taylor et al 2004). The mechanism of decreased nitric oxide bioavailability in African American patients may be due to oxidative stress caused by upregulation of NAD(P)H-dependent oxidases and subsequent increases in production of superoxide (). can react with nitric oxide, decreasing its bioavailability and increasing production of the oxidant peroxynitrite (ONOO−) (Kalinowski et al 2004). Mason and colleagues compared the activity of nebivolol and atenolol on nitric oxide release from endothelial cells of age-matched African American and Caucasian donors with comparable cardiovascular risk histories (Mason et al 2005). Levels of nitric oxide, as well as ONOO− and , the primary components of nitroxidative and oxidative stress in the vascular system, were measured to assess endothelial function. At baseline, release of nitric oxide was 5 times slower, and release of both ONOO− and was 2–4 times faster in African Americans compared to Caucasians. While atenolol had no effect on nitric oxide, ONOO−, and levels in either white or black patients, nebivolol treatment increased nitric oxide and reduced ONOO− and levels in African Americans to similar levels documented in Caucasian patients.
Pharmacokinetics and drug interactions
Table 1 summarizes the general pharmacokinetic properties of nebivolol and other beta-adrenergic blockers typically used in the management of heart failure (Frishman and Alwarshetty 2002; Eon Labs, Inc. 2004; AstraZeneca LP 2006; GlaxoSmithKline 2007). Nebivolol is rapidly absorbed following oral administration, reaching peak plasma concentrations within 0.5–4 hours after a dose (Sule and Frishman 2006). Food has a minimal impact on absorption and therefore nebivolol may be taken without regard to meals (Shaw et al 2003a). Nebivolol is extensively metabolized via hydroxylation in the hepatic system to active and inactive metabolites. The oral bioavailability of nebivolol is dependent on cytochrome P450 2D6 genetic polymorphism and so ranges from 12% in extensive metabolizers to 96% in poor metabolizers. Similarly, the half-life of nebivolol is approximately 10 hours in extensive metabolizers but can be prolonged up to 30–50 hours in poor metabolizers (Van Peer et al 1991; A. Menarini Pharmaceuticals 2005). Despite genetic differences in metabolism of nebivolol, the clinical response to the drug appears to be similar (Lefebvre et al 2006). Nebivolol displays linear kinetics across a dose range of 2.5–20 mg, demonstrated by dose-proportional changes in maximum concentrations (Cmax) and area under the drug concentration curve (AUC) (Shaw 2003b). The average volume of distribution of nebivolol is 10 L/kg and this does not appear to be affected by patient weight (Cheymol et al 1997). Less than 1% of the drug is excreted unchanged in the urine and so adjustments of doses in patients with chronic renal failure are unnecessary (A. Menarini Pharmaceuticals 2005).
Table 1.
Pharmacokinetic characteristics of beta-adrenergic blockers used in the management of heart failure
| Characteristic | Bisoprolol (Zebeta®) | Carvedilol (Coreg®) | Metoprolol succinate (Toprol XL®) | Nebivolol |
|---|---|---|---|---|
| Absorption | ||||
| Bioavailability | 80% | 25%–35% | 50% | 12%–96% |
| First-pass elimination | Small | Significant | Moderate | Variablec |
| Effect of food | None | Decreases rate but not extent of absorption | None | None |
| Protein binding | 30% | 95%–98% | 12% | 98% |
| Half-life (hours) | 9–12 | 6–10 | 3–7 | 10–30 |
| Hepatic metabolism | 50% to inactive metabolites via N-dealkylation and O-dealkylation | Extensive primarily by CYP450 2D6 and 2C9 to active and inactive metabolitesa,b | Extensive via CYP450 2D6 to inactive metabolitesa | Extensive via CYP450 2D6 to active and inactive metabolitesa |
| Renal excretion | 50% as unchanged drug, 50% as metabolites | <2% as unchanged drug | 95%, <5% as unchanged drug | <1% unchanged in urine |
| Other excretion | <2% in feces | Primarily in bile and feces | Minimal |
CYP450 = cytochrome P450.
Carvedilol is metabolized to a lesser extent by CYP 450 3A4, 2C19, 1A2, and 2E1.
Bioavailability and first-pass elimination are dependant on cytochrome P450 2D6 genetic polymorphism.
Nebivolol is highly protein bound intravascularly, predominately to albumin. Studies assessing drug interactions with nebivolol in healthy volunteers have found no significant interactions with spironolactone, hydrochlorothiazide, digoxin, warfarin, losartan, and ramipril (Lawrence et al 2003; Morton et al 2003, 2005; Lawrence et al 2005a, b, c). Co-administration with cimetidine, a potent inhibitor of cytochrome P450 3A4, increased the bioavailability of nebivolol, however this interaction did not influence the extent to which nebivolol reduced heart rate and blood pressure (Kamali et al 1997). Similarly, fluoxetine, a cytochrome P450 2D6 inhibitor, resulted in peak plasma concentrations of nebivolol that were three times higher than normal (Shaw 2005). Although the clinical impact of cytochrome P450 drug interactions with nebivolol is unclear, caution should be exercised when inhibitors or inducers of 2D6 and 3A4 are used in conjunction with this agent. At this time, it is also unknown whether nebivolol is a substrate of p-glycoprotein and if there is a risk of drug interactions at this protein.
Clinical studies
Earlier studies assessing the utility of nebivolol in chronic heart failure were limited by small patient populations. These studies did suggest, however, that nebivolol would improve left ventricular function and mechanics; improve patient functional capacity assessed by New York heart association (NYHA) classification; and would at least have a stabilizing effect on exercise capacity (Uhlir et al 1997; Brehm et al 2002). More recently, the ENECA (efficacy of nebivolol in the treatment of elderly patients with chronic heart failure as add-on therapy to ACE inhibitors or angiotensin II receptor blockers, diuretics, and/or digitalis) study performed by Edes and colleagues evaluated whether nebivolol therapy improves left ventricular ejection fraction (LVEF) compared with placebo in 260 patients with chronic heart failure (Edes et al 2005). The study design also included a secondary endpoint to assess the safety and tolerability of nebivolol in elderly patients, defined in the study as age greater than 65. In addition to the age requirement, patients qualified for enrollment in the study if they met the following criteria: NYHA class II, III, or IV; LVEF less than or equal to 35%; stable clinical status; and stable therapy for at least 2 weeks prior to randomization with angiotensin-converting-enzyme inhibitors (ACEIs) and/or angiotensin receptor blockers (ARBs), diuretics, and/or digitalis. Patients were randomized to therapy with nebivolol 1.25 mg daily, titrated to a target dose of 10 mg, or placebo and followed for a period of 8 months. Intention to treat analysis showed that nebivolol therapy significantly increased LVEF compared with placebo (improvement of 6.51 ± 9.15% vs 3.97 ± 9.2% from baseline, respectively; p = 0.027), and this was consistent across all subgroups examined. Quality of life and changes in NYHA functional class were not significantly improved with nebivolol therapy in this trial. While nebivolol was generally well tolerated in this elderly population and did not result in an increased number of patients experiencing adverse events compared with placebo (81 vs 78, respectively; p = 0899), drug-related adverse events were more commonly reported with nebivolol vs placebo (40 vs 14; p < 0.0001). The most frequent of these were hypotension, bradycardia, and dizziness. Despite proving in the ENECA study that nebivolol is superior to placebo in improving surrogate endpoints of chronic heart failure, this study was underpowered to assess the effect of nebivolol on clinical endpoints such as overall survival, cardiovascular death, or need for cardiovascular hospital admission.
Subsequent to the ENECA study, Flather and colleagues published the results of the SENIORS (study of the effects of nebivolol intervention on outcomes and rehospitalization in seniors with heart failure) trial (Flather et al 2005). This was the first and is the only randomized, double-blind, placebo-controlled trial to date assessing the benefit of nebivolol therapy on morbidity and mortality. The trial enrolled 2135 elderly patients, defined as 70 years of age or older, with a clinical history of heart failure, defined as a documented LVEF less than or equal to 35% within the previous 6 months or hospitalization with a discharge diagnosis of chronic heart failure within the previous 12 months. Patients were enrolled provided they were not currently receiving therapy with a beta-adrenergic blocker or had a contraindication to treatment. The primary outcome of the SENIORS trial was a composite of all-cause mortality or cardiovascular hospital admission. In the nebivolol arm, doses of 1.25 mg daily were initiated and titrated over a 16 week period to a target dose of 10 mg once daily. Over a mean treatment period of 21 months, 31.1% of patients in the nebivolol group reached the primary endpoint compared with 35.3% in the placebo group (hazard ration [HR] 0.86, 95% confidence interval [CI] 0.74–0.99, p = 0.039). These results imply that 24 patients with chronic heart failure would need to be treated with nebivolol for approximately 2 years to prevent one death or cardiovascular hospital admission. Of note, this benefit was observed as early as 6 months and was independent of baseline therapy with diuretics, ACEIs, digoxin, and/or spironolactone which were used by approximately 85%, 82%, 40%, and 38% of patients enrolled, respectively. Subgroup analysis determined that nebivolol was efficacious regardless of age, gender, ejection fraction, diabetes, or prior myocardial infarction. At the present time, the SENIORS trial is the only assessment of beta-adrenergic blocker therapy in an elderly population with chronic heart failure. This may be of particular importance in clinical practice since the prevalence of heart failure increases with age, from 2% to 3% at age 65 years to greater than 80% in patients aged 80 years and above (Hunt et al 2005). Additionally, treatment of elderly patients with beta-adrenergic blockers can be more challenging due to desensitization of beta-adrenergic receptors and variable pharmacokinetic responses that occur with age (potentially decreased absorption, metabolism and excretion) (Tregaskis and McDevitt 1990; Frishman and Alwarshetty 2002). While adverse outcomes have been documented when standard therapy for heart failure is insufficient, it is important to individualize therapy for each individual patient (Komajda et al 2005).
There are very few head-to-head comparisons of beta-adrenergic blockers for the treatment of chronic heart failure. Nebivolol has been compared with carvedilol in two small trials. Patrianakos and colleagues assessed the effects of carvedilol and nebivolol on left ventricular function and exercise capacity at 3 and 12 months. Seventy-two patients with NYHA class II or III heart failure, specifically non-ischemic dilated cardiomyopathy documented by a LVEF of less than 45% on echocardiogram within the previous 6 months (Patrianakos et al 2005) were included. Patients were randomized to double-blind therapy with either carvedilol 3.125 mg twice daily or nebivolol 1.25 mg once daily, with titration to carvedilol 25 mg twice daily or nebivolol 5 mg daily as tolerated. Additional requirements for enrollment included stable therapy with an ACEI or ARB for at least 4 weeks prior to randomization with no new drug therapies initiated within 6 weeks prior to randomization. No patients enrolled in the study had received prior treatment with a beta-adrenergic blocker. At 3 and 12 months, both nebivolol and carvedilol caused significant improvements in LVEF compared with baseline. Intergroup comparisons, however, revealed that carvedilol provided a greater change in LVEF than nebivolol at these time points (3 months: absolute improvement of 7.4% vs 4.8%; relative improvement of 32.1% ± 34.9% vs 15.3% ± 15.9%, mean difference −16.7% ± 16.5%, 95% CI −29.9 to −3.4, p = 0.004; 12 months: absolute improvement of 8.8% vs 6.1%; relative improvement of 35.5% ± 31.9% vs 20.7% ± 19.1%, mean difference −14.7% ± 6.4%, 95% CI −27.8 to −1.8, p = 0.02). Both agents significantly decreased left ventricular end-systolic volumes at 3 and 12 months and although only carvedilol improved left ventricular end-diastolic volumes compared to baseline, intergroup analysis showed no statistically significant differences in left ventricular volumes across the treatment period. Diastolic function, assessed by ventricular relaxation and filling patterns, was significantly improved at 12 months with both nebivolol and carvedilol therapy; however, only carvedilol demonstrated a benefit as early as 3 months.
Exercise duration, measured in seconds, significantly improved at 12 months with both nebivolol (894 ± 381 at baseline vs 994 ± 396 at 12 months; p = 0.01, 95% CI −181 to −18) and carvedilol (982 ± 475 at baseline vs 1124 ± 427 at 12 months; p = 0.01, 95% CI −248 to −36), with no statistically significant differences observed between the two groups. Of note, there was an initial decline in exercise capacity detected at 3 months with nebivolol (894 ± 381 at baseline vs 795 ± 392 at 3 months; p = 0.07, 95% CI −12 to −209). Although this was not statistically significant, this effect was not seen in the carvedilol group (982 ± 475 at baseline vs 1025 ± 419 at 3 months; p = 0.26, 95% CI −120 to −33) and compared with nebivolol, exercise capacity at 3 months was significantly better with carvedilol therapy (p = 0.002, 95% CI 0.03–0.48). One explanation for the initial decline in exercise capacity with nebivolol could be too rapid titration of the drug to target doses, which was accomplished over 4 weeks, a much faster titration than used in the SENIORS trial. Although, this study appears more favorable for carvedilol, a subsequent trial published by Lombardo and colleagues found conflicting results (Lombardo et al 2006). A similar patient population, 70 patients with NYHA class II or III heart failure and LVEF less than or equal to 40%, were randomized to carvedilol and nebivolol at similar doses used in the aforementioned trial. Patients were evaluated at baseline, 3, and 6 months, but data for baseline and 6 months only were reported. In contrast to the study by Patrianakos and colleagues, increases in LVEF and decreases in left-ventricular end-systolic volumes observed at 6 months were not statistically different from baseline; nor was there a difference observed between groups. Both carvedilol and nebivolol showed a trend towards an increased exercise capacity at 6 months and there was no reported decline in exercise capacity with nebivolol at earlier assessments. Both of these trials enrolled a small number of patients and evaluated surrogate endpoints. The study by Patrianakos and colleagues was performed in patients with non-ischemic dilated cardiomyopathy so extrapolation to the general heart failure population is inappropriate given that ischemic heart disease is one of the most common causes of chronic heart failure. Additionally, target doses of nebivolol 5 mg used in these comparator trials is lower than the 10 mg target dose used in the ENECA study and SENIORS trial discussed above. Any differences between nebivolol and carvedilol on clinical endpoints of mortality and hospitalizations for heart failure cannot be inferred from these trials. Larger trials with head-to-head comparisons of nebivolol, carvedilol, metoprolol succinate, and bisoprolol are needed to further establish if one agent is any more beneficial than the others in increasing survival and decreasing hospitalizations for acute decompensated heart failure.
Tolerability
Clinical trial data suggest that nebivolol is generally well tolerated. Placebo-controlled trials have reported increased incidences of drug-related adverse events such as hypotension, bradycardia, and dizziness but this should be anticipated based on the mechanism of drug action (Uhlir et al 1997; Brehm et al 2002; Edes et al 2005; Flather et al 2005). When compared with other agents in its class, however, these adverse events have not been shown to occur any more frequently with nebivolol, and in fact there is some evidence to suggest that bradycardia occurs less frequently with nebivolol than with alternate beta-adrenergic blockers in the first few weeks of treatment (Van Neuten et al 1998; Czuriga et al 2003; Grassi et al 2003). The mechanism for this has not been well described and, due to the short-term duration of the clinical studies, it is unclear if this response would be sustained with long-term therapy.
Nebivolol does not appear to impair insulin sensitivity, glucose levels or lipoprotein levels and seems to have a more favorable effect on these metabolic parameters than other beta-adrenergic blockers (Fogari et al 1997; Pesant et al 1999; Poirier et al 2001; Rizos et al 2003; Celik et al 2006; Peter et al 2006). Laboratory assessments of kidney function, liver function and hematology tests before and during therapy with nebivolol indicate no adverse effects on each of these systems (Edes et al 2005).
Conclusion
Nebivolol is currently marketed in Europe for the treatment of hypertension and heart failure and is under FDA review for use in the US. Evidence shows that nebivolol, titrated to a maximum dose of 10 mg, is a potentially promising therapeutic option for the treatment of chronic heart failure when added to standard therapy. Its unique mechanism of selectively blocking beta1-receptors and decreasing peripheral vascular resistance by enhancing nitric oxide bioavailability distinguish it from other agents in its class; however the clinical significance of this still needs to be defined. Since not all beta-adrenergic blockers have proved to be effective for the treatment of heart failure, the addition of nebivolol to the current armamentarium of carvedilol, metoprolol succinate, and bisoprolol is encouraging and provides more options for individualizing patient therapy. Specifically, and in light of recent evidence for other agents known to work via the nitric oxide pathway, nebivolol may prove to be more useful than other beta-adrenergic blockers in African American patients and those suspected of having decreased nitric oxide bioavailability as an underlying pathophysiology of disease (Taylor et al 2004). Large-scale clinical trials are needed, however, to test this hypothesis. Additionally, without head-to-head trials assessing mortality or hospitalizations for decompensated heart failure with nebivolol, it is premature to comment on which beta-adrenergic blocker is preferred for heart failure management. Nebivolol is the only agent to date that has evidence supporting use of beta-adrenergic blockers in the treatment of elderly patients with chronic heart failure. At this time, numerous clinical studies with nebivolol are in progress and include: an assessment of the role of nebivolol in the treatment of diastolic heart failure; use of nebivolol in African American patients with hypertension; and a comparison of nebivolol and metoprolol in patients with subclinical left ventricular dysfunction (The Menarini Group 2007).
References
- A. Menarini Pharmaceuticals UK Ltd. Nebilet 5 mg tablets: prescribing information (UK) [Online] 2005. Accessed 26 November 2006. URL: http://emc.medicines.org.uk.
- AstraZeneca LP. Toprol-XL (metoprolol succinate): prescribing information (USA) [online] 2006. Accessed 25 March 2007. URL: http://www.astrazeneca-us.com/pi/toprol-xl.pdf.
- Bristow MR, Nelson P, Minobe W, et al. Characterization of ±1-adrenergic receptor selectivity of nebivolol and various other beta-blockers in human myocardium [abstract] J Hypertens. 2005;18:A51–2. [Google Scholar]
- Brixius K, Bundkirchen A, Bolck B, et al. Nebivolol, bucindolol, metoprolol and carvedilol are devoid of intrinsic sympathomimetic activity in human myocardium. Br J Pharmacol. 2001;133:1330–8. doi: 10.1038/sj.bjp.0704188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowman AJ, Chen CP, Ford GA. Nitric oxide mediated venodilator effects of nebivolol. Br J Clin Pharmacol. 1994;38:199–204. doi: 10.1111/j.1365-2125.1994.tb04342.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brehm BR, Wolf SC, Gorner S, et al. Effect of nebivolol on left ventricular function in patients with chronic heart failure: a pilot study. Eur J Heart Fail. 2002;4:757–63. doi: 10.1016/s1388-9842(02)00113-7. [DOI] [PubMed] [Google Scholar]
- Celik T, Lyisoy A, Kursaklioglu H, et al. Comparative effects of nebivolol and metoprolol on oxidative stress, insulin resistance, plasma adiponectin and soluble P-selectin levels in hypertensive patients. J Hypertens. 2006;24:591–6. doi: 10.1097/01.hjh.0000209993.26057.de. [DOI] [PubMed] [Google Scholar]
- Cheymol G, Woestenborgh R, Snoeck E, et al. Pharmacokinetic study and cardiovascular monitoring of nebivolol in normal and obese subjects. Eur J Clin Pharmacol. 1997;51:493–8. doi: 10.1007/s002280050237. [DOI] [PubMed] [Google Scholar]
- CIBIS-II Investigators and Committees. The Cardiac Insufficiency Bisoprolol Study II (CIBIS-II) Lancet. 1999;353:9–13. [PubMed] [Google Scholar]
- Cockcroft JR, Chowienczyk PJ, Brett SE, et al. Nebivolol vasodilates human forearm vasculature: evidence for an L-arginine/NO-dependent mechanism. J Pharmacol Exp Ther. 1995;374:1067–71. [PubMed] [Google Scholar]
- Cominacini L, Pasini AF, Garbin U, et al. Nebivolol and its 4-keto derivative increase nitric oxide in endothelial cells by reducing its oxidative inactivation. J Am Coll Cardiol. 2003;42:1838–44. doi: 10.1016/j.jacc.2003.06.011. [DOI] [PubMed] [Google Scholar]
- Czuriga I, Riecansky I, Bodnar J, et al. Comparison of new cardioselective beta-blocker nebivolol with bisoprolol in hypertension: the nebivolol, bisoprolol multicenter study (NEBIS) Cardiovasc Drugs Ther. 2003;17:257–63. doi: 10.1023/a:1026180325278. [DOI] [PubMed] [Google Scholar]
- Dessy C, Saliez J, Ghisdal P, et al. Endothelial β3-adrenoreceptors mediate nitric oxide-dependent vasorelaxation of coronary microvessels in response to the third-generation β-blocker nebivolol. Circulation. 2005;112:1198–205. doi: 10.1161/CIRCULATIONAHA.104.532960. [DOI] [PubMed] [Google Scholar]
- Edes I, Gasior Z, Wita K, et al. Effects of nebivolol on left ventricular function in elderly patients with chronic heart failure: results of the ENECA study. Eur J Heart Fail. 2005;7:631–9. doi: 10.1016/j.ejheart.2004.10.015. [DOI] [PubMed] [Google Scholar]
- Eon Labs, Inc. Bisoprolol fumurate and hydrochlorothiazide tablets: prescribing information (USA) 2004 [Google Scholar]
- Flather MD, Shibata MC, Coats AJS, et al. Randomized trial to determine the effect of nebivolol on mortality and cardiovascular hospital admission in elderly patients with heart failure (SENIORS) Eur Heart J. 2005;26:215–25. doi: 10.1093/eurheartj/ehi115. [DOI] [PubMed] [Google Scholar]
- Fogari R, Zoppi A, Lazzari P, et al. Comparative effects of nebivolol and atenolol on blood pressure and insulin sensitivity in hypertensive subjects with type II diabetes. J Hum Hypertens. 1997;11:753–7. doi: 10.1038/sj.jhh.1000533. [DOI] [PubMed] [Google Scholar]
- Frishman WH, Alwarshetty M. β-adrenergic blockers in systemic hypertension: pharmacokinetic considerations related to the current guidelines. Clin Pharmacokinet. 2002;41:505–16. doi: 10.2165/00003088-200241070-00004. [DOI] [PubMed] [Google Scholar]
- Gauthier C, Leblais V, Lester K, et al. The negative inotropic effect of β3-adrenoreceptor stimulation is mediated by activation of a nitric oxide synthase pathway in human ventricle. J Clin Invest. 1998;102:1377–84. doi: 10.1172/JCI2191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GlaxoSmithKline Coreg (carvedilol): prescribing information (USA) [online] 2007. Accessed 25 March 2007. URL: http://us.gsk.com/products/assets/us_coreg.pdf.
- Gosgnach W, Boixel C, Nevo N, et al. Nebivolol induces calcium-dependent signaling in endothelial cells by a possible β-adrenergic pathway. J Cardiovasc Pharmacol. 2001;38:191–9. doi: 10.1097/00005344-200108000-00004. [DOI] [PubMed] [Google Scholar]
- Grassi G, Trevano FG, Facchini A, et al. Efficacy and tolerability profile of nebivolol vs. atenolol in mild-moderate essential hypertension: a double-blind randomized multi-centre trial. Blood Press Suppl. 2003;2:35–40. [PubMed] [Google Scholar]
- Hjalmarson A, Goldstein S, Fagerberg B, et al. Effect of metoprolol CR/XL in chronic heart failure: Metoprolol CR/XL Randomised Intervention Trial in Congestive Heart Failure (MERIT-HF) Lancet. 1999;353:2001–7. [PubMed] [Google Scholar]
- Hunt SA, Abraham WT, Chin MH, et al. ACC/AHA 2005 guideline update for the diagnosis and management of chronic heart failure in the adult: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Update the 2001 Guidelines for the Evaluation and Management of Heart Failure) [online] 2005. American College of Cardiology Website. URL: http://www.acc.org/clinical/guidelines/failure//index.pdf. [DOI] [PubMed]
- Ignarro LJ. Experimental evidences of nitric oxide-dependent vasodilatory activity of nebivolol, a third-generation β-blocker. Blood Press Suppl. 2004;1:2–16. [PubMed] [Google Scholar]
- Janssen PML, Zeitz O, Rahman A, et al. Protective role of nebivolol in hydroxyl radical induced injury. J Cardiovasc Pharmacol. 2001;38(Suppl 3):S17–23. doi: 10.1097/00005344-200112003-00004. [DOI] [PubMed] [Google Scholar]
- Jessup M, Brozena S. Heart failure. N Engl J Med. 2003;348:2007–18. doi: 10.1056/NEJMra021498. [DOI] [PubMed] [Google Scholar]
- Kalinowski L, Dobrucki IT, Malinski T. Race-specific differences in endothelial function: predisposition of African Americans to vascular diseases. Circulation. 2004;109:2511–17. doi: 10.1161/01.CIR.0000129087.81352.7A. [DOI] [PubMed] [Google Scholar]
- Kamali F, Howes A, Thomas SHL, et al. A pharmacokinetic and pharmacodynamic interaction study between nebivolol and the H2-receptor antagonists cimetidine and ranitidine. Br J Clin Pharmacol. 1997;43:201–4. doi: 10.1046/j.1365-2125.1997.54212.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinlay S, Creager MA, Fukumoto M, et al. Endothelium-derived nitric oxide regulates arterial elasticity in human arteries in vivo. Hypertension. 2001;38:1049–53. doi: 10.1161/hy1101.095329. [DOI] [PubMed] [Google Scholar]
- Komajda M, Lapuerta P, Herman N, et al. Adherence to guidelines is a predictor of outcome in chronic heart failure, the MAHLER survey. Eur Heart J. 2005;26:1653–9. doi: 10.1093/eurheartj/ehi251. [DOI] [PubMed] [Google Scholar]
- Lawrence TE, Liu S, Bland TM, et al. Single-dose pharmacokinetics and anticoagulant activity of warfarin is unaffected by nebivolol in healthy volunteers [abstract] Clin Pharmacol Ther. 2005a;77:P39. [Google Scholar]
- Lawrence TE, Liu S, Fisher J, et al. No interaction between nebivolol and digoxin in healthy volunteers [abstract] Clin Pharmacol Ther. 2005b;77:P76. [Google Scholar]
- Lawrence TE, Chien C, Liu S, et al. No effect of concomitant administration of nebivolol and losartan in healthy volunteers genotyped for CYP2D6 status [abstract] Clin Pharmacol Ther. 2005c;77:P82. [Google Scholar]
- Lawrence TE, Liu S, Donnelly CM, et al. A phase I open-label multiple dose study assessing the pharmacokinetic interaction of hydrochlorothiazide and nebivolol HCl in healthy volunteers. AAPS PharmSci [online journal] 2003;5 Abstract T2332. Accessed 30 November 2006. URL: http://www.aapsj.org/abstracts/am_abstracts2002.asp. [Google Scholar]
- Lefebvre J, Poirier L, Poirier P, et al. The influence of CYP2D6 phenotype on the clinical response of nebivolol in patients with essential hypertension. Br J Clin Pharmacol. 2006 doi: 10.1111/j.1365-2125.2006.02796.x. published online 10 November 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lekakis JP, Protogerou A, Papamichael C, et al. Effect of nebivolol and atenolol on brachial artery flow-mediated vasodilation in patients with coronary artery disease. Cardiovasc Drugs Ther. 2005;19:277–81. doi: 10.1007/s10557-005-3117-9. [DOI] [PubMed] [Google Scholar]
- Lombardo RMR, Reina C, Abrignani MG, et al. Effects of nebivolol versus carvedilol on left ventricular function in patients with chronic heart failure and reduced left ventricular systolic function. Am J Cardiovasc Drugs. 2006;6:259–63. doi: 10.2165/00129784-200606040-00006. [DOI] [PubMed] [Google Scholar]
- McMurray J, Cohen-Solal A, Dietz R, et al. Practical recommendations for the use of ACE inhibitors, beta-blockers, aldosterone antagonists and angiotensin receptor blockers in heart failure: Putting guidelines into practice. Eur J Heart Fail. 2005;7:710–21. doi: 10.1016/j.ejheart.2005.07.002. [DOI] [PubMed] [Google Scholar]
- Mason RP. Nitric oxide mechanisms in the pathogenesis of global risk. J Clin Hypertens. 2006;8(8 Suppl 2):31–8. doi: 10.1111/j.1524-6175.2006.05838.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason RP, Kalinowski L, Jacob RF, et al. Nebivolol reduces nitroxidative stress and restores nitric oxide bioavailability in endothelium of Black Americans. Circulation. 2005;112:3795–801. doi: 10.1161/CIRCULATIONAHA.105.556233. [DOI] [PubMed] [Google Scholar]
- Morton T, Tu HC, Liu S, et al. A phase I open-label multiple-dose study of the pharmacokinetic interaction between nebivolol HCl and spironolactone in healthy volunteers. AAPS PharmSci [online journal] 2003;5 Abstract T2333. Accessed 30 November 2006. URL: http://www.aapsj.org/abstracts/am_abstracts2002.asp. [Google Scholar]
- Moncada S, Higgs A. The L-arginine-nitric oxide pathway. N Engl J Med. 1993;329:2002–12. doi: 10.1056/NEJM199312303292706. [DOI] [PubMed] [Google Scholar]
- Morton TL, Liu S, Phillips JM, et al. Pharmacokinetics of nebivolol and ramipril are not affected by coadministration [abstract] Clin Pharmacol Ther. 2005;77:P77. [Google Scholar]
- Opie LH, Commerford PJ, Gersh BJ, et al. Controversies in ventricular remodeling. Lancet. 2006;367:356–67. doi: 10.1016/S0140-6736(06)68074-4. [DOI] [PubMed] [Google Scholar]
- Packer M, Bristow MR, Cohn JN, et al. The effect of carvedilol on morbidity and mortality in patients with chronic heart failure. N Engl J Med. 1996;334:1349–55. doi: 10.1056/NEJM199605233342101. [DOI] [PubMed] [Google Scholar]
- Packer M, Coats AJS, Fowler MB, et al. Effect of carvedilol on survival in severe chronic heart failure. N Engl J Med. 2001;344:1651–8. doi: 10.1056/NEJM200105313442201. [DOI] [PubMed] [Google Scholar]
- Pasini AF, Garbin U, Nava MC, et al. Nebivolol decreases oxidative stress in essential hypertensive patients and increases nitric oxide by reducing its oxidative inactivation. J Hypertens. 2005;23:589–96. doi: 10.1097/01.hjh.0000160216.86597.ff. [DOI] [PubMed] [Google Scholar]
- Panza JA, Quyyumi AA, Brush JE, et al. Abnormal endothelium-dependent vascular relaxation in patients with essential hypertension. N Engl J Med. 1990;323:22–7. doi: 10.1056/NEJM199007053230105. [DOI] [PubMed] [Google Scholar]
- Patrianakos AP, Parthenakis FI, Mavrakis HE, et al. Comparative efficacy of nebivolol versus carvedilol on left ventricular function and exercise capacity in patients with nonischemic dilated cardiomyopathy. A 12-month study. Am Heart J. 2005;150(985):e9–e18. doi: 10.1016/j.ahj.2005.07.024. [DOI] [PubMed] [Google Scholar]
- Pesant Y, Marc-Aurele J, Bielmann P, et al. Metabolic and antihypertensive effects of nebivolol and atenolol in normometabolic patients with mild-moderate hypertension. Am J Ther. 1999;6:137–47. doi: 10.1097/00045391-199905000-00004. [DOI] [PubMed] [Google Scholar]
- Peter P, Martin U, Sharma A, et al. Effect of treatment with nebivolol on parameters of oxidative stress in type 2 diabetics with mild to moderate hypertension. J Clin Pharm Ther. 2006;31:153–9. doi: 10.1111/j.1365-2710.2006.00718.x. [DOI] [PubMed] [Google Scholar]
- Poirier L, Cleroux J, Nadeau A, et al. Effects of nebivolol and atenolol on insulin sensitivity and haemodynamics in hypertensive patients. J Hypertens. 2001;19:1429–35. doi: 10.1097/00004872-200108000-00011. [DOI] [PubMed] [Google Scholar]
- Rizos E, Bairaktari E, Kostoula A, et al. The combination of nebivolol plus pravastatin is associated with a more beneficial metabolic profile compared with that of atenolol plus pravastatin in hypertensive patients with dyslipidemia: a pilot study. J Cardiovasc Pharmacol Ther. 2003;8:127–34. doi: 10.1177/107424840300800206. [DOI] [PubMed] [Google Scholar]
- Shaw AA, Bland TM, Tu HC, et al. Single-dose, relative bioavailability and food effect study of nebivolol hydrochloride in healthy volunteers characterized according to their metabolizing status. AAPS PharmSci [online journal] 2003a;5 Abstract W5238. Accessed 30 November 2006. URL: http://www.aapsj.org/abstracts/am_abstracts2002.asp. [Google Scholar]
- Shaw AA, Bland TM, Tu HC, et al. Single-dose, dose-proportionality pharmacokinetic study of nebivolol hydrochloride in healthy volunteers characterized according to their metabolizing status. AAPS PharmSci [online journal] 2003b;5 Abstract M1327. Accessed 30 November 2006 URL: http://www.aapsj.org/abstracts/am_abstracts2002.asp. [Google Scholar]
- Shaw AA, Liu S, Zachwieja LF, et al. Effect of chronic administration of fluoxetine on the pharmacokinetics of nebivolol [abstract] Clin Pharmacol Ther. 2005;77:P38. [Google Scholar]
- Sule SS, Frishman W. Nebivolol: new therapy update. Cardiol Rev. 2006;14:259–64. doi: 10.1097/01.crd.0000223651.03023.8e. [DOI] [PubMed] [Google Scholar]
- Taylor AL, Ziesche S, Yancy, et al. Combination of isosorbide dinitrate and hydralazine in blacks with heart failure. N Engl J Med. 2004;351:2049–57. doi: 10.1056/NEJMoa042934. [DOI] [PubMed] [Google Scholar]
- Tregaskis BF, McDevitt DG. β-adrenoceptor-blocking drugs in the elderly. J Cardiovasc Pharmacol. 1990;16(Suppl 5):S25–S28. [PubMed] [Google Scholar]
- Uhlir O, Dvorak I, Gregor P, et al. Nebivolol in the treatment of cardiac failure: a double-blind controlled clinical trial. J Card Fail. 1997;3:271–6. doi: 10.1016/s1071-9164(97)90026-9. [DOI] [PubMed] [Google Scholar]
- The Menarini Group. Clinical Trial Registry [online] 2007. Accessed 2 April 2007 URL: http://www.menarini.com/english/clinical_trials/clinical_studies.htm.
- Swedberg K, Cleland J, Dargie H, et al. Guidelines for the diagnosis and treatment of chronic heart failure: full text (update 2005): the Task Force for the diagnosis and treatment of CHF of the European Society of Cardiology [online] 2005. Accessed 12 November 2006 URL: http://www.escardio.org/knowledge/guidelines/Chronic_Heart_Failure.htm.
- Taylor AL, Ziesche S, Yancy C, et al. Combination of isosorbide dinitrate and hydralazine in blacks with heart failure. N Engl J Med. 2004;351:2049–57. doi: 10.1056/NEJMoa042934. [DOI] [PubMed] [Google Scholar]
- Van Bortel LM, De Hoon JN, Kool MJ, et al. Pharmacological properties of nebivolol in man. Eur J Clin Pharmacol. 1997;51:379–84. doi: 10.1007/s002280050217. [DOI] [PubMed] [Google Scholar]
- Van Neuten L, De Cree J. Nebivolol: comparison of the effects of dl-nebivolol, d-nebivolol, l-nebivolol, atenolol, and placebo on exercise-induced increases in heart rate and systolic blood pressure. Cardiovasc Drugs Ther. 1998;12:339–44. doi: 10.1023/a:1007760515117. [DOI] [PubMed] [Google Scholar]
- Van Neuten L, Taylor FR, Robertson JIS, et al. Nebivolol vs. atenolol and placebo in essential hypertension: a double-blind randomized trial. J Hum Hypertens. 12:135–40. doi: 10.1038/sj.jhh.1000571. [DOI] [PubMed] [Google Scholar]
- Van Peer A, Snoeck E, Woestenborghs R, et al. Clinical pharmacology of nebivolol. A review. Drug Invest. 1991;3(Suppl 1):25–30. [Google Scholar]
- Veverka A, Nuzum DS, Jolly JL. Nebivolol: a third-generation β-adrenergic blocker. Ann Pharmacother. 2006;40:1353–60. doi: 10.1345/aph.1G708. [DOI] [PubMed] [Google Scholar]