Abstract
Hypertensive diabetes individuals are at higher risk for cardiovascular events and progression to end stage renal disease. Several well conducted clinical trials indicate that aggressive treatment of hypertension in individual with diabetes reduces these complications. Combinations of two or more antihypertensive drugs are frequently required to reach the target blood pressure and to improve the cardiovascular and renal outcomes in these patients. There are physiological and clinical rationales for renin-angiotensin system blockade in hypertensive diabetics. Trandolapril/verapamil sustained released (SR) is a fixed-dose combination of trandolapril and a sustained release formulation of verapamil and indicated in treatment of hypertension in patients who require more than one drug to reach target blood pressure. The antihypertensive efficacy of trandolapril/verapamil SR has been evaluated extensively in large trials. In the INVEST trial, a verapamil SR-based treatment strategy that included trandolapril in most patients was effective in reducing the primary outcome in hypertensive patients with coronary artery disease. The new onset of diabetes was also significantly lower in the verapamil SR/trandolapril treatment group in comparison with those on the atenolol/hydroclorothiazide treatment group. The BErgamo NEphrologic DIabetes Complications Trial (BENEDICT) documented that in hypertensive diabetes and normoalbuminuria, trandolapril plus verapamil or trandolapril alone delayed the onset of microalbuminuria independent of their blood pressure-reducing effect. Thus, trandolapril/verapamil is an effective option for treatment of hypertensive diabetes patients requiring more than one agent to achieve target blood pressure.
Keywords: diabetes mellitus, hypertension, trandolapril, verapamil SR
Introduction
Diabetes is a rapidly growing health problem worldwide, related in part to improved living conditions and increasing rate of obesity (World Health Report 2003). It is estimated that approximately 5% of people in the general population of most industrialized societies have diabetes mellitus and that an additional 3%–5% have either undiagnosed diabetes or impaired glucose tolerance. According to the World Health Organization, worldwide the number of people living with diabetes is projected to increased from 172 million in 2000 (prevalence: 2.8%) to 366 million (prevalence: 4.4%) in 2030 (Wild et al 2004). Hypertension is a common co-morbid condition in diabetes and found in 20%–60% of patients with diabetes (American Diabetes Association 2004). Prevalence of hypertension in the diabetic population is 1.5–3 times higher than in the age- and weight-adjusted non-diabetic group (Hypertension in Diabetes Study 1993). In type 2 diabetes, hypertension is often present as a part of metabolic syndrome, while in type 1 diabetes it may herald the onset of diabetic kidney disease. The hypertensive diabetic individuals are at a 2- to 4-fold greater risk of vascular complications, such as coronary artery disease, cerebrovascular accident, and death compared with age-matched control subjects and with the patients with type 2 diabetes mellitus but normal blood pressure. Similarly, they also have a 7-fold greater risk of progression to end stage renal disease (ESRD) (Perneger et al 1994; Beckman 2002). Reduction of high blood pressure reduces cardiovascular morbidity and mortality and delays the progression to ESRD. Indeed, various studies has shown that lowering blood pressure in high risk patients with diabetes reduces overall mortality (Haansoon et al 1998; UK Prospective Diabetes Study Group 1998; Waeber 2003), death from stroke (Curb 1996), and cardiovascular events (Haansoon et al 1998; Tatti et al 1998) and slows the progression of renal disease in patients with type 2 diabetes mellitus (Brenner et al 2001; Parving et al 2001; Berl et al 2003). Consensus statements and guidelines from various international authorities recommend initiation of pharmacological therapy with the goal of reduction of blood pressure to <130/80 mmHg (National Kidney Foundation 2002; American Diabetes Association 2004) and/or <130/85 mmHg (Chobanian et al 2003) and consider diabetes as a risk equivalent similar to history of myocardial infarction (Chobanian et al 2003) because of the increased risk of cardiovascular events in these groups of patients. Evidence has shown that achieving this goal requires multiple drug antihypertensive therapy. Indeed, because of the fact that blood pressure reduction to recommended goal is often difficult to achieve with monotherapy, the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure (JNC) (Chobanian et al 2003) suggests initial use of a two-drug combination, even in non-diabetic patients. Greater efficacy is likely to be achieved when two or more antihypertensive agents with complementary modes of action are used to reduce blood pressure. This approach is also likely to reduce adverse effects of drugs, as both drugs can be given at a lower dose than either drug as monotherapy. In addition, combination of two fixed-dose agents in a single capsule or tablet is expected to increase compliance (Sica 2002). In treatment of hypertensive diabetic patients adverse effects on metabolic control and insulin sensitivity is an issue to be considered. Calcium channel blockers and angiotensin-converting enzyme (ACE) inhibitors are considered lack of undesirable effect on glucose tolerance and insulin sensitivity; rather potentially improve insulin sensitivity (Sutter et al 1995; Holzgreve et al 2003).
Trandolapril/verapamil sustained released (SR) is a fixed-dose combination of trandolapril, and a sustained release formulation of verapamil (Abbott Laboratories 2003a). This article provides an overview of trandolapril/verapamil SR in the treatment of hypertensive diabetic patients and its effectiveness on reduction of microalbuminuria in hypertensive type 2 diabetic patients.
Pharmacodynamics properties of trandolapril/verapamil SR
Trandolapril is an ethyl ester pro-drug of a nonsufyldril ACE inhibitor. It is converted to trandolaprilat by de-esterfication. The active metabolite is 8 times more active as an inhibitor of ACE. Trandolapril inhibits the circulating and tissue ACE, leading to inhibition of conversion of angiotensin I to angiotensin II that results in a decrease in vasoconstriction, a decrease in aldoesteron secretion, and an increase in plasma renin (Muijsers et al 2002). Although, the principal mechanism of trandolapril is thought to be through the renin-angiotensin-aldosteron system, trandolapril is effective in low renin hypertension also (Abbott Laboratories 2003a). Verapamil, an L-type calcium channel blocker, exerts its pharmacological effect by blocking the calcium influx through calcium channel. This effect leads to dilatation of peripheral vessels, thereby decreasing systemic vascular resistance and blood pressure. It also dilates coronary blood vessels (Muijsers et al 2002).
Trandolapril/verapamil SR is an oral fixed-dosed combination of slow release verapamil hydrochloride, and an immediate-release formulation of trandolapril. It is indicated in the treatment of hypertension in a patient who requires more than one agent to achieve blood pressure target. Studies of verapamil with various ACE inhibitors indicate that the combination should be the therapy of choice in hypertensive patients with diabetes or nephropathy (Haegert et al 1987; Bakris et al 1992; Choi et al 2005). The complementary pharmacological action of ACE inhibitor and calcium antagonist on efferent and afferent arterioles also results in a beneficial effect on glomerular function. It also exerts additive inhibition of sodium reabsorption via inhibition of aldosterone secretion and a direct tubular effect. The combination of trandolapril and verapamil may also provide additional benefit in inducing the regression of left ventricular hypertrophy (Widimsky 2000; Raynolds et al 2005). Combination therapy also significantly increases left ventricular ejection fraction and left ventricular wall motion index in hypertensive patients (Widimsky 2000) and duration of exercise time in patients with coronary heart disease and left heart failure. It also leads to an improvement in the ratio of exercise to rest rate-pressure product and a decrease in the number of angina attacks (Widimsky 2000; Raynolds et al 2005). The trandolapril/verapamil treatment was also found to reduce incidence of cardiac events in comparison with trandolapril therapy in patients with congestive heart failure after acute myocardial infarction (Hansen et al 1997). The combination therapy significantly reduced death, re-infarction, unstable angina, or readmission due to increasing congestive heart failure. Similarly, the combination therapy was also found to have a positive effect on aortic elastic properties (Breithaupt-Grogler et al 1998; Topouchian et al 1999; Romos et al 2001). In the patients with hypertension and diabetes mellitus, trandolapril/verapamil combination does not adversely affect glucose and lipid metabolism (Fernández et al 1996; Schneider et al 1996; Holzgreve et al 2003). Rather, data from short-term and long-term studies indicate that ACE inhibitors may actually improve insulin sensitivity and decrease the risk of hypertensive diabetic patients (Bakris et al 1992; The Heart Outcome Prevention Evaluation Study Investigators 2000; Sowers et al 2000). As worsening/inadequate glycemic control has been shown to be a strong risk factor for both micro- and macro-vascular complications in diabetes, antihypertensive drug without adverse effects on glucose metabolism, such as trandolapril/verapamil combination, in hypertensive diabetic patients offers an advantage provided blood pressure control is adequate.
Pharmacokinetics trandolapril/verapamil SR
There is no known kinetics interaction between verapamil and trandolapril or trandolaprilate. The single agent pharmacokinetic parameters of these two drugs should be applicable to a combination product also. However, when administered concomitantly, trandolapril increases the peak plasma concentration (Cmax) of verapamil and norverapamil but time to Cmax (Tmax) remains unaffected (Abbott Laboratories 2003a).
Trandolapril component
The pharmacokinetic parameters of single and multiple doses of trandolapril in healthy volunteers are summarized in Table 1. However, there are very few data on pharmacokinetics of trandolapril/verapamil in patients with hypertension, more so for the hypertensive diabetic patients. Most of the information is derived from the manufacturer’s information (Abbott Laboratories 2002b, 2003a). After oral administration trandolapril is absorbed rapidly. Although, absorption is 40%–60%, absolute bioavailability of trandolapril is 10% of the oral dose. The absolute bioavailability of trandolaprilate is 70% (Abbott Laboratories 2003b). Food does not interfere with absorption of the drug. Cmax of trandolapril after a single oral dose is proportional to the dose administered and occurs at around a half to 1 hour (Bevan et al 1993; Arner et al 1994; Lenfant et al 1994). After the oral dose the Cmax of the trandolaprilate is dose proportional, but inversely related. Tmax ranges from 4 to 8 hours (Bevan et al 1993; Arner et al 1994; Lenfant et al 1994). The elimination half-life (t1/2) is less than 1 hour for trandolapril, whereas it is about 75 hours for trandolaprilate (Bevan et al 1993; Arner et al 1994; Lenfant et al 1994; Siepman et al 1997). Plasma protein binding of both trandolapril and trandolaprilate is more than 80% (Abbott Laboratories 2003b).
Table 1.
Pharmacokinetic properties of trandolapril (T) in healthy volunteers
| Dosage | Duration | Cmaxa (mg/mL) | Tmaxb (hour) | AUC ng/mL (hour) | T1/2 (hour) |
|---|---|---|---|---|---|
| T 0.5 mg | Single dose | 0.43 (0.1)* | 1# | 0.4 (0.11)* | 0.71 (0.08)* |
| T 1 mg | Single dose | 0.86 (0.1)* | 0.5# | 0.95 (0.15)* | 0.74 (0.09)* |
| T 2 mg | Single dose | 1.68 (0.33)* | 0.5# | 1.86 (0.3)* | 0.68 (0.05)* |
| T 4 mg | Single dose | 3.32 (0.56)* | 0.5# | 3.64* (0.44)* | 0.76 (0.13)* |
| T 2 mg | qd for 10 days | 3.19 (0.33)* | 1# | 3.67 (0.97)* | 0.68 (0.07)* |
Cmax, peak plasma concentration.
Tmax, time to Cmax
Mean.
Median.
Trandolapril is de-esterified to trandolaprilate and this biotransformation takes place mainly in liver (Abbott Laboratories 2003b). Steady state is reached after about 4 days with multiple oral doses. The effective half-life calculated from accumulation is 16–24 hours (Abbott Laboratories 2003b). The drug is eliminated from the body predominantly (2/3rd) by fecal route, and one third in the urine. Most of the radioactivity of orally administered radiolabeled trandolapril is excreted after 48 hours and elimination is complete (~99%) by 7 days (Wiserman et al 1994)
Renal clearance of trandolapril and trandolaprilate exhibit a linear correlation with creatinine clearance and the clearance of trandolapril and trandolaprilate decrease with decreasing renal function (Abbott Laboratories 2002b, 2003b). Two studies have evaluated the pharmacokinetics of trandolapril and trandolaprilate in patients with chronic renal failure and compared that with volunteers (Bevan et al 1993; Danielson et al 1994). Compared with normal subjects, the plasma concentration of trandolapril and trandolaprilate are approximately 2-fold greater and renal clearance is reduced by about 85% in patient with creatinine clearance below 30 mL/min and in patients on hemodialysis (Abbott Laboratories 2003b). In people with moderate to severe hepatic impairment the plasma concentration of trandolapril is increased by 9-fold. The plasma concentration of trandolaprilate is also increased but to a lesser extent. However, inhibition of ACE activity was not affected (Abbott Laboratories 2002b, 2003b). In people above 65 years of age, the bioavailability of trandolapril is increased by 25%. However, dose adjustment is not recommended (Abbott Laboratories 2003b; Raynolds et al 2005).
Verapamil component
Similar to the immediate-release formulation, approximately 90% of the administered sustained released formulation is absorbed (Abbott Laboratories 2003a). However, the rate of absorption is delayed. Because of first-pass metabolism of verapamil, the absolute bioavailability of the drug ranges from 20% to 35% (Abbott Laboratories 2002a, 2003a). Following administration of verapamil SR 240 mg, the mean peak plasma concentration is reached at 5 hours, while that of its active metabolite norverapamil in around 6 hours (Abbott Laboratories 2002a, 2003b). Steady state plasma concentration is reached 3–4 days after multiple oral doses of verapamil. Verapamil is 90% bound to plasma proteins (Abbott Laboratories 2003b). Verapamil undergoes extensive first-pass metabolism in the liver by the P450 cytochrome system (Abbott Laboratories 2002b). Out of 12 metabolites of verapamil, except the primary active metabolite norverapamil, all other metabolites are present in trace amount (Abbott Laboratories 2002a). The mean elimination half-life is around 8 hours following multiple doses administration (Abbott Laboratories 2002b). Most of the administered verapamil is excreted as metabolites in urine (70%) and feces (16%). Three to four percent of administered dose is excreted unchanged (Abbott Laboratories 2003b). As verapamil undergoes extensive first-pass metabolism, presence of hepatic dysfunction increases the bioavailability and elimination half-life of verapamil (Abbott Laboratories 2002b). In patient with severe hepatic dysfunction, the clearance is reduced to 30%, while terminal elimination half-life is prolonged by 4–16 hours (Abbott Laboratories 2002a, 2003a). The pharmacokinetics and elimination of verapamil is not altered by impairment of kidney function (Abbott Laboratories 2002a; Danielson et al 1994). Compared with younger subjects, the bioavailability of verapamil and its metabolite norverapamil is increased by 87% and 77%, respectively, in the elderly (Abbott Laboratories 2003a).
The trandolapril/verapamil SR combination has not been evaluated in subjects with hepatic or renal impairment. In Europe, it is contraindicated in patients with creatinine clearance of less than 10 mL/min, patients receiving hemodialysis, and patients with severe hepatic impairment, cirrhosis, and ascites (Abbott Laboratories 2002b, 2003a), while in the US, caution is required to use the combination in patients with hepatic impairment (Abbott Laboratories 2003a). The special instructions for the individual compounds of the combination of trandolapril and verapamil SR apply to the combination product also (Abbott Laboratories 2002b, 2003a).
In the elderly, systemic availability of trandolapril/verapamil SR is higher than in younger subjects; therefore blood pressure of some elderly patients might be lowered more than that of younger subjects (Abbott Laboratories 2002b)
Drug interactions
There are several important pharmacodynamic and pharmacokinetic interactions between these two classes of drugs, as with a number of other drugs (Abbott Laboratories 2002a, 2003b; Sweetman 2005). Although co-prescription of trandolapril/verapamil SR with potassium-sparing diuretics or supplementation of potassium may cause hyperkalemia due to trandolapril, the use of dantrolin with verapamil may cause malignant hyperthermia, and is therefore not recommended (Abbott Laboratories 2002b, 2003b; Sweetman 2005). A numbers of drugs have a potential pharmacodynamic interaction with trandolapril or verapamil SR; therefore, close monitoring or dose adjustment is needed (Abbott Laboratories 2002a; Abbott Laboratories 2002b; Abbott Laboratories 2003b; Abbott Laboratories 2003b). Few important pharmacokinetic interactions are tabulated (Table 2). Trandolapril and its active metabolite do not have clinically significant interactions with furosemide and warfarin (Abbott Laboratories 2003b). The details of the pharmacodynamic and pharmacokinetic interactions of the combination may be found in the manufacturer’s prescribing information (Haegert et al 1987; Abbott Laboratories 2002b; Abbott Laboratories 2003b; Sweetman 2005).
Table 2.
Pharmacokinetic interaction between oral trandolapril or verapamil SR and other agents when administered concomitantly (only clinically relevant interactions are cited)
| Effects of verapamil-SR on pharmacokinetics of | |
|---|---|
| Carbamazapine | Increases the plasma concentration of carbamazapin |
| Cyclosporine | Increases the serum concentration of cyclosporine |
| Digoxin | Increases the serum concentration of digoxin |
| Ethanol | Verapamil decreases the elimination of ethanol |
| Metoprololo/propanolol | Verapamil decreases the clearance |
| Quinidine | Increases the serum concentration of quinidine |
| Effects of trandolapril on | |
| Lithium | Trandolapril (ACE inhibitors) increase serum concentration of lithium |
| Effects of other agents on verapamil SR pharmacokinetics | |
| Cimetidine | Increase in verapamil clearance is possible |
| Erythromycin | Increases plasma level of verapamil due to inhibition of CYP3A4 inhibition |
| Grapefruit juice | Increases plasma concentration of verapamil |
| Phenobarbitone | Increases verapamil clearance |
| Rifampicine | Decreases verapamil bioavailability due to CYP3A4 induction |
| Ritonavir | Increases plasma level of verapamil due to inhibition of CYP3A4 inhibition |
| Effects of other agents on trandolapril pharmacokinetics | |
| Antacids | Decreases the bioavailability of trandolapril |
Therapeutic efficacy
The antihypertensive efficacy of trandolapril/verapamil SR has been evaluated extensively in large randomized, double-blind or open-labeled blinded endpoint, multicenter studies (de leeuw PW et al 1997; DeQuattro V 1997; The Veratran study group 1997; Viskoper 1997; Kalberge et al 2000; Papine et al 2003). In the INVEST trial (Papine et al 2003) a verapamil SR-based treatment strategy that included trandolapril in most patients was effective in reducing the primary outcome in hypertensive patients with coronary artery disease (Papine et al 2003). Several subgroup analyses of INVEST (Bakris et al 2004; Mancia et al 2004; Reid et al 2005) have been published. Of note, the risk of new onset diabetes was lower in those receiving verapamil SR and trandolapril therapy. INVEST was a randomized, open-label, blinded, endpoint study that involved 22,576 patients aged ≥50 years with hypertension and coexisting coronary artery disease (CAD). A total of 16,176 patients were non-diabetic hypertensives with CAD at the time of entry to the trial. The primary outcome – all-cause mortality, non-fatal stroke, and non-fatal myocardial infarction – was compared between two treatment strategies: a calcium channel blocker (verapamil SR)-based strategy and beta blocker (atenolol)-based startegy. As most older hypertensives need two or more antihypertensive drugs to control blood pressure, INVEST was intended to compare the multidrug strategies rather than individual agents. To reach the blood pressure goals targeted according to JNC VI at the time of initiation of the trial, adding sequentially prespecified antihypertensive medications to both treatment arms was allowed in the trial. The verapamil arm allowed for the addition of the ACE inhibitor trandolapril or of a co-formulated tablet containing verapamil SR and trandolapril. The diuretic hydrochlorothiazide could then be added if needed. The beta-blocker arm allowed for the addition of hydrochlorothiazide, with trandolapril added as required to reach goal. ACE inhibitor therapy was recommended for all patients with diabetes or renal disease, regardless of treatment strategy.
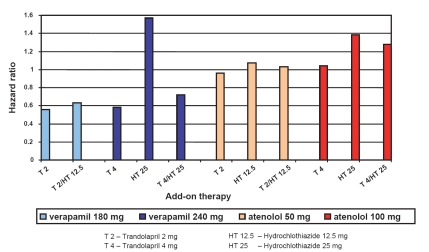
Among the patients who did not have diabetes at baseline (8098 in the verapamil SR group and 8078 in the atenolol group), the new onset of diabetes was significantly lower (7.03%) in the verapamil SR/trandolapril treatment group in comparison with those on the atenolol/hydroclorothiazide treatment group (8.23%) (Figure 1). Treatment with hydrochlorothiazide 25 mg daily was associated with new diabetes in both strategies, whereas increased exposure to the ACE inhibitor trandolapril in verapamil SR strategy appeared to be associated with more protection from the atenolol/hydrochlorothiazide strategy (Abuissa 2005). However, it is difficult to draw a conclusion about the contribution of any single agent because the trial was a comparison of both arms with more than one drug regimen (Alderman 2003). So it is also not possible to evaluate the independent role of verapamil in INVEST, and it cannot be excluded that the benefit is driven by the trandolapril alone, as it is more likely that the ACE inhibitor prevents diabetes by blocking the renin angiotensin system. While antihypertensive treatment strategy in the diabetes cohort of the INVEST trial was compared between the verapamil SR-based (n = 3169) treatment arm and the atenolol-based (n = 3231) treatment arm, there was no significant difference in the primary outcome between these two treatment groups.
Figure 1.
Among the patients who did not have diabetes at baseline (8098 in the verapamil SR group and 8078 in the atenolol group) addition of trandolapril in verapamil SR strategy was associated with more protection from the development of new onset diabetes than that in the atenolol/hydrochlorothiazide strategy arm. When trandolapril 2 mg was added to verapamil 180 mg and trandolapril 4 mg to verapamil 240 mg the hazard ratio for development of new onset diabetes was 0.56 (0.98–1.64, confidence interval [CI] 95%), and 0.58 (0.44–0.78, CI 95%) respectively.
Bakris et al (1998) reported for the first time that combination of a non-dihydorcalcium channel blocker (verapamil) with an ACE inhibitor (trandolapril) achieved better proteinuria reduction in comparison with either agent alone in a randomized, open-labeled, parallel group study. Mean arterial pressure was lower by 3–4 mmHg in the combination group than in the group receiving either agent alone. The effect of combinations of antihypertensive drugs on metabolic control and albuminuria in type 2 diabetic patients has also been studied in the TRAVEND trial. The group of patients receiving the verapamil/trandolapril combination had better metabolic control than enalapril/hydrochlorothiazide group (0.45% difference in HbA1c levels; p = 0.04) (Fernández et al 2001). The efficacy related to reduction of albuminuria and blood pressure was comparable in both groups.
A fixed combination of trandolapril/verapamil was compared with trandolapril alone or placebo to attain the required blood pressure goal in previously untreated type 2 diabetic patients presenting with either high normal blood pressure or first stage of isolated hypertension in a randomized, double-blind, placebo-controlled, comparative multicenter study (Ruilope et al 2004). A total of 393 patients completed the study. Blood pressure reduction was better in patients treated with either trandolapril or fixed combination of verapamil/trandolapril than in the placebo group. The mean difference in systolic blood pressure from placebo was 7.1 mmHg (3.3–10.9, 95% CI, p < 0.001) for trandolapril and 7.8 mmHg (3.9–11.6, 95% CI, p < 0.001) for the fixed-dose combination group with no statistically significant difference between the two groups. Combination treatment was also more effective in reducing the diastolic blood pressure than placebo. No significant difference was found between the fixed-combination verapamil/trandolapril and trandolapril with regards to control of systolic blood pressure, but diastolic blood pressure control was significantly higher in the fixed-combination verapamil/trandolapril group (88%) when compared with trandolapril alone (79.1%) or placebo (63.5%).
The issues of metabolic effects of antihypertensive therapy on diabetic patients were also evaluated in various trials. There is increasing concern regarding the metabolic effects of antihypertensive drug therapy and their impact on cardiovascular risk reduction resulting from the treatment of hypertension (Sowers 1995). Studies by Berne et al (1991) have indicated that ACE inhibitors improve glucose use and insulin sensitivity in hypertensive patients with type 2 diabetes. The metabolic, antihypertensive, and albuminuria-modifying effects of the trandolapril/verapamil combination compared with those of a β-blocker low-dose diuretic combination (atenolol/chlortalidone) in hypertensive type 2 diabetic patients was investigated in 2 separate studies (Schneider et al 1996; Ruilope 2002). The two approaches produced similar decrease in mean supine clinic blood pressure (BP), upright clinic BP, and ambulatory daytime BP. The trandolapril/verapamil combination was found to be metabolically neutral, while the atenolol/chlortalidone combination further aggravated insulin resistance. This indicates that the trandolapril/verapamil combination is a potentially valuable therapy for hypertension accompanying type 2 diabetes, since the benefits of the ACE inhibitor may be amplified by providing superior BP control with added renal and cardiovascular protection.
In a further study, in patients with hypertension and type 2 diabetes, the effects of either fixed-dose combination of trandolapril/verapamil or a combination of atenolol/clorthalidone, on glycosylated hemoglobin (HbA1c) and other metabolic parameters was evaluated. The trandolapril/verapamil group had lower HbA1c concentration compared with patients treated with atenolol/chlortalidone (7.8 vs 8.6%; p = 0.001), demonstrating a significantly more favorable profile on glycemic control (Holzgreve et al 2003). The data of all these studies make the combination trandolapril/verapamil very attractive in hypertensive diabetic patients.
The high prevalence of diabetes, the increased rate of cardiovascular and renal complications, and the beneficial effect of antihypertensive therapy make primary prevention efforts a high priority. Hypertensive diabetes individuals are at high risk of developing cardiovascular disease and progression to ESRD. Hypertension, in addition, to obesity, hyperglycemia, dyslipidemia, and microalbuminuria, is a component of the metabolic syndrome – a syndrome sustained by decreased insulin activity that almost invariably precedes or accompanies the onset of type 2 diabetes and is independently associated with excessive cardiovascular morbidity and mortality (Flack et al 1991). Now, numerous studies have documented that the albumin excretion in the urine above the normal range is clearly associated with heightened risk of cardiovascular events and further progression of kidney disease. Every year 2%–5% of the type 2 diabetic patients develop microalbuminuria (Gall 1997; Alder et al 2003) and, unlike type 1 diabetes mellitus, it is seldom reversible in type 2 diabetic patients and progresses to overt proteniuria in 20%–40% of patients (Mogensen 1984; Nelson et al 1991). Forty to fifty percent of patients with type 2 diabetes having microalbuminuria eventually die of cardiovascular disease (Alder et al 2003). Therefore, it is quite reasonable to target the treatment to modify the development of microalbuminuria at early stage of diabetes, as well as control blood pressure in hypertensive diabetes to limit the cardiovascular and renal disease. The Bergamo Nephrologic Diabetes Complications Trial (BENEDICT [Ruggenenti et al 2004]) was designed to test the hypothesis that combination therapy with the ACE inhibitor trandolapril and non-dihydropyridine calcium channel blocker verapamil reduced the incidence of persistent microalbuminuria in hypertensive type 2 diabetic patients with no clinical evidence of diabetic nephropathy. The secondary objectives involved comparisons of trandolapril or verapamil with placebo in providing renal protection in hypertensive diabetic patients.
In the BENEDICT study a total of 1204 patients with hypertension (systolic or diastolic blood pressure more than 130 or 85 mmHg, respectively, or concomitant antihypertensive therapy), type 2 diabetes and normal urinary excretion (<20 mg/min in at least 2 of 3 consecutive overnight urine collections) were randomly assigned, using 2 × 2 factorial design, to receive in a double blind manner for at least 3 years one of the following study drugs: I, a non-dihydropyridine calcium channel blocker (ndCCB): verapamil SR, 240 mg/day; II, an ACE inhibitor: trandolapril 2 mg/day; III, the combination of verapamil SR, 180 mg/day plus trandolapril 2 mg/day: VeraTran; and IV, placebo. The target blood pressure after randomization and throughout the whole study period was less than 130/80 mmHg for all the treatment groups. Other antihypertensive drugs (with the exception of renin-angiotensin system inhibitors and ndCCBs different from the study drugs) could be used to achieve and maintain systolic and diastolic blood pressure consistently below 140/90 mmHg (subsequently amended to below 130/80 mmHg at randomization and below 120/80 on follow up (The BENEDICT Group 2003; Ruggenenti et al 2004). Baseline characteristics and simultaneous treatments of patients randomized in the four treatment groups were comparable. Patients were to be maintained in metabolic control (target HbA1c <7%). Patients were excluded if they had concomitant non-diabetic renal disease or heart failure (NYHA-IV or on ACE inhibition therapy), cardiovascular events (stroke, transient ischemic attack, unstable angina) in the last 3 months before randomization, severe hypertension (diastolic blood pressure ≥115), systemic diseases, or any major clinical condition that jeopardize study participation. Patients with specific contraindication to the study drug were also excluded.
The BENEDICT study found that over 4 years of follow-up, trandolapril alone, or trandolapril/verapamil SR (VeraTran) delayed the onset of microalbuminuria by a factor of 2.6 and 2.1, respectively, while verapamil had no significant effect (Ruggenenti et al 2004). Thus the incidence of microalbuminuria versus placebo was reduced by 60% (hazard ratio [95% CI]: 0.39 [0.21–0.73]) with VeraTran and by 50% with trandolapril (Hazard ratio [95% CI]: 0.49 [0.27–0.90]), but was not appreciably affected by verapamil (Figure 2). This indicates that the apparent advantage of ACE inhibitors (trandolapril in BENEDICT) over other antihypertensive agents includes also a protective effect on the kidney against the development of microalbuminuria, at least in type 2 diabetic patients. Moreover, finding that this effect was significant even after adjustment for baseline and follow-up systolic and diastolic blood pressure provided evidence of a specific renoprotective effect of ACE inhibition therapy against the development of microalbuminuria which was independent of the level of achieved blood pressure control.
Figure 2.
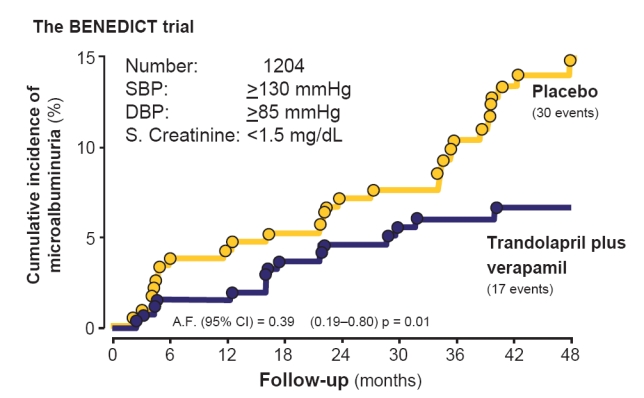
The BErgamo NEphrologic DIabetes Complications Trial (BENEDICT) is a multicenter double-blind, randomized study designed to assess whether the angiotensin converting enzyme inhibitor trandolapril and the non-dihydropyridine calcium-channel blocker verapamil, alone or in combination, prevent microalbuminuria in subjects with hypertension, type 2 diabetes mellitus, and normal urinary albumin excretion. The Kaplan-Meier curves show the percentages of subjects with microalbuminuria during treatment with trandolapril/verapamil or placebo. The difference between the two groups adjusted for pre-specified baseline covariates was significant (p = 0.01) according to the accelerated failure (A.F.)-time model.
Abbreviations: DBP, diastolic blood pressure; SBP, systolic blood pressure.
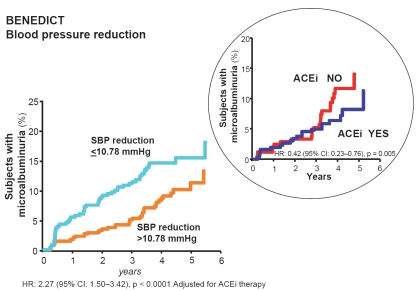
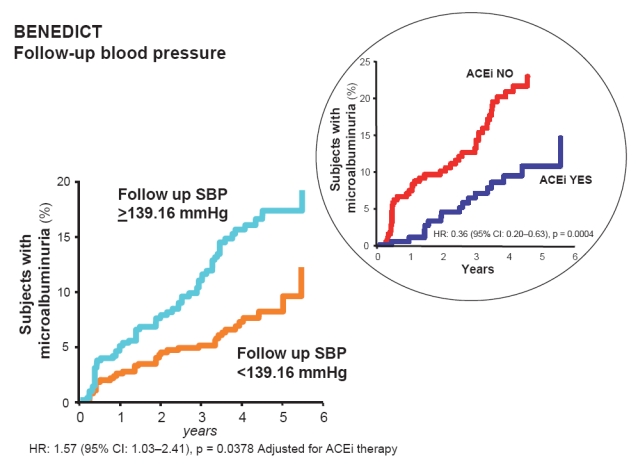
Recently the post-hoc analysis of the BENEDICT trial was reported (Ruggenenti et al 2006). Of 1204 patients in the original study cohort, 1180 patients were included in the post-hoc analysis. Twenty-four patients were excluded because either they reached an endpoint, stopped regular study follow-up or blood pressure recording was inadequate for the analysis. Overall, the systolic blood pressure was decreased 6.5 ± 7.63% and diastolic blood pressure was decreased 6.4 ± 7.47% from baseline to follow-up. Baseline mean arterial pressure (MAP) and pulse pressure were 108.6 ± 8.33 mmHg and 63.3 ± 12.64 mmHg: they decreased by 6.5 ± 6.83% and 5.3 ± 14.70%, to 101.2 ± 6.47/58.9 ± 9.99 mmHg on follow-up. The findings from a post-hoc analysis suggest that in patients with type 2 diabetes and hypertension, effective blood pressure reduction has a specific and independent protective effect against the development of microalbuminuria. Nevertheless, trandolapril had a further protective effect, in particular when blood pressure was poorly controlled, whereas the ndCCB therapy was ineffective at any level of achieved blood pressure (Figure 3). The finding that the risk of developing microalbuminuria was not associated with baseline blood pressure provided consistent evidence that the lower incidence of microalbuminuria observed with more effective blood pressure reduction reflected a benefit of treatment and not simply less severe hypertension at study entry (Figure 4). Therefore, these results extend to the very early stages of diabetic renal disease previous evidence of a renoprotective effect of blood pressure control in people with diabetes and established nephropathy (Mogensen 1976; Dillon 1993; Bakris et al 2003; Pohl et al 2005; Remuzzi et al 2006). Of interest, patients with systolic, diastolic, mean blood pressure, and pulse pressure reduction above the medians compared with those with corresponding blood pressure reductions below the medians, were more frequently on ACE inhibitor therapy with trandolapril/verapamil or trandolapril alone and, on the contrary, were less frequently on treatment with concomitant antihypertensive medications such as diuretics, betablockers, ndCCBs, and sympatholytic agents (Table 3). The risk reduction achieved by ACE inhibitor therapy in patients with systolic, MAP, and pulse pressure below the median was significant even after adjustment for baseline covariates and concomitant treatment with ndCCBs. A similar trend was observed for diastolic blood pressure.
Figure 3.
Patients who developed microalbuminuria throughout the study period of the BENEDICT trial according to follow-up systolic blood pressure (SBP). These are patients with type 2 diabetes, arterial hypertension, and normoalbuminuria at baseline. Effective SBP reduction below the median (<139.16 mmHg) has specific and independent protective effects against the development of microalbuminuria. The risk reduction for microalbuminuria that was achieved by angiotensin converting enzyme inhibitor (ACEi) therapy in patients with follow-up SBP above the median (≥139.16 mmHg) was highly significant even after adjustment for baseline covariates and concomitant treatment with non-dihydropyridine calcium channel blockers. Thus ACEi therapy had a further protective effect, in particular when SBP was less effectively controlled (inset).
Figure 4.
In the BENEDICT study, the extent of systolic blood pressure (SBP) reduction had a specific and independent effect against the development of microalbuminuria. Angiotensin converting enzyme inhibitor (ACEi) therapy had a further protective effect, in particular when the SBP was less effectively controlled (inset).
Table 3.
Number (%) of patients on different antihypertensive treatments on trial according to systolic blood pressure (SBP), diastolic BP (DBP), mean arterial pressure (MAP), and pulse pressure reduction above or below the median
| Systolic BP | Diastolic BP | MAP | Pulse pressure | |||||
|---|---|---|---|---|---|---|---|---|
| below | above | below | above | below | above | below | above | |
| ACEi YES | 319 (53.9) | 273 (46.4)* | 323 (54.6) | 269 (45.8)** | 316 (53.3) | 276 (47.0)* | 317 (53.6) | 275 (46.8)* |
| ndCCB YES | 293 (49.5) | 297 (50.5) | 300 (50.7) | 290 (49.3) | 303 (51.1) | 287 (48.9) | 283 (47.8) | 307 (52.2) |
| VeraTran | 158 (26.7) | 135 (23.0) | 157 (26.5) | 136 (23.1) | 160 (27.0) | 133 (22.7) | 153 (25.8) | 140 (23.8) |
| Trandolapril | 161 (27.2) | 138 (23.5) | 166 (28.0) | 133 (22.6)* | 156 (26.3) | 143 (24.4) | 164 (27.7) | 135 (23.0) |
| Verapamil | 135 (22.8) | 162 (27.6) | 143 (24.2) | 154 (26.2) | 143 (24.1) | 154 (26.2) | 130 (22.0) | 67 (28.4)* |
| Placebo | 138 (23.3) | 152 (26.0) | 126 (21.3) | 165 (28.1)** | 134 (22.6) | 157 (26.8) | 145 (24.5) | 146 (24.8) |
| Diuretic | 97 (16.4) | 132 (22.5)* | 95 (16.1) | 134 (22.8)** | 98 (16.5) | 131 (22.3)* | 105 (17.7) | 124 (21.1) |
| Beta-blocker | 44 (7.4) | 59 (10.0) | 47 (7.9) | 56 (9.5) | 45 (7.6) | 58 (9.9) | 51 (8.6) | 52 (8.8) |
| dCCB | 142 (24.0) | 185 (31.5)** | 145 (24.5) | 182 (31.0)* | 137 (23.1) | 190 (32.4)*** | 160 (27.0) | 167 (28.4) |
| Sympatholytic | 265 (44.8) | 292 (49.7) | 250 (42.2) | 307 (52.2)*** | 256 (43.2) | 301 (51.3)** | 276 (46.6) | 281 (47.8) |
| agent | ||||||||
p ≤ 0.001
p ≤ 0.01
p < 0.05 versus below.
Abbreviations: ACEi, angiotensin converting enzyme inhibitor; dCCB, dihydropyridine calcium channel blocker; ndCCB, non-dihydropyridine calcium channel blocker.
A major practical problem, however, is that the recommended target of 130 mmHg for systolic blood pressure (Chobanian et al 2003) is seldom achievable in patients with type 2 diabetes, even when several blood-pressure-lowering medications are used in combination (Brenner et al 2001; Berl et al 2003; Ruggenenti et al 2004, 2006). Sixty-two percent of BENEDICT patients received one or more antihypertensive drugs in addition to the study drugs and all of them were recommended a low sodium intake. Despite this, only 14% of them achieved a systolic blood pressure of 130 mmHg or less. However, of note the highest proportion of patients on target was observed among those on combined trandolapril/verapamil treatment who, notably, less frequently required concomitant treatment with other antihypertensive medications. This is of great significance as hypertension is an independent and strong risk factor for cardiovascular disease events and renal disease progression in diabetic patients with hypertension, and concern for compliance with multiple drugs is an issue. Since microalbuminuria is a strong predictor of kidney failure and cardiovascular morbidity/mortality, the specific protective effect of ACE inhibitor therapy against the development of microalbuminuria should be taken into consideration in treatment guidelines for the practising physician (Dinneen 1997; Ruggenenti et al 2006). Indeed, most guidelines recommend any agent in patients with diabetes and hypertension and without nephropathy, and only ACE inhibitors or angiotensin II receptor blockers once nephropathy occurs (National Kidney Foundation 2002). This largely rests on data from hypertension trials showing that blood pressure reduction limits cardiovascular morbidity and mortality regardless of the antihypertensive agent used to achieve the target blood pressure, and overlooks that only ACE inhibitors have been proven to reduce the onset of microalbuminuria in hypertensive patients with diabetes. The post-hoc analysis of BENEDICT, consistent with previous evidence from BENEDICT (Ruggenenti et al 2004) and other trials (Strippoli et al 2005), provide the evidence that compared with other agents, ACE inhibitors have an incremental benefit on renal outcomes and so should be the treatment of choice in hypertensive patients with diabetes.
Conclusion
Effective blood pressure control and ACE inhibitor therapy are both key components of cardiovascular and renoprotective treatments in hypertensive type 2 diabetic patients. The combination of an ACE inhibitor and an ndCCB may help achieve optimal blood pressure control while limiting the need for concomitant antihypertensive medications that may adversely affect the metabolic control and the overall cardiovascular risk profile of people with diabetes. Trandolapril/verapamil SR is an effective and well tolerated combination therapy for both patients with hypertension and type 2 diabetes mellitus. In BENEDICT, trandolapril alone, or trandolapril/verapamil delayed the onset of microalbuminuria in more than 40% of patients compared with placebo in hypertensive diabetic patients. The effect of verapamil alone was similar to that of placebo. In the INVEST trial, ndCCB-based treatment strategy, in which most of the patients received ACE inhibitor (trandolapril), was more effective in reducing new onset diabetes in patients 50 years and older with hypertension and CAD. This indicates that the apparent advantage of ACE inhibitors over other antihypertensive agents includes also a protective effect on the kidney against the development of microalbuminuria, at least in type 2 diabetic patients, as well as development of new onset diabetes in hypertensive patients. Because microalbuminuria is a strong predictor of kidney failure and cardiovascular morbidity/mortality, the specific protective effect of ACE inhibition therapy against the development of microalbuminuria should be taken into consideration in the treatment guidelines for the practising physician. Whether an antihypertensive regimen including such a combination drug may effectively limit the excess morbidity and mortality associated with type 2 diabetes is worth investigating in prospective randomized trials.
References
- Abbott Laboratories. Isoptin SR (verapamil HCL) US prescribing information. North Chicago (IL): Abbott Laboratories; 2002a. [Google Scholar]
- Abbott Laboratories. Tarka 180/2 mg capsules: summery of product characteristics 2002 [online] 2002b Accessed 2006 Jun 22. URL: http//emc.medicine.org.uk.
- Abbott Laboratories, North Chicago (IL), USA. TARKA® (trandolapril/verapamil hydrochloride ER tablets): prescribing information 2003 [online] 2003a Accessed 2006 Jun 22. URL: http//www.abbott.com/corporate/product_list.cfm.
- Abbott Laboratories, North Chicago (IL), USA. Mavik® (trandolapril tablets): prescribing information 2003 [online] 2003b Accessed 2006 Jun 22. URL: http//www.rxabbott.com/pdf/mavik.pdf.
- Abuissa H, Bell DSH, O’Keefe JH., Jr Strategies to prevent type 2 diabetes. Curr Med Res Opin. 2005;21:1107–14. doi: 10.1185/030079905X50606. [DOI] [PubMed] [Google Scholar]
- Alder AI, Stevens RJ, Manley SE, et al. Development and progression of nephropathy in type 2 diabetes: the United Kingdom Prospective Diabetes Study (UKPDS 64) Kidney Int. 2003;63:225–32. doi: 10.1046/j.1523-1755.2003.00712.x. [DOI] [PubMed] [Google Scholar]
- Alderman MH. The return on INVEST. JAMA. 2003;290:2859–61. doi: 10.1001/jama.290.21.2859. [DOI] [PubMed] [Google Scholar]
- American Diabetes Association. Hypertension management in adults with diabetes. Diabetes Care. 2004;27:s65–7. doi: 10.2337/diacare.27.2007.s65. [DOI] [PubMed] [Google Scholar]
- Arner P, Wade A, Engfeldt P, et al. J Cardiovasc Pharmacol. Suppl 4. Vol. 23. 1994. Pharmacokinetics and Pharmacodynamics of trandolapril after repeated administration of 2 mg to young and elderly patients with mild-to-moderate hypertension; pp. s44–9. [PubMed] [Google Scholar]
- Bakris GL, Burnhill BW, Sadler R, et al., editors. Kidney Int. Vol. 41. 1992. Treatment of arterial hypertension in diabetic humans: importance of therapeutic selection; pp. 912–19. [DOI] [PubMed] [Google Scholar]
- Bakris GL, Gaxiola E, Messerli FH, et al., editors. Clinical outcome in the diabetes cohort on International Verapamil SR-Trandolapril study. Hypertension. 2004;44:637–42. doi: 10.1161/01.HYP.0000143851.23721.26. [DOI] [PubMed] [Google Scholar]
- Bakris GL, Weir MR, DeQuattro V, et al. Effects of an ACE inhibitor/calcium antagonist combination on protenuria in diabetic nephropathy. Kidney Int. 1998;54:1283–9. doi: 10.1046/j.1523-1755.1998.00083.x. [DOI] [PubMed] [Google Scholar]
- Bakris GL, Weir MR, Shanifar S, et al. Effects of blood pressure level on progression of diabetic nephropathy: results from the RENAAL study. Arch Intern Med. 2003;163:1555–65. doi: 10.1001/archinte.163.13.1555. [DOI] [PubMed] [Google Scholar]
- Beckman JA, Creager MA, Libby P. Diabetes and atherosclerosis: Epidemiology, pathophysiology, and management. JAMA. 2002;287:2570–81. doi: 10.1001/jama.287.19.2570. [DOI] [PubMed] [Google Scholar]
- Berl T, Hunsicker LG, Lewis JB, et al. for the Collaborative Study Group. Cardiovascular outcomes in the Irbesartan Diabetic Nephropathy Trial of patients with type 2 diabetes and overt nephropathy. Ann Intern Med. 2003;138:542–9. doi: 10.7326/0003-4819-138-7-200304010-00010. [DOI] [PubMed] [Google Scholar]
- Berne C, Pollare T, Lithell H. Effects of antihypertensive treatment on insulin sensitivity with special reference to ACE-inhibitors. Diabetes Care. 1991;14(Suppl 4):39–47. doi: 10.2337/diacare.14.4.39. [DOI] [PubMed] [Google Scholar]
- Bevan EG, McInnes GT, Aldigier JC, et al. Effect of renal function on the pharmacokinetics and pharmacodynamics of trandolapril. Br J Clin Pharmacol. 1993;35:128–35. [PMC free article] [PubMed] [Google Scholar]
- Brenner BM, Cooper ME, de Zeeuw D, et al. RENAAL Study Investigators: Effects of losartan on renal and cardiovascular outcomes in patients with type 2 diabetes and nephropathy. N Engl J Med. 2001;345:861–9. doi: 10.1056/NEJMoa011161. [DOI] [PubMed] [Google Scholar]
- Breithaupt-Grogler K, Gerhardt G, Lehmann G, et al. Blood presuure and aortic elastic properties: verapamil SR/trandolapril compared to metoprolol/hydrochlorthiazide combination thearapy. Int J Clin Pharmacol Ther. 1998;36:425–31. [PubMed] [Google Scholar]
- Chobanian AV, Bakris GL, Black HR, et al. Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. National Heart, Lung, and Blood Institute. National High Blood Pressure Education Program Coordinating Committee. Seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Hypertension. 2003;42:1206–125. doi: 10.1161/01.HYP.0000107251.49515.c2. [DOI] [PubMed] [Google Scholar]
- Choi KL, Bakris GL. Hypertension treatment Guidelines: practical implications. Semin Nephrol. 2005;25:198–209. doi: 10.1016/j.semnephrol.2005.02.004. [DOI] [PubMed] [Google Scholar]
- Curb JD, Pressel SL, Cutler JA, et al. Effect of diuretic based antihypertensive treatment on cardiovascular disease risk in older diabetes patients with isolated systolic hypertension. Systolic Hyperetension in the Elderly Program Cooperative Research Group. JAMA. 1996;276:1886–92. [PubMed] [Google Scholar]
- Danielson B, Querin S, LaRochelle P, et al. Pharmacokinetic and pharmacodynamics of trandolapril after repeated administration of 2 mg to patients with chronic renal failure and healthy control subjects. J Cardiovasc Pharmacol. 1994;23(Suppl 4):s50–9. [PubMed] [Google Scholar]
- de leeuw PW, Notter T, Zilles P. Comparison of different fixed antihypertensive combination drugs: a double blind, placebo-controlled, parallel group study. J Hypertens. 1997;15:87–91. doi: 10.1097/00004872-199715010-00009. [DOI] [PubMed] [Google Scholar]
- DeQuattro V, Lee D Trandolapril Study Group. Fixed dose combination therapy with trandolapril and verapamil SR is effective in primary hypertension. Am J Hypertens. 1997;10:138s–45s. doi: 10.1016/s0895-7061(97)00102-7. [DOI] [PubMed] [Google Scholar]
- Dillon JJ. The quantitative relationship between treated blood pressure and progression of diabetic renal disease. Am J Kidney Dis. 1993;22:798–802. doi: 10.1016/s0272-6386(12)70337-2. [DOI] [PubMed] [Google Scholar]
- Dinneen SF, Gerstein HC. The association of microalbuminuria and mortality in non-insulin-dependent diabetes mellitus. A systematic overview of the literature. Arch Intern Med. 1997;157:1413–18. [PubMed] [Google Scholar]
- Fernández R, Puig JG, Rodríguez-Pérez JC, et al. Effect of two antihypertensive combinations on metabolic control in type-2 diabetic hypertensive patients with albuminuria: a randomised, double-blind study. J Hum Hypertens. 2001;15:849–56. doi: 10.1038/sj.jhh.1001279. [DOI] [PubMed] [Google Scholar]
- Flack JM, Sowers JR. Epidemiologic and clinical aspects of insulin resistance and hyperinsulinemia. Am J Med. 1991;91:11S–21S. doi: 10.1016/0002-9343(91)90058-6. [DOI] [PubMed] [Google Scholar]
- Gall MA, Hougaard P, Borch-Johnsen K, et al. Risk factors for development of incipient and overt diabetic nephropathyin patients with non insulin dependant diabetes mellitus: prospective observational study. BMJ. 1997;314:783–88. doi: 10.1136/bmj.314.7083.783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haansoon L, Zanchetti A, Carruthers SG, et al. Effects of intensive blood-pressure lowering and low-dose aspirin in patients with hypertension: principal results of the Hypertension Optimal Treatment (HOT) randomized trial. HOT study group. Lancet. 1998;351:1755–62. doi: 10.1016/s0140-6736(98)04311-6. [DOI] [PubMed] [Google Scholar]
- Haegert AM, Swales JD. The combination of verapamil and captopril in the treatment of essential hypertension. Pharmacotherapeutica. 1987;5(1) [PubMed] [Google Scholar]
- Hansen JF, Hangerup L, Sigurd B, et al. Cardiac events rate after myocaridal infarction in patients treated with verapamil and trandolapril versus trandolapril alone. Am J Cardiol. 1997;15:738–41. doi: 10.1016/s0002-9149(96)00860-0. [DOI] [PubMed] [Google Scholar]
- Harris MI, Flegal KM, Cowie CC, et al. Prevalence of diabetes, impaired fasting glucose, and impaired glucose tolerance in U.S. adults. The Third National Health and Nutrition Examination Survey, 1988-1994. Diabetes Care. 1998;21:518–24. doi: 10.2337/diacare.21.4.518. [DOI] [PubMed] [Google Scholar]
- Holzgreve H, Nakov R, Beck K, Janka HU. Antihypertensive therapy with verapamil sr plus trandolapril versus atenolol plus chlorthalidone on glycemic control. Am J Hypertens. 2003;16:381–6. doi: 10.1016/s0895-7061(03)00062-1. [DOI] [PubMed] [Google Scholar]
- Hypertension in Diabetes Study (HDS) Prevalence of hypertension in newly presenting type 2 diabetic patients and the association of risk factors for cardiovascular and diabetic complicatins. J Hypertens. 1993;11:309–17. doi: 10.1097/00004872-199303000-00012. [DOI] [PubMed] [Google Scholar]
- Kalberge BE, Andrup M, Oden A on behalf of the Swidish Tarka Trialists. Efficacy and safety of a new long-acting drug combination, trandolapril/verapamil as compared to monotherapy in primary hypertension. Blood Press. 2000;9:140–5. doi: 10.1080/080370500453483. [DOI] [PubMed] [Google Scholar]
- Lenfant B, Mauren M, Bryce T, et al. Trandolapril: Pharmacokinetics of single oral dose in healthy male volunteers. J Cardiovasc Pharmacol. 1994;23(Suppl 4):s238–43. [PubMed] [Google Scholar]
- Mancia G, Cooper-DeHoff RM, Hewkin A, et al. Association of BP control and frequency of mortality and morbidity in hypertensive patients with CAD - The International Verapamil SR-Trandolapril Study [abstract no. p-229] Am J Hypertens. 2004;15:116a. [Google Scholar]
- Mogensen CE. Progression of nephropathy in long-term diabetics with proteinuria and effect of initial anti-hypertensive treatment. Scand J Clin Lab Invest. 1976;36:383–8. doi: 10.1080/00365517609055274. [DOI] [PubMed] [Google Scholar]
- Mogensen CE. Microalbuminuria predicts clinical proteiuria and early mortality in maturity-onset diabetes. N Engl J Med. 1984;310:356–60. doi: 10.1056/NEJM198402093100605. [DOI] [PubMed] [Google Scholar]
- Muijsers RBR, Curan MP, Erry CM. Fixed combination trandolapril/verapamil sustained-release: a review of its use in essential hypertension. Drugs. 2002;62:2539–67. doi: 10.2165/00003495-200262170-00014. [DOI] [PubMed] [Google Scholar]
- National Kidney Foundation. K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification and stratification. Am J Kidney Dis. 2002;39(Suppl 1):S1–266. [PubMed] [Google Scholar]
- Nelson RG, Knowler WC, Pettitt DJ, et al. Assessing risk of overt nephropathy in diabetic patients from albumin excretion in untimed urine specimen. Arch Inttrn Med. 1991;151:1761–5. [PubMed] [Google Scholar]
- Papine MD, Handberg EM, Cooper-DeHoff RM, et al. A calcium antagonist vs a non-calcium antagonist hypertension treatment strategy for patients with coronary artery disease. The International Verapamil SR-Trandolapril Study: a randomized controlled trial. JAMA. 2003;290:2805–16. doi: 10.1001/jama.290.21.2805. [DOI] [PubMed] [Google Scholar]
- Parving HH, Lehnert H, Brochner-Mortensen J, et al. The effect of Irbesartan on the development of diabetic nephropathy in patients with type 2 diabetes. N Engl J Med. 2001;345:870–78. doi: 10.1056/NEJMoa011489. [DOI] [PubMed] [Google Scholar]
- Perneger TV, Brancati F, Whelton PK, et al. End-stage renal disease attributable to diabetes mellitus. Ann Intern Med. 1994;121:912–18. doi: 10.7326/0003-4819-121-12-199412150-00002. [DOI] [PubMed] [Google Scholar]
- Pohl MA, Blumenthal S, Cordonnier DJ, et al. Independent and additive impact of blood pressure control and angiotensin II receptor blockade on renal outcomes in the irbesartan diabetic nephropathy trial: clinical implications and limitations. J Am Soc Nephrol. 2005;16:3027–37. doi: 10.1681/ASN.2004110919. [DOI] [PubMed] [Google Scholar]
- Raynolds NA, Wagstaff AJ, Keam SJ. Trandolapril/verapamil sustained released: a review of its use in the treatment of Eessential hypertension. Drugs. 2005;65:1893–1914. doi: 10.2165/00003495-200565130-00011. [DOI] [PubMed] [Google Scholar]
- Reid LD, Tueth MJ, Handberg E, et al. A study of antihypertensive drug and depressive symptoms (SADD-Sx) in patients treated with calcium antagonist versus an atenolol hyepretension Treatment Strategy in the International Verapamil SR-Trandolapril Study (INVEST) Psychosom Med. 2005;67:398–406. doi: 10.1097/01.psy.0000160468.69451.7f. [DOI] [PubMed] [Google Scholar]
- Remuzzi G, Macia M, Ruggenenti P. Prevention and treatment of diabetic renal disease in type 2 diabetes: the BENEDICT study. J Am Soc Nephrol. 2006;17:S90–7. doi: 10.1681/ASN.2005121324. [DOI] [PubMed] [Google Scholar]
- Romos F, Baglovo H, Sanchez R. Comparative double blind study at fixed dose of trandolapril+verapamil vs. atenolol+chorthalidon in mild to moderate hypertensive patients [abstract no. p3185] J Hypertens. 2001;19(Supp 12):s236. [Google Scholar]
- Ruggenenti P, Fassi A, Ilieva AP, et al. for the Bergamo Nephrologic Diabetes Complications Trial (BENEDICT) Investigators. Preventing microalbuminuria in type 2 diabetes. N Engl J Med. 2004;351:1941–51. doi: 10.1056/NEJMoa042167. [DOI] [PubMed] [Google Scholar]
- Ruggenenti P, Perna A, Ganeva M, et al. Impact of blood pressure control and ace inhibitor therapy on newly onset microalbuminuria in type 2 diabetes: a post-hoc analysis of the BENEDICT trial. J AM Soc Nephrol. 2006;17:3472–81. doi: 10.1681/ASN.2006060560. [DOI] [PubMed] [Google Scholar]
- Ruggenenti P, Remuzzi G. Time to abandon microalbuminuria? Kidney Int. 2006;70:1214–22. doi: 10.1038/sj.ki.5001729. [DOI] [PubMed] [Google Scholar]
- Ruilope LM. Dissociation between blood pressure reduction and fall in proteinuria in primary renal disease: a randomised double-blind trial. J Hypertens. 2002;20:729–37. doi: 10.1097/00004872-200204000-00032. [DOI] [PubMed] [Google Scholar]
- Ruilope LM, Usan L, Segura J, et al. Intervention at lower blood pressure levels to achieve target goals in type 2 diabetes: PRADID (PResión Arterial en DIabéticos tipo Dos) study. J Hypertens. 2004;22:217–22. doi: 10.1097/00004872-200401000-00032. [DOI] [PubMed] [Google Scholar]
- Schneider M, Lerch M, Papiri M, et al. Metabolic neutrality of combined verapamil-trandolapril treatment in contrast to beta-blocker-low-dose chlorthalodone treatment in hypertesnsive type 2 diabetes. J Hypertens. 1996;14:669–77. doi: 10.1097/00004872-199605000-00018. [DOI] [PubMed] [Google Scholar]
- Sica DA. Rationale for fixed-dose combination in the treatment of hypertension: the cycle repeats. Drugs. 2002;62:443–62. doi: 10.2165/00003495-200262030-00003. [DOI] [PubMed] [Google Scholar]
- Siepman M, Rao BR, Kirch W. Disposition, elimination and haemodynamic effects of varapamil and trandolapril in patients with fatty liver disease. Clin Drug Invest. 1997;14:376–82. doi: 10.2165/00044011-199510060-00004. [DOI] [PubMed] [Google Scholar]
- Sowers JR. Effects of ACE inhibitors and calcium channel blockers on insulin sensitivity and other components of the syndrome. Nephrol Dial Transplant. 1995;10(Suppl 9):52–5. [PubMed] [Google Scholar]
- Sowers J, Bakris GL. Antihypertensive therapy and risk of type 2 diabetes mellitus. N Engl J Med. 2000;342:969–70. doi: 10.1056/NEJM200003303421310. [DOI] [PubMed] [Google Scholar]
- Strippoli GFM, Craig M, Schena FP, et al. Antihypertensive agents for primary prevention of diabetic nephropathy. J Am Soc Nephrol. 2005;16:3081–91. doi: 10.1681/ASN.2004080634. [DOI] [PubMed] [Google Scholar]
- Sutter PM, Vettr W. Metabolic effects of antihypertensive drugs. J Hypertens. 1995;13(Suppl 4):s11–s17. doi: 10.1097/00004872-199512002-00003. [DOI] [PubMed] [Google Scholar]
- Sweetman S, editor. Martindale: the complete drug reference. London: Pharmaceutical press; 2005. Electronic version. Dantrolin sodium [online]. Accessed 2005 December 2. URL: http://www.medicinecomplete.com/mc/martindales. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tatti P, Pahor M, Byington RP, et al. Outcome results of fosinopril versus amlodipine cardiovascular events randomized trial (FACET) in patients with hypertension and NIDDM. Diabetes Care. 1998;21:597–603. doi: 10.2337/diacare.21.4.597. [DOI] [PubMed] [Google Scholar]
- The BENEDICT Group. The BErgamo NEphrologic DIabetes Complications Trial (BENEDICT): Design and baselines group. Control Clin Trials. 2003;24:442–61. doi: 10.1016/s0197-2456(03)00028-x. [DOI] [PubMed] [Google Scholar]
- The Heart Outcome Prevention Evaluation Study Investigators. Effects of an angiotensine-converting-anzyme inhibitor, ramipril, on cardiovascular events in high-risk patients. N Engl J Med. 2000;342:145–53. doi: 10.1056/NEJM200001203420301. [DOI] [PubMed] [Google Scholar]
- The Veratran study group. The effects of verapamil SR trandolapril and their fix combination on 24-h blood pressure: the Veratran Study. Am J Hypertens. 1997;10:492–9. doi: 10.1016/s0895-7061(96)00486-4. [DOI] [PubMed] [Google Scholar]
- Topouchian J, Asmar R, Sayegh F, et al. Changes in arterial structure and function under trandolapril-verapamil combination in hypertension. Stroke. 1999;30:1056–64. doi: 10.1161/01.str.30.5.1056. [DOI] [PubMed] [Google Scholar]
- UK Prospective Diabetes Study Group. Tight blood pressure control and risk of macrovascular and microvascular complications in type 2 diabetes: UKPDS. BMJ. 1998;317:703–13. [PMC free article] [PubMed] [Google Scholar]
- Viskoper RJ, Compagnone D, Dies R, et al. Verapamil and trandolapril alone and fixed combination on 24-hour ambulatory blood pressure profile of patients with moderate essential hypertesnsion. Curr Ther Res Clin Exp. 1997;58:343–51. [Google Scholar]
- Waeber B. Trails in isolated systolic hypertension: an update. Curr Hypertension Rep. 2003;5:329–36. doi: 10.1007/s11906-003-0042-9. [DOI] [PubMed] [Google Scholar]
- Widimsky J. The fixed combination of verapamil SR/trandolapril. Expert Opin Pharmacother. 2000;1:515–35. doi: 10.1517/14656566.1.3.515. [DOI] [PubMed] [Google Scholar]
- Wiserman LR, McTavish D. Trandolapril: A review of its pharmacodynamic and pharmacokinetic properties, and therapeutic use in essential hypertension. Drugs. 1994;48(1):71–90. doi: 10.2165/00003495-199448010-00007. [DOI] [PubMed] [Google Scholar]
- Wild S, Roglic G, Green A, et al. Global prevalence of diabetes: estimates for the year 2000 and projections for 2030. Diabetes Care. 2004;27:1047–53. doi: 10.2337/diacare.27.5.1047. [DOI] [PubMed] [Google Scholar]
- World Health Report. Sharing the Future. Neglected Global Epidemics: three growing threats in Report of World Health Organization, Geneva 2003. 2003 [Google Scholar]