Abstract
The physiological role of the renin angiotensin aldosterone system (RAAS) is to maintain the integrity of the cardiovascular system. The effect of angiotensin II is mediated via the angiotensin type I receptor (AT1) resulting in vasoconstriction, sodium retention and myocyte growth changes. This causes myocardial remodeling which eventually leads to left ventricular hypertrophy, dilation and dysfunction. Inhibition of the RAAS with angiotensin converting enzyme (ACE) inhibitors after acute myocardial infarction has been shown to reduce cardiovascular morbidity and mortality. Angiotensin receptor blockers (ARBs) specifically inhibit the AT1 receptor. It has not been known until the performance of the VALIANT (valsartan in acute myocardial infarction trial) whether blockade of the angiotensin receptor with an ARB or combination of an ACE inhibitor and ARB leads to similar outcomes as an ACE inhibitor. The VALIANT trial demonstrated equal efficacy and non-inferiority of the ARB valsartan 160 mg bid compared with captopril 50 mg tds, when administered to high risk patients with left ventricular dysfunction or heart failure in the immediate post myocardial infarction period. The combination therapy showed no incremental benefit over ACE inhibition or an ARB alone and resulted in increased adverse effects. This review examines the role of valsartan in left ventricular dysfunction post myocardial infarction. We also discuss pharmacokinetics, dosing, side effects, and usage in the elderly.
Keywords: valsartan, heart failure, left ventricular dysfunction, myocardial infarction
Introduction
The renin angiotensin aldosterone system (RAAS) is an endocrine system, which generates an effector hormone called angiotensin II. The effects of angiotensin II are mediated through its stimulation of AT1 and AT2 receptors. Angiotensin receptor blockers (ARBs) and angiotensin converting enzyme (ACE) inhibitors are antagonists of the RAAS recommended for use in the management of patients with heart failure post myocardial infarction (Antman et al 2004). The 2004 American college of cardiology/American heart association (ACC/AHA) gave ARBs a Class I recommendation in patients who were intolerant of ACE inhibitors and who had an acute myocardial infarction with an left ventricular ejection fraction less than 40% or clinical (or radiological) evidence of heart failure (Antman et al 2004).
Renin angiotensin aldosterone system (RAAS)
The RAAS is responsible for maintaining the integrity of the cardiovascular system (see Figure 1). The ACE catalyzes the conversion of angiotensin I to angiotensin II. Homeostasis of sodium and the extracellular fluid volume as well as vasoconstriction occur through direct action of angiotensin II on the AT1 (angiotensin I) receptor. In addition the ACE is also responsible for the degradation of bradykinin which is a potent vasodilator (Goodfriend et al 1996). Stimulation of the AT2 receptor has also been shown to induce vasodilation and natriuresis (Goodfriend et al 1996). This effect is in contrast to stimulation of the AT1 receptor which causes vasoconstriction and sodium retention. In heart failure a vicious cycle prevails in which the RAAS activity is increased. This results in increased angiotensin II that perpetuates vasoconstriction, left ventricular hypertrophy, endothelial dysfunction, and myocardial remodeling (see Table 1). Aldosterone and catecholamine increases also maintain hemodynamics.
Figure 1.
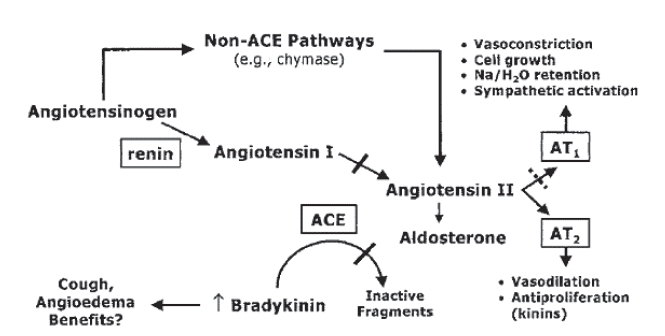
The renin angiotensin aldosterone system. Reproduced with permission from McMurray JJ, Pfeffer MA, Swedberg K, et al. 2004. Which inhibitor of the renin-angiotensin system should be used in chronic heart failure and acute myocardial infarction? Circulation, 110:3281–8. Copyright © 2004. Massachusetts Medical Society. All rights reserved.
Table 1.
The effects of angiotensin II on the heart in left ventricular dysfunction
| Potent vasoconstriction |
| Sympathetic nervous system activation |
| Vasopressin release |
| Endothelin activation |
| Platelet aggregation |
| Thrombosis due to increased plasminogen activator inhibitor-1 (PAI-1) |
| Myocardial remodeling |
| –myocyte hypertrophy |
| –collagen deposition |
| Aldosterone release |
ACE inhibitors work by inhibiting kininase II and degradation of bradykinin which results in elevated levels of bradykinin. Increased bradykinin leads to vasodilation via the release of endothelial nitric oxide but is also responsible for ACE inhibitor intolerance with cough. ARBs act by a different mechanism than ACE inhibitors by blocking the binding of angiotensin II to the AT1 receptor (Goodfriend et al 1996). The production of angiotensin II is unaffected. Bradykinin is metabolized in its normal fashion and this may at least partly explain the lower frequency of cough than with ACE inhibitors.
Angiotensin II may be generated by intramyocardial tissue angiotensin converting enzyme pathways (tissue ACE) or non-ACE pathways (chymase), which are not inhibited by ACE inhibitors (Colucci 2006).
ARBs have beneficial effects by blocking the AT1 receptor in heart failure. The effect of heart failure on the AT1 receptor is down-regulation and reduced gene expression. This results in enhanced local activity of angiotensin II (Haywood et al 1997; Asano et al 2006). Increased ACE activity and intra-myocardial ACE binding sites have been demonstrated in heart failure (Zisman et al 1998). The intra-myocardial renin angiotensin system may be an important mechanism for the development of left ventricular hypertrophy and ventricular dilation producing the myocardial remodeling that accompanies heart failure (Dzau 1993).
ACE inhibitor use post myocardial infarction is associated with stabilization of heart size and delayed progression in the remodeling that results in systolic and diastolic dysfunction (Mitchell et al 1882; Hayashida et al 1993). ACE inhibitors have been shown to retard the progression of heart failure, improve survival, and reduce ventricular remodeling post myocardial infarction (Rutherford et al 1994; Pfeffer 1995; Hunt et al 2001). ACE inhibitors in patients with myocardial infarction also reduce the occurrence of cardiovascular events in high-risk patients with signs of heart failure (Pfeffer et al 2003). International guidelines recommend ACE inhibitors as first-line therapy (Hunt et al 2001).
Angiotensin receptor blocker versus ACE inhibitor
ARBs have been shown to be as effective as ACE inhibitors in the management of hypertension, congestive heart failure and chronic renal failure (McMurray et al 2003). However, there are fundamental differences between these two classes of drugs. In addition to the differential effect of kinins, inhibition of angiotensin II formation with an ACE inhibitor diminishes the activity of both the AT1 and AT2 receptors. ARBs selectively block AT1 receptor activity with no blocking effect on the AT2 receptor. The blockade of AT1 receptor results in enhanced stimulation of the AT2 receptor. Further cardiovascular protection may be afforded from the enhanced AT2 receptor activity via vasodilation and fibrinolytic mechanisms (Horiuchi et al 1999).
The Val-HeFT trial evaluated the addition of valsartan to standard therapy for congestive heart failure, including ACE inhibitors. There was a significant reduction in the risk of hospital admissions for worsening heart failure, and death, in this cohort with the use of valsartan (Cohn and Tognoni 2001). In a subset analysis, 366 patients who were not receiving ACE inhibitors were randomly assigned to either valsartan or placebo. At approximately 2 years, valsartan significantly reduced both all-cause mortality (17% vs 27%, p = 0.01) and a combined mortality and morbidity end point (25% vs 43%, p <0.001) (Maggioni et al 2002). Patients in the Val-HeFT trial treated with valsartan had a smaller rise in serum noradrenalin than those treated with placebo (12 vs 41 pg/mL) (Latini et al 2002).
The efficacy and safety of ARBs in acute myocardial infarction has recently been evaluated with the ARB losartan in the optimal trial in myocardial infarction with the angiotensin II antagonist losartan (OPTIMAAL) trial in patients with myocardial infarction with the angiotensin II antagonist losartan and valsartan in the valsartan in acute myocardial infarction trial (VALIANT) trials (Pfeffer et al 2000, 2003). In the OPTIMAAL trial the effects of losartan (50 mg per day) were compared with the ACE inhibitor captopril (50 mg 3 times per day) for morbidity and mortality after acute myocardial infarction. The results showed a non-significant difference in total mortality in favor of captopril. It is important to note that in this particular trial a sub-optimal dose of losartan was used and may have been responsible for the apparent inferiority of losartan (Dickstein et al 2002).
VALIANT was a double blinded, multicenter international trial that compared treatment with valsartan alone, or in combination with captopril (Pfeffer et al 2000, 2003). The target dose of valsartan was 160 mg twice daily. The cohort included high risk patients with evidence of systolic dysfunction or heart failure 12 hours to 10 days post myocardial infarction and were followed up for 2 years. The results showed equal efficacy of valsartan monotherapy versus captopril monotherapy. Valsartan was neither superior nor inferior to captopril for the primary endpoint of mortality: 19.5% captopril vs 19.9% valsartan (hazard ration [HR] = 1.00; 97.5%, confidence interval [CI] 0.90–1.11; p = 0.98); and the composite end point of cardiovascular death, myocardial infarction, and heart failure in the valsartan group as compared with the captopril group was close to one (Pfeffer et al 2003) (HR = 0.96; p = 0.198). The combination showed no beneficial effects and was associated with more adverse effects (see next section).
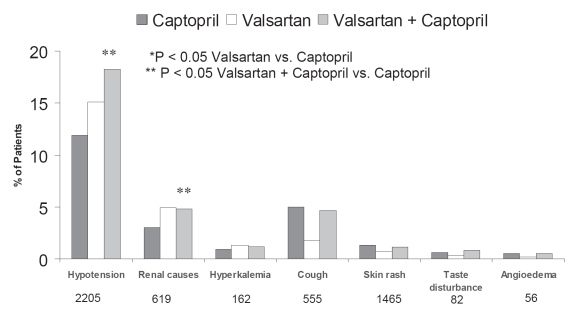
Cough, rash, and taste disturbance were more common in the captopril group. Angioedema was infrequent and did not differ among the groups (Figure 3).
Figure 3.
Adverse experience leading to dose reduction in the VALIANT trial. Adapted with permission from table 3 in Pfeffer MA, McMurray JJV, Velazquez EJ, et al. 2003. Valsartan, captopril, or both in myocardial infarction complicated by heart failure, left ventricular dysfunction, or both [Erratum in N Engl J Med, 2004. 350:203]. N Engl J Med, 349:1893–906. Copyright © Massachusetts Medical Society. All rights reserved.
The combination showed no beneficial effects and was associated with more adverse effects (see next section).
A recent analysis of the VALIANT trial shows that valsartan was as effective as captopril in decreasing the frequency of atherosclerotic events such as fatal and non-fatal myocardial infarction, angina, revascularization, and stroke (McMurray et al 2003).
Angiotensin receptor blocker–ACE inhibitor combination therapy
The intramyocardial enzymatic pathway may produce angiotensin II through a mechanism that bypasses the effects of ACE inhibitors. This alternative pathway using myocardial chymase is upregulated in heart failure (Menard et al 1997). Angiotensin II mediates its activity via the AT1 receptor, which is selectively blocked by ARBs. Combined treatment with an ACE inhibitor and an ARB may therefore block more efficiently the effects of angiotensin II (Menard et al 1997).
In an animal study of heart failure produced by rapid atrial pacing, the ACE inhibitor benazipril resulted in improvement in myocyte beta-adrenergic response with reduction in the degree of left ventricular dilation, reduction in the levels of circulating catecholamines, and improvement in myocyte shortening velocity and global ventricular function (Spinale et al 1997a, b). The combination of benazipril and valsartan resulted in changes in the above parameters that were greater than those obtained with ACE inhibition alone. In rats combination therapy has been shown to reduce the deleterious effects of exercise on post myocardial infarction remodeling (Mankad et al 2001). There is therefore a theoretical basis for combination therapy to be more effective than either class alone.
In the VALIANT trial, the valsartan-and-captopril combination group had the most drug-related adverse events and did not increase survival nor decrease morbidity compared to monotherapy (Figure 2). Combination therapy therefore has no current role in the management of patients early post myocardial infarction (Pfeffer et al 2003).
Figure 2.
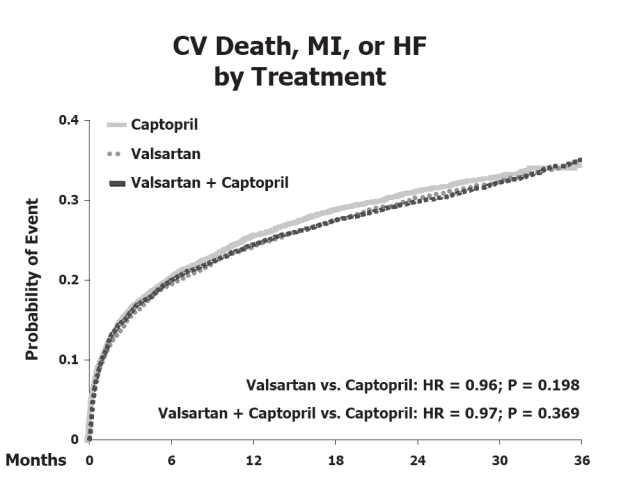
Cardiovascular death, myocardial infarction or heart failure by treatment in the VALIANT trial. Reproduced from Pfeffer MA, McMurray JJV, Velazquez EJ, et al. 2003. Valsartan, captopril, or both in myocardial infarction complicated by heart failure, left ventricular dysfunction, or both [Erratum in N Engl J Med, 2004. 350:203]. N Engl J Med, 349:1893–906. Copyright © Massachusetts Medical Society. All rights reserved.
Adverse effects in the valsartan group were more common (compared with the captopril monotherapy group) with a higher risk of developing hypotension (15.1% vs 11.9%; p < 0.05), renal dysfunction (4.9% vs 3.0%; p < 0.05), and hyperkalemia (1.3% vs 0.9%; p = n/s) (Figure 3). Dose reductions and permanent discontinuations of study medication for renal causes were more frequent in the valsartan and the combination groups.
Valsartan in the elderly
Sizable proportions of patients with myocardial infarction are elderly and have a disproportionately high mortality and morbidity. The numbers of elderly patients are increasing and their outcomes remain poor. In the VALIANT trial age was an independent predictor of mortality and heart failure, with each 10-year age increase being associated with an HR of 1.49 for mortality and an odds ratio (OR) of 1.38 for readmission with heart failure (White et al 2005). Hypotension, renal dysfunction, and hyperkalemia were more common in elderly patients receiving valsartan. Elderly patients had good compliance with the study medications but were prescribed lower doses of captopril, valsartan, and combination therapy than younger patients. In the doses used, captopril and valsartan monotherapy achieved similar mortality and morbidity outcomes in elderly patients.
Valsartan doses
In patients with left ventricular dysfunction after myocardial infarction the recommended starting dose is 20 mg twice daily then titrated to a target of 160 mg twice daily as tolerated. This may be initiated in stable patients 12 hours following acute myocardial infarction. Close monitoring for hypotension, hyperkalemia, and renal dysfunction is required.
Pharmacokinetics
Valsartan has a half-life of 6 hours (Chiolero and Burnier 2006). It is largely protein bound to albumin (95%) and undergoes significant first pass metabolism in the liver with a low bioavailability of approximately 25%. Peak plasma levels occur 2 hours after dosing. Valsartan is excreted as an inactive metabolite in the feces and less than a fifth in the urine as unchanged drug. Studies evaluating the pharmacokinetics of valsartan in elderly patients (over 70 years of age) showed decreased plasma clearance and a slightly prolonged elimination half-life. Dosing should be carefully titrated in the elderly in order to achieve the desired effect (Sioufi et al 1998).
Contraindications
There are a number of contraindications to valsartan, including hypersensitivity to either valsartan or other angiotensin receptor antagonists, bilateral renal artery stenosis, and pregnancy. Valsartan must be discontinued once pregnancy is detected, as medications that act on the RAAS have a number of fetal effects including hypotension, neonatal skull hypoplasia, anuria, renal failure, and death.
Precautions with valsartan
During the initiation of therapy, hypotension and hyperkalemia may occur, particularly in patients with heart failure or post myocardial infarction patients. A small dose should be used in patients who are volume depleted with correction of the dehydration first. Deterioration in renal function can occur with initiation and renal function should be monitored closely especially in patients with severe heart failure. Changes in renal function should be anticipated and dosage adjustments of valsartan or concomitant medications may be needed. Valsartan should be used with caution in patients with unilateral renal artery stenosis and pre-existing renal insufficiency. Initiation of low doses is necessary in low output states and low doses are recommended in patients with significant hepatic dysfunction.
Conclusion
Valsartan is an important addition to the armamentarium of drugs that have been shown to be effective in patients with heart failure post myocardial infarction. The addition of valsartan to ACE inhibitors early after myocardial infarction has no current role but valsartan has an important role as an alternative to ACE inhibitors when ACE inhibitors are not tolerated.
Acknowledgments
We are grateful to Barbara Semb and Nicky Hashfield for secretarial assistance.
References
- Antman EM, Anbe DT, Armstrong PW, et al. ACC/AHA guidelines for the management of patients with ST-elevation myocardial infarction—executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Revise the 1999 Guidelines for the Management of Patients With A Myocardial Infarction) Circulation. 2004;110:588–636. doi: 10.1161/01.CIR.0000134791.68010.FA. [DOI] [PubMed] [Google Scholar]
- Asano K, Dutcher DL, Port JD, et al. Selective downregulation of the angiotensin II AT1-receptor subtype in the failing human ventricular myocardium. Circulation. 2006;95:1193–200. doi: 10.1161/01.cir.95.5.1193. [DOI] [PubMed] [Google Scholar]
- Chiolero A, Burnier M. Pharmacology of valsartan, an angiotensin II receptor antagonist. Expert Opin Investig Drugs. 2006;7:1915–25. doi: 10.1517/13543784.7.11.1915. [DOI] [PubMed] [Google Scholar]
- Cohn JN, Tognoni G. A randomized trial of the angiotensin-receptor blocker valsartan in chronic heart failure. N Engl J Med. 2001;345:1667–75. doi: 10.1056/NEJMoa010713. [DOI] [PubMed] [Google Scholar]
- Colucci WS. Pathophysiologic and clinical considerations in the treatment of heart failure: An overview. In: Antman EM, editor. Cardiovascular Therapeutics. 2. Philadelphia: W.B.Saunders Company; 2006. pp. 293–324. [Google Scholar]
- Dickstein K, Kjekshus J, et al. And the OPTIMAAL Steering Committee. Effects of losartan and captopril on mortality and morbidity in high-risk patients after acute myocardial infarction:the OPTIMAAL randomised trial. Lancet. 2002;360:752–60. doi: 10.1016/s0140-6736(02)09895-1. [DOI] [PubMed] [Google Scholar]
- Dzau VJ. Tissue renin-angiotensin system in myocardial hypertrophy and failure. Arch Intern Med. 1993;153:937–42. [PubMed] [Google Scholar]
- Goodfriend TL, Elliott ME, Catt KJ. Angiotensin receptors and their antagonists. N Engl J Med. 1996;334:1649–54. doi: 10.1056/NEJM199606203342507. [DOI] [PubMed] [Google Scholar]
- Hayashida W, van Eyll C, Rousseau MF, et al. Regional remodeling and nonuniform changes in diastolic function in patients with left ventricular dysfunction:modification by long-term enalapril treatment. J Am Coll Cardiol. 1993;22:1403–10. doi: 10.1016/0735-1097(93)90550-k. [DOI] [PubMed] [Google Scholar]
- Haywood GA, Gullestad L, Katsuya T, et al. AT1 and AT2 angiotensin receptor gene expression in human heart failure. Circulation. 1997;95:1201–6. doi: 10.1161/01.cir.95.5.1201. [DOI] [PubMed] [Google Scholar]
- Horiuchi M, Akishita M, Dzau VJ. Recent progress in angiotensin II type 2 receptor research in the cardiovascular system. Hypertension. 1999;33:613–21. doi: 10.1161/01.hyp.33.2.613. [DOI] [PubMed] [Google Scholar]
- Hunt SA, Baker DW, Chin MH, et al. ACC/AHA guidelines for the evaluation and management of chronic heart failure in the adult:executive summary. A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee to revise the 1995 Guidelines for the Evaluation and Management of Heart Failure) J Am Coll Cardiol. 2001;38:2101–13. doi: 10.1016/s0735-1097(01)01683-7. [DOI] [PubMed] [Google Scholar]
- Latini R, Masson S, Anand I, et al. Effects of valsartan on circulating brain natriuretic peptide and norepinephrine in symptomatic chronic heart failure:the Valsartan Heart Failure Trial (Val-HeFT) Circulation. 2002;106:2454–8. doi: 10.1161/01.cir.0000036747.68104.ac. [DOI] [PubMed] [Google Scholar]
- Maggioni AP, Anand I, Gottlieb SO, et al. Effects of valsartan on morbidity and mortality in patients with heart failure not receiving angiotensin-converting enzyme inhibitors. J Am Coll Cardiol. 2002;40:1414–21. doi: 10.1016/s0735-1097(02)02304-5. [DOI] [PubMed] [Google Scholar]
- Mankad S, D’Amato TA, Reichek N, et al. Combined angiotensin II receptor antagonism and angiotensin-converting enzyme inhibition further attenuates postinfarction left ventricular remodeling. Circulation. 2001;103:2845–50. doi: 10.1161/01.cir.103.23.2845. [DOI] [PubMed] [Google Scholar]
- McMurray JJ, Pfeffer MA, Swedberg K, et al. Which inhibitor of the renin-angiotensin system should be used in chronic heart failure and acute myocardial infarction? Circulation. 2004;110:3281–8. doi: 10.1161/01.CIR.0000147274.83071.68. [DOI] [PubMed] [Google Scholar]
- McMurray JJV, Ostergren J, Swedberg K, et al. Effects of candesartan in patients with chronic heart failure and reduced left-ventricular systolic function taking angiotensin-converting-enzyme inhibitors:the CHARM-Added trial. Lancet. 2003;362:767–71. doi: 10.1016/S0140-6736(03)14283-3. [DOI] [PubMed] [Google Scholar]
- Menard J, Campbell DJ, Azizi M, et al. Synergistic effects of ACE inhibition and Ang II antagonism on blood pressure, cardiac weight and renin in spontaneously hypertensive rats. Circulation. 1997;96:3072–8. doi: 10.1161/01.cir.96.9.3072. [DOI] [PubMed] [Google Scholar]
- Mitchell GF, Lamas GA, Vaughan DE, et al. Left ventricular remodeling in the year following first anterior myocardial infarction:a quantitative analysis of contractile segment length and ventricular shape. J Am Coll Cardiol. 1992;19:1136–44. doi: 10.1016/0735-1097(92)90314-d. [DOI] [PubMed] [Google Scholar]
- Pfeffer MA. ACE inhibition in acute myocardial infarction [editorial] N Engl J Med. 1995;332:118–20. doi: 10.1056/NEJM199501123320210. [DOI] [PubMed] [Google Scholar]
- Pfeffer MA, McMurray J, Leizorovicz A, et al. Valsartan in Acute Myocardial Infarction trial (VALIANT):rationale and design. Am Heart J. 2000;140:727–50. doi: 10.1067/mhj.2000.108832. [DOI] [PubMed] [Google Scholar]
- Pfeffer MA, McMurray JJV, Velazquez EJ, et al. Valsartan, captopril, or both in myocardial infarction complicated by heart failure, left ventricular dysfunction, or both [Erratum in N Engl J Med, 2004. 350:203] N Engl J Med. 2003;349:1893–906. doi: 10.1056/NEJMoa032292. [DOI] [PubMed] [Google Scholar]
- Rutherford JD, Pfeffer MA, Moye LA, et al. Effects of captopril on ischaemic events after myocardial infarction. Results of the survival and ventricular enlargement trial. SAVE investigators. Circulation. 1994;90:1731–8. doi: 10.1161/01.cir.90.4.1731. [DOI] [PubMed] [Google Scholar]
- Sioufi A, Marfil F, Jaouen A, et al. The effect of age on the pharmacokinetics of valsartan. Biopharm Drug Dispos. 1998;19:237–44. doi: 10.1002/(sici)1099-081x(199805)19:4<237::aid-bdd100>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- Spinale FG, de Gasparo M, Whitebread S, et al. Modulation of the renin-angiotensin pathway through enzyme inhibition and specific receptor blockade in pacing-induced heart failure. I. Effects on left ventricular performance and neurohormonal systems. Circulation. 1997;96:2385–96. doi: 10.1161/01.cir.96.7.2385. [DOI] [PubMed] [Google Scholar]
- Spinale FG, Mukherjee R, Iannini JP, et al. Modulation of the renin-angiotensin pathway through enzyme inhibition and specific receptor blockade in pacing-induced heart failure. II. Effects on myocyte contractile processes. Circulation. 1997;96:2397–406. doi: 10.1161/01.cir.96.7.2397. [DOI] [PubMed] [Google Scholar]
- White HD, Aylward PEG, Huang Z, et al. Mortality and morbidity remain high despite captopril and/or valsartan therapy in elderly patients with left ventricular systolic dysfunction, heart failure, or both following acute myocardial infarction:results from the Valsartan In Acute Myocardial Infarction (VALIANT) trial. Circulation. 2005;112:3391–9. doi: 10.1161/CIRCULATIONAHA.105.551143. [DOI] [PubMed] [Google Scholar]
- Zisman LS, Asano K, Dutcher DL, et al. Differential regulation of cardiac angiotensin converting enzyme binding sites and AT1 receptor density in the failing human heart. Circulation. 1998;98:1741. doi: 10.1161/01.cir.98.17.1735. [DOI] [PubMed] [Google Scholar]