Abstract
Type 2 diabetes is widespread and its prevalence is increasing rapidly. In the US alone, approximately 41 million individuals have prediabetes, placing them at high risk for the development of diabetes. The pathogenesis of type 2 diabetes involves inadequate insulin secretion and resistance to the action of insulin. Suggestive data link insulin resistance and accompanying hyperglycemia to an excess of abdominal adipose tissue, a link that appears to be mediated partially by adipocyte secretion of multiple adipokines that mediate inflammation, thrombosis, atherogenesis, hypertension, and insulin resistance. The adipokine adiponectin has reduced expression in obesity and appears to be protective against the development of type 2 diabetes. Current recommendations to prevent type 2 diabetes center on lifestyle modifications, such as diet and exercise. Clinical trials have established the efficacy of lifestyle intervention, as well as pharmacologic interventions that target glycemic control or fat metabolism. However, diabetes did develop in a substantial percentage of individuals who received intensive intervention in these trials. Thus there is an unmet need for additional strategies in high-risk individuals. Recent data suggest thiazolidinediones and blockade of the endocannabinoid system represent novel therapeutic approaches that may be used for the prevention of diabetes.
Keywords: cardiometabolic risk, abdominal obesity, dyslipidemia, diabetes, insulin resistance, endocannabinoid system
Burden of diabetes
Type 2 diabetes is the most common metabolic disorder worldwide (Goldstein 2003), and its prevalence is growing at an alarming rate in both developed and developing countries (Wild et al 2004; Yach et al 2006). This growth has been related to the increased prevalence of obesity (defined as body mass index [BMI] ≥30 kg/m2), a primary driver in the development of type 2 diabetes, as well as an independent health problem (Centers for Disease Control and Prevention 2005; Diabetes Research Working Group 1999; Yach et al 2006). Moreover, an estimated 41 million people in the US currently have prediabetes, defined as impaired fasting glucose (IFG) or impaired glucose tolerance (IGT). These individuals have a high risk for the development of diabetes (Centers for Disease Control and Prevention 2006).
Type 2 diabetes is associated with considerable morbidity and mortality, which can lead to substantial personal and societal costs (Yach et al 2006). In 2002, in the US alone, direct and indirect costs attributable to diabetes were estimated at US$132 billion by the American Diabetes Association (ADA) (Hogan et al 2003). This estimate does not include many intangible costs, such as pain and suffering. Cardiovascular disease (CVD) is the leading cause of death among diabetics, and is responsible for much of the increase in diabetes-related morbidity and mortality. CVD-related mortality is 2–4 times higher among diabetics (Centers for Disease Control and Prevention 2005). Atherosclerosis, hypertension, and stroke are common problems affecting individuals with diabetes, all of which correlate highly with the presence of obesity (Centers for Disease Control and Prevention 2005; Glendening et al 2005).
A cluster of interrelated cardiometabolic risk factors is closely related to the development of type 2 diabetes and cardiovascular disease. Current views suggest that cardiometabolic risk factors represent a continuum of disease risks – not merely the presence or absence of a distinct disease entity (Eckel et al 2006). Obesity, hyperglycemia and insulin resistance, dyslipidemia, inflammation, and hypertension represent interrelated therapeutic targets in the battle against the increasing prevalence of type 2 diabetes (Eckel et al 2006).
Obesity, insulin resistance, and progression to diabetes
The classification and pathogenesis of type 2 diabetes involves abnormalities of glucose and lipid metabolism, including inadequate insulin secretion from pancreatic β cells and resistance to the action of insulin (ADA 2006; Goldstein 2003). There is epidemiologic and physiologic evidence linking insulin resistance and hyperglycemia (which precedes and characterizes the development of type 2 diabetes) to the presence of abdominal obesity (Diabetes Research Working Group 1999; Sharma 2006). An association between intra-abdominal adipose tissue and insulin resistance has been demonstrated in animal models and in human subjects (Raz et al 2005), and increased abdominal adipose tissue greatly increases the risk of developing IGT and insulin resistance in individuals with normal glucose tolerance at baseline (Hayashi et al 2003).
The underlying mechanisms involve the increased flux of free fatty acids (FFAs) to the liver, pancreas, and other tissues, and subsequent deposition of triglycerides (TG) (Lewis et al 2002; Raz et al 2005). This process is related to excessive release by adipose tissue of assorted bioactive substances known as adipokines (Chandran et al 2003), the combined actions of which trigger a chronic inflammatory state that contributes to the development of insulin resistance (Xu et al 2003). Elevated circulating FFA levels cause tissues to become resistant to the action of insulin. Hyperinsulinemia results as a compensatory mechanism to maintain glucose tolerance under these conditions, a situation that can lead to pancreatic β cell damage and the development or worsening of glucose tolerance and diabetes (Lewis et al 2002; Zraika et al 2002; Goldstein 2003; Haber et al 2003; Raz et al 2005). In addition to adverse pancreatic effects, the excess glucose and fat in the blood can lead to additional organ and vascular damage, which underlies much of the morbidity and mortality associated with diabetes (Deedwania and Fonseca 2005; Centers for Disease Control and Prevention 2006).
The mechanisms by which abdominal obesity contribute to cardiometabolic risk appear to involve the endocrine activity of adipose tissue (Kershaw and Flier 2004). Adipose tissue and specifically abdominal adipose cells secrete a number of cytokines and adipokines that can have deleterious cardiometabolic effects. These include effects on glucose control, lipid profile, increased thrombotic risk, and increased inflammatory state (Lewis et al 2002; Goldstein 2003). Secreted substances include (but are not limited to) C-reactive protein (CRP), a marker of inflammation and cardiovascular risk (Berg and Scherer 2005; Natali et al 2006), and the inflammatory cytokines interleukin-6 (IL-6) and tumor necrosis factor-alpha (TNF-α) (Matsuzawa 2006; Natali et al 2006; Sharma 2006). These cytokines in turn induce expression of the adipocyte-derived secretory protein resistin, which has been implicated in induction of the inflammatory cascade that contributes to the development of insulin resistance (Lehrke et al 2004). Additional adipokines that play a role in cardiometabolic risk include the thrombotic and fibrinolytic factors plasminogen-activator inhibitor type 1 (PAI-1) and fibrinogen (Matsuzawa 2006; Natali et al 2006; Sharma 2006), and components of the renin-angiotensin system (RAS) that are involved in the pathogenesis of hypertension, endothelial dysfunction, and the development of insulin resistance (Reaven et al 1996; Caglayan et al 2005; Sharma 2006).
Adiponectin, a serum protein synthesized exclusively by adipocytes, plays a role in cardiometabolic pathology (Scherer et al 1995; Hu et al 1996). This adipokine is a modulator of insulin sensitization, lipid metabolism, and inflammatory states (Chandran et al 2003; Kadowaki et al 2006; Matsuzawa 2006). In contrast to many of the inflammatory adipokines related to atherogenesis, and induction of insulin resistance, adiponectin has reduced expression under conditions of abdominal obesity, type 2 diabetes, and insulin resistance (Weyer et al 2001; Chandran et al 2003; Schulze et al 2004; Schulze et al 2005). Increased adiponectin levels are associated with lower hyperglycemia, dyslipidemia, and inflammation in diabetic individuals, and appear protective against the development of type 2 diabetes in individuals at risk (Lindsay et al 2002; Krakoff et al 2003; Spranger et al 2003). Low adiponectin levels are independently predictive of eventual type 2 diabetes even in apparently healthy (non-obese) individuals (Lindsay et al 2002; Spranger et al 2003) and in patients with coronary artery disease and IFG (Knobler et al 2006). Genetic variability is attributable to plasma adiponectin levels and may be an independent cardiovascular risk factor in diabetic individuals (Qi et al 2006). Although the mechanisms underlying the protective role of adiponectin are under investigation, several models have been proposed. These include adiponectin-mediated modification of the insulin receptor in skeletal muscle, leading to enhanced signaling; increased FFA oxidation in skeletal muscle and liver, leading to a decrease in FFA flux; and decreased vascular inflammation through adiponectin-mediated effects on monocyte adhesion and on vascular proliferation of smooth muscle cells (Chandran et al 2003). Abdominal obesity and resultant adipokine dysregulation may be potential therapeutic targets through lifestyle and/or pharmacologic approaches aimed at delaying or preventing the onset of type 2 diabetes in high-risk individuals.
Preventing the progression or onset of diabetes
Prediabetes (or IGT) is a major risk factor for diabetes, as are obesity, physical inactivity, and insulin resistance. It is well established that the risk for development or progression of type 2 diabetes can be modified through lifestyle changes and/or pharmacotherapy. A number of well-designed clinical trials evaluating different strategies to prevent or delay type 2 diabetes have shown that lifestyle modifications and/or therapy with glucose-lowering agents used to treat this form of diabetes can significantly prevent or delay its onset in individuals with IGT, irrespective of obesity (Sherwin et al 2003; Klein et al 2004). The results of major, randomized, controlled clinical studies on the prevention of type 2 diabetes are summarized in Figure 1.
Figure 1.
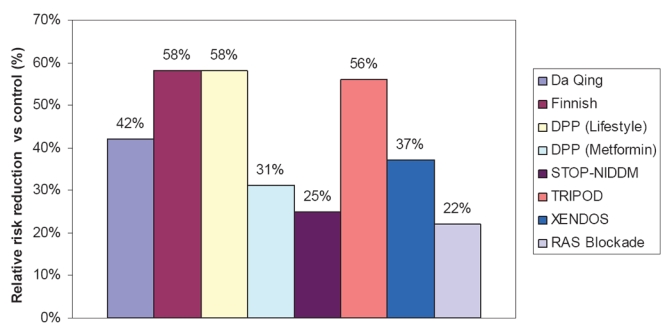
Summary of the relative risk reduction (%) of new-onset type 2 diabetes in randomized, controlled clinical trials of prevention. Studies shown are the Da Qing IGT and Diabetes Study, which evaluated diet and exercise (Pan et al 1997); the Finnish Diabetes Prevention Study, which evaluated diet and exercise (Tuomilehto et al 2001); the Diabetes Prevention Program (DPP) studies, which evaluated diet and exercise or metformin (Knowler et al 2002); the Study To Prevent Non-Insulin-Dependent Diabetes Mellitus (STOP-NIDDM), which evaluated acarbose; Troglitazone in Prevention of Diabetes (TRIPOD), which evaluated Troglitazone; XENical in the Prevention of Diabetes in Obese Subjects (XENDOS), which evaluated the gastrointestinal lipase inhibitor orlistat (Torgerson et al 2004); and a meta-analysis of 10 trials of RAS blockade with either ACE inhibitors or ARBs (Scheen 2004a). (See text for study details.)
Intensive lifestyle modification
The Da Qing IGT and Diabetes Study (Pan et al 1997) was an early long-term trial evaluating the impact of diet and/or exercise on the development of type 2 diabetes in more than 110,660 individuals with IGT. After 6 years, the cumulative incidence of diabetes was 68% in the control group, compared with 44% in the diet group, 41% in the exercise group, and 46% in the diet-plus-exercise group. The benefit of lifestyle modification was similar for the lean and overweight subgroups.
The Finnish study (Tuomilehto et al 2001) compared the impact of lifestyle modification involving intensive individualized diet counseling and increased physical activity (intervention group) with that of brief diet and exercise counseling (control group) in 522 obese males with IGT. After an average follow-up of 3.2 years, there was a 58% relative reduction in the incidence of diabetes in the intervention group compared with the control group. There was a clear correlation between diabetes risk reduction and the extent to which weight and activity goals were achieved. The impact of lifestyle intervention on insulin sensitivity and secretion, as measured using frequently sampled intravenous glucose tolerance testing, was evaluated after 4 years in a subset of these patients (Uusitupa et al 2003). There was a strong correlation between 4-year changes in insulin sensitivity and changes in weight. Insulin sensitivity improved by 64% among patients in the highest tertile of weight loss (a loss of −8% to −17%) and deteriorated by 24% among patients who gained weight (weight change of −1.4% to +10%). The acute insulin response declined significantly among patients in the control group (ie, no intensive lifestyle modification). Importantly, insulin secretion remained stable (ie, did not worsen) among patients with IGT at baseline who were able to lose weight.
Intensive lifestyle modification and/or pharmacologic intervention with glucose-lowering agents
The Diabetes Prevention Program (DPP) is one of the largest and most extensive ongoing clinical trials evaluating this issue (Knowler et al 2002). Intensive nutrition and exercise counseling, metformin therapy, and placebo were compared among 3234 obese individuals with IGT in a randomized, controlled format. The latter 2 interventions were combined with standard diet and exercise recommendations. Following the 2.8-year follow-up, there was a 58% relative reduction in the progression to diabetes with lifestyle modification (compared with controls), and a 31% relative reduction in progression in the metformin group.
Pharmacologic intervention with glucose-lowering agents
Thiazolinedione (troglitazone) also has been evaluated as a preventive agent. The TRIPOD study monitored 236 Hispanic women with a history of previous gestational diabetes who were randomized to receive either troglitazone or placebo (Buchanan et al 2002). After the median follow-up of 30 months, a 56% relative reduction in progression to diabetes in the troglitazone group was noted. Protection from diabetes was associated with a preservation of pancreatic β cell compensation for insulin resistance, as measured by acute insulin response to intravenous glucose administration and whole-body insulin sensitivity. The DPP group conducted a long-term comparison of treatment with metformin, troglitazone, placebo, or intensive lifestyle intervention in 2343 patients with IGT (Knowler et al 2005). During the study, concerns arose over the potential liver toxicity of troglitazone, leading to discontinuation of this study arm and the withdrawal of troglitazone from clinical use. Prior to troglitazone discontinuation (mean time of 0.9 years), the incidence of diabetes was lower (3.0 cases per 100 person-years) than that observed for individuals receiving placebo, metformin, or intensive lifestyle intervention (12.0, 6.7, and 5.1 cases per 100 person-years, respectively). This lower incidence was statistically significant for the troglitazone-treated group versus both the placebo and metformin groups, but was not significant in comparison with lifestyle intervention (p < 0.001, p = 0.02, and p = 0.18, respectively). This protective effect observed in the troglitazone group persisted for 8 months after discontinuation of the study drug.
The Study To Prevent Non-Insulin-Dependent Diabetes Mellitus (STOP-NIDDM) was a randomized, placebo-controlled trial designed to evaluate the α-glucosidase inhibitor acarbose in the prevention or delay of type 2 diabetes in 1368 individuals with IGT (Chiasson et al 2002). All patients were instructed to follow a weight-reducing or maintaining diet and were encouraged to exercise regularly. After the mean follow-up period of 3.3 years, diabetes had developed in 32% of patients treated with acarbose and in 42% of placebo subjects, yielding a relative hazard rate of 0.75 with acarbose treatment (p = 0.0015). Acarbose also significantly increased reversion of IGT to normal glucose tolerance (p < 0.0001). This treatment generally was well tolerated; the most common adverse events that occurred during acarbose treatment were gastrointestinal effects such as flatulence and diarrhea.
The DREAM (Diabetes REduction Assessment with ramipril and rosiglitazone Medication) trial prospectively assessed whether treatment with the thiazolidinedione rosiglitazone could reduce the frequency of diabetes in 5269 individuals with IGT, IFG, or both (Gerstein et al 2006). All participants were given information on diet and lifestyle modifications to delay or prevent diabetes. After the median follow-up of 3 years, diabetes or death was the outcome for 11.6% of rosiglitazone recipients and 26.0% of placebo subjects (p < 0.0001). The incidence of regression to normoglycemia (defined as 2-hour and fasting plasma glucose concentrations of <7.8 mmol/L and <6.1 mmol/L, respectively) was significantly greater in the rosiglitazone group (p < 0.0001 vs placebo).
Pharmacologic intervention with lipase inhibitor
The XENical in the Prevention of Diabetes in Obese Subjects (XENDOS) trial was a 4-year, randomized, double-blind, placebo-controlled study conducted in 3,305 obese individuals to evaluate the efficacy of lifestyle changes in conjunction with either the gastrointestinal lipase inhibitor orlistat or placebo in preventing or delaying type 2 diabetes (Torgerson et al 2004). Only 21% of patients had IGT at the time of study entry. After 4 years of treatment, a 37% reduction in the risk of developing diabetes was noted for the orlistat group (cumulative incidence: 6% and 9% with orlistat and placebo, respectively; p = 0.0032). This risk reduction was accompanied by a significantly greater mean weight loss in the orlistat group compared with placebo (5.8 vs 3.0 kg, respectively; p < 0.001). Further analysis suggested that the reduced risk of developing diabetes could be explained by an effect on the group with IGT at baseline.
Underlying mechanisms of prevention
A number of studies have identified mechanistic bases for the benefits of lifestyle and/or pharmacologic intervention in the delay or prevention of diabetes. As expected, the benefits appear to be mediated by effects on multiple potentially causative emerging risk factors related to inflammation, adipokine dysregulation, fibrinolysis, insulin resistance, and glucose metabolism. For example, lifestyle intervention in subjects participating in the Finnish Diabetes Prevention Study was associated with decreased expression of PAI-1 and fibrinogen, both mediators of fibrinolysis (Hamalainen et al 2005).
The thiazolidinediones troglitazone, rosiglitazone, and pioglitazone (Ghanim et al 2006; Samaha et al 2006; Szapary et al 2006) have early anti-inflammatory effects, which are associated with decreased lipolysis and FFA flux, improved insulin signaling and sensitization, increased adiponectin expression, and improved lipid profiles (eg, elevated high-density lipoprotein [HDL] cholesterol levels and favorable changes in lipoprotein particle size). These beneficial effects of thiazolidinediones on cardiometabolic risk factors have been observed in obese and non-obese patients with type 2 diabetes (Chiquette et al 2004; Ghanim et al 2006) and in nondiabetic patients with metabolic syndrome (Samaha et al 2006; Szapary et al 2006). This interrelationship of factors confirms the concept of metabolic risk being a continuum of disease processes and not merely the absence or presence of one or all of these cardiometabolic risk factors.
Renin-angiotensin blockade
Large randomized clinical trials have demonstrated that blockade of the RAS with angiotensin-converting enzyme (ACE) inhibition or angiotensin receptor blockers (ARBs) can reduce the incidence of new-onset type 2 diabetes. A recent meta-analysis of 10 randomized clinical trials involving more than 76,000 patients assessed the potential prevention of diabetes through RAS blockade (Scheen 2004a), including 5 trials of ACE inhibitors (enalapril, lisinopril, captopril, and ramipril) and 4 of ARBs (losartan, candesartan, and valsartan). Among all these trials, new cases of type 2 diabetes were found in 7.4% of patients treated with an ACE inhibitor or ARB, compared with 9.6% of control patients. This corresponded to a 22% relative risk reduction of developing type 2 diabetes (p < 0.00001). Despite these positive findings on the preventive effects of ACE inhibitors, it was recently reported that ramipril (included as a treatment arm in the DREAM study) failed to reduce the incidence of the primary outcome (diabetes or death) among participants with IFG or IGT over the median 3-year treatment period (Bosch et al 2006). However, individuals receiving ramipril in this study were more likely to have normal fasting glucose levels or glucose tolerance at study endpoint than those receiving placebo. This suggests that the study may not have had adequate power to discern a difference; a longer or larger study might be required for detecting an effect of the ACE inhibitor on the incidence of diabetes.
The mechanisms involved in the actions of ACE inhibitors and ARBs in diabetes prevention are not fully understood. The multiple physiologic actions of angiotensin II may in part explain this complexity (Deedwania and Fonseca 2005). It is likely that the beneficial actions of RAS blockade involve improvement in both insulin sensitivity and insulin secretion through the impact of multiple mediators on insulin action and receptor signaling, muscle pancreatic islet blood flow, sympathetic nervous activity, adipokine production, and lipid metabolism (Scheen 2004b). For example, it was recently shown that losartan improves insulin sensitivity by increasing adiponectin production and decreasing TNF-α production (Park et al 2006). There is also evidence that elevated angiotensin II produced by large insulin-resistant adipocytes may inhibit the recruitment of pre-adipocytes, resulting in increased lipid storage in muscle and decreased insulin sensitivity (Sharma et al 2002).
Impact of dietary choice
Both large randomized trials (The Finnish and DPP studies) and many smaller ones have demonstrated a significant impact of intensive lifestyle modification using conventional dietary recommendations for overweight or obese persons at risk for the development of diabetes; such diets are low in saturated fats and total fat intake, and rich in complex carbohydrates (Knowler et al 2002; Tuomilehto et al 2001; Klein et al 2004). However, there has been growing interest in low-carbohydrate diets as therapy for obesity. Overall, recent prospective trials indicate similar weight-loss efficacy over the long term with low-fat and carbohydrate-restricted diets (Samaha 2005). The combined results of 5 recent randomized controlled trials indicate that subjects receiving a low-carbohydrate, high-fat diet achieved greater short- but not long-term weight loss than those receiving a conventional diet (Klein et al 2004). However, some data suggest that high-fat diets, while having similar effects on weight, may have more favorable effects on lipid profile and glycemic status in obese patients. In a 6-month study in 79 severely obese patients with a high prevalence of metabolic syndrome or diabetes, a carbohydrate-restricted diet was associated with relative improvement in insulin sensitivity and TG levels compared with a low-fat diet (Samaha et al 2003). A carbohydrate-restricted diet also was associated with more favorable effects on lipoprotein subfractions and on inflammation as measured by CRP levels in these patients (Seshadri et al 2004). In a 1-year follow-up of the latter study, weight loss was still similar for the 2 groups, and effects on atherogenic dyslipidemia and glycemia had remained more favorable in the group on a low-carbohydrate diet (Stern et al 2004). Although these findings are compelling, outcomes studies evaluating the impact of carbohydrate-restricted diet on diabetes development and other cardiovascular outcomes are needed before recommendations regarding such a diet are warranted.
Drugs with potential application to prevention
Pharmacologic intervention enhancing incretin action
The incretin hormone glucagon-like peptide-1 (GLP-1) has demonstrated a variety of antidiabetic effects, including stimulation of insulin secretion, inhibition of glucagon secretion, delay of gastric emptying, suppression of appetite, promotion of β cell proliferation, and inhibition of β cell apoptosis (Drucker 2006). Native GLP-1 has limited clinical use because it is rapidly inactivated by the enzyme dipeptidyl peptidase-4 (DPP-4), resulting in a half-life of <2 minutes. To overcome this obstacle, GLP-1 analogues have been developed with low affinity for DPP-4 as well as inhibitors of DPP-4. The GLP-1 mimetic exenatide and the DPP-4 inhibitor sitagliptin are now available for clinical use. Preclinical evidence suggests that exenatide may delay onset or prevent development of diabetes (Wang and Brubaker 2002; Stoffers et al 2003), although these findings need confirmation in clinical studies.
Exenatide has demonstrated efficacy in randomized controlled studies when used as adjunct therapy in patients with type 2 diabetes and inadequate glycemic control while receiving metformin (n = 336) (DeFronzo et al 2005), a sulfonylurea (n = 377) (Buse et al 2004), or metformin/sulfonylurea (n = 733) (Kendall et al 2005). The recommended dosages are 5 or 10 µg subcutaneously, twice daily. In addition to improving glycemic control, treatment with exenatide for 30 weeks reduced body weight by 0.9–1.6 kg with the lower dose and by 1.6–2.8 kg with the higher dose, compared with reductions of 0.3–0.9 kg in placebo recipients (Buse et al 2004; DeFronzo et al 2005; Kendall et al 2005). In each study, dose-related nausea was the most common adverse event in exenatide-treated patients (low dose, 36%–39%; high dose, 45%–51%). No correlation between change in body weight and nausea was observed in any of the studies.
Sitagliptin was recently approved as monotherapy, or in combination with metformin or a peroxisome proliferator-activated receptor gamma agonist, to improve glycemic control in patients with type 2 diabetes. The recommended oral dosage of sitagliptin is 100 mg once daily, with dosage adjustments required for patients with moderate or severe end-stage renal disease. Pharmacokinetic studies support once-daily dosing and show a lack of interaction between sitagliptin and metformin (Bergman et al 2006; Herman et al 2005, 2006). Based on prescribing information, treatment with sitagliptin improves glycemic control (Januvia™ prescribing information 2006). No significant effects on body weight were reported, although the duration of treatment (up to 24 weeks) was shorter than that for exenatide. The most common adverse events were nasopharyngitis (5%) in patients receiving sitagliptin alone, and upper respiratory tract infection (6%) and headache (5%) in patients receiving sitagliptin/pioglitazone (Januvia™ prescribing information 2006).
Pharmacologic intervention inhibiting serotonin and norepinephrine reuptake
Numerous clinical studies have been conducted with the serotonin and norepinephrine reuptake inhibitor sibutramine, which is thought to induce weight loss by increasing satiety and possibly energy expenditure (Poston and Foreyt 2004). Sibutramine is indicated for the management of obesity in conjunction with a reduced-calorie diet. It is administered orally at dosages of 5–15 mg once daily. The effects of sibutramine on body weight are well established, as described below. However, no study has assessed whether this agent can delay or prevent the development of type 2 diabetes.
In a large systematic review involving 29 randomized controlled trials of obese patients who were healthy (n = 3604) or who had type 2 diabetes (n = 654), hyperlipidemia (n = 341), hypertension (n = 340), or obstructive sleep apnea (n = 40), sibutramine 10–20 mg/day was associated with a placebo-subtracted mean weight loss of 2.8 kg in 8- to 12-week studies, 5.16 kg in 16- to 24-week studies, and 4.5 kg in 44- to 54-week studies (Arterburn et al 2004). No dose-response was observed. Body-weight reductions were consistent across the different study populations. A recent meta-analysis of 8 randomized, double-blind, placebo-controlled trials, which was restricted to obese patients with type 2 diabetes (n = 1093), yielded similar benefits with regard to body-weight reductions (Vettor et al 2005). In both analyses, cardiovascular and metabolic findings were more modest and included increases in heart rate and blood pressure, improvements in triglyceride and HDL cholesterol levels and, among patients with type 2 diabetes, improvements in glycemic control (Arterburn et al 2004; Vettor et al 2005). The cardiovascular effects of sibutramine necessitate monitoring of blood pressure and heart rate prior to initiating therapy and at regular intervals during therapy; sibutramine should not be used in patients with a history of coronary artery disease, congestive heart failure, arrhythmias, or stroke (Meridia® prescribing information 2006).
Blockade of the endocannabinoid (EC) system
Blockade of the EC system represents a novel therapeutic modality that targets intra-abdominal obesity, glycemic regulation, and related cardiometabolic risk factors. Animal studies have shown that this system plays a role in energy intake and homeostasis as well as excessive food intake (De Petrocellis et al 2004; Di Marzo et al 2004). The EC system is overactivated in obesity (Di Marzo et al 2001), and there is growing evidence that its activity may impact a number of cardiometabolic parameters associated with obesity (Despres et al 2006; John et al 2006).
Promising preclinical findings have led to the development of selective CB1 antagonists for clinical use. Several are currently in development, with one (rimonabant) now in Phase III testing (Antel et al 2006). The efficacy and safety of CB1 blockade have been investigated in patients with obesity, with or without co-morbidities, in a clinical development program (Rimonabant in Obesity; RIO) (Lebovitz et al 2006) involving 4 large trials; the results of 3 trials have been published in full (Despres et al 2005; Van Gaal et al 2005; Pi-Sunyer et al 2006; Scheen et al 2006).
Treatment with rimonabant 20 mg for 1 year induced a consistent reduction in waist circumference and weight in all 4 trials (Despres et al 2005; Van Gaal et al 2005; Pi-Sunyer et al 2006; Scheen et al 2006). Improvements in HDL and TG levels also were observed and, as in the animal studies, much of this effect (approximately 50%) was not attributable to the weight decrease. These benefits were maintained throughout the second year of follow-up.
The potential benefit of rimonabant therapy on glycemic and other cardiometabolic parameters has been investigated in RIO-Diabetes (Scheen et al 2006), which involved 1045 patients with type 2 diabetes. In addition to the significant reduction in weight and waist circumference observed in all RIO studies, rimonabant 20 mg/day for 1 year produced a 0.7% reduction in hemoglobin A1c (HbA1C) versus placebo, with 43% of patients achieving HbA1C levels below 6.5%. Again, much of this effect was independent of weight change. Consistent with the other RIO trials, improvements from baseline in cardiometabolic parameters also were noted, including beneficial effects on HDL cholesterol and TG.
To explore the association between abdominal obesity and the development of diabetes, pooled data from RIO-Europe, RIO-North America, and RIO-Lipids were used to assess the effects of rimonabant on the development of diabetes in obese individuals who were classified with prediabetes at study enrolment (Rosenstock 2005). Prediabetes, or IFG, was defined as fasting glucose levels >5.6 mmol/L (100 mg/dL) and <7 mmol/L (126 mg/dL). This analysis involved 1290 patients participating in the 3 studies. Significant reductions were observed for fasting insulin levels (−2.7 MIU/mL; p < 0.001 vs placebo) and for homeostasis model assessment of insulin resistance (HOMA-IR) (−0.8%; p = 0.002 vs placebo). A trend toward halting or delaying the progression of IFG was suggested by the greater percentage of patients whose fasting glucose level became normal (46% vs 39% for rimonabant 20 mg vs placebo, respectively). In addition, a smaller percentage of rimonabant recipients progressed to type 2 diabetes (3.6% vs 4.9% for rimonabant 20 mg vs placebo, respectively); however, statistical analyses of these comparisons were not provided. The potential of rimonabant to delay or prevent the onset of type 2 diabetes is undergoing more extensive study in a clinical trial of patients with prediabetes (US National Institutes of Health 2006).
Conclusion
Although lifestyle intervention has proven successful in clinical trials, there are barriers to its long-term success for many individuals, such as cost, communication with healthcare providers, and the difficulty in maintaining substantial lifestyle changes long term (Vijan et al 2005). In the DPP studies, 49% and 74% of lifestyle participants met their weight loss and activity goals, respectively, at the end of 6 months; however, the success rates declined to 37% and 67%, respectively, over the 3.2-year follow-up period (Wing et al 2004). Despite the reduced risk that accompanies even modest weight loss (eg, 5% of body weight) (Klein et al 2004), diabetes developed in a substantial number of patients with active intervention in the randomized clinical trials (Figure 1). There is a current medical need for additional preventive intervention strategies in overweight and obese patients, particularly those at high risk for the development of type 2 diabetes.
Current guidelines do not advocate specific preventive pharmacologic therapy for prediabetes, although aggressive therapy for patients with cardiovascular risk factors (eg, hypertension) is recommended and has been beneficial in preventing type 2 diabetes in these patients. More aggressive preventive therapy, the adoption of novel therapeutic approaches, and possibly modification of current treatment guidelines in patients at high risk should be considered as strategies to reduce the growing burden of type 2 diabetes.
Acknowledgments
Funding for editorial support was provided by Sanofi-Aventis US.
References
- American Diabetes Association. Standards of medical care in diabetes- – 2006. Diabetes Care. 2006;29(Suppl 1):42. [PubMed] [Google Scholar]
- Antel J, Gregory PC, Nordheim U. CB1 cannabinoid receptor antagonists for treatment of obesity and prevention of comorbid metabolic disorders. J Med Chem. 2006;49:4008–16. doi: 10.1021/jm058238r. [DOI] [PubMed] [Google Scholar]
- Arterburn DE, Crane PK, Veenstra DL. The efficacy and safety of sibutramine for weight loss: a systematic review. Arch Intern Med. 2004;164:994–1003. doi: 10.1001/archinte.164.9.994. [DOI] [PubMed] [Google Scholar]
- Berg AH, Scherer PE. Adipose tissue, inflammation, and cardiovascular disease. Circ Res. 2005;96:939–49. doi: 10.1161/01.RES.0000163635.62927.34. [DOI] [PubMed] [Google Scholar]
- Bergman AJ, Stevens C, Zhou Y, et al. Pharmacokinetic and pharmacodynamic properties of multiple oral doses of sitagliptin, a dipeptidyl peptidase-IV inhibitor: a double-blind, randomized, placebo-controlled study in healthy male volunteers. Clin Ther. 2006;28:55–72. doi: 10.1016/j.clinthera.2006.01.015. [DOI] [PubMed] [Google Scholar]
- Bosch J, Yusuf S, Gerstein HC, et al. Effect of ramipril on the incidence of diabetes. N Engl J Med. 2006;355:1551–62. doi: 10.1056/NEJMoa065061. [DOI] [PubMed] [Google Scholar]
- Buchanan TA, Xiang AH, Peters RK, et al. Preservation of pancreatic beta-cell function and prevention of type 2 diabetes by pharmacological treatment of insulin resistance in high-risk hispanic women. Diabetes. 2002;51:2796–803. doi: 10.2337/diabetes.51.9.2796. [DOI] [PubMed] [Google Scholar]
- Buse JB, Henry RR, Han J, et al. Effects of exenatide (exendin-4) on glycemic control over 30 weeks in sulfonylurea-treated patients with type 2 diabetes. Diabetes Care. 2004;27:2628–35. doi: 10.2337/diacare.27.11.2628. [DOI] [PubMed] [Google Scholar]
- Caglayan E, Blaschke F, Takata Y, et al. Metabolic syndrome-interdependence of the cardiovascular and metabolic pathways. Curr Opin Pharmacol. 2005;5:135–42. doi: 10.1016/j.coph.2005.01.008. [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention. National diabetes fact sheet: general information and national estimates on diabetes in the United States, 2005. Atlanta, GA: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention; 2005. [Google Scholar]
- Centers for Disease Control and Prevention. Diabetes: Disabling, deadly, and on the rise. U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, Coordinating Center for Health Promotion; 2006. [Google Scholar]
- Chandran M, Phillips SA, Ciaraldi T, et al. Adiponectin: more than just another fat cell hormone? Diabetes Care. 2003;26:2442–50. doi: 10.2337/diacare.26.8.2442. [DOI] [PubMed] [Google Scholar]
- Chiasson JL, Josse RG, Gomis R, et al. Acarbose for prevention of type 2 diabetes mellitus: the STOP-NIDDM randomised trial. Lancet. 2002;359:2072–7. doi: 10.1016/S0140-6736(02)08905-5. [DOI] [PubMed] [Google Scholar]
- Chiquette E, Ramirez G, Defronzo R. A meta-analysis comparing the effect of thiazolidinediones on cardiovascular risk factors. Arch Intern Med. 2004;164:2097–104. doi: 10.1001/archinte.164.19.2097. [DOI] [PubMed] [Google Scholar]
- De Petrocellis L, Cascio MG, Di MV. The endocannabinoid system: a general view and latest additions. Br J Pharmacol. 2004;141:765–74. doi: 10.1038/sj.bjp.0705666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deedwania PC, Fonseca VA. Diabetes, prediabetes, and cardiovascular risk: shifting the paradigm. Am J Med. 2005;118:939–47. doi: 10.1016/j.amjmed.2005.05.018. [DOI] [PubMed] [Google Scholar]
- DeFronzo RA, Ratner RE, Han J, et al. Effects of exenatide (exendin-4) on glycemic control and weight over 30 weeks in metformin-treated patients with type 2 diabetes. Diabetes Care. 2005;28:1092–100. doi: 10.2337/diacare.28.5.1092. [DOI] [PubMed] [Google Scholar]
- Despres JP, Golay A, Sjostrom L. Effects of rimonabant on metabolic risk factors in overweight patients with dyslipidemia. N Engl J Med. 2005;353:2121–34. doi: 10.1056/NEJMoa044537. [DOI] [PubMed] [Google Scholar]
- Despres JP, Lemieux I, Almeras N. Contribution of CB1 blockade to the management of high-risk abdominal obesity. Int J Obes (Lond) 2006;30(Suppl 1):S44–S52. doi: 10.1038/sj.ijo.0803278. [DOI] [PubMed] [Google Scholar]
- Di Marzo V, Bifulco M, De Petrocellis L. The endocannabinoid system and its therapeutic exploitation. Nat Rev Drug Discov. 2004;3:771–84. doi: 10.1038/nrd1495. [DOI] [PubMed] [Google Scholar]
- Di Marzo V, Goparaju SK, Wang L, et al. Leptin-regulated endocannabinoids are involved in maintaining food intake. Nature. 2001;410:822–5. doi: 10.1038/35071088. [DOI] [PubMed] [Google Scholar]
- Diabetes Research Working Group. NIH Publication No. 99–4398. National Institutes of Health; 1999. Conquering diabetes. A strategic plan for the 21st century. [Google Scholar]
- Drucker DJ. The biology of incretin hormones. Cell Metab. 2006;3:153–65. doi: 10.1016/j.cmet.2006.01.004. [DOI] [PubMed] [Google Scholar]
- Eckel RH, Kahn R, Robertson RM, et al. Preventing cardiovascular disease and diabetes: a call to action from the American Diabetes Association and the American Heart Association. Circulation. 2006;113:2943–6. doi: 10.1161/CIRCULATIONAHA.106.176583. [DOI] [PubMed] [Google Scholar]
- Gerstein HC, Yusuf S, Bosch J, et al. Effect of rosiglitazone on the frequency of diabetes in patients with impaired glucose tolerance or impaired fasting glucose: a randomised controlled trial. Lancet. 2006;368:1096–105. doi: 10.1016/S0140-6736(06)69420-8. [DOI] [PubMed] [Google Scholar]
- Ghanim H, Dhindsa S, Aljada A, et al. Low-dose rosiglitazone exerts an antiinflammatory effect with an increase in adiponectin independently of free fatty acid fall and insulin sensitization in obese type 2 diabetics. J Clin Endocrinol Metab. 2006;91:3553–8. doi: 10.1210/jc.2005-2609. [DOI] [PubMed] [Google Scholar]
- Glendening PN, Hearne SA, Segal LM, et al. F as in fat: How obesity policies are failing in America. Trust for America’s Health. 2005 Available at: http://healthyamericans.org/reports/obesity2005/Obesity2005Report.pdf.
- Goldstein BJ. Insulin resistance: from benign to type 2 diabetes mellitus. Rev Cardiovasc Med. 2003;4(Suppl 6):S3–10. [PubMed] [Google Scholar]
- Haber EP, Ximenes HM, Procopio J, et al. Pleiotropic effects of fatty acids on pancreatic beta-cells. J Cell Physiol. 2003;194:1–12. doi: 10.1002/jcp.10187. [DOI] [PubMed] [Google Scholar]
- Hamalainen H, Ronnemaa T, Virtanen A, et al. Improved fibrinolysis by an intensive lifestyle intervention in subjects with impaired glucose tolerance. The Finnish Diabetes Prevention Study. Diabetologia. 2005;48:2248–53. doi: 10.1007/s00125-005-1938-5. [DOI] [PubMed] [Google Scholar]
- Hayashi T, Boyko EJ, Leonetti DL, et al. Visceral adiposity and the risk of impaired glucose tolerance: a prospective study among Japanese Americans. Diabetes Care. 2003;26:650–5. doi: 10.2337/diacare.26.3.650. [DOI] [PubMed] [Google Scholar]
- Herman GA, Bergman A, Yi B, et al. Tolerability and pharmacokinetics of metformin and the dipeptidyl peptidase-4 inhibitor sitagliptin when co-administered in patients with type 2 diabetes. Curr Med Res Opin. 2006;22:1939–47. doi: 10.1185/030079906X132587. [DOI] [PubMed] [Google Scholar]
- Herman GA, Stevens C, Van Dyck K, et al. Pharmacokinetics and pharmacodynamics of sitagliptin, an inhibitor of dipeptidyl peptidase IV, in healthy subjects: results from two randomized, double-blind, placebo-controlled studies with single oral doses. Clin Pharmacol Ther. 2005;78:675–88. doi: 10.1016/j.clpt.2005.09.002. [DOI] [PubMed] [Google Scholar]
- Hogan P, Dall T, Nikolov P. Economic costs of diabetes in the US in 2002. Diabetes Care. 2003;26:917–32. doi: 10.2337/diacare.26.3.917. [DOI] [PubMed] [Google Scholar]
- Hu E, Liang P, Spiegelman BM. AdipoQ is a novel adipose-specific gene dysregulated in obesity. J Biol Chem. 1996;271:10697–703. doi: 10.1074/jbc.271.18.10697. [DOI] [PubMed] [Google Scholar]
- Januvia™ Januvia™ (sitagliptin) prescribing information. Merck & Co. Inc [online] 2006 URL: http://www.merck.com/product/usa/pi_circulars/j/januvia/januvia_pi.pdf.
- John BJ, Irukulla S, Abulafi AM, et al. Systematic review: adipose tissue, obesity and gastrointestinal diseases. Aliment Pharmacol Ther. 2006;23:1511–23. doi: 10.1111/j.1365-2036.2006.02915.x. [DOI] [PubMed] [Google Scholar]
- Kadowaki T, Yamauchi T, Kubota N, et al. Adiponectin and adiponectin receptors in insulin resistance, diabetes, and the metabolic syndrome. J Clin Invest. 2006;116:1784–92. doi: 10.1172/JCI29126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kendall DM, Riddle MC, Rosenstock J, et al. Effects of exenatide (exendin-4) on glycemic control over 30 weeks in patients with type 2 diabetes treated with metformin and a sulfonylurea. Diabetes Care. 2005;28:1083–91. doi: 10.2337/diacare.28.5.1083. [DOI] [PubMed] [Google Scholar]
- Kershaw EE, Flier JS. Adipose tissue as an endocrine organ. J Clin Endocrinol Metab. 2004;89:2548–56. doi: 10.1210/jc.2004-0395. [DOI] [PubMed] [Google Scholar]
- Klein S, Sheard NF, Pi-Sunyer X, et al. Weight management through lifestyle modification for the prevention and management of type 2 diabetes: rationale and strategies: a statement of the American Diabetes Association, the North American Association for the Study of Obesity, and the American Society for Clinical Nutrition. Diabetes Care. 2004;27:2067–73. doi: 10.2337/diacare.27.8.2067. [DOI] [PubMed] [Google Scholar]
- Knobler H, Benderly M, Boyko V, et al. Adiponectin and the development of diabetes in patients with coronary artery disease and impaired fasting glucose. Eur J Endocrinol. 2006;154:87–92. doi: 10.1530/eje.1.02054. [DOI] [PubMed] [Google Scholar]
- Knowler WC, Barrett-Connor E, Fowler SE, et al. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 2002;346:393–403. doi: 10.1056/NEJMoa012512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knowler WC, Hamman RF, Edelstein SL, et al. Prevention of type 2 diabetes with troglitazone in the Diabetes Prevention Program. Diabetes. 2005;54:1150–6. doi: 10.2337/diabetes.54.4.1150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krakoff J, Funahashi T, Stehouwer CD, et al. Inflammatory markers, adiponectin, and risk of type 2 diabetes in the Pima Indian. Diabetes Care. 2003;26:1745–51. doi: 10.2337/diacare.26.6.1745. [DOI] [PubMed] [Google Scholar]
- Lebovitz HE, Austin MM, Blonde L, et al. ACE/AACE consensus conference on the implementation of outpatient management of diabetes mellitus: consensus conference recommendations. Endocr Pract. 2006;12(Suppl 1):6–12. doi: 10.4158/EP.12.S1.6. [DOI] [PubMed] [Google Scholar]
- Lehrke M, Reilly MP, Millington SC, et al. An inflammatory cascade leading to hyperresistinemia in humans. PLoS Med. 2004;1:161–8. doi: 10.1371/journal.pmed.0010045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis GF, Carpentier A, Adeli K, et al. Disordered fat storage and mobilization in the pathogenesis of insulin resistance and type 2 diabetes. Endocr Rev. 2002;23:201–29. doi: 10.1210/edrv.23.2.0461. [DOI] [PubMed] [Google Scholar]
- Lindsay RS, Funahashi T, Hanson RL, et al. Adiponectin and development of type 2 diabetes in the Pima Indian population. Lancet. 2002;360:57–8. doi: 10.1016/S0140-6736(02)09335-2. [DOI] [PubMed] [Google Scholar]
- Matsuzawa Y. Therapy Insight: adipocytokines in metabolic syndrome and related cardiovascular disease. Nat Clin Pract Cardiovasc Med. 2006;3:35–42. doi: 10.1038/ncpcardio0380. [DOI] [PubMed] [Google Scholar]
- Meridia® Meridia® (sibutramine hydrochloride monohydrate) prescribing information. Abbott Laboratories [online] 2006 URL: http://rxabbott.com/pdf/meridia.pdf.
- Natali A, Toschi E, Baldeweg S, et al. Clustering of insulin resistance with vascular dysfunction and low-grade inflammation in type 2 diabetes. Diabetes. 2006;55:1133–40. doi: 10.2337/diabetes.55.04.06.db05-1076. [DOI] [PubMed] [Google Scholar]
- Pan XR, Li GW, Hu YH, et al. Effects of diet and exercise in preventing NIDDM in people with impaired glucose tolerance. The Da Qing IGT and Diabetes Study. Diabetes Care. 1997;20:537–44. doi: 10.2337/diacare.20.4.537. [DOI] [PubMed] [Google Scholar]
- Park H, Hasegawa G, Obayashi H, et al. Relationship between insulin resistance and inflammatory markers and anti-inflammatory effect of losartan in patients with type 2 diabetes and hypertension. Clin Chim Acta. 2006;374:129–34. doi: 10.1016/j.cca.2006.06.004. [DOI] [PubMed] [Google Scholar]
- Pi-Sunyer FX, Aronne LJ, Heshmati HM, et al. Effect of rimonabant, a cannabinoid-1 receptor blocker, on weight and cardiometabolic risk factors in overweight or obese patients: RIO-North America: a randomized controlled trial. JAMA. 2006;295:761–75. doi: 10.1001/jama.295.7.761. [DOI] [PubMed] [Google Scholar]
- Poston WS, Foreyt JP. Sibutramine and the management of obesity. Expert Opin Pharmacother. 2004;5:633–42. doi: 10.1517/14656566.5.3.633. [DOI] [PubMed] [Google Scholar]
- Qi L, Doria A, Manson JE, et al. Adiponectin genetic variability, plasma adiponectin, and cardiovascular risk in patients with type 2 diabetes. Diabetes. 2006;55:1512–6. doi: 10.2337/db05-1520. [DOI] [PubMed] [Google Scholar]
- Raz I, Eldor R, Cernea S, et al. Diabetes: insulin resistance and derangements in lipid metabolism. Cure through intervention in fat transport and storage. Diabetes Metab Res Rev. 2005;21:3–14. doi: 10.1002/dmrr.493. [DOI] [PubMed] [Google Scholar]
- Reaven GM, Lithell H, Landsberg L. Hypertension and associated metabolic abnormalities--the role of insulin resistance and the sympathoadrenal system. N Engl J Med. 1996;334:374–81. doi: 10.1056/NEJM199602083340607. [DOI] [PubMed] [Google Scholar]
- Rosenstock J. The potential of rimonabant in prediabetes: Pooled 1-year results from the RIO-Lipids, RIO-Europe and RIO-North American studies [abstract]. Presented at the 65th Annual Meeting of the American Diabetes Association; 2005. Abstract 13-LB. [Google Scholar]
- Samaha FF. Effect of very high-fat diets on body weight, lipoproteins, and glycemic status in the obese. Curr Atheroscler Rep. 2005;7:412–20. doi: 10.1007/s11883-005-0057-6. [DOI] [PubMed] [Google Scholar]
- Samaha FF, Iqbal N, Seshadri P, et al. A low-carbohydrate as compared with a low-fat diet in severe obesity. N Engl J Med. 2003;348:2074–81. doi: 10.1056/NEJMoa022637. [DOI] [PubMed] [Google Scholar]
- Samaha FF, Szapary PO, Iqbal N, et al. Effects of rosiglitazone on lipids, adipokines, and inflammatory markers in nondiabetic patients with low high-density lipoprotein cholesterol and metabolic syndrome. Arterioscler Thromb Vasc Biol. 2006;26:624–30. doi: 10.1161/01.ATV.0000200136.56716.30. [DOI] [PubMed] [Google Scholar]
- Scheen A, Finer N, Hollander P, et al. Rimonabant improves cardiometabolic risk factors in overweight/obese patients with type 2 diabetes irrespective of background oral antidiabetic therapy (metformin or sulfonylureas) [abstract] Diabetes. 2006;55(Suppl 1):A133–4. Abstract 560-P. [Google Scholar]
- Scheen AJ. Renin-angiotensin system inhibition prevents type 2 diabetes mellitus. Part 1. A meta-analysis of randomised clinical trials. Diabetes Metab. 2004a;30:487–96. doi: 10.1016/s1262-3636(07)70146-5. [DOI] [PubMed] [Google Scholar]
- Scheen AJ. Renin-angiotensin system inhibition prevents type 2 diabetes mellitus. Part 2. Overview of physiological and biochemical mechanisms. Diabetes Metab. 2004b;30:498–505. doi: 10.1016/s1262-3636(07)70147-7. [DOI] [PubMed] [Google Scholar]
- Scherer PE, Williams S, Fogliano M, et al. A novel serum protein similar to C1q, produced exclusively in adipocytes. J Biol Chem. 1995;270:26746–9. doi: 10.1074/jbc.270.45.26746. [DOI] [PubMed] [Google Scholar]
- Schulze MB, Rimm EB, Shai I, et al. Relationship between adiponectin and glycemic control, blood lipids, and inflammatory markers in men with type 2 diabetes. Diabetes Care. 2004;27:1680–7. doi: 10.2337/diacare.27.7.1680. [DOI] [PubMed] [Google Scholar]
- Schulze MB, Shai I, Rimm EB, et al. Adiponectin and future coronary heart disease events among men with type 2 diabetes. Diabetes. 2005;54:534–9. doi: 10.2337/diabetes.54.2.534. [DOI] [PubMed] [Google Scholar]
- Seshadri P, Iqbal N, Stern L, et al. A randomized study comparing the effects of a low-carbohydrate diet and a conventional diet on lipoprotein subfractions and C-reactive protein levels in patients with severe obesity. Am J Med. 2004;117:398–405. doi: 10.1016/j.amjmed.2004.04.009. [DOI] [PubMed] [Google Scholar]
- Sharma AM. The obese patient with diabetes mellitus: from research targets to treatment options. Am J Med. 2006;119:S17–23. doi: 10.1016/j.amjmed.2006.01.010. [DOI] [PubMed] [Google Scholar]
- Sharma AM, Janke J, Gorzelniak K, et al. Angiotensin blockade prevents type 2 diabetes by formation of fat cells. Hypertension. 2002;40:609–11. doi: 10.1161/01.hyp.0000036448.44066.53. [DOI] [PubMed] [Google Scholar]
- Sherwin RS, Anderson RM, Buse JB, et al. The prevention or delay of type 2 diabetes. Diabetes Care. 2003;26(Suppl 1):S62–9. doi: 10.2337/diacare.26.2007.s62. [DOI] [PubMed] [Google Scholar]
- Spranger J, Kroke A, Mohlig M, et al. Adiponectin and protection against type 2 diabetes mellitus. Lancet. 2003;361:226–8. doi: 10.1016/S0140-6736(03)12255-6. [DOI] [PubMed] [Google Scholar]
- Stern L, Iqbal N, Seshadri P, et al. The effects of low-carbohydrate versus conventional weight loss diets in severely obese adults: one-year follow-up of a randomized trial. Ann Intern Med. 2004;140:778–85. doi: 10.7326/0003-4819-140-10-200405180-00007. [DOI] [PubMed] [Google Scholar]
- Stoffers DA, Desai BM, DeLeon DD, et al. Neonatal exendin-4 prevents the development of diabetes in the intrauterine growth retarded rat. Diabetes. 2003;52:734–40. doi: 10.2337/diabetes.52.3.734. [DOI] [PubMed] [Google Scholar]
- Szapary PO, Bloedon LT, Samaha FF, et al. Effects of pioglitazone on lipoproteins, inflammatory markers, and adipokines in nondiabetic patients with metabolic syndrome. Arterioscler Thromb Vasc Biol. 2006;26:182–8. doi: 10.1161/01.ATV.0000195790.24531.4f. [DOI] [PubMed] [Google Scholar]
- Torgerson JS, Hauptman J, Boldrin MN, et al. XENical in the prevention of diabetes in obese subjects (XENDOS) study: a randomized study of orlistat as an adjunct to lifestyle changes for the prevention of type 2 diabetes in obese patients. Diabetes Care. 2004;27:155–61. doi: 10.2337/diacare.27.1.155. [DOI] [PubMed] [Google Scholar]
- Tuomilehto J, Lindstrom J, Eriksson JG, et al. Prevention of type 2 diabetes mellitus by changes in lifestyle among subjects with impaired glucose tolerance. N Engl J Med. 2001;344:1343–50. doi: 10.1056/NEJM200105033441801. [DOI] [PubMed] [Google Scholar]
- US National Institutes of Health. ClinicalTrials.gov. 2006. [online] Accessed 17 October 2006. URL: http://www.clinicaltrials.gov/ct/show/NCT00325650;jsessionid=DE34C9537828718F3BB395432F702959?order=4.
- Uusitupa M, Lindi V, Louheranta A, et al. Long-term improvement in insulin sensitivity by changing lifestyles of people with impaired glucose tolerance: 4-year results from the Finnish Diabetes Prevention Study. Diabetes. 2003;52:2532–8. doi: 10.2337/diabetes.52.10.2532. [DOI] [PubMed] [Google Scholar]
- Van Gaal LF, Rissanen AM, Scheen AJ, et al. Effects of the cannabinoid-1 receptor blocker rimonabant on weight reduction and cardiovascular risk factors in overweight patients: 1-year experience from the RIO-Europe study. Lancet. 2005;365:1389–97. doi: 10.1016/S0140-6736(05)66374-X. [DOI] [PubMed] [Google Scholar]
- Vettor R, Serra R, Fabris R, et al. Effect of sibutramine on weight management and metabolic control in type 2 diabetes: a meta-analysis of clinical studies. Diabetes Care. 2005;28:942–9. doi: 10.2337/diacare.28.4.942. [DOI] [PubMed] [Google Scholar]
- Vijan S, Stuart NS, Fitzgerald JT, et al. Barriers to following dietary recommendations in Type 2 diabetes. Diabet Med. 2005;22:32–8. doi: 10.1111/j.1464-5491.2004.01342.x. [DOI] [PubMed] [Google Scholar]
- Wang Q, Brubaker PL. Glucagon-like peptide-1 treatment delays the onset of diabetes in 8 week-old db/db mice. Diabetologia. 2002;45:1263–73. doi: 10.1007/s00125-002-0828-3. [DOI] [PubMed] [Google Scholar]
- Weyer C, Funahashi T, Tanaka S, et al. Hypoadiponectinemia in obesity and type 2 diabetes: close association with insulin resistance and hyperinsulinemia. J Clin Endocrinol Metab. 2001;86:1930–5. doi: 10.1210/jcem.86.5.7463. [DOI] [PubMed] [Google Scholar]
- Wild S, Roglic G, Green A, et al. Global prevalence of diabetes: estimates for the year 2000 and projections for 2030. Diabetes Care. 2004;27:1047–53. doi: 10.2337/diacare.27.5.1047. [DOI] [PubMed] [Google Scholar]
- Wing RR, Hamman RF, Bray GA, et al. Achieving weight and activity goals among diabetes prevention program lifestyle participants. Obes Res. 2004;12:1426–34. doi: 10.1038/oby.2004.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu H, Barnes GT, Yang Q, et al. Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance. J Clin Invest. 2003;112:1821–30. doi: 10.1172/JCI19451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yach D, Stuckler D, Brownell KD. Epidemiologic and economic consequences of the global epidemics of obesity and diabetes. Nat Med. 2006;12:62–6. doi: 10.1038/nm0106-62. [DOI] [PubMed] [Google Scholar]
- Zraika S, Dunlop M, Proietto J, et al. Effects of free fatty acids on insulin secretion in obesity. Obes Rev. 2002;3:103–12. doi: 10.1046/j.1467-789x.2002.00062.x. [DOI] [PubMed] [Google Scholar]


