Abstract
Currently, intracerebral hemorrhage (ICH) has the highest mortality rate of all stroke subtypes (Counsell et al 1995; Qureshi et al 2005). Hematoma growth is a principal cause of early neurological deterioration. Prospective and retrospective studies indicate that up to 38% hematoma expansion is noted within three hours of ICH onset and that hematoma volume is an important predictor of 30-day mortality (Brott et al 1997; Qureshi et al 2005). This article will review current standard of care measures for ICH patients and new research directed at early hemostatic therapy and minimally invasive surgery.
Keywords: ICH, hemostatic therapy, recombinant factor VII, surgical management
Intracerebral hemorrhage
An intracerebral hemorrhage (ICH) account for only 15% of all strokes but it is one of the most disabling forms of stroke (Counsell et al 1995; Qureshi et al 2005). Greater than one third of patients with intracerebral hemorrhage (ICH) will not survive and only twenty percent of patients will regain functional independence (Counsell et al 1995). This high rate of morbidity and mortality has prompted investigations for new medical and surgical therapies for intracerebral hemorrhage.
Primary ICH develops in the absence of any underlying vascular malformation or coagulopathy. Primary intracerebral hemorrhage is more common than secondary intracerebral hemorrhage. Hypertensive arteriosclerosis and cerebral amyloid angiopathy (CAA) are responsible for 80% of primary hemorrhages (Sutherland and Auer 2006). At times it may be difficult to identify the underlying etiology because poorly controlled hypertension is often identified in most ICH patients. Patients with CAA-related ICH are more likely to be older and the volume of hemorrhage is usually > 30 cc (Ritter et al 2005). Hypertension related ICH is frequently seen in younger patients, involving the basal ganglia, and the volume of blood is usually < 30 cc (Lang et al 2001). However these characteristics are nonspecific and histopathological studies are needed to confirm a definitive diagnosis of CAA or hypertension related ICH. Hypertension causes high pressure within the Circle of Willis resulting in smooth cell proliferation followed by smooth muscle cell death. This may explain why hypertension related ICH are frequently located deep within the basal ganglia, thalamus (Figure 1), cerebellum, pons and rarely the neocortex (Campbell and Toach 1981; Sutherland and Auer 2006). In contrast, preferential amyloid deposition within leptomeningeal and intraparenchymal cortical vessels may explain the reason for large superficial lobar hemorrhages with amyloid angiopathy (Auer and Sutherland 2005). It is important to identify those afflicted with cerebral amyloid angiopathy because of the high risk of recurrent lobar hemorrhage and predisposition for symptomatic hemorrhage with anticoagulants and thrombolytics (Rosand and Greenberg 2000).
Figure 1.
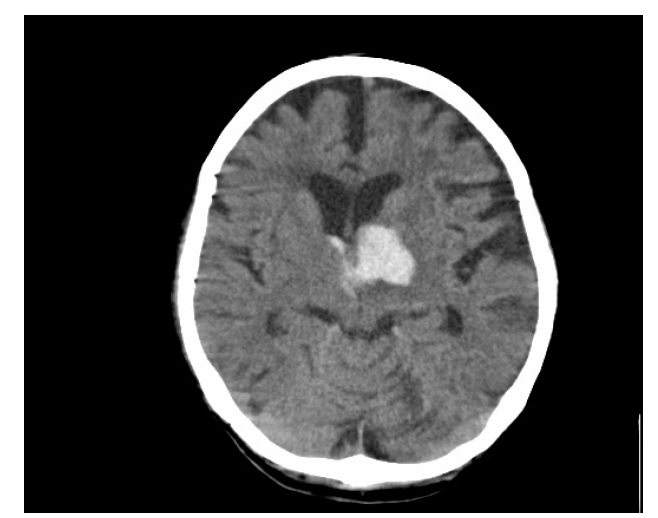
CT scan showing hemorrhage in the left thalamus secondary to hypertension.
Secondary ICH is due to underlying vascular malformation, hemorrhagic conversion of an ischemic stroke, coagulopathy, intracranial tumor, etc. Arteriovenous malformations and cavernous malformations account for majority of underlying vascular malformations (Sutherland and Auer 2006). An AVM (Figure 2) is usually a singular lesion composed of an abnormal direct connection between distal arteries and veins. AVMs account for only 2% of all ICH but are associated with an 18% annual rebleed risk (Al-Shahi and Warlow 2001). Cavernous malformations are composed of sinusoidal vessels and are typically located in within the supratentorial white matter. The annual risk of recurrent hemorrhage is only 4.5% (Konziolka and Bernstein 1987). Intracranial aneurysms usually present with subarachnoid hemorrhage but anterior communicating artery and middle cerebral artery may also have a parenchymal hemorrhagic component near the interhemispheric fissure and perisylvian region respectively (Wintermark and Chaalaron 2003). Embolic ischemic strokes can often demonstrate hemorrhagic conversion without significant mass effect (Ott and Zamani 1986). Sinus thrombosis should be suspected in patients with signs and symptoms suggestive of increased intracranial pressure and radiographic evidence of superficial cortical or bilateral symmetric hemorrhages (Canhoe and Ferro 2005). An underlying cogenial or acquired coagulopathy causing platelet or coagulation cascade dysfunction can result in ICH. Cogenial disorders account for Hemophilia A, Hemophilia B, and other rare diseases. Acquired coagulopathy may be attributed to longstanding liver disease, renal disease, malignancy, or medication. Particular attention has been directed towards oral anticoagulant (OAT) associated hemorrhage due to greater risk for hematoma expansion as well as increased 30 day morbidity and mortality rates (Flibotte et al 2004; Roquer et al 2005; Toyoda et al 2005; Steiner and Rosand 2006). Metastatic tumors account for less than ten percent of ICH located near the grey white junction with significant mass effect. The primary malignancy is usually melanoma, choriocarninoma, renal carcinoma, or thyroid carcinoma (Kondziolka and Berstein 1987).
Figure 2.

Axial T2- weighted MR image showing multiple abnormal flow void (arrow) signals indicating presence of an arteriovenous malformation in the left temporal lobe.
Clinical presentation
The classic presentation of ICH is sudden onset of a focal neurological deficit that progresses over minutes to hours with accompanying headache, nausea, vomiting, decreased consciousness, and elevated blood pressure. Rarely patients present with symptoms upon awakening from sleep. Neurologic deficits are related to the site of parenchymal hemorrhage. Thus, ataxia is the initial deficit noted in cerebellar hemorrhage, whereas weakness may be the initial symptom with a basal ganglia hemorrhage. Early progression of neurologic deficits and decreased level of consciousness can be expected in 50% of patients with ICH. The progression of neurological deficits in many patients with an ICH is frequently due to ongoing bleeding and enlargement of the hematoma during the first few hours (Kazui et al 1996; Brott et al 1997; Fujii et al 1998). Compared with patients with ischemic stroke, headache and vomiting at onset of symptoms is observed three times more often in patients with ICH (Gorlick et al 1986; Rathore et al 2002). Despite the differences in clinical presentation between hemorrhagic and ischemic strokes, brain imaging is required to definitively diagnose intracerebral hemorrhage.
Diagnosis
Computed tomography (CT) is more widely available so CT of the brain has become the initial diagnostic test of choice for ICH. However, recent studies suggest MRI and CT are equally efficacious in diagnosing hyperacute ICH (<6 hours) (Fiebach et al 2004; Kidwell et al 2004). In addition, in 2004, Fiebach and colleagues conducted a multicenter study and concluded that visual identification of ICH is not difficult with MRI with mean sensitivities = 95% with expert readers as well as final-year medical students (Fiebach et al 2004). MRI and magnetic resonance angiography (MRA) can also help elucidate any underlying cause of the hemorrhage. Sometimes the pattern and topography of bleeding can give important clues about a secondary cause of ICH. For example, subarachnoid blood should raise suspicion for a ruptured aneurysm, multiple inferior frontal and temporal hemorrhages may be seen after head trauma, and fluid levels within the hematoma suggest an underlying coagulopathy (Figure 3). Active contrast extravasation into the hematoma seen with CT angiography may predict hematoma expansion and is predictive of poor outcome (Becker et al 1999; Murai et al 1999). Angiography is not required for older hypertensive patients with hemorrhages in deep subcortical structures with no findings suggestive of an underlying structural lesion. Secondary intracerebral hemorrhage should be suspected in patients <45 years of age, no risks for hypertensive hemorrhage, presence of subarachnoid hemorrhage, prominent vascular structures, perisylvian or interhemispheric hemorrhage and angiography should be pursued. Angiography should always be considered in young non-hypertensive patients with ICH who have no obvious explanation for their hemorrhage, or when the only risk factor is cocaine or sympathomimetic-drug use (Halpin et al 1994; Griffiths et al 1997; Zhu et al 1997; Broderick and Adams 1999).
Figure 3.

CT scan showing large left parietal lobe lobar hemorrhage with a fluid level (arrow) after the patient received r-TPA.
Management
Emergency management
ICH is a neurological emergency and initial management should be focused on assessing the patients airway, breathing capability, blood pressure and signs of increased intracranial pressure. The patient should be intubated based on risk of aspiration, impending ventilatory failure (PaO2 < 60 mmHg or pCO2 > 50 mmHg), and signs of increased intracranial pressure. (Broderick et al 1999) Emergency measures for ICP control are appropriate for stuporous or comatose patients, or those who present acutely with clinical signs of brainstem herniation. The head should be elevated to 30 degrees, 1.0–1.5 g/kg of 20% mannitol should be given by a rapid infusion, and the patient should be hyperventilated to a pCO2 of 30–35 mmHg. (Allen and Ward 1998) These measures are designed to lower ICP as quickly as possible prior to a definitive neurosurgical procedure (craniotomy, ventriculostomy, or placement of an ICP monitor) can be done. A number of these patients will present after a fall so particular attention should be directed to lacerations, skeletal fractures, stabilization of the cervical spine.
Blood pressure
Elevated blood pressure is seen in 46%–56% of patients with ICH. (Dandapani et al 1995) It remains unclear if elevated blood pressure directly causes hematoma expansion but studies have shown elevated systolic, diastolic, and mean arterial pressure are associated with a poor outcome in ICH (Terrayama et al 1997; Leonardi-Bee et al 2002; Vemmos et al 2004). However, physicians have been reluctant to treat hypertension in ICH patients because the fear of overaggressive treatment of blood pressure may decrease cerebral perfusion pressure and theoretically worsen brain injury, particularly in the setting of increased intracranial pressure. In 1999, a special group consisting of healthcare professionals from the American Heart Association Stroke Council addressed these 2 rational theoretical concerns while attempting to write guidelines for the management of intracerebral hemorrhage. The task force recommended maintaining a mean arterial pressure below 130 mmHg in patients with a history of hypertension (level of evidence V, grade C recommendation). In patients with elevated ICP who have an ICP monitor, cerebral perfusion pressure (MAP–ICP) should be kept >70 mmHg (level of evidence V, grade C recommendation) (Broderick et al 1999).
Early hemostatic therapy
In the past, early neurologic deterioration in ICH was attributed to edema and mass effect around the hematoma. Pathological, CT, and SPECT studies suggest that continuous rebleeding into congested damaged tissue is associated with poor clinical outcome and is now an exciting new target of treatment (Fisher 1971; Kazui et al 1996; Fujii et al 1998; Becker et al 1999; Mayer 2005). Recent interest in hemostatic therapy is based on early hematoma growth often seen within six hours of onset of ICH in 14%–38% of patients (Kazui et al 1996; Brott et al 1997; Fujii et al 1998; Flibotte et al 2004; Roquer et al 2005). Initial efforts should be directed towards identifying thrombolytic, antiplatelet or anticoagulant use and reversing their effects. The biologic half-life of recombinant tissue plasminogen activator (rt-PA) at the site of the thrombus is limited to 45 minutes and accordingly hemorrhagic complications from rt-PA occur within the first few hours of use. Information is scarce to guide recommendations about treatment of hemorrhagic complications of thrombolytic therapy (levels of evidence III through V). According to guidelines devised by the American Heart Association Stroke Council, if bleeding is suspected the following measures should be taken: (1) blood should be drawn to measure the patient’s hematocrit, hemoglobin, partial thromboplastin time, prothrombin time/INR, platelet count, and fibrinogen (2) blood should be typed and cross-matched if transfusions are needed (at least 4 U of packed red blood cells, 4–6 U of cryoprecipitate or fresh frozen plasma, and 1 U of single donor platelets) (Adams et al 1996). These therapies should be made available for urgent administration. The risk of intracerebral hemorrhage with heparin is related to the level of anticoagulation. Heparin can be inactivated by 1 mg of protamine sulfate for every 100 IU of heparin administered (Wakefield and Stanley 1996). FFP should not be used to correct heparin related coagulopathy because FFP contains heparin binding antithrombin III (AT-III) which may prolong the anticoagulated status (Badjatia and Rosand 2005). Warfarin prevents recycling of vitamin K and indirectly inhibits synthesis of vitamin K dependent coagulation factors. Replenishing vitamin K via the oral or intravenous route helps reverse the effect of warfarin but an effective response may be delayed over 24 hours. Concominant use of vitamin K with FFP, cryoprecipitate, or clotting factor concentrates are recommended to hasten reversal of warfarin induced coagulopathy. Considering the short half-life of coagulation factors at least 5–20 mg of vitamin K is required to sustain reversal of anticoagulation. Intravenous administration of vitamin K should be limited due to concerns of allergic and anaphylactic reactions. In an acute setting, vitamin K should not be administered subcutaneously because reversal of anticoagulation is neither rapid nor reliable (Steiner et al 2006). However, the variable content of vitamin K-dependent clotting factors in FFP and the effects of dilution have raised concerns that a coagulopathic state may persist despite correction of the international normalized ratio (INR) (Makris et al 1997). It is not clear at this time whether prothrombin complex concentrate is more reliable than FFP in repleting coagulation factors but it has proven to correct the INR faster than FFP which reduces the incidence and extent of hematoma expansion (Fredriksson et al 1992; Huttner et al 2006). Use of antiplatelet agents prior to ICH is a risk factor for continuous bleeding and poor outcome so it is reasonable to treat these patients with platelet infusions and desmopressin (Janssen and van der Meulen 1996; Saloheimo et al 2006).
Antifibrinolytic agents such as e-aminocaproic, tranexemic acid, aprotinin, and activated recombinant Factor VII (rFVIIa) have been receiving attention for early hemostatic therapy in patients with no underlying coagulopathy. However, rFVIIa is the only agent whose role in treating primary ICH has been evaluated in the randomized placebo control trial. The Novoseven Phase II trial was an international, multicenter, double-blinded trial that clearly demonstrated a reduction in early hematoma expansion in patients administered rFVIIa within 4 hours of symptom onset compared with placebo. In fact, the hemostatic effect was more pronounced with incremental doses of rFVIIa (Mayer et al 2005). Despite these promising results, early results from the Phase III Fast trial showed use of rFVIIa did not alter severe disability or mortality rates at 90 days (Forbes 2007). Complete results from the Phase III FAST trial are expected later this year.
Management of ICP
Elevated ICP is defined as intracranial pressure >20 mmHg for over 5 minutes. Large volume ICH is commonly associated with high ICP and brain tissue shifts related to ICP gradients. This problem can be exacerbated by intraventricular hemorrhage, which leads to acute obstructive hydrocephalus. The therapeutic goal of treating elevated ICP is to maintain ICP < 20 mmHg while maintaining cerebral perfusion pressure >70 mmHg. When ICP is monitored, use of a standard management algorithm results in better control, fewer interventions, and shorter duration of therapy. Initially, acute and sustained increase in ICP should prompt a repeat CT to assess the need for a definitive neurosurgical procedure. An intravenous sedative such as propofol (0.6–6.0 mg/kg/h) or fentanyl (0.5–3.0 μg/kg/h) should be given to the agitated patient to attain a motionless state. Thereafter, therapy should be directed at controlling blood pressure with vasopressors such as dopamine and phenylephrine if the CPP is < 70 mmHg or with antihypertensive agents if the CPP is > 70 mmHg. If ICP does not respond to sedation and cerebral perfusion management, osmotic agents and hyperventilation should be considered (Mckinley et al 1999). Of the 3 osmotic agents frequently used (mannitol, glycerol, and sorbitol), each has characteristic advantages and disadvantages. Sorbitol and glycerol are metabolized by the liver and interfere with glucose metabolism. However, sorbitol is infrequently used due to a short half life and poor penetration into the cerebrospinal fluid (CSF). Glycerol has a half-life less than one hour but it penetrates into the cerebrospinal fluid the best. Mannitol is commonly used because it is renally metabolized, has a half-life up to 4 hours, and achieves intermediate concentrations within the CSF (Nau 2000). Large ICH associated with elevated intracranial pressure refractory to these measures is fatal in most patients but a barbiturate coma may considered as a last resort to try to reduce intracranial pressure (Broderick et al 1999; Mckinley et al 1999). Corticosteroids are not recommended in the management of ICH because they have been proven to offer no benefit in randomized trials (Tellez and Bauer 1973; Poungvarin et al 1987).
Ventricular drains should be used in patients with or at risk for hydrocephalus. Drainage can be initiated and terminated according to clinical performance and ICP values. The volume of IVH strongly affects morbidity and mortality at 30-days (Tuhrim et al 1988). Preliminary studies with urokinase have suggested use of intraventricular thrombolysis within 72 hours of IVH may help drain the blood filled ventricles, speed clot resolution and decrease 30-day mortality rate (Naff et al 2000; Naff et al 2004). Patients are currently being recruited for Phase III trials assessing thrombolytic use in intraparenchymal and intraventricular hemorrhage.
Anticonvulsant therapy
The 30-day risk of seizures after ICH is about 8%. Seizures most commonly occur at the onset of hemorrhage and may even be the presenting symptom. Lobar location is an independent predictor of early seizures (Passero et al 2003). Although, no randomised trial has addressed the efficacy of prophylactic antiepileptic in ICH patients, the Stroke Council of the American Heart Association suggest prophylactic antiepileptic treatment may be considered for 1 month in patients with intracerebral hemorrhage and discontinued if no seizures are noted (Broderick et al 1999; Temkin 2001). Acute management of seizures entail administering intravenous lorazepam (0.05–0.10 mg/kg) followed by an intravenous loading dose of phenytoin or fosphenytoin (15–20 mg/kg), valproic acid (15–45 mg/kg), or phenobarbital (15–20 mg/kg).
Fever control
Fever after ICH is common and should be treated aggressively because it is independently associated with a poor outcome (Schwarcz et al 2001). Sustained fever in excess of 38.3 °C (101.0 °F) should be treated with acetaminophen and cooling blankets. Patients should be physically examined and should undergo laboratory testing or imaging to determine the source of infection. Fever of neurologic origin is diagnosis of exclusion and may be seen when blood extends into the subarachnoid or intraventricular (Commichau and Scarmeas 2003). Intracerebral hemorrhage patients with persistent fever that is refractory to acetaminophen and without infectious cause may require cooling devices to become normothermic. Adhesive surface-cooling systems and endovascular heat-exchange catheters are better at maintaining normothermia than conventional treatment. However, it is still unclear whether maintaining normothermia will improve clinical outcome (Dringer 2004).
Deep venous thrombosis prophylaxis
Immobilized state due to limb paresis predisposes ICH patients for deep vein thrombosis and pulmonary embolism. Intermittent pneumatic compression devices and elastic stockings should be placed on admission (Lacut et al 2005). A small prospective trial by Boeer and colleagues using low-dose heparin on hospital day 2 to prevent thromboebolic complications in ICH patients significantly lowered the incidence of pulmonary embolism and no increase in rebleeding was observed (Boeer et al 1991).
Surgical management
Numerous surgical trials since the 1960s offered conflicting results and until recently no firm conclusions could be reached regarding the operative management of intracerebral hemorrhage. In 1995 randomization for the landmark Surgical Trial in Intracerebral Hemorrhage (STICH) had commenced. This trial was an international, multicenter trial that randomized 1033 patients with spontaneous supratentorial intracerebral hemorrhage within twenty-four hours to early surgery or conservative best medical therapy. Size, location, and volume of hemorrhage were similar in both treatment groups. Patients randomized to early surgery had their hematoma evacuated within twenty-four hours of randomization by the method of choice of the designated neurosurgeon. In 77% of cases, craniotomy was the surgical procedure and the remainder of cases had hematoma removal by burr hole, endoscopy, or stereotaxy in similar numbers. Thus, the STICH Trial is primarily a trial of craniotomy for ICH removal and left the role of less invasive surgery to remove ICH unanswered. Structured postal questionnaires were used to assess outcomes with the Glasgow Coma Scale, modified Rankin Scale, Barthel index, and mortality at 6-months. Overall, the STICH trial revealed no benefit from early craniotomy in supratentorial intracerebral hemorrhage when compared to initial conservative management. Of the prespecified subgroups that were examined, patients with an ICH within a centimeter of the cortical surface showed a benefit for early surgery. However, the statistical testing of this subgroup was not adjusted for in the multiple subgroup comparisons in this trial. In addition, early surgery was delayed with median time from onset to treatment for early surgery group was 30 hours and that may have affected the outcome (Broderick 2005; Mendelow et al 2005).
In contrast, infratentorial hemorrhages seem to benefit from early surgery. Most neurosurgeons believe cerebellar hemorrhages greater than 3 centimeters benefit from early surgical intervention because of the significant risk of brainstem compression and obstructive hydrocephalus within 24 hours (Ott et al 1974).
New areas of surgical research are focused on combination of minimally invasive surgery and and clot lysis with r-tPA to remove intracerebral hemorrhage. Small preliminary trials have demonstrated that stereotactic aspiration and thrombolysis spontaneous intracerebral hemorrhage appears to be safe and effective in the reduction of ICH volume (Teernstra et al 2003; Barrett et al 2005; Vespa et al 2005). The National Institute of Health (NIH) has sponsored the Minimally Invasive Surgery Plus rtPA for Intracerebral Hemorrhage Evacuation (MISTIE) trial to determine the safety of using a combination of minimally invasive surgery and clot lysis with rt-PA to remove supratentorial primary ICH and compare efficacy to conventional medical management. The MISTIE trial is an open-label randomized treatment trial which is currently enrolling patients at multiple centers within the United States (NIH 2001).
Conclusion
Currently, no specific therapies improve the outcome after ICH. Although rFVIIa limits hematoma expansion, early Phase III results failed to show reduction in severe disability or mortality rates at 90 days. New trials evaluating the safety of the combination of minimally invasive surgery and clot lysis with r-tPA to remove intracerebral hemorrhage are currently underway.
References
- Adams HP, Brott TG, et al. Guidelines for thrombolytic therapy for acute stroke: a supplement to the guidelines for the management of patients with acute ischemic stroke. A statement for healthcare professionals from a special writing group of the Stroke Council, American Heart Association. Circulation. 1996;94:1167–74. doi: 10.1161/01.cir.94.5.1167. [DOI] [PubMed] [Google Scholar]
- Allen CH, Ward JD. An evidence-based approach to management of increased intracranial pressure. Crit Care Clin. 1998;14:485–95. doi: 10.1016/s0749-0704(05)70012-9. [DOI] [PubMed] [Google Scholar]
- Al-Shahi R, Warlow C. A systematic review of the frequency and prognosis of the arteriovenous malformation of the brain in adults. Brain. 2001;124:1900–26. doi: 10.1093/brain/124.10.1900. [DOI] [PubMed] [Google Scholar]
- Auer RN, Sutherland GR. Primary intracerebral hemorrhage: pathophysiology. Can J Neurol Sci. 2005;32:S3–12. [PubMed] [Google Scholar]
- Badjatia N, Rosand J. Intracerebral Hemorrhage. The Neurologist. 2005;11:311–24. doi: 10.1097/01.nrl.0000178757.68551.26. [DOI] [PubMed] [Google Scholar]
- Barrett RJ, Hyssain R, et al. Frameless stereotactic aspiration and thrombolysis of spontaneous intracerebral hemorrhage. Neurocrit Care. 2005;3:237–45. doi: 10.1385/NCC:3:3:237. [DOI] [PubMed] [Google Scholar]
- Becker KJ, Baxter AB, et al. Extravasation of radiographic contrast is an independent predictor of death in primary intracerebral hemorrhage. Stroke. 1999;30:2025–32. doi: 10.1161/01.str.30.10.2025. [DOI] [PubMed] [Google Scholar]
- Boeer A, Voth E, et al. Early heparin therapy in patients with spontaneous intracerebral haemorrhage. J Neurol Neurosurg Psychiatry. 1991;54:466–7. doi: 10.1136/jnnp.54.5.466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broderick JP, Adams HP, et al. Guidelines for the management of spontaneous intracerebral hemorrhage: a statement for health professionals from a special writing group of the Stroke Council, American Heart Association. Stroke. 1999;30:905–15. doi: 10.1161/01.str.30.4.905. [DOI] [PubMed] [Google Scholar]
- Broderick JP. The STICH trial: what does it tell us and where do we go from here? Stroke. 2005;36:1619–20. doi: 10.1161/01.STR.0000170714.43167.34. [DOI] [PubMed] [Google Scholar]
- Brott T, Broderick J, et al. Early hemorrhage growth in patients with intracerebral hemorrhage. Stroke. 1997;28:1–5. doi: 10.1161/01.str.28.1.1. [DOI] [PubMed] [Google Scholar]
- Campbell GJ, Roach M. Fenestrations in the internal elastic lamina at bifurcations of human cerebral arteries. Stroke. 1981;12:489–96. doi: 10.1161/01.str.12.4.489. [DOI] [PubMed] [Google Scholar]
- Canhoe P, Ferro JM. Causes and predictors of death in cerebral venous thrombosis. Stroke. 2005;6:1720–5. doi: 10.1161/01.STR.0000173152.84438.1c. [DOI] [PubMed] [Google Scholar]
- Commichau C, Scarmeas N. Risk factors for fever in the neurologic intensive care unit. Neurology. 2003;60:837–41. doi: 10.1212/01.wnl.0000047344.28843.eb. [DOI] [PubMed] [Google Scholar]
- Counsell C, Boonyakarnkul S, et al. Primary intracerebral hemorrhage in the Oxfordshire Community Stroke Project. Cerebrovasc Dis. 1995;5:26–34. [Google Scholar]
- Dandapani BK, Suzuki S, et al. Relation of blood pressure and outcome in intracerebral hemorrhage. Stroke. 1995;26:21–4. doi: 10.1161/01.str.26.1.21. [DOI] [PubMed] [Google Scholar]
- Dringer MN. Treatment of fever in the neurologic intensive care unit with a catheter-based heat exchange system. Crit Care Med. 2004;32:559–64. doi: 10.1097/01.CCM.0000108868.97433.3F. [DOI] [PubMed] [Google Scholar]
- Fiebach JJ, Schellinger PD, et al. Stroke magnetic resonance imaging is accurate in hyperacute intracerebral hemorrhage: a multicenter study on the validity of stroke imaging. Stroke. 2004;35:502–6. doi: 10.1161/01.STR.0000114203.75678.88. [DOI] [PubMed] [Google Scholar]
- Fisher CM. Pathological observations in hypertensive cerebral hemorrhage. J Neuropathol Exp Neurol. 1971;30:536–50. doi: 10.1097/00005072-197107000-00015. [DOI] [PubMed] [Google Scholar]
- Flibotte JJ, Hagan N, et al. Warfarin, hematoma expansion, and outcome of intracerebral hemorrhage. Neurology. 63:1059–64. doi: 10.1212/01.wnl.0000138428.40673.83. [DOI] [PubMed] [Google Scholar]
- Forbes Novo Nordisk won’t seek clearance for NovoSeven for bleeding in the brain. 2007. Accessed 11 Apr 2007. URL: http://http://www.forbes.com/markets/feeds/afx/2007/02/26/afx3460856.html.
- Fredriksson K, Norrving B, et al. Emergency reversal of anticoagulation after intracerebral hemorrhage. Stroke. 1992;23:972–7. doi: 10.1161/01.str.23.7.972. [DOI] [PubMed] [Google Scholar]
- Fujii Y, Takeuchi S, et al. Multivariate analysis of predictors of hematoma enlargement in spontaneous intracerebral hemorrhage. Stroke. 1998;29:1160–6. doi: 10.1161/01.str.29.6.1160. [DOI] [PubMed] [Google Scholar]
- Gorelick PB, Caplan LR, et al. Headache in acute cerebrovascular disease. Neurology. 1986;36:1445–50. doi: 10.1212/wnl.36.11.1445. [DOI] [PubMed] [Google Scholar]
- Griffiths PD, Beveridge CJ, et al. Angiography in non-traumatic brain haematoma. An analysis of 100 cases. Acta Radiol. 1997;38:797–802. doi: 10.1080/02841859709172413. [DOI] [PubMed] [Google Scholar]
- Halpin SF, Britton JA, et al. A Prospective evaluation of cerebral angiography and computed tomography in cerebral haematoma. J Neurol Neurosurg Psychiatry. 1994;57:1180–6. doi: 10.1136/jnnp.57.10.1180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huttner HH, Schellinger PD, et al. Hematoma growth and outcome in treated neurocritical care patients with intracerebral hemorrhage related to oral anticoagulant therapy: comparison of acute treatment strategies using vitamin K fresh frozen plasma and prothrombin complex concentrates. Stroke. 2006;37:1465–70. doi: 10.1161/01.STR.0000221786.81354.d6. [DOI] [PubMed] [Google Scholar]
- Janssen MJ, van der Meulen J. The bleeding risk in chronic haemodialysis: preventive strategies in high-risk patients. Neth J Med. 1996;48:198–207. doi: 10.1016/0300-2977(96)00005-8. [DOI] [PubMed] [Google Scholar]
- Kazui S, Naritomi H, et al. Enlargement of spontaneous intracerebral hemorrhage. Incidence and time course. Stroke. 1996;27:1783–7. doi: 10.1161/01.str.27.10.1783. [DOI] [PubMed] [Google Scholar]
- Kidwell CS, Chalela JA, et al. Comparison of MRI and CT for detection of acute intracerebral hemorrhage. JAMA. 2004;292:1823–30. doi: 10.1001/jama.292.15.1823. [DOI] [PubMed] [Google Scholar]
- Kondziolka D, Lunsford LD. The natural history of cerebral cavernous malformations. J Neurosurg. 1995;83:820–4. doi: 10.3171/jns.1995.83.5.0820. [DOI] [PubMed] [Google Scholar]
- Kondziolka D, Bernstein M, et al. Significance of hemorrhage into brain tumors: clinicopathologic study. J Neurosurg. 1987;67:852–7. doi: 10.3171/jns.1987.67.6.0852. [DOI] [PubMed] [Google Scholar]
- Lacut K, Bressollette L, et al. Prevention of venous thrombosis in patients with acute intracerebral hemorrhage. Neurology. 2005;65:865–9. doi: 10.1212/01.wnl.0000176073.80532.a2. [DOI] [PubMed] [Google Scholar]
- Lang EW, Ren Ya Z, et al. Stroke pattern interpretation: the variability of hypertensive versus amyloid angiopathy hemorrhage. Cerbrovasc Dis. 2001;12:121–30. doi: 10.1159/000047691. [DOI] [PubMed] [Google Scholar]
- Leonardi-Bee J, Bath PM, et al. Blood pressure and clinical outcomes in the International Stroke Trial. Stroke. 2002;33:1315–20. doi: 10.1161/01.str.0000014509.11540.66. [DOI] [PubMed] [Google Scholar]
- Makris M, Greaves M, et al. Emergency oral anticoagulant reversal: the relative efficacy of infusions of fresh frozen plasma and clotting factor concentrate on correction of the coagulopathy. Thromb Haemost. 1997;77:477–80. [PubMed] [Google Scholar]
- Mayer SA. Ultra-early Hemoastatic therapy for primary intracerebral hemmorhage: a review. Can J Neurol Sci. 2005;S2:S31–7. [PubMed] [Google Scholar]
- Mayer SA, Brun NC, et al. Recombinant activated factor VII for acute intracerebral hemorrhage. NEJM. 2005;352:777–85. doi: 10.1056/NEJMoa042991. [DOI] [PubMed] [Google Scholar]
- Mayer S, Commichau C, et al. Clinical trial of an air-circulating cooling blanket for fever control in critically ill neurologic patients. Neurology. 2001;56:292–8. doi: 10.1212/wnl.56.3.292. [DOI] [PubMed] [Google Scholar]
- Mayer S, Ligenelli A, et al. Perilesional blood flow and edema formation in acute intracerebral hemorrhage. Stroke. 1998;29:1791–8. doi: 10.1161/01.str.29.9.1791. [DOI] [PubMed] [Google Scholar]
- McKinley BA, Parmley CL, et al. Standardized management of intracranial pressure: a preliminary clinical trial. J Trauma. 1999;46:271–9. doi: 10.1097/00005373-199902000-00013. [DOI] [PubMed] [Google Scholar]
- Mendelow AD, Gregson BA, et al. Early surgery versus initial conservative treatment in patients with spontaneous supratentorial intracerebral haemotomas in the International Surgical Trial in Intracerebral Haemmorhage (STICH): a randomized trial. Lancet. 2005;365:387–97. doi: 10.1016/S0140-6736(05)17826-X. [DOI] [PubMed] [Google Scholar]
- Murai Y, Takagi R, et al. Three-dimensional computerized tomography angiography in patients with hyperacute intracerebral hemorrhage. J Neurosurg. 1999;91:424–31. doi: 10.3171/jns.1999.91.3.0424. [DOI] [PubMed] [Google Scholar]
- Naff N, Hanley D, et al. Intraventricular thrombolysis speeds blood clot resolution: results of a pilot, prospective, randomized, double-blind, controlled trial. Neurosurgery. 2004;54:577–83. doi: 10.1227/01.neu.0000108422.10842.60. [DOI] [PubMed] [Google Scholar]
- Naff N, Carhuapoma JR, et al. Treatment of intraventricular hemorrhage with urokinase: effects on 30-Day survival. Stroke. 2000;31:841–7. doi: 10.1161/01.str.31.4.841. [DOI] [PubMed] [Google Scholar]
- National Institute of Health. NIH clinical trials registry [online] 2001. Accessed 26 Sep 2006. URL: http://http://www.clinicaltrials.gov.
- Nau R. Osmotherapy for elevated intracranial pressure: a critical reappraisal. Clinical Pharmokinetics. 2000;38:23–40. doi: 10.2165/00003088-200038010-00002. [DOI] [PubMed] [Google Scholar]
- Ott BR, Zamani A, et al. The clinical spectrum of hemmorhagic infarction. Stroke. 1986;17:630–7. doi: 10.1161/01.str.17.4.630. [DOI] [PubMed] [Google Scholar]
- Ott KH, Kase CS, et al. Cerebellar hemorrhage: diagnosis and treatment a review of 56 cases. Arch Neurol. 1974;31:160–7. doi: 10.1001/archneur.1974.00490390042003. [DOI] [PubMed] [Google Scholar]
- Passero S, Rocchi R, et al. Seizures after spontaneous supratentorial intracerebral hemorrhage. Epilepsia. 2002;43:1175–80. doi: 10.1046/j.1528-1157.2002.00302.x. [DOI] [PubMed] [Google Scholar]
- Poungvarin N, Bhoopat W, et al. Effects of dexamethasone in primary supratentorial intracerebral hemorrhage. NEJM. 1987;316:1229–33. doi: 10.1056/NEJM198705143162001. [DOI] [PubMed] [Google Scholar]
- Powers WJ, Zazulia AR. The use of positron emission tomography in cerebrovascular disease. Neuroimaging Clin N Am. 2003;13:741–58. doi: 10.1016/s1052-5149(03)00091-1. [DOI] [PubMed] [Google Scholar]
- Qureshi AI, Mohammed YM, et al. A prospective multi-center study to evaluate the feasibility and safety of aggressive antihypertensive treatment in patients with acute intracerebral hemorrhage. J Intensive Care Med. 2005;20:34–42. doi: 10.1177/0885066604271619. [DOI] [PubMed] [Google Scholar]
- Qureshi AI, Tuhrim S, et al. Spontaneous intracerebral hemorrhage. N Engl J Med. 2001;344:1450–60. doi: 10.1056/NEJM200105103441907. [DOI] [PubMed] [Google Scholar]
- Rabinstein AA, Atkinson JL, et al. Emergency craniotomy in patients worsening due to expanded cerebral hematoma: to what purpose? Neurology. 2002;58:1367–72. doi: 10.1212/wnl.58.9.1367. [DOI] [PubMed] [Google Scholar]
- Rathore SS, Hinn AR, et al. Characterization of incident stroke signs and symptoms: findings from the atherosclerosis risk in communities study. Stroke. 2002;33:2718–21. doi: 10.1161/01.str.0000035286.87503.31. [DOI] [PubMed] [Google Scholar]
- Ritter MA, Droste DW, et al. Role of cerebral amyloid angiopathy in intracerebral hemorrhage in hypertensive patients. Neurology. 2005;64:1233–7. doi: 10.1212/01.WNL.0000156522.93403.C3. [DOI] [PubMed] [Google Scholar]
- Roquer J, Rodriguez CA, et al. Previous antiplatelet therapy is an independent predictor of 30-day mortality after spontaneous supratentorial intracerebral hemorrhage. J Neurol. 2005;252:412–16. doi: 10.1007/s00415-005-0659-5. [DOI] [PubMed] [Google Scholar]
- Rosand J, Greenberg SM. Cerebral amyloid angiopathy. Neurologist. 2000;6:315–25. [Google Scholar]
- Saloheimo P, Ahonen M, et al. Regular aspirin-use preceding the onset of primary intracerebral hemorrhage is an independent predictor for death. Stroke. 2006;37:129–33. doi: 10.1161/01.STR.0000196991.03618.31. [DOI] [PubMed] [Google Scholar]
- Schwarcz S, Hafner K, et al. Incidence and prognostic significance of fever following intracerebral hemorrhage. Neurology. 2001;54:354–61. doi: 10.1212/wnl.54.2.354. [DOI] [PubMed] [Google Scholar]
- Smith EE, Eicher F. Cerebral amyloid angiopathy and lobar intracerebral hemorrhage. Arch Neurol. 2006;63:148–51. doi: 10.1001/archneur.63.1.148. [DOI] [PubMed] [Google Scholar]
- Steiner T, Rosand J, et al. Intracerebral hemorrhage associated with oral anticoagulant therapy. Stroke. 2006;37:256–62. doi: 10.1161/01.STR.0000196989.09900.f8. [DOI] [PubMed] [Google Scholar]
- Sutherland GR, Auer RN. Primary intracerebal hemorrhage. J Clin Neuroscience. 2006;13:511–17. doi: 10.1016/j.jocn.2004.12.012. [DOI] [PubMed] [Google Scholar]
- Teernstra OP, Evers SM, et al. Stereotactic treatment of intracerebral hematoma by means of a plasminogen activator: a multicenter randomized controlled trial (SICHPA) Stroke. 2003;34:968–74. doi: 10.1161/01.STR.0000063367.52044.40. [DOI] [PubMed] [Google Scholar]
- Tellez H, Bauer RB. Dexamethasone as treatment in cerebrovascular disease. 1. A controlled study in intracerebral hemorrhage. Stroke. 1973;4:541–6. doi: 10.1161/01.str.4.4.541. [DOI] [PubMed] [Google Scholar]
- Temkin NR. Antiepileptogenesis and seizure prevention trials with antiepileptic drugs: meta-analysis of controlled trials. Epilepsia. 2001;42:515–24. doi: 10.1046/j.1528-1157.2001.28900.x. [DOI] [PubMed] [Google Scholar]
- Terayama Y, Tanahashi N, et al. Prognostic value of admission blood pressure in patients with intracerebral hemorrhage. Stroke. 1997;28:1185–8. doi: 10.1161/01.str.28.6.1185. [DOI] [PubMed] [Google Scholar]
- Toyoda K, Okado Y, et al. Antiplatelet therapy contributes to acute deterioration of intracerebral hemorrhage. Neurology. 2005;65:1000–4. doi: 10.1212/01.wnl.0000179178.37713.69. [DOI] [PubMed] [Google Scholar]
- Tuhrim S, Dambrosia JM, et al. Prediction of intracerebral hemorrhage survival. Ann Neurol. 1988;24:258–63. doi: 10.1002/ana.410240213. [DOI] [PubMed] [Google Scholar]
- Vemmos KN, Tsivgoulis G, et al. U-shaped relationship between mortality and admission blood pressure in patients with acute stroke. J Intern Med. 2004;255:257–65. doi: 10.1046/j.1365-2796.2003.01291.x. [DOI] [PubMed] [Google Scholar]
- Vespa P, McArthur D, et al. Frameless stereotactic aspiration and thrombolysis of deep intracerebral hemorrhage is associated with reduction of hemorrhage volume and neurological improvement. Neurocrit Care. 2005;2:274–81. doi: 10.1385/NCC:2:3:274. [DOI] [PubMed] [Google Scholar]
- Wakefield TW, Stanley JC. Intraoperative heparin anticoagulation and its reversal. Semin Vasc Surg. 1996;9:296–302. [PubMed] [Google Scholar]
- Wintermark MUA, Chaalaron M, et al. Multislice computerized tomopgraphy angiography in the evaluation of intracranial aneurysms: a comparison with intrarterial digital subtraction angiography. J Neurosurg. 2003;98:828–36. doi: 10.3171/jns.2003.98.4.0828. [DOI] [PubMed] [Google Scholar]
- Zhu XL, Chan MS, Poon WS. Spontaneous intracranial hemorrhage: which patients need diagnostic cerebral angiography? A prospective study of 206 cases and review of the literature. Stroke. 1997;28:1406–9. doi: 10.1161/01.str.28.7.1406. [DOI] [PubMed] [Google Scholar]


