Abstract
Appropriate tools are necessary for predicting cardiovascular events in patients with diabetes mellitus because of their high incidence. In this study, we assessed whether a combination of brain natriuretic peptide (BNP) and C-reactive protein (CRP) measurement were useful prognosticators in patients with type 2 diabetes mellitus. One hundred and nine patients with type 2 diabetes mellitus, aged 52 to 93 years, were examined at outpatient clinics for blood, urinary samples, and echocardiography. They were then followed prospectively. During the average follow-up period of 30 months (range, 3 to 37), 15 patients (14%) had cardiovascular events: This was the first event in 5 patients and a recurrence in 10. Cox regression analysis showed that the past event (hazard ratio [HR] 4.819 [95% confidence interval (CI): 1.299–17.881]; p = 0.019) and plasma BNP level (HR 1.007 [95% CI: 1.002–1.012]; p = 0.010] were independently significant factors for the cardiovascular events during the follow-up period. Patients with plasma BNP ≥53 pg/mL and CRP ≥0.95 mg/dL demonstrated the highest incidence in cardiovascular event, compared to those categorized into either or both low levels of BNP and CRP. This study suggests that combination of plasma BNP and CRP measurement provides the additive prognostic information of cardiovascular events in patients with type 2 diabetes mellitus.
Keywords: diabetes mellitus, natriuretic peptide, inflammation, mortality and morbidity
Introduction
Cardiovascular disease is the leading cause of morbidity and mortality in diabetic patients (ADA et al 1999). Diabetes mellitus has been reported to be associated with coronary artery disease, left ventricular (LV) hypertrophy, and systolic and/or diastolic dysfunction (Poirier et al 2001). A recent study showed that 14% of patients with type 2 diabetic mellitus develop congestive heart failure (Nichols et al 2004). Accordingly, the risk of cardiovascular diseases in diabetic patients is two- to three-fold that among non-diabetic population (Garcia et al 1974). As the symptoms diabetic patients complain of are often discordant with the severity of myocardial ischemia and dysfunction, we need reliable screening methods for cardiac function and for predicting cardiovascular events to reduce the morbidity and mortality (Janand-Delenne et al 1999; Struthers and Morris 2002; Fang et al 2005).
Brain natriuretic peptide (BNP), a 32-amino-acid hormone, is synthesized mainly in the cardiac myocardium in response to ventricular dilation and pressure overload (Yoshimura et al 1993; Maeda et al 1998). The plasma levels of BNP are elevated in patients with congestive heart failure in proportion to the degree of LV dysfunction and the severity of symptoms (Tsutamoto et al 1997). In addition, the plasma level of BNP has been reported to independently predict cardiovascular events and death, even in patients without heart failure (de Lemos et al 2001; Wang et al 2004). Thus, measuring the plasma BNP concentration has been recognized as clinically useful for screening the heart diseases with a cost benefit value (Ogawa et al 2002). C-reactive protein (CRP), an acute phase reactant produced in the liver, is increased in inflammatory states. Epidemiological studies have shown that minor CRP elevation is associated with the major cardiovascular risk (Ridker et al 2000; Danesh et al 2004; Wakugawa et al 2006) and lowering of CRP levels by pharmacological intervention is beneficial for the secondary prevention of coronary artery disease (Ridker et al 2005). Furthermore, accumulating evidence suggests a link between hyperglycemia and inflammation in diabetic patients (Schulze et al 2004).
Although BNP and CRP measurements are recognized for their usefulness for detecting and predicting cardiovascular events, their utility has not reached its maximum potential. Therefore, we hypothesized whether the blood levels of the two molecules provide the additive value for predicting cardiovascular events in patients with type 2 diabetes mellitus.
Methods
Study population
This study was approved by the Human Investigation Review Committee of the University of Miyazaki (No. 126) and conformed with the principles outlined in the Declaration of Helsinki (Cardiovasc Res 1997; 35: 2–4). Written informed consent was obtained from all participants. One hundred and nine patients with type 2 diabetes mellitus (71 ± 10 years old; male, 50%) were recruited from November 2003 to May 2005 at the outpatient clinics of University of Miyazaki and affiliated hospitals. The cardiologists examined whether they had cardiovascular diseases such as coronary artery disease, heart failure, stroke and transient ischemia attack. Out of 109 patients, 29 of them had the cardiovascular disease; 11 stroke, 2 transient ischemia attack, 3 angina pectoris, 6 myocardial infarction, and 7 patients had congestive heart failure. All patients had been stable within 3 months prior to entry of this study. Diabetes mellitus was defined according to the statement of the American Diabetes Association: fasting plasma glucose level ≥126 mg/dL, two-hour postprandial glucose ≥200 mg/dL, or receiving any antidiabetes medicine. Hypertension was defined as systolic blood pressure ≥140 mmHg, diastolic blood pressure ≥90 mm Hg or taking any antihypertensive medicine and hyperlipidemia as fasting plasma cholesterol level ≥220 mg/dL, triglyceride ≥150 mg/dL, or taking any medicine for hyperlipidemia. Insulin resistance was assessed by the homeostasis model assessment (HOMA)-R index calculated using the formula of fasting serum insulin (mU/L) × fasting plasma glucose (mg/dL)/405. Subjects with inflammatory diseases such as infection or rheumatoid arthritis were excluded from this study.
Laboratory parameters
All samples were obtained at the outpatient clinics in the morning. At initial evaluations, 5 ml of blood sample was collected with EDTA-2Na (1.5 mg/mL blood). Plasma BNP levels were measured using an immunoradiometric assay or chemiluminescence enzyme immunoassay (Shionoria BNP or MI02 Shionogi BNP; Shionogi Inc., Osaka, Japan); identical values of BNP levels were obtained by these two methods (r = 0.981). Serum levels of CRP were determined by the latex agglutination test with a measurement range of 0.01 to 40 mg/dL. The level of HbA1c was measured by the immuno-turbidimetric method or high performance liquid chromatography (ARKRAY HA-8160), and total cholesterol and triglyceride by an enzymatic method. The first, morning-voided urine was collected and the urinary level of albumin was analyzed by immuno-turbidity method using an anti-human albumin antibody.
Echocardiography
Two-dimensional, M-mode and color flow Doppler echocardiograms were obtained with an instrument operating at 2.5 MHz. Two-dimensional imaging was performed in a standard fashion with the parasternal long- or short-axis and apical two- or four-chamber views. Left atrial and LV end-diastolic or systolic dimensions were obtained by an M-mode view. After careful analysis of regional contractile abnormalities, LV ejection fraction was determined by either biplane apical views with a modified Simpson’s method or the formula of (LV end-diastolic dimension)2-(LV systolic dimension)2/(LV end-diastolic dimension)2 when the wall motion was normal. LV mass index was estimated by its dimension and wall thickness according to the method of Devereux and associates (1986). To determine the ratio of E to A velocity and deceleration time, a 4 × 4 mm sample volume was placed at the tips of the mitral leaflets and the transmitral pulsed Doppler waves were recorded at three consecutive cardiac cycles.
Endpoint
The participants were monitored regularly for occurrence of the cardiovascular events by examining the clinical records and files. The cardiovascular events were sudden death, heart failure, unstable angina pectoris, myocardial infarction, and stroke. The criteria used to diagnose the cardiovascular events are as follows: heart failure, according to Framingham Heart Study congestive heart failure criteria (Margolis et al 1974); acute myocardial infarction, typical electrocardiographic changes and elevated cardiac enzyme levels; stroke, a neurological deficit with sudden or rapid onset that persisted for 24 hours or longer. The diagnosis of angina pectoris was confirmed by coronary angiography showing atherosclerotic narrowing of coronary arteries.
Statistical analysis
Data were expressed as mean ± SD or median with 25 and 75 percentiles. Unadjusted and adjusted association of BNP or CRP with the other variables was evaluated using Spearman’s correlation coefficient and linear regression, respectively. For adjusted association, a model with parameters significantly correlated by the unadjusted association was fitted, and then the other variables were added to see if there was significant residual association. A Cox proportional hazards model was used to test the significance of any variables as predictors of the cardiovascular events in the study subjects. The covariates included in this model were history of cardiovascular events, age, gender, current smoking, body mass index, HOMA-R index, systolic blood pressure, total cholesterol, serum creatinine, urinary albumin, BNP, CRP, LV ejection fraction and medicines, such as angiotensin converting enzyme inhibitor, angiotensin II type 1 receptor blocker, β-blocker, aspirin, thiazolidinedione, and statin. Results are presented as relative risks with 95% confidence intervals. Receiver-operating characteristic (ROC) curves were constructed to examine the predictive values of plasma BNP and CRP concentrations for the cardiovascular events. SPSS version 11.0 (SPSS Japan, Tokyo, Japan) was used for all the analyses, and statistical significance was accepted at p < 0.05.
Results
Patients’ characteristics
Baseline of the clinical characteristics and echocardiographic parameters of the study participants are shown in Tables 1 and 2, respectively. Plasma BNP level was distributed between 1.8 and 526.6 pg/mL, and 15.6% of the patients exceeded 100 pg/mL. Plasma CRP level was distributed between 0.00 and 2.17 mg/dL, and 39% of those exceeded 0.1 mg/dL. Twenty eight percent of patients had received angiotensin converting enzyme inhibitor or angiotensin II type 1 receptor blocker. By echocardiogram, only 1.9% of patients had LV ejection fractions less than 40%. Table 3 shows the variables considered as potentially associated with BNP or CRP. Several parameters, such as age, HOMA-R, creatinine, LA size, LV mass index, and LV ejection fraction were associated with BNP. CRP was associated with metabolic parameters, such as BMI, HOMA-R and HDL-C level as well as urinary albumin, LA size, and EV ejection fraction. As shown in Table 4, BNP was independently associated with LA size, LV ejection fraction, gender, urinary albumin, serum creatinine, and BMI. Meanwhile, CRP was independently associated with LV ejection fraction and HDL-C.
Table 1.
Clinical characteristics of the patients (n = 109)
| Parameter | |
|---|---|
| Age, y | 71 ± 10 |
| Male (%) | 50 |
| Body mass index (kg/m2) | 24 ± 4 |
| Current smoker (%) | 17 |
| Hypertension (%) | 77 |
| Hyperlipidemia (%) | 53 |
| Atrial fibrillation (%) | 6 |
| Valvular diseases (%) | 10 |
| History of cardiovascular diesases (N/C/H) | 80/13/16 |
| Systolic blood pressure (mmHg) | 137 ± 16 |
| Diastolic blood pressure (mmHg) | 71 ± 11 |
| Fasting blood glucose (mg/dL) | 137 ± 44 |
| Hemoglobin A1c (%) | 6.6 ± 1.5 |
| Total cholesterol (mg/dL) | 204 ± 35 |
| Triglyceride (mg/dL) | 113 [86, 158] |
| Serum creatinine (mg/dL) | 0.8 ± 0.3 |
| Urinary albumin (mg/gCreatinine) | 33 [13, 139] |
| BNP (pg/mL) | 26.7 [14.3, 57.2] |
| CRP (mg/dL) | 0.10 [0.04, 0.20] |
| Sulfonylurea (%) | 48 |
| Biguanide (%) | 3 |
| α-glycosidase inhibitor (%) | 34 |
| thiazolidinedione (%) | 6 |
| Glinide (%) | 8 |
| ACEI (%) | 17 |
| ARB (%) | 11 |
| β-blocker (%) | 16 |
| Calcium channel blocker (%) | 55 |
| Diuretics (%) | 9 |
| Aspirin (%) | 13 |
| Statin (%) | 24 |
Notes: Data are presented as means ± SD, percentage or median [25, 75 percentiles] of the values.
Abbreviations: ACEI, angiotensin converting enzyme inhibitor; ARB, angiotensin II type 1 receptor blocker; N, C and H indicates none of any past cardiovascular diseases, cerebral infarction/hemorrhage or heart diseases, such as angina pectoris, myocardial infarction and heart failure.
Table 2.
Echocardiographic parameters
| Size of left atrium (cm) | 3.2 ± 0.7 |
| Left ventricular diastolic dimension (cm) | 4.2 ± 0.8 |
| Left ventricular systolic dimension (cm) | 2.7 ± 0.6 |
| Left ventricular mass (g/m2) | 132 ± 46 |
| Left ventricular ejection fraction (%) | 66 ± 10 |
| Ratio of E to A velocity | 0.9 ± 0.5 |
| Deceleration time (ms) | 211 ± 63 |
Values are means ± SD
Table 3.
Association of BNP or CRP with the other variables
| BNP | CRP | |||
|---|---|---|---|---|
| Spearman CC | p value | Spearman CC | p value | |
| Age | 0.445 | <0.001 | 0.049 | 0.61 |
| BMI | −0.079 | 0.411 | 0.370 | <0.001 |
| HOMA-R | −0.206 | 0.033 | 0.254 | 0.008 |
| HDL-C | −0.136 | 0.158 | −0.260 | 0.006 |
| Urinary albumin | 0.130 | 0.18 | 0.224 | 0.02 |
| Creatinine | 0.306 | 0.001 | 0.119 | 0.219 |
| LA size | 0.268 | 0.006 | 0.206 | 0.038 |
| LV mass index | 0.330 | 0.001 | 0.121 | 0.23 |
| LVEF | −0.231 | 0.018 | −0.212 | 0.031 |
Abbreviations: CC, correlation coefficient; BMI, body mass index; HOMA, homeostasis model assessment; LA, left atrial; LV, left ventricular; EF, ejection fraction.
Table 4.
Linear regression analysis for BNP and CRP
| Dependent variable | Independent varible | Regression coefficient | p value |
|---|---|---|---|
| BNP | LA size | 0.321 | <0.001 |
| LVEF | −0.282 | 0.001 | |
| Gender | 0.334 | 0.001 | |
| Urinary albumin | 2.875 | 0.005 | |
| Serum creatinine | 2.751 | 0.007 | |
| BMI | −0.179 | 0.037 | |
| CRP | LVEF | −3.338 | 0.001 |
| HDL-C | −2.682 | 0.009 |
Note: For BNP, age, gender, body mass index (BMI), urinary albumin, serum creatinine, left atrial (LA) size, left ventricular ejection fraction (LVEF), LV mass index and CRP were included as the independent variables. For CRP, age, BMI, homeostasis model assessment (HOMA)-R, plasma levels for total cholesterol, triglyceride, HDL-C and LVEF were included as independent variables.
Occurrence of cardiovascular events
During the average follow-up period of 30 months (range, 3 to 37), 15 patients (14%) had cardiovascular events. Five patients had the first events of unstable angina pectoris in 1, acute myocardial infarction in 2, and congestive heart failure in 2. Ten patients had recurrences: sudden death in 1 and ventricular fibrillation in 1, and hospitaization due to congestive heart failure in 4, cerebral infarction in 3 and unstable angina pectoris in 1.
Univariate and multivariate regression analyses
Table 5 shows the results of univariate and multivariate Cox regression analyses using step-wise variable selection. In univariate regression analysis, the history of the cardiovascular events, BNP, LV ejection fraction, use of ACE inhibitor, serum creatinine, and CRP level were extracted as significant factors for the events during the follow-up period. In multivariate analysis, the history and BNP remained independent.
Table 5.
Univariate and multivariate cox regression analysis
| Univariate | Multivariate | |||||
| Variables | HR | 95% CI | HR | 95% CI | ||
| Past event | 8.535 | 2.997–24.32 | <0.001 | 4.819 | 1.299–17.881 | 0.019 |
| BNP(10 pg/mL) | 1.008 | 1.005–1.011 | <0.001 | 1.007 | 1.002–1.012 | 0.01 |
| LVEF (%) | 0.92 | 0.881–0.960 | <0.001 | |||
| ACEI use | 0.201 | 0.077–0.522 | <0.001 | |||
| Creatinine(mg/dL) | 7.846 | 2.723–22.60 | <0.001 | |||
| CRP(0.1 mg/dL) | 3.002 | 1.416–6.364 | 0.004 | |||
Note: Univariate and multivariate models were constructed by Cox regression with forward step wise variable selection. Covariates included in the analysis were as follows: age, gender, body mass index, history of past cardiovascular event, hypertension, hyperlipidemia, fast blood sugar, HbA1c, urinary albumin, BNP, serum creatinine, CRP and LV ejection fraction (LVEF), LV mass index, use of angiotensin converting enzyme inhibitor (ACEI), angiotensin II type 1 receptor blocker, β-blocker, statin, thiazolidinedione and/or aspirin. Hazard ratios (HR) are for an increment in the unit or number shown in parenthesis.
Identification of subgroups of patients with high risk of events
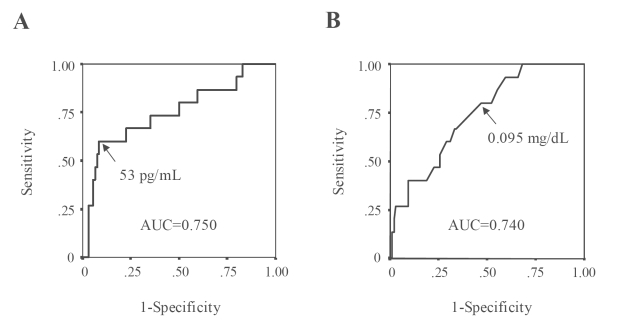
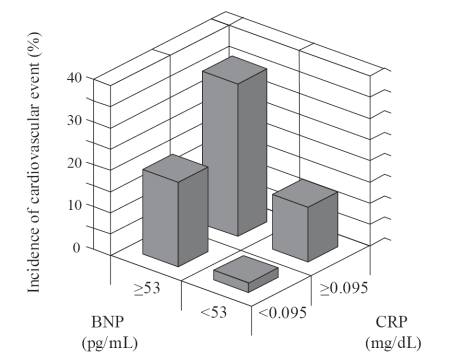
Figure 1 shows the sensitivity and specificity of various cut-off points of BNP or CRP for predicting the cardiovascular events by ROC curves. On each curve, the potential optimal point was indicated. According to the basis on the optimal values of BNP and CRP, the patients were divided into four categories; group I, BNP <53 pg/mL and CRP <0.095 mg/dL; group II, BNP ≥53 pg/mL and CRP ≥0.095 mg/dL; group III, BNP<53 pg/mL and CRP ≥0.095 mg/dL; group IV, BNP ≥53 pg/mL and CRP ≥ 0.095 mg/dL. With this combination, patients with BNP ≥53 pg/mL and CRP ≥0.095 mg/dL had the highest incidence of cardiovascular event among the respective groups (Figure 2).
Figure 1.
ROC curves and the sensitivity/specificity of BNP (A) or CRP (B) level for predicting the cardiovascular events in diabetic patients. Areas under the ROC curve (AUC) for BNP or CRP are shown in the diagrams.
Figure 2.
Incidence of cardiovascular events after 30 months of follow-up by categories of BNP and CRP at baseline. Group I (n = 45), BNP <53 pg/mL and CRP <0.095 mg/dL; group II (n = 10), BNP ≥53 pg/mL and CRP <0.095 mg/dL; group III (n = 32), BNP <53 pg/mL and CRP ≥0.095 mg/dL; group IV (n = 22), BNP ≥53 pg/mL, and CRP ≥0.095 mg/dL.
Discussion
Once patients with diabetes mellitus develop clinically overt coronary artery disease, their prognosis is particularly poor irrespective of the control of blood glucose levels (UK Prospective Diabetes Study Group 1998; Howard et al 2006). As the prevalence of underlying coronary artery disease, stroke and heart failure in diabetic patients is higher than in the non-diabetic population (Sprafka et al 1991), screening and assessment of cardiovascular risk in diabetic patients are of importance.
Firstly, this study shows the significance of understanding medical history in the diabetic patients, as those with a past event had a high incidence of recurrence. Indeed, it was the strongest independent factor for predicting the events during the follow-up period. In patients enrolled, 5.6% developed the first event, while 34% for the recurrence. It seems that these patients had a high occurrence of cardiovascular events in this study. These results are reported to be dependent on the study population, with the range between 10%–22% during patient follow-up (Booth et al 2006; Yoshimura et al 2006; Wilcox et al 2007). It is of note that cardiovascular event increases up to 50% in those who had suffered from myocardial infarction (Murcia et al 2004). Age is also strong risk factor for cardiovascular disease in people with diabetes, especially in those that have a history of myocardial infarction (Booth et al 2006). Blockade of angiotensin II with the angiotensin converting enzyme inhibitor or angiotensin II type 1 receptor blocker seems to be beneficial among patients with diabetes mellitus in reducing subsequent risks of cardiovascular events. However this was not frequently within this study. Thus, our data demonstrates that elderly patients with diabetes mellitus are inclined to experience cardiovascular events. In order to decrease the likelihood of future events, more accurate diagnostic tools to assess the risk and intensive medical treatments would be necessary.
Secondly, this data is evidence that the plasma level of BNP was useful parameter to predict the future event in diabetic patients. The increased level of plasma BNP was associated with enlarged size in LA and reduced systolic function in LV. This supports the notion that the structural or functional changes of heart are important determinants for BNP production (Maeda et al 1998; de Lemos et al 2001).
According to Dawson and colleagues (2005), increased plasma BNP concentration is associated with cardiovascular risk even in subjects with mean BNP levels lower than 30 pg/mL, who had normal LV systolic functions and no history of coronary heart disease. Epsteyn and colleagues (2003) report the extended utility of BNP measurement for screening the latent LV dysfunction in patients with diabetes mellitus. On the other hand, CRP has been recognized as a factor closely associated with the development of cardiovascular diseases (Ridker et al 2000; Danesh et al 2004; Wakugawa et al 2006) and in line with this, high CRP levels indicate an increased risk of cardiovascular events among men with type 2 diabetes mellitus (Schulze et al. 2004). Meanwhile, Howard and colleagues (2006) reported that the risk of cardiovascular events in diabetic patients depends on concomitant metabolic disorders. In line with the previous studies (Shah et al 2006; Zuliani et al 2007), our data showed that the plasma level of CRP was independently associated with low HDL-C and LV ejection.
As BNP levels were independently affected by age, gender and renal function, clinical settings to screen the LV dysfunction might be limited. In addition, BNP levels are believed to increase with thiazolidinedione treatment (Ogawa et al 2003), however the medication had little influence on the predictive value of BNP in this study (data not shown). On the other hand, CRP is not disease-specific, and its concentration might elevate as a consequence of infection, inflammation and undefined reasons as well as atherosclerosis-related risk factors (Miller et al 2007). At this point, it remains to be elucidated whether measurement of CRP can be useful in general clinical practice to evaluate the risk of cardiovascular diseases. Regardless of these limitations, this study shows that elevation in the plasma levels of either BNP or CRP revealed the higher incidence of morbidity, compared with the patient category with low concentration of both markers. Importantly, patients categorized into both high-BNP and -CRP levels demonstrated the highest incidence in the cardiovascular event. Our findings are supported by two studies in which the combined measurements of N-terminal proBNP (or BNP) and CRP have been reported to provide an additive predictive value in patients with stable coronary heart disease (Ndrepepa et al 2006) and dilated cardiomyopathy (Ishikawa et al 2006). Taken together, this data supports and extends the hypothesis that elevated BNP and CRP levels reflect an increased risk of cardiovascular events. Therefore the combined use of BNP and CRP measurements would provide better identification of cardiovascular risk in patients with type 2 diabetes mellitus, compared with the use of each alone. BNP levels may reflect alterations in cardiac structure and function, while CRP might be associated with subtle vascular inflammation. A number of studies have shown the utility of BNP and CRP as diagnostic markers for screening cardiovascular risk to recognize the disease progression and to see the effect of medical treatment. However, the information is still too limited to use BNP and CRP as diagnostic markers regularly, for example to measure the levels of blood sugar and HbA1c, in diabetic patients. Further studies are necessary to determine whether BNP and CRP levels are useful for monitoring the occult cardiac dysfunction and vascular inflammation in general clinical practice.
This was an observation study in which a small number of participants were examined, and the hypothesis raised in this study may need to be tested in a larger number of patients with longer study periods. Also, because the average age was greater than seventy years, we did not assess the physical activity/daily exercise, which has been reported to be associated with mortality in men with diabetes mellitus (Wei et al 2000). Furthermore, threshold values obtained from the ROC curves are dependent on the study population, and might have a different optimal point in another set of patients.
Acknowledgments
The authors are greatly thankful to Drs. Shigeru Nakamura, Naozumi Otsuka, Masashi Seita for helping to recruit the patients.
References
- [ADA] American Diabetes Association, The National Heart, Lung, and Blood Institute, the juvenile diabetes foundation international, et al. Diabetes mellitus: a major risk factor for cardiovascular disease. A joint editorial statement by the american diabetes association; The national heart, lung, and blood institute; The juvenile diabetes foundation international; The national institute of diabetes and digestive and kidney diseases; and The american heart association. Circulation. 1999;100:1132–3. doi: 10.1161/01.cir.100.10.1132. [DOI] [PubMed] [Google Scholar]
- Booth GL, Kapral MK, Fung K, et al. Relation between age and cardiovascular disease in men and women with diabetes compared with non-diabetic people: a population-based retrospective cohort study. Lancet. 2006;368:29–36. doi: 10.1016/S0140-6736(06)68967-8. [DOI] [PubMed] [Google Scholar]
- Danesh J, Wheeler JG, Hirschfield GM, et al. C-reactive protein and other circulating markers of inflammation in the prediction of coronary heart disease. N Engl J Med. 2004;350:1387–97. doi: 10.1056/NEJMoa032804. [DOI] [PubMed] [Google Scholar]
- Dawson A, Jeyaseelan S, Morris AD, et al. B-type natriuretic peptide as an alternative way of assessing total cardiovascular risk in patients with diabetes mellitus. Am J Cardiol. 2005;96:933–4. doi: 10.1016/j.amjcard.2005.05.050. [DOI] [PubMed] [Google Scholar]
- de Lemos JA, Morrow DA, Bentley JH, et al. The prognostic value of B-type natriuretic peptide in patients with acute coronary syndromes. N Engl J Med. 2001;345:1014–21. doi: 10.1056/NEJMoa011053. [DOI] [PubMed] [Google Scholar]
- Devereux RB, Alonso DR, Lutas EM, et al. Echocardiographic assessment of left ventricular hypertrophy: comparison to necropsy findings. Am J Cardiol. 1986;57:450–8. doi: 10.1016/0002-9149(86)90771-x. [DOI] [PubMed] [Google Scholar]
- Epshteyn V, Morrison K, Krishnaswamy P, et al. Utility of B-type natriuretic peptide as a screen for left ventricular dysfunction in patients with diabetes. Diabetes Care. 2003;26:2081–7. doi: 10.2337/diacare.26.7.2081. [DOI] [PubMed] [Google Scholar]
- Fang ZY, Schull-Meade R, Leano R, et al. Screening for heart disease in diabetic subjects. Am Heart J. 2005;149:349–54. doi: 10.1016/j.ahj.2004.06.021. [DOI] [PubMed] [Google Scholar]
- Garcia MJ, McNamara PM, Gordon T, et al. Morbidity and mortality in diabetics in the Framingham population. Sixteen year follow-up study. Diabetes. 1974;23:105–11. doi: 10.2337/diab.23.2.105. [DOI] [PubMed] [Google Scholar]
- Howard BV, Best LG, Galloway JM, et al. Coronary heart disease risk equivalence in diabetes depends on concomitant risk factors. Diabetes Care. 2006;29:391–7. doi: 10.2337/diacare.29.02.06.dc05-1299. [DOI] [PubMed] [Google Scholar]
- Ishikawa C, Tsutamoto T, Fujii M, et al. Prediction of mortality by high-sensitivity C-reactive protein and brain natriuretic peptide in patients with dilated cardiomyopathy. Circ J. 2006;70:857–63. doi: 10.1253/circj.70.857. [DOI] [PubMed] [Google Scholar]
- Janand-Delenne B, Savin B, Habib G, et al. Silent myocardial ischemia in patients with diabetes: who to screen. Diabetes Care. 1999;22:1396–1400. doi: 10.2337/diacare.22.9.1396. [DOI] [PubMed] [Google Scholar]
- Maeda K, Tsutamoto T, Wada A, et al. Plasma brain natriuretic peptide as a biochemical marker of high left ventricular end-diastolic pressure in patients with symptomatic left ventricular dysfunction. Am Heart J. 1998;135:825–32. doi: 10.1016/s0002-8703(98)70041-9. [DOI] [PubMed] [Google Scholar]
- Margolis JR, Gillum RF, Feinleib M, et al. Community surveillance for coronary heart disease: the Framingham Cardiovascular Disease Survey. Methods and preliminary results. Am J Epidemiol. 1974;100:425–36. doi: 10.1093/oxfordjournals.aje.a112054. [DOI] [PubMed] [Google Scholar]
- Miller VM, Redfield MM, McConnell JP. Use of BNP and CRP as biomarkers in assessing cardiovascular disease: diagnosis versus risk. Curr Vasc Pharmacol. 2007;5:15–25. doi: 10.2174/157016107779317251. [DOI] [PubMed] [Google Scholar]
- Murcia AM, Hennekens CH, Lamas GA, et al. Impact of diabetes on mortality in patients with myocardial infarction and left ventricular dysfunction. Arch Intern Med. 2004;164:2273–9. doi: 10.1001/archinte.164.20.2273. [DOI] [PubMed] [Google Scholar]
- Ndrepepa G, Kastrati A, Braun S, et al. N-terminal probrain natriuretic peptide and C-reactive protein in stable coronary heart disease. Am J Med. 2006;119:e1–8. doi: 10.1016/j.amjmed.2005.10.060. [DOI] [PubMed] [Google Scholar]
- Nichols GA, Gullion CM, Koro CE, et al. The incidence of congestive heart failure in type 2 diabetes: an update. Diabetes Care. 2004;27:1879–84. doi: 10.2337/diacare.27.8.1879. [DOI] [PubMed] [Google Scholar]
- Ogawa K, Oida A, Sugimura H, et al. Clinical significance of blood brain natriuretic peptide level measurement in the detection of heart disease in untreated outpatients: comparison of electrocardiography, chest radiography and echocardiography. Circ J. 2002;66:122–6. doi: 10.1253/circj.66.122. [DOI] [PubMed] [Google Scholar]
- Ogawa S, Takeuchi K, Ito S. Plasma BNP levels in the treatment of type 2 diabetes with pioglitazone. J Clin Endocrinol Metab. 2003;88:3393–6. doi: 10.1210/jc.2002-021765. [DOI] [PubMed] [Google Scholar]
- Poirier P, Bogaty P, Garneau C, et al. Diastolic dysfunction in normotensive men with well-controlled type 2 diabetes: importance of maneuvers in echocardiographic screening for preclinical diabetic cardiomyopathy. Diabetes Care. 2001;24:5–10. doi: 10.2337/diacare.24.1.5. [DOI] [PubMed] [Google Scholar]
- Ridker PM, Cannon CP, Morrow D, et al. C-reactive protein levels and outcomes after statin therapy. N Engl J Med. 2005;352:20–8. doi: 10.1056/NEJMoa042378. [DOI] [PubMed] [Google Scholar]
- Ridker PM, Hennekens CH, Buring JE, et al. C-reactive protein and other markers of inflammation in the prediction of cardiovascular disease in women. N Engl J Med. 2000;342:836–43. doi: 10.1056/NEJM200003233421202. [DOI] [PubMed] [Google Scholar]
- Schulze MB, Rimm EB, Li T, et al. C-reactive protein and incident cardiovascular events among men with diabetes. Diabetes Care. 2004;27:889–94. doi: 10.2337/diacare.27.4.889. [DOI] [PubMed] [Google Scholar]
- Shah SJ, Marcus GM, Gerber IL, et al. High-sensitivity C-reactive protein and parameters of left ventricular dysfunction. J Card Fail. 2006;12:61–5. doi: 10.1016/j.cardfail.2005.08.003. [DOI] [PubMed] [Google Scholar]
- Sprafka JM, Burke GL, Folsom AR, et al. Trends in prevalence of diabetes mellitus in patients with myocardial infarction and effect of diabetes on survival. The Minnesota Heart Survey. Diabetes Care. 1991;14:537–43. doi: 10.2337/diacare.14.7.537. [DOI] [PubMed] [Google Scholar]
- Struthers AD, Morris AD. Screening for and treating left-ventricular abnormalities in diabetes mellitus: a new way of reducing cardiac death. Lancet. 2002;359:1430–2. doi: 10.1016/S0140-6736(02)08358-7. [DOI] [PubMed] [Google Scholar]
- Tsutamoto T, Wada A, Maeda K, et al. Attenuation of compensation of endogenous cardiac natriuretic peptide system in chronic heart failure: prognostic role of plasma brain natriuretic peptide concentration in patients with chronic symptomatic left ventricular dysfunction. Circulation. 1997;96:509–16. doi: 10.1161/01.cir.96.2.509. [DOI] [PubMed] [Google Scholar]
- UK Prospective Diabetes Study Group. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33) Lancet. 1998;352:837–53. [PubMed] [Google Scholar]
- Wakugawa Y, Kiyohara Y, Tanizaki Y, et al. C-reactive protein and risk of first-ever ischemic and hemorrhagic stroke in a general Japanese population: the Hisayama Study. Stroke. 2006;37:27–32. doi: 10.1161/01.STR.0000194958.88216.87. [DOI] [PubMed] [Google Scholar]
- Wang TJ, Larson MG, Levy D, et al. Plasma natriuretic peptide levels and the risk of cardiovascular events and death. N Engl J Med. 2004;350:655–63. doi: 10.1056/NEJMoa031994. [DOI] [PubMed] [Google Scholar]
- Wei M, Gibbons LW, Kampert JB, et al. Low cardiorespiratory fitness and physical inactivity as predictors of mortality in men with type 2 diabetes. Ann Intern Med. 2000;132:605–11. doi: 10.7326/0003-4819-132-8-200004180-00002. [DOI] [PubMed] [Google Scholar]
- Wilcox R, Bousser MG, Betteridge DJ, et al. Effects of pioglitazone in patients with type 2 diabetes with or without previous stroke: results from PROactive (PROspective pioglitAzone Clinical Trial In macroVascular Events 04) Stroke. 2007;38:865–73. doi: 10.1161/01.STR.0000257974.06317.49. [DOI] [PubMed] [Google Scholar]
- Yoshimura T, Suzuki E, Egawa K, et al. Low blood flow estimates in lower-leg arteries predict cardiovascular events in Japanese patients with type 2 diabetes with normal ankle-brachial indexes. Diabetes Care. 2006;29:1884–90. doi: 10.2337/dc06-0142. [DOI] [PubMed] [Google Scholar]
- Yoshimura M, Yasue H, Okumura K, et al. Different secretion patterns of atrial natriuretic peptide and brain natriuretic peptide in patients with congestive heart failure. Circulation. 1993;87:464–9. doi: 10.1161/01.cir.87.2.464. [DOI] [PubMed] [Google Scholar]
- Zuliani G, Volpato S, Blè A, et al. High interleukin-6 plasma levels are associated with low HDL-C levels in community-dwelling older adults: the InChianti study. Atherosclerosis. 2007;192:384–90. doi: 10.1016/j.atherosclerosis.2006.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]