Abstract
Bisoprolol fumarate is a highly selective beta-1 receptor blocker. Bisoprolol has been extensively studied in three large mortality trials in stable chronic heart failure (CHF) patients. The CIBIS trial enrolled 641 patients and demonstrated the good tolerability of bisoprolol in a large CHF population, without evidence for any harmful effect. The CIBIS-II study was the first large randomized, double-blind, placebo-controlled study demonstrating in 2647 patients a dramatic reduction in mortality with a beta-blocking agent in CHF patients. CIBIS-III demonstrated in 1010 patients the equivalence of 2 different therapeutic strategies in de novo CHF patients. There was no difference in morbidity and mortality between sub-groups of patients receiving first bisoprolol or enalapril. These three trials also demonstrated the good tolerability of bisoprolol fumarate. Other studies were either limited in number of patients or not randomized. However, these studies confirmed the good tolerability of bisoprolol in CHF patients, even in elderly population. Bisoprolol fumarate is a selective beta-1 receptor blocker that significantly reduced morbidity and mortality in stable CHF patients. Bisoprolol is well tolerated with few significant side effects in different large trials.
Keywords: bisoprolol, chronic heart failure, beta-blocker
Introduction
Chronic heart failure (CHF) represents a major health problem and is one of the leading causes of hospitalization, in particular in elderly patients. Medical treatment had significantly improved during the last decade and several studies have demonstrated that angiotensin converting enzyme inhibitors (ACEI) and beta-blocker therapy are now the cornerstone of the treatment of patients with CHF (The SOLVD Investigators, 1991, 1992; Pfeffer et al 1992; CIBIS-II investigators and Committees 1999; MERIT-HF study group 1999; Packer et al 2001; Flather et al 2005). In the 1970s, Waagstein and collaborators first reported, in uncontrolled studies, that a treatment with a beta-blocker may dramatically improve symptoms and ventricular function in patients with mild to severe heart failure due to idiopathic dilated cardiomyopathy (Waagstein et al 1975; Swedberg et al 1979). Recently, different mortality trials have clearly demonstrated the beneficial effects of beta-blocker therapy. However, other trials did not demonstrate a significant mortality reduction (The Beta-Blocker Evaluation of Survival Trial Investigators 2001) or showed different effects between beta-blockers (Poole-Wilson et al 2003), leading international guidelines to recommend only 4 beta-blockers for CHF: bisoprolol, metoprolol succinate, carvedilol, and nebivolol (Hunt et al 2005; Swedberg et al 2005). Beta-blocker agents represent a large heterogeneous family with one important difference concerning receptor selectivity. In CHF, three recommended drugs are beta-1 adrenoreceptor blockers, namely bisoprolol, metoprolol succinate, and nebivolol, and one, carvedilol, is a beta-1–beta-2 adrenoreceptor blocker with additional alpha-1 vasodilatory activity. We will not focus our review on the comparison of these different beta-blockers. This review will summarize the results of different studies with bisoprolol in stable CHF patients, and particularly the different Cardiac Insufficiency Bisoprolol Studies (CIBIS).
Pharmacokinetics of bisoprolol fumarate
Bisoprolol fumarate is a beta-1 receptor blocker, very freely soluble in water, with a molecular weight of 383.48 kDa (Leopold et al 1986; McGavin and Keating 2002). Bisoprolol is well absorbed after oral administration with a bioavailability of 90% and has a low plasma protein binding (30%). Food intake does not modify its biodisponibility. Bisoprolol is metabolized in the liver in inactive metabolites (50%) and eliminated (50%) via renal excretion without metabolisation. The plasma elimination half-life ranges from 10 to 12 hours. The pharmacokinetics of bisoprolol is minimally changed in patients with hepatic impairment or with a creatinine clearance between 10 and 30 mL/min. In patients with severe renal impairment (creatinine clearance <10 mL/min), the exposure to bisoprolol is increased 2-fold. The plasma elimination half-life increases to 24.2 hours in the latter case (Kirch et al 1987).
There is limited information available on the pharmacokinetics of bisoprolol in patients with stable heart failure. In NYHA class III patients, receiving a chronic treatment of 10 mg/day, peak plasma concentrations were 78% higher, with a plasma elimination half-life reaching 17 hours.
Beta-receptor selectivity
Different experimental studies have demonstrated that bisoprolol fumarate is one of the most selective beta-1 adrenoreceptor blockers, with a 19-fold higher affinity for the beta-1 receptor than for the beta-2 receptor (Wellstein et al 1986; Smith and Teilter 1999). Even at higher dose, there is no beta-2 blockade effect. Nebivolol is 3.5 times more beta-1 adrenoreceptor selective than bisoprolol in human myocardium and in vitro study (Bundkirchen et al 2003).
In a randomized, double-blind, placebo-controlled, cross-over study in 12 patients with stable angina pectoris and non-asthmatic chronic obstructive lung disease, a single dose of 100 mg of atenolol mg was compared with 20 mg of bisoprolol. Both drugs had a similar effect on heart rate but airway resistance increased with atenolol, whereas was unchanged with bisoprolol compared with placebo (Dorow et al 1986). Similar results were found in another randomized, double-blind, placebo-controlled, cross-over study in 12 hypertensive asthmatic patients, comparing the airway resistance after the administration of 10 or 20 mg of bisoprolol, 100 mg of atenolol, or placebo (Chatterjee 1986).
Efficacy studies: CIBIS trials
Bisoprolol fumarate has the advantage to have been studied largely in 3 major mortality trials in stable CHF patients, demonstrating the important benefits of this beta-1 blocking agent and its good tolerability.
CIBIS
CIBIS was the first randomized, double-blind, placebo-controlled study with the primary objective of evaluate the impact of bisoprolol on mortality in patients with heart failure (CIBIS Investigators and Committees 1994). The inclusion criteria were ambulatory CHF patients in NYHA class III-IV, with a left ventricular ejection fraction (LVEF) <40%, receiving diuretics and vasodilator therapy and not registered on a waiting list for heart transplantation. At this time, the tolerability of a chronic beta-blocker therapy was unknown in a large population. An important prerequisite before inclusion was the need of a clinical phase of stability, without any episode of heart failure decompensation and the absence of major modification of heart failure therapy in the last 3 weeks before randomization. The initial dose of bisoprolol was 1.25 mg once daily, which could be increased 48 hours later to 2.5 mg/day and 1 month after to the maximal dose of 5 mg/day. It was not a forced titration procedure and each investigator was free to give to their patient, according to their clinical status, one of the four doses: 1.25, 2.5, 3.75, or 5 mg/day.
CIBIS enrolled 641 patients with CHF, in NYHA class III in the vast majority (609, 95%). Clinical characteristics are summarized in Table 1. During a mean duration of follow-up of 1.9 ± 0.1 years, there were 120 deaths, 67 (20.9%) in the placebo group and 53 (16.6%) in the bisoprolol group (Figure 1). This difference was not statistically significant with a risk reduction of 0.80 (0.56–1.15).
Table 1.
Clinical characteristics of the different CIBISa trials
| CIBIS | CIBIS II | CIBIS III | |
|---|---|---|---|
| n | 641 | 2647 | 1010 |
| Age, years (mean) | 59 | 61 | 72 |
| Female | 17% | 19% | 32% |
| NYHA class III/IV | 95%/5% | 83%/17% | 50%/0% |
| Ischemic heart failure | 55% | 50% | 62% |
| Idiopathic dilated cardiomyopathy | 36% | 12% | 10% |
| ACEI | 90% | 96% | – |
| Diuretics | 100% | 99% | 90% |
| Heart rate, bpm | 83 ± 1.5 | 81 ± 15 | 79 ± 13 |
| Systolic blood pressure, mmHg | 126 | 130 ± 19 | 134 ± 17 |
| Diastolic blood pressure, mmHg | 78 | 80 ± 11 | 80 ± 10 |
| LVEF (%) | 25 ± 0.9 | 27 ± 6 | 28.8 ± 5 |
| Mean dose bisoprolol, mg | 3.8 ± 0.2 |
for CIBIS, quantitative results are presented as mean ± SEM.
Abbreviations: ACEI, angiotensin converting enzyme inhibitor; CIBIS, Cardiac Insufficiency Bisoprolol Studies; LVEF, left ventricular ejection fraction.
Figure 1.
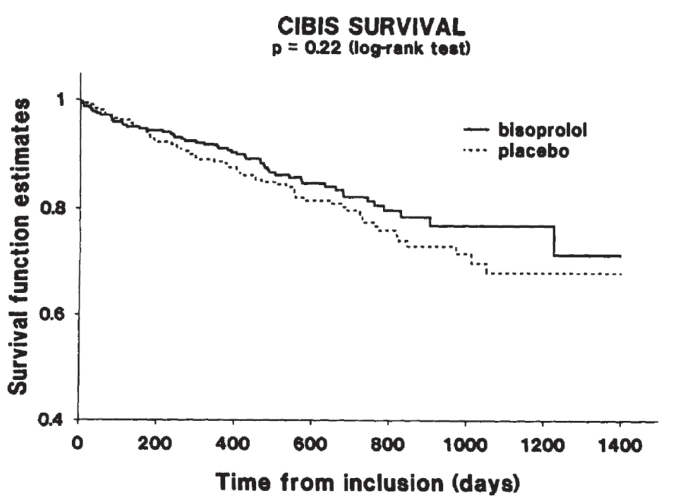
Survival curves in CIBIS patients.
Despite this negative result, CIBIS was a very important study demonstrating the good tolerability of bisoprolol in a large CHF population, without evidence for any harmful effect of the beta-blocker therapy. Bisoprolol decreased the rate of hospitalization for worsening heart failure (107 (17%) compared with 154 (24%) in the placebo group, p < 0.001). Moreover, more patients in the bisoprolol group than in the placebo group improved their functional status. At the end of the trial, 21% of the patients receiving bisoprolol had an improvement of at least 1 class, compared with 15% in the placebo group (p < 0.03). Deterioration of at least 1 NYHA class was similar in the two sub-groups (13% vs 11%).
CIBIS-II
The CIBIS-II study was the first large randomized, double-blind, placebo-controlled study demonstrating a dramatic reduction in mortality with a beta-blocking agent in CHF patients (CIBIS-II investigators and Committees 1999). The equivalent study for the ACE-I was the CONSENSUS trial with enalapril (The CONSENSUS Trial Study Group 1987). CIBIS-II enrolled 2647 class III-IV stable CHF patients with a LVEF <35%, who were receiving diuretics and vasodilator therapy. In contrast to the CIBIS study, bisoprolol titration was forced to the maximal tolerated dose, with the highest possible dose of 10 mg/day. The clinical characteristics of the CIBIS-II population are summarized in Table 1. Bisoprolol induced a significant heart rate reduction (of 9.8 ± 14.7 beats/min), with a limited effect on blood pressure (systolic blood pressure reduction of 4.1 ± 16.4 mmHg with bisoprolol but of 2.3 ± 16.4 mmHg with placebo) (Lechat et al 2001).
The study was prematurely stopped because of the significant mortality benefit associated with bisoprolol (Figure 2). After a mean follow-up period of 1.3 years, there were 384 deaths, with 228 (17.3%) deaths in the placebo arm and 156 (11.8%) in the bisoprolol arm (hazard ratio (HR) of 0.66 [0.54–0.81]). Bisoprolol significantly reduced cardiovascular mortality (HR: 0.71 [0.56–0.9]), sudden cardiac death (HR: 0.56 [0.39–0.80]), hospital admission (HR: 0.80 [0.71–0.91]), and hospital admission for worsening heart failure (HR: 0.64 [0.53–0.79]) compared with placebo. The magnitude of the benefit was similar in NYHA sub-classes, and independent of the etiology of heart failure. However, in patients with atrial fibrillation (n = 521, 20%), bisoprolol did not decrease total mortality when compared with placebo. This result must be taken with caution because of the limited number of patients with atrial fibrillation and the retrospective nature of the analysis (Lechat et al 2001). Although heart rate at baseline and heart rate reduction were independently associated with survival, there was no interaction with bisoprolol, suggesting that the beneficial effect of bisoprolol was not influenced by these two parameters, and in particular by the extent of the heart rate reduction (Lechat et al 2001). In CIBIS, retrospective analysis suggested that patients with a LVEF ≤20% benefit more from bisoprolol than other patients (Funck-Brentano et al 2000). However, this was not confirmed in CIBIS-II, where the beneficial effect of bisoprolol was independent of the level of left ventricular dysfunction.
Figure 2.
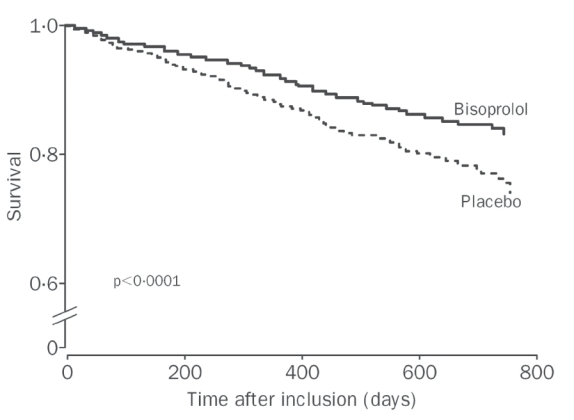
Survival curves in CIBIS-II patients.
CIBIS-III
The third important study with bisoprolol in CHF patients was designed to determine what drug to initiate in a de novo, stable, CHF patient, either ACEI or beta-blocker (Willenheimer et al 2005). Because the beneficial effects of ACEI have been demonstrated first (The CONSENSUS Trial Study Group 1987; The SOLVD Investigators 1991, 1992; Pfeffer et al 1992), all the subsequent studies have studied the impact on mortality of a new drug or device on top of ACEI (Pitt et al 1999; Cleland et al 2005). This question is of importance since the effects of beta-blockers in CHF patients are observed quickly after their introduction, in particular with a reduction of sudden cardiac death, which is the most prevalent cause of death in this population. Moreover, instead of ACEI that blocks one system, beta-blockers effectively inhibit 2 systems, the sympathetic system and the renin-angiotensin system.
CIBIS-III was a multicenter, prospective, randomized, open label, blinded end-point evaluation study, with 2 parallel groups (Figure 3). Inclusion criteria were different from the previous CIBIS studies. Eligible patients were patients older than 65 years, in NYHA class II or III, with a LVEF ≤35% and of course receiving neither ACEI nor beta-blocker. All patients were clinically stable for at least 7 days before the inclusion.
Figure 3.
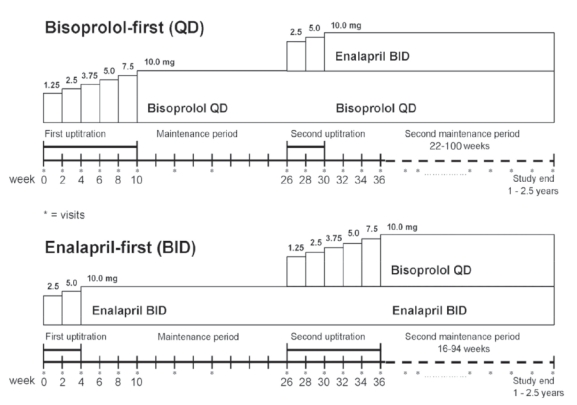
Study design of CIBIS-III. Double titration with monotherapy and combination phases for each arm, bisoprolol-first and enalapril-first.
As in CIBIS-II, the titration was forced, depending on patient’s tolerance, for both drugs with a target dose of 10 mg/day for bisoprolol and of 20 mg/day for enalapril. After the titration, there was a 6-month monotherapy period, followed by a new titration in order to have the combination of enalapril and bisoprolol for a combined period. The primary end-point was the combination of all-cause mortality or all-cause hospitalization. CIBIS-III was designed as a non-inferiority trial comparing the impact on the primary end-point of the initiation of bisoprolol first compared with enalapril.
CIBIS-III enrolled 1010 patients who were followed during a mean period of 1.22 ± 0.42 years. Clinical characteristics of the study population are presented in Table 1. Because of the different inclusion criteria, some characteristics of CIBIS-III patients were different compared with those of patient enrolled in previous CIBIS studies. Patients in CIBIS-III were older, more often female, and less symptomatic (no NYHA class IV patients and half of the population was in NYHA class II). The results did not demonstrate any significant difference between the two strategies (Figure 4). There were 178 patients with the primary end-point in the bisoprolol-first group and 186 in the enalapril-first group (35.2 vs 36.8%). At the end of the monotherapy period, 109 bisoprolol-first patients had a primary end-point compared with 108 enalapril-first patients. There were fewer deaths in the bisoprolol-first group than in the enalapril-first group, but the difference was not statistically significant (65 vs 73, HR: 0.88 [0.63–1.22], p = 0.44). There was a non-significant increase in the number of patients having a hospitalization for worsening CHF in the bisoprolol-first group compared with the enalapril-first group (63 vs 51 patients, respectively, HR = 1.25 [0.87–1.81], p = 0.23).
Figure 4.
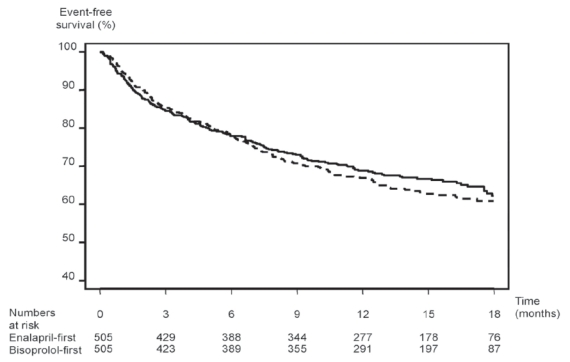
Kaplan-Meier curves of the combined primary end-point (death or hospitalization) in CIBIS-III patients. Intention-to-treat analysis.
We can conclude, from the results of the CIBIS-III trial, that there was no difference in terms of efficacy and safety between the two strategies of treatment initiation in stable CHF patients.
Non-mortality studies
There are few other studies with bisoprolol in CHF patients, but all these studies are either small or not randomized.
A small study analyzed the impact of bisoprolol on LVEF using magnetic resonance imaging (Dubach et al 2002). It was a randomized, double-blind study in 28 patients with a mean age of 57 years, 13 receiving bisoprolol and 15 placebo. Eight patients in each group had an ischemic cardiomyopathy and others had a dilated cardiomyopathy. All the patients were receiving ACEI and 24 were taking diuretics. The mean dose of bisoprolol was 7.19 mg/day. At baseline, at 6 months, and at 1 year after the introduction of bisoprolol, patients performed a cardiopulmonary exercise test and a magnetic resonance imaging of the heart. Bisoprolol produced a significant reduction in heart rate associated with a non-significant increase in peak oxygen consumption. Left ventricular ejection fraction improved at 1 year only in the bisoprolol sub-group, from 25 ± 7% to 36.2 ± 9% (p < 0.05).
We performed an observational study in consecutive stable patients with CHF and LVEF <40% (de Groote et al 2004). All the patients received maximal tolerated doses of renin inhibitors and were clinically stable at least 2 months before the introduction of bisoprolol. All the patients performed a cardiopulmonary exercise test, and underwent a radionuclide angiography, before and 3 months after maximal tolerated doses of bisoprolol had been reached. Blood samples were drawn for hormonal determinations. We included 201 patients, with a mean age of 54 ± 12 years; 34% had ischemic cardiomyopathy and the vast majority was in NYHA class I or II (75%). The mean dose of bisoprolol was 8.8 ± 2.4 mg/day. Bisoprolol was associated with an improvement in NHYA functional class, and a significant decrease in heart rate without any effect on blood pressure. There was a small significant improvement in peak oxygen consumption (from 16.1 ± 5 to 16.8 ± 5.5 mL/min/kg, p = 0.015) with a significant decrease in peak expiratory exchange ratio for carbon dioxide production (from 38 ± 7.4 to 34 ± 6.7, p = 0.005). This suggests an improvement in the exercise ventilatory efficacy with bisoprolol. LVEF improved from 31 ± 11 to 41 ± 13% (p < 0.0001). This favorable effect was associated with a reduction in ventricular volumes and with an improvement in the left ventricular filling function. Right ventricular ejection fraction also significantly improved with bisoprolol. Finally, plasma levels of type A and type B natriuretic peptides and norepinephrine were significantly reduced with bisoprolol. Of course, one of the biggest limitations of the study is the lack of a control group. However, at this time it was not ethical to give placebo to CHF patients.
In addition, another observational study in 87 CHF patients showed that beta-blockade improved LVEF in the majority of patients. However, significant improvement in LVEF did not enhance functional capacity consistently in CHF patients (Ennezat et al 2005).
Doses
In CIBIS and CIBIS-II, mean doses were significantly greater in the placebo arm compared with the bisoprolol arm. In CIBIS, respective doses were 4.5 ± 0.1 and 3.8 ± 0.2 mg/day. Half of the patients received 5 mg of bisoprolol in CIBIS, and 43% reached 10 mg in CIBIS-II and 67% at least 5 mg/day. In CIBIS-III, during the monotherapy period, 65% of the patients reached the target dose in the bisoprolol-first group compared with 84% in the enalapril-first group. At the end of the study, in the bisoprolol-first group, 65% of the patients had the target dose of bisoprolol and 67% the target dose of enalapril. In the enalapril-first group, respective percentages were 54% and 77%.
A retrospective analysis looking at the doses achieved after the forced titration in CIBIS-II revealed that patients in the lower tertile of doses were older, more often in NYHA class IV, and had a lower blood pressure. However, the beneficial effect of bisoprolol was similar whatever the dose received (Simon et al 2003).
Tolerability of bisoprolol
Curiously, no precise information on non-serious adverse events is available from the three CIBIS studies. Bisoprolol is well tolerated. In CIBIS, percentages of patients with non-serious adverse events were similar in the 2 arms: 26% in the placebo group and 23% in the bisoprolol group. Two cases of sinus bradycardia and 2 cases of atrioventricular blockade were recorded in the bisoprolol group. Significant hypotension was recorded in 3 patients in the placebo group and in 5 in the bisoprolol group.
In CIBIS-II, the percentage of premature treatment withdrawal was also similar in the 2 arms (15%), but there were more bradycardia with bisoprolol (14 vs 2, p < 0.004). The main cause of permanent treatment withdrawal was patient’s or investigator’s personal decision. Age (≥68 years) and heart rate at inclusion were both independent predictors of permanent treatment withdrawal. In patients with a heart rate <72 beats/min at inclusion, the risk of permanent bisopropol withdrawal was 1.97 (1.38–2.80). Of importance, the beneficial mortality effect of bisoprolol was lost in patients having a permanent treatment withdrawal (Funck-Brentano et al 2001).
In CIBIS-III, during the monotherapy period, 35 patients (6.9%) had a permanent bisoprolol withdrawal compared with 49 (9.7%) with enalapril. During the combination period, in the bisoprolol-first group, 19 patients (4.2%) had a permanent withdrawal of bisoprolol and 47 patients (10.4%) a permanent withdrawal of enalapril. In the enalapril-first group, corresponding values were 24 (5.5%) and 16 (3.7%).
Different post-hoc analyses were performed from the CIBIS-II study population (Erdmann et al 2001). In CIBIS-II, a significant and similar mortality reduction with bisoprolol was observed in the 539 CHF elderly patients (≥71 years) compared with younger patients (HR: 0.68 [0.48–0.97]). Although sudden death was not significantly reduced in the elderly population, rates of pump failure death and CHF hospitalizations were reduced, with a similar permanent treatment withdrawal as compared with younger patients. These results were confirmed by the meta-analysis of both CIBIS trials (Leizorovicz et al 2002).
Using the Cockroft Gault equation, 849 patients (32%) had renal impairment with a creatinine clearance <60 mL/min (Erdmann et al 2001). These patients had a similar benefit with bisoprolol compared with patients who had a greater creatinine clearance. However, the rate of permanent treatment withdrawal was significantly higher in patients with renal impairment, reaching almost 25% in patients with a creatinine clairance <60 mL/min and 40% in the 63 patients with a creatinine clearance <30 mL/min.
Some other studies have looked at the tolerability of bisoprolol in CHF patients. A small study showed a similar tolerability of the initiation of carvedilol and bisoprolol in 87 patients (Galatius et al 2004).
An observational study in elderly CHF patients analyzed the tolerability of bisoprolol. Patients included were older than 70 years, in chronic NYHA class II or III, receiving diuretics and a renin system inhibitor and having a LVEF <40%. As for CIBIS, the inclusion in the study required a period of 6 weeks of clinical stability before the introduction of bisoprolol (Baxter et al 2002). Baxter et al enrolled 51 patients with a mean age of 78 years, with 23 women. After the first dose of 1.25 mg of bisoprolol, the majority of the patients had a hypotension (86%); in 28 of these patients, blood pressure fell below 100 mmHg and in 16 patients the blood pressure fall was greater than 20 mmHg but with a systolic blood pressure >100 mmHg. Of interest, despite a great frequency of hypotension, only 4 patients, all having a blood pressure <100 mmHg, experienced symptoms and complained dizziness.
During the titration period, 35 patients tolerated bisoprolol (69%) with a mean dose of 7.6 mg/day. The mean reason for withdrawal was hypotension in 7 patients (including the 4 previous patients) and fatigue (3 other patients). Bradycardia was not a cause for bisoprolol withdrawal. Twenty-one patients tolerated 10 mg/day of bisoprolol and 9 received less than 5 mg/day. The main reasons for not reaching the target dose of 10 mg/day were hypotension in 7 cases, fatigue in 5 cases, and bradycardia in 1 case.
In conclusion, this study showed that in elderly CHF patients, hypotension is the major symptom leading to treatment withdrawal or to keep low doses of bisoprolol. However, if bisoprolol was well tolerated, it was possible to reach the target dose of 10 mg/day without problem.
Another study looked at the tolerability of bisoprolol after its initiation by the primary care physicians (Schuchert 2005). This prospective study included 328 patients with stable CHF receiving diuretics and renin inhibitors. Mean age was 63 ± 10 years, and 145 patients were in NYHA class III and 1 in class IV. The maximal tolerated dose was 7.2 ± 3.15 mg/day, 61% of the patients receiving at least 7.5 mg of bisoprolol. NYHA class significantly improved, from 2.4 ± 0.5 to 1.8 ± 0.6 (p < 0.0001) at the end of the 24 week study period. At the end, 74% of the patients had an improvement and only 5% a worsening in functional class. Bisoprolol was withdrawn in 57 patients (17%), of whom 40 related to adverse events. No patient had symptomatic bradycardia. This study demonstrated that bisoprolol could safely be introduced by primary care physician who did not have the same level of experience in CHF than physicians involved in the large mortality trials.
In summary, bisoprolol is well tolerated, even if in elderly patients treatment withdrawal seems to be more frequent than that observed in the large mortality trials. The main reason for not reaching the target dose of bisoprolol or for bisoprolol withdrawal is hypotension.
However, all the previous studies have excluded patients with resting heart rate <60 beats/min, patients with resting systolic blood pressure <100 mmHg and other main contra-indications to beta-blocker such as asthmatic patients.
In the future, it will be important to have more information about tolerance of bisoprolol in some subgroups of patients, in particular elderly patients (>75 years), patients with severe renal failure, patients with chronic obstructive pulmonary diseases, and patients in NYHA class IV.
Another important question is the management of CHF patients receiving chronic bisoprolol therapy and hospitalized for cardiac decompensation. Currently, the management of these patients depends on their clinical status and the experience of the practitioner. There are three possibilities: no modification, reduction of the doses of beta-blocker (and it is often a half reduction of the dose), or transitory interruption of the beta-blocker. An ongoing French study, B-Convinced, will try to answer to this important question. This study was designed as a non-inferiority trial comparing two strategies after an acute CHF decompensation: to stop or to pursue the beta-blocker.
Finally, another unresolved question is to know how long the beneficial effects of beta-blocker therapy in CHF patients will be maintained.
Conclusions
Bisoprolol fumarate is a potent, highly selective beta-1 adrenergic blocker. Large mortality trials have clearly demonstrated the beneficial effects of bisoprolol on mortality and on morbidity compared with placebo. These favorable effects are associated with a reverse remodelling of the left ventricle and a significant improvement in LVEF. Finally, bisoprolol is well tolerated, with a limited number of side effects leading to its permanent withdrawal.
References
- Baxter AJ, Spensley A, Hildreth A, et al. Beta blockers in older persons with heart failure: tolerability and impact on quality of life. Heart. 2002;88:611–4. doi: 10.1136/heart.88.6.611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bundkirchen A, Brixius K, Bolck B, et al. Beta 1-adrenoceptor selectivity of nebivolol and bisoprolol. A comparison of [3H]CGP 12.177 and [125I]iodocyanopindolol binding studies. Eur J Pharmacol. 2003;460:19–26. doi: 10.1016/s0014-2999(02)02875-3. [DOI] [PubMed] [Google Scholar]
- Chatterjee SS. The cardioselective and hypotensive effects of bisoprolol in hypertensive asthmatics. J Cardiovasc Pharmacol. 1986;8(Suppl 11):S74–7. doi: 10.1097/00005344-198511001-00013. [DOI] [PubMed] [Google Scholar]
- CIBIS-II Investigators and Committees. The Cardiac Insufficiency Bisoprolol Study II (CIBIS-II): a randomised trial. The Lancet. 1999;353:9–13. [PubMed] [Google Scholar]
- CIBIS Investigators and Committees. A randomized trial of beta-blockade in heart failure. The Cardiac Insufficiency Bisoprolol Study (CIBIS) Circulation. 1994;90:1765–73. doi: 10.1161/01.cir.90.4.1765. [DOI] [PubMed] [Google Scholar]
- Cleland JG, Daubert JC, Erdmann E, et al. The effect of cardiac resynchronization on morbidity and mortality in heart failure. N Engl J Med. 2005;352:1539–49. doi: 10.1056/NEJMoa050496. [DOI] [PubMed] [Google Scholar]
- de Groote P, Delour P, Lamblin N, et al. Effects of bisoprolol in patients with stable congestive heart failure. Ann Cardiol Angeiol (Paris) 2004;53:167–70. doi: 10.1016/j.ancard.2004.03.003. [DOI] [PubMed] [Google Scholar]
- Dorow P, Bethge H, Tonnesmann U. Effects of single oral doses of bisoprolol and atenolol on airway function in nonasthmatic chronic obstructive lung disease and angina pectoris. Eur J Clin Pharmacol. 1986;31:143–7. doi: 10.1007/BF00606650. [DOI] [PubMed] [Google Scholar]
- Dubach P, Myers J, Bonetti P, et al. Effects of bisoprolol fumarate on left ventricular size, function, and exercise capacity in patients with heart failure: analysis with magnetic resonance myocardial tagging. Am Heart J. 2002;143:676–83. doi: 10.1067/mhj.2002.121269. [DOI] [PubMed] [Google Scholar]
- Ennezat PV, Ennezat CA, Vijayaraman P, et al. Dissociation between improvement in left ventricular performance and functional class in patients with chronic heart failure. J Cardiovasc Pharmacol. 2005;46:262–8. doi: 10.1097/01.fjc.0000175235.33949.c4. [DOI] [PubMed] [Google Scholar]
- Erdmann E, Lechat P, Verkenne P, et al. Results from post-hoc analyses of the CIBIS II trial: effect of bisoprolol in high-risk patient groups with chronic heart failure. Eur J Heart Fail. 2001;3:469–79. doi: 10.1016/s1388-9842(01)00174-x. [DOI] [PubMed] [Google Scholar]
- Flather MD, Shibata MC, Coats AJ, et al. Randomized trial to determine the effect of nebivolol on mortality and cardiovascular hospital admission in elderly patients with heart failure (SENIORS) Eur Heart J. 2005;26:215–25. doi: 10.1093/eurheartj/ehi115. [DOI] [PubMed] [Google Scholar]
- Funck-Brentano C, Lancar R, Hansen S, et al. Predictors of medical events and of their competitive interactions in the Cardiac Insufficiency Bisoprolol Study 2 (CIBIS-2) Am Heart J. 2001;142:989–97. doi: 10.1067/mhj.2001.118741. [DOI] [PubMed] [Google Scholar]
- Funck-Brentano C, Lancar R, Le Heuzey JY, et al. Predictors of medical events in patients enrolled in the cardiac insufficiency bisoprolol study (CIBIS): a study of the interactions between beta-blocker therapy and occurrence of critical events using analysis of competitive risks. Am Heart J. 2000;139:262–71. doi: 10.1067/mhj.2000.101491. [DOI] [PubMed] [Google Scholar]
- Galatius S, Gustafsson F, Atar D, et al. Tolerability of beta-blocker initiation and titration with bisoprolol and carvedilol in congestive heart failure — a randomized comparison. Cardiology. 2004;102:160–5. doi: 10.1159/000080485. [DOI] [PubMed] [Google Scholar]
- Hunt SA, Abraham WT, Chin MH, et al. ACC/AHA 2005 Guideline Update for the Diagnosis and Management of Chronic Heart Failure in the Adult: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Update the 2001 Guidelines for the Evaluation and Management of Heart Failure): developed in collaboration with the American College of Chest Physicians and the International Society for Heart and Lung Transplantation: endorsed by the Heart Rhythm Society. Circulation. 2005;112:e154–235. doi: 10.1161/CIRCULATIONAHA.105.167586. [DOI] [PubMed] [Google Scholar]
- Kirch W, Rose I, Demers HG, et al. Pharmacokinetics of bisoprolol during repeated oral administration to healthy volunteers and patients with kidney or liver disease. Clin Pharmacokinet. 1987;13:110–7. doi: 10.2165/00003088-198713020-00003. [DOI] [PubMed] [Google Scholar]
- Lechat P, Hulot JS, Escolano S, et al. Heart rate and cardiac rhythm relationships with bisoprolol benefit in chronic heart failure in CIBIS II Trial. Circulation. 2001;103:1428–33. doi: 10.1161/01.cir.103.10.1428. [DOI] [PubMed] [Google Scholar]
- Leizorovicz A, Lechat P, Cucherat M, et al. Bisoprolol for the treatment of chronic heart failure: a meta-analysis on individual data of two placebo-controlled studies—CIBIS and CIBIS II. Cardiac Insufficiency Bisoprolol Study. Am Heart J. 2002;143:301–7. doi: 10.1067/mhj.2002.120768. [DOI] [PubMed] [Google Scholar]
- Leopold G, Pabst J, Ungethum W, et al. Basic pharmacokinetics of bisoprolol, a new highly beta 1-selective adrenoceptor antagonist. J Clin Pharmacol. 1986;26:616–21. doi: 10.1002/j.1552-4604.1986.tb02959.x. [DOI] [PubMed] [Google Scholar]
- McGavin JK, Keating GM. Bisoprolol: a review of its use in chronic heart failure. Drugs. 2002;62:2677–96. doi: 10.2165/00003495-200262180-00017. [DOI] [PubMed] [Google Scholar]
- MERIT-HF study group. Effect of metoprolol CR/XL in chronic heart failure: Metoprolol CR/XL randomised intervention trial in congestive heart failure (MERIT-HF) The Lancet. 1999;353:2001–7. [PubMed] [Google Scholar]
- Packer M, Coats AJ, Fowler MB, et al. Effect of carvedilol on survival in severe chronic heart failure. N Engl J Med. 2001;344:1651–8. doi: 10.1056/NEJM200105313442201. [DOI] [PubMed] [Google Scholar]
- Pfeffer MA, Braunwald E, Moye LA, et al. Effect of captopril on mortality and morbidity in patients with left ventricular dysfunction after myocardial infarction. Results of the survival and ventricular enlargement trial. The SAVE Investigators. N Engl J Med. 1992;327:669–77. doi: 10.1056/NEJM199209033271001. [DOI] [PubMed] [Google Scholar]
- Pitt B, Zannad F, Remme WJ, et al. for the Randomized Aldactone Evaluation Study Investigators. The effect of spironolactone on morbidity and mortality in patients with severe heart failure. N Engl J Med. 1999;341:709–17. doi: 10.1056/NEJM199909023411001. [DOI] [PubMed] [Google Scholar]
- Poole-Wilson PA, Swedberg K, Cleland JG, et al. Comparison of carvedilol and metoprolol on clinical outcomes in patients with chronic heart failure in the Carvedilol Or Metoprolol European Trial (COMET): randomised controlled trial. Lancet. 2003;362:7–13. doi: 10.1016/S0140-6736(03)13800-7. [DOI] [PubMed] [Google Scholar]
- Schuchert A. Effects of bisoprolol treatment for chronic heart failure initiated and followed up by primary care physicians. Eur J Heart Fail. 2005;7:604–11. doi: 10.1016/j.ejheart.2004.03.008. [DOI] [PubMed] [Google Scholar]
- Simon T, Mary-Krause M, Funck-Brentano C, et al. Bisoprolol dose-response relationship in patients with congestive heart failure: a sub-group analysis in the cardiac insufficiency bisoprolol study(CIBIS II) Eur Heart J. 2003;24:552–9. doi: 10.1016/s0195-668x(02)00743-1. [DOI] [PubMed] [Google Scholar]
- Smith C, Teitler M. Beta-blocker selectivity at cloned human beta 1- and beta 2-adrenergic receptors. Cardiovasc Drugs Ther. 1999;13:123–6. doi: 10.1023/a:1007784109255. [DOI] [PubMed] [Google Scholar]
- Swedberg K, Cleland J, Dargie H, et al. Guidelines for the diagnosis and treatment of chronic heart failure: executive summary (update 2005): The Task Force for the Diagnosis and Treatment of Chronic Heart Failure of the European Society of Cardiology. Eur Heart J. 2005;26:1115–40. doi: 10.1093/eurheartj/ehi204. [DOI] [PubMed] [Google Scholar]
- Swedberg K, Hjalmarson A, Waagstein F, et al. Prolongation of survival in congestive cardiomyopathy by beta-receptor blockade. Lancet. 1979;1:1374–6. doi: 10.1016/s0140-6736(79)92010-5. [DOI] [PubMed] [Google Scholar]
- The Beta-Blocker Evaluation of Survival Trial Investigators. A trial of the beta-blocker bucindolol in patients with advanced chronic heart failure. N Engl J Med. 2001;344:1659–67. doi: 10.1056/NEJM200105313442202. [DOI] [PubMed] [Google Scholar]
- The CONSENSUS Trial Study Group. Effects of enalapril on mortality in severe congestive heart failure. Results of the Cooperative North Scandinavian Enalapril Survival Study (CONSENSUS) N Engl J Med. 1987;316:1429–35. doi: 10.1056/NEJM198706043162301. [DOI] [PubMed] [Google Scholar]
- The SOLVD Investigators. Effect of enalapril on survival in patients with reduced left ventricular ejection fractions and congestive heart failure. The SOLVD Investigators. N Engl J Med. 1991;325:293–302. doi: 10.1056/NEJM199108013250501. [DOI] [PubMed] [Google Scholar]
- The SOLVD Investigators. Effect of enalapril on mortality and the development of heart failure in asymptomatic patients with reduced left ventricular ejection fractions. The SOLVD Investigattors. N Engl J Med. 1992;327:685–91. doi: 10.1056/NEJM199209033271003. [DOI] [PubMed] [Google Scholar]
- Waagstein F, Hjalmarson A, Varnauskas E, et al. Effect of chronic beta-adrenergic receptor blockade in congestive cardiomyopathy. Br Heart J. 1975;37:1022–36. doi: 10.1136/hrt.37.10.1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wellstein A, Palm D, Belz GG, et al. Concentration kinetics of propranolol, bisoprolol, and atenolol in humans assessed with chemical detection and a subtype-selective beta-adrenoceptor assay. J Cardiovasc Pharmacol. 1986;8(Suppl 11):S41–5. doi: 10.1097/00005344-198511001-00007. [DOI] [PubMed] [Google Scholar]
- Willenheimer R, van Veldhuisen DJ, Silke B, et al. Effect on survival and hospitalization of initiating treatment for chronic heart failure with bisoprolol followed by enalapril, as compared with the opposite sequence: results of the randomized Cardiac Insufficiency Bisoprolol Study (CIBIS) III. Circulation. 2005;112:2426–35. doi: 10.1161/CIRCULATIONAHA.105.582320. [DOI] [PubMed] [Google Scholar]


